Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Peptides Synthesis
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Purification of RBM Antibodies
2.5. Microscale Thermophoresis (MST)
2.6. Protein Thermal Shift Assay
2.7. Statistical Analysis
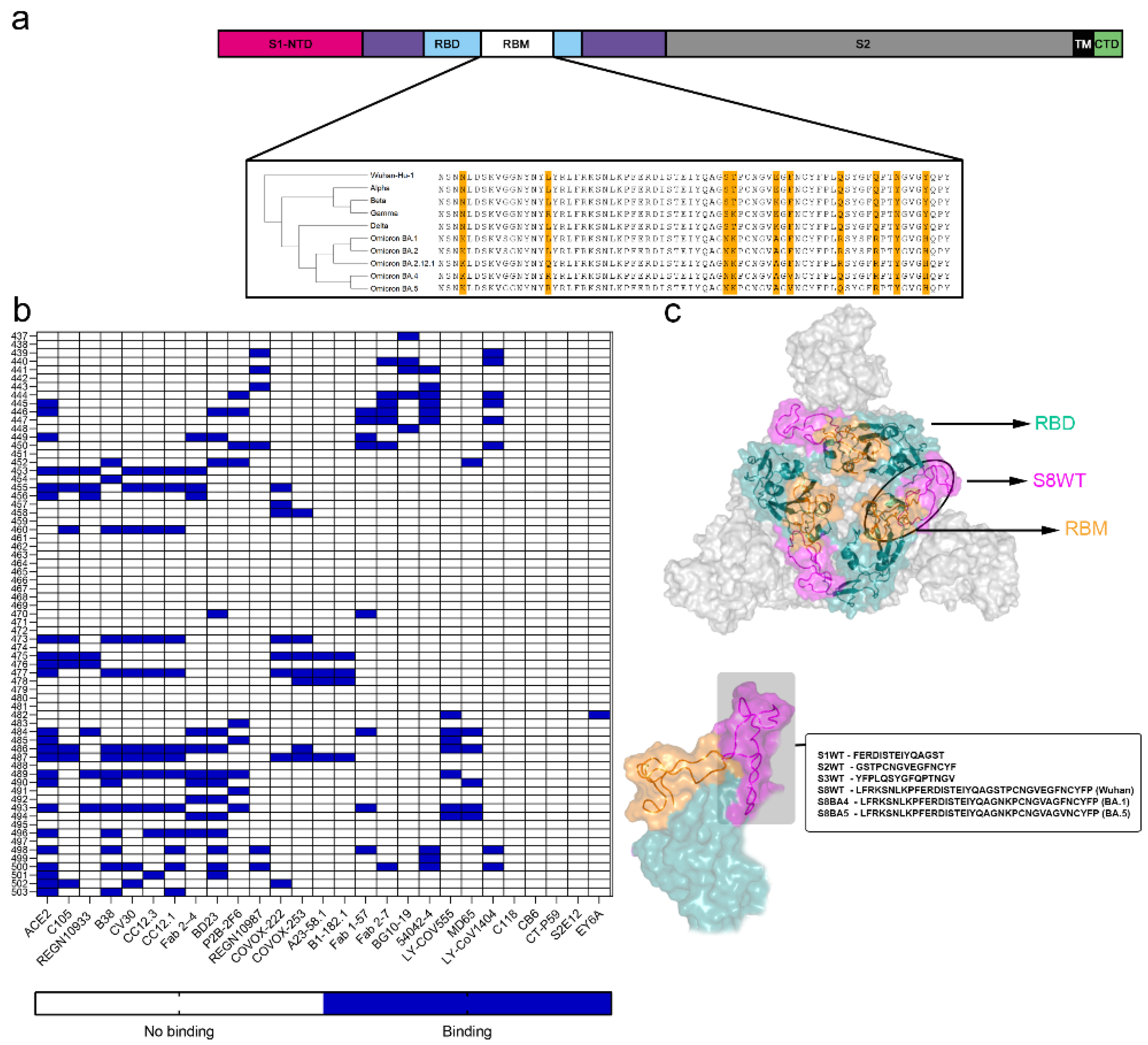
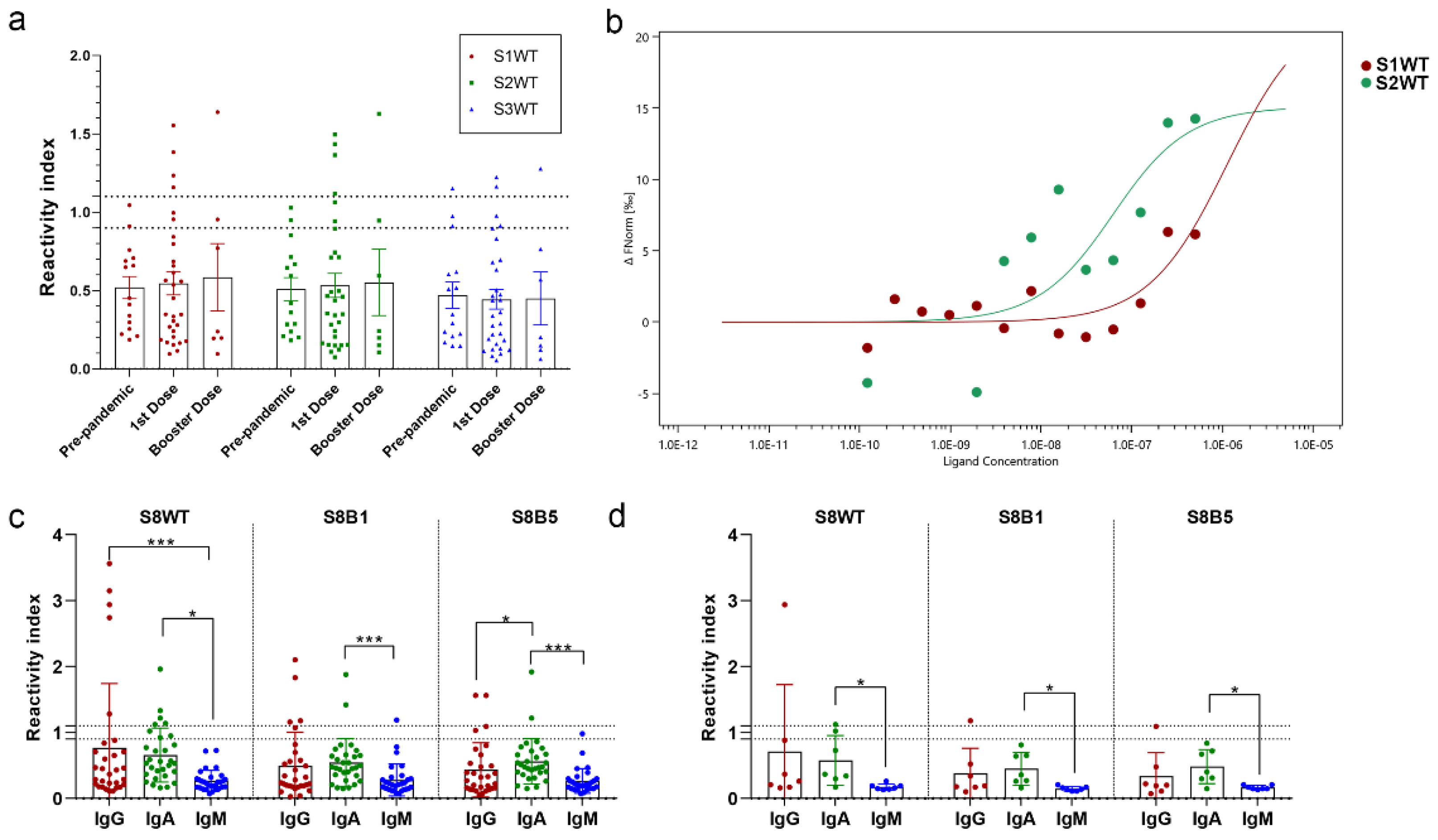
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal: M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, e7423. [CrossRef]
- Lenharo, M. WHO declares end to COVID-19’s emergency phase. Nature 2023, d41586-023-01559-z. [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021, 19, 409–424. [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A Neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [CrossRef]
- Huang, Q.; Han, X.; Yan, J. Structure-based neutralizing mechanisms for SARS-CoV-2 antibodies. Emerg Microbes Infect 2022, 11, 2412–2422. [CrossRef]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 Spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024-1042.e21. [CrossRef]
- Deshpande, A.; Harris, B.D.; Martinez-Sobrido, L.; Kobie, J.J.; Walter, M.R. Epitope classification and RBD binding properties of neutralizing antibodies against SARS-CoV-2 variants of concern. Front Immuno 2021, 12, 691715. [CrossRef]
- Gasser, R.; Cloutier, M.; Prévost, J.; Fink, C.; Ducas, É.; Ding, S.; Dussault, N.; Landry, P.; Tremblay, T.; Laforce-Lavoie, A.; et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep 2021, 34, 108790. [CrossRef]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020, 26, 845–848. [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J Allergy Clin Immunol 2010, 125, S41–S52. [CrossRef]
- Ferrante, A.; Beard, L.J.; Feldman, R.G. IgG subclass distribution of antibodies to bacterial and viral antigens. Ped Infect Dis J 1990, 9, 516.
- Korobova, Z.R.; Zueva, E.V.; Arsentieva, N.A.; Batsunov, O.K.; Liubimova, N.E.; Khamitova, I.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Savin, T.V.; Stanevich, O.V.; et al. Changes in anti-SARS-CoV-2 IgG subclasses over time and in association with disease severity. Viruses 2022, 14, 941. [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system? Biomolecules 2023, 13, 1338. [CrossRef]
- Almanza, G.; Clark, A.E.; Kouznetsova, V.; Olmedillas, E.; Castro, A.; Tsigelny, I.F.; Wu, Y.; Gao, G.F.; Leibel, S.L.; Bray, W.; et al. Structure-selected RBM immunogens prime polyclonal memory responses that neutralize SARS-CoV-2 variants of concern. PLoS Pathog 2022, 18, e1010686. [CrossRef]
- Pratesi, F.; Errante, F.; Pacini, L.; Peña-Moreno, I.C.; Quiceno, S.; Carotenuto, A.; Balam, S.; Konaté, D.; Diakité, M.M.; Aréva-lo-Herrera, M.; et al. A SARS–CoV-2 spike receptor binding motif peptide induces anti-spike antibodies in mice and is recognized by COVID-19 patients. Front. Immunol 2022, 13, 879946. [CrossRef]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat Rev Immunol 2023, 23, 189–199. [CrossRef]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 transmission. Nature 2022, 602, 294-299. [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T., Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [CrossRef]
- Bachmann, M.F.; Mohsen, M.O.; Zha, L.; Vogel, M.; Speiser, D.E. SARS-CoV-2 Structural features may explain limited neutralizing-antibody responses. npj Vaccines (Basel) 2021, 6, 1–5. [CrossRef]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; de Pina, J.S.; da Silva, F.R. Epitope mapping of the diphtheria toxin and development of an ELISA-specific diagnostic assay. Vaccines (Basel) 2021, 9, 313. [CrossRef]
- Gomes, L.R.; Durans, A.M.; Napoleão-Pêgo, P.; Waterman, J.A.; Freitas, M.S.; De Sá, N.B.R.; Pereira, L.V.; Furtado, J.S.; Aquino, R.G.; Machado, M.C.R.; et al. Multiepitope proteins for the differential detection of IgG antibodies against RBD of the spike protein and non-RBD regions of SARS-CoV-2. Vaccines (Basel) 2021, 9, 986. [CrossRef]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Bottino, C.C.G.; Pinho, R.T.; Provance-Jr, D.W.; De-Simone, S.G. Trypanosoma cruzi presenilin-like transmembrane aspartyl protease: characterization and cellular localization. Biomolecules 2020, 10, 1564. [CrossRef]
- De-Simone, S.G.; Nascimento, H.J.; Prado, I.C.; Aguiar, A.S.; Melgarejo, A.R.; Pina, J.L.S.; Ferreira, P.F.; Provance, D.W. Puri-fication of equine IgG3 by lectin affinity and an interaction analysis via microscale thermophoresis. Anal Biochem 2018, 561–562, 27–31. [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 1205–1211. [CrossRef]
- Bachmann, M.F.; Mohsen, M.O.; Zha, L.; Vogel, M.; Speiser, D.E. SARS-CoV-2 Structural features may explain limited neutralizing-antibody responses. npj Vaccines (Basel) 2021, 6, 1–5. [CrossRef]
- Li, Y.; Lai, D.; Zhang, H.; Jiang, H.; Tian, X.; Ma, M.; Qi, H.; Meng, Q.; Guo, S.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell Mol Immunol 2020, 17, 1095–1097. [CrossRef]
- Sun, C.; Xie, C.; Bu, G.-L.; Zhong, L.-Y.; Zeng, M.-S. Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants. Sig Transduct Target Ther 2022, 7, 202. [CrossRef]
- Lupala, C.S.; Ye, Y.; Chen, H.; Su, X.-D.; Liu, H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem Biophys Res Communi 2022, 590, 34–41. [CrossRef]
- Chen, W.-H.; Hotez, P.J.; Bottazzi, M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum Vaccine Immunother 2020, 16, 1239–1242. [CrossRef]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O.; et al. AP205 VLPs based on dimerized capsid proteins accommodate RBM domain of SARS-CoV-2 and serve as an attractive vaccine candidate. Vaccines (Basel) 2021, 9, 403. [CrossRef]
- Castro, A.; Ozturk, K.; Zanetti, M.; Carter, H. In silico analysis suggests less effective MHC-II Presentation of SARS-CoV-2 RBM Peptides: Implication for neutralizing antibody responses. PLoS ONE 2021, 16, e0246731. [CrossRef]
- Vainio, O.; Toivanen, P.; Toivanen, A. Major histocompatibility complex and cell cooperation. Poul Sci 1987, 66, 795–801. [CrossRef]
- Lani, R.; Senin, N.A.; AbuBakar, S.; Hassandarvish, P. Knowledge of SARS-CoV-2 epitopes and population HLA types is important in the design of COVID-19 vaccines. Vaccines (Basel) 2022, 10, 1606. [CrossRef]
- Bertinetto, F. E.; Magistroni, P.; Mazzola; G. A., Costa, C.; Elena, G.; Alizzi, S.; Scozzari, G.; Migliore, E.; Galassi, C.; Ciccone, G.; et al. The humoral and cellular response to mRNA SARS-CoV-2 vaccine is influenced by HLA polymorphisms. HLA 2023, 102, 301–315. [CrossRef]
- Mentzer, A. J.; Connor, D.; Bibi, S.; Chelysheva, I.; Clutterbuck, E.A.; Demissie, T.; Dinesh, T.; Edwards, N. J.; Felle, S.; Feng, S.; et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat Med 2023, 29, 147–157. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




