Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Participants
| VARIABLE | MANN- WHITNEY U |
STANDARD ERROR | STANDARDIZED TEST STATISTICS |
ASYMP. SIG. (2-TAILED) |
EXACT SIG. [2*(1-TAILED SIG.)] |
|---|---|---|---|---|---|
| Age | 48 | 13.05 | -.15 | .88 | .91 |
| Laterality quotient | 53.5 | 12.61 | .28 | .78 | .8 |
| Extraversion | 48 | 13.04 | -.15 | .88 | .91 |
| Agreeableness | 59 | 12.98 | .69 | .49 | .53 |
| Coscientiousness | 47 | 12.75 | -.24 | .81 | .85 |
| Emotional stability | 41 | 13.06 | -.69 | .49 | .53 |
| Openness | 34.5 | 12.97 | -1.2 | .23 | .25 |
| High Sensitive Trait | 49 | 13.22 | -.08 | .94 | .97 |
| BIS | 65.5 | 13.16 | 1.18 | .24 | .25 |
| BAS-D | 35.5 | 13.12 | -1.11 | .27 | .28 |
| BAS-FS | 42 | 13.08 | -.61 | .54 | .58 |
| BAS-RR | 40 | 13.08 | -.77 | .45 | .48 |
| Mind wandering | 62.5 | 13.19 | .95 | .34 | .35 |
| Absorption | 37.5 | 13.13 | -.95 | .34 | .35 |
2.2. Procedure

2.3. Instruments
2.3.1. Handedness
2.3.2. Personality Measures
2.3.3. Mood assessment
2.3.4. State Mind-wandering assessment
2.3.5. Pranayama technique
3. Results
3.1. Descriptive statistics
3.1.2. Mood scales
3.1.3. Mind-wandering
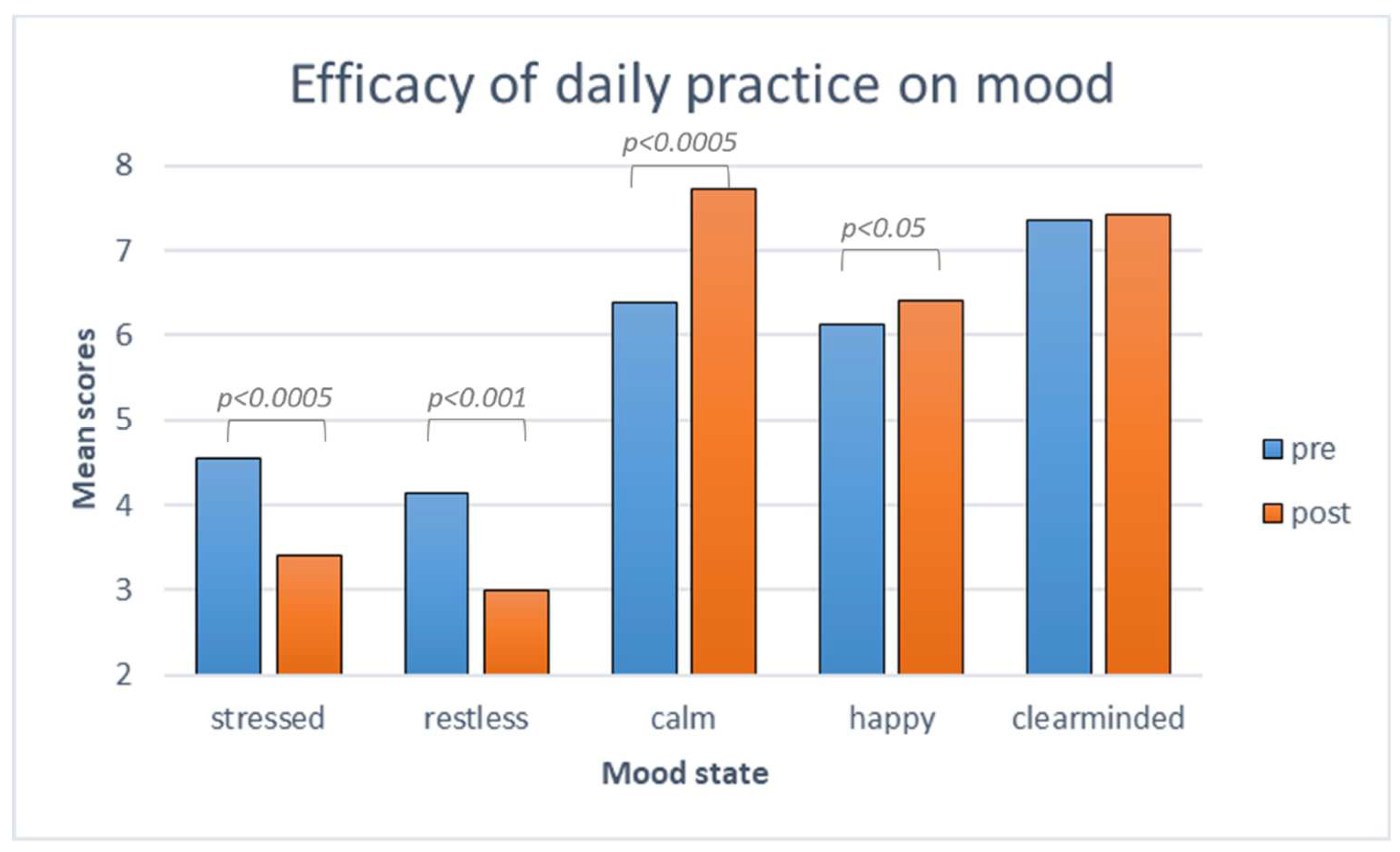
3.2. The efficacy of UNB
3.2.1. The efficacy of daily practice over mood states
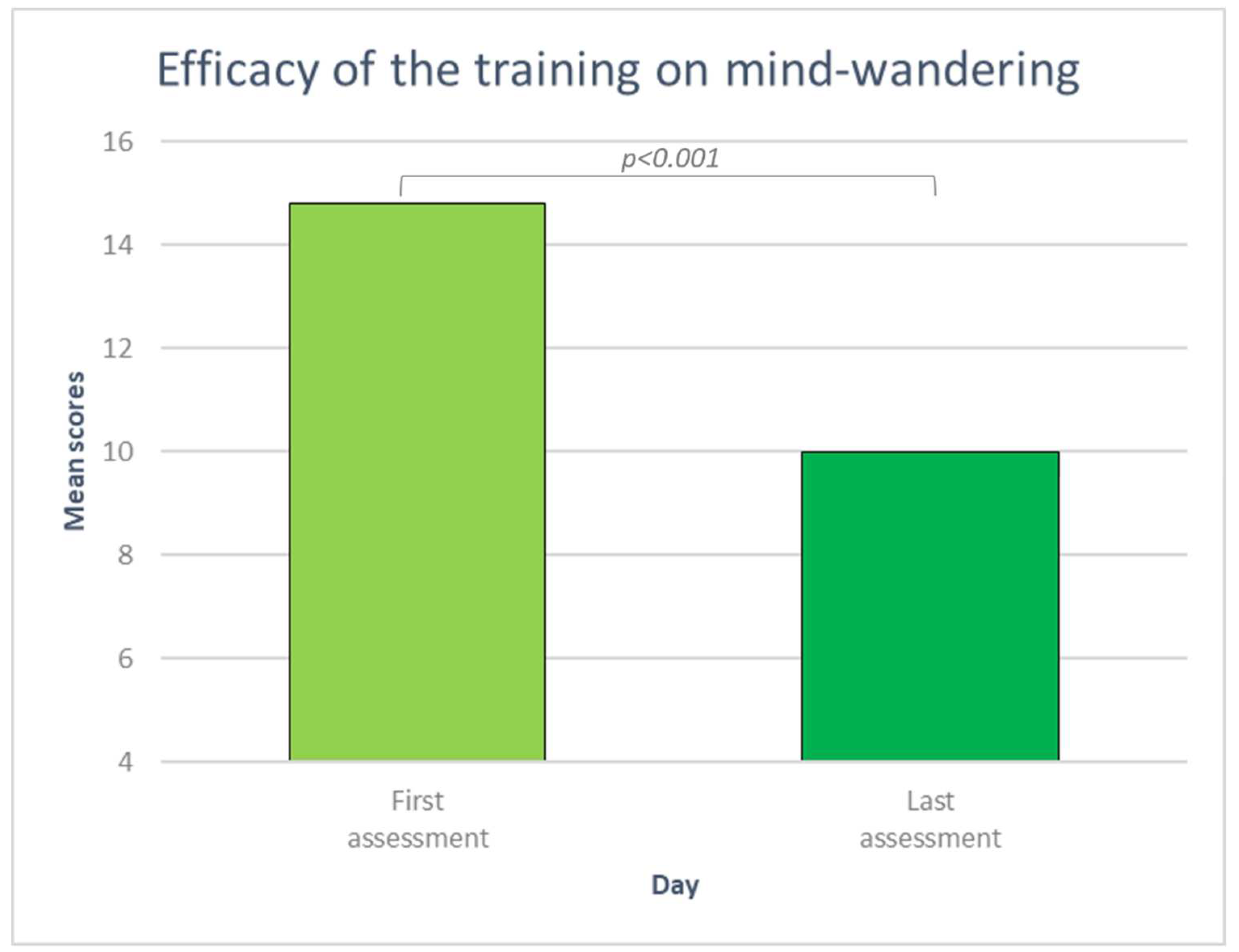
3.2.2. Mind-wandering
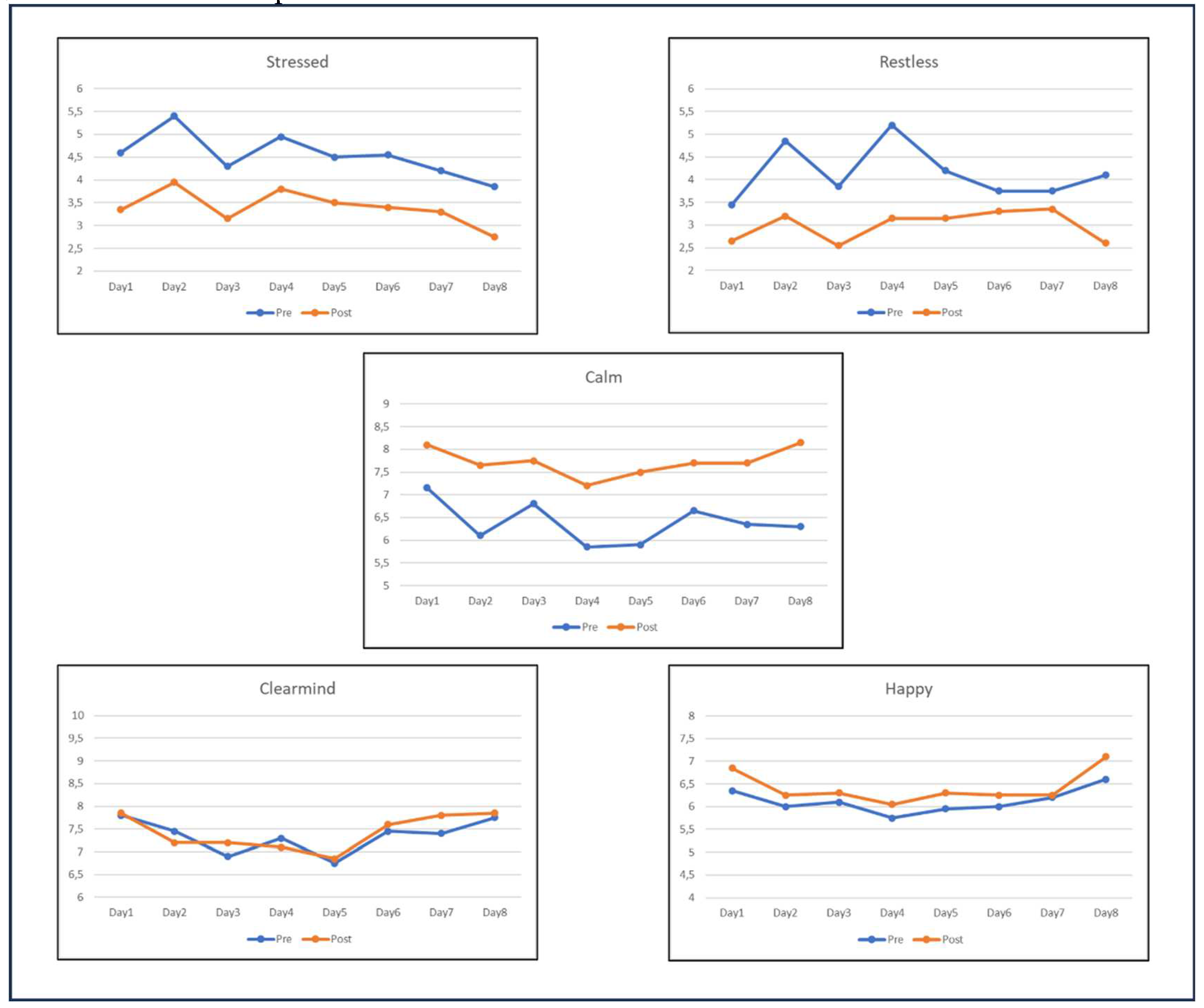
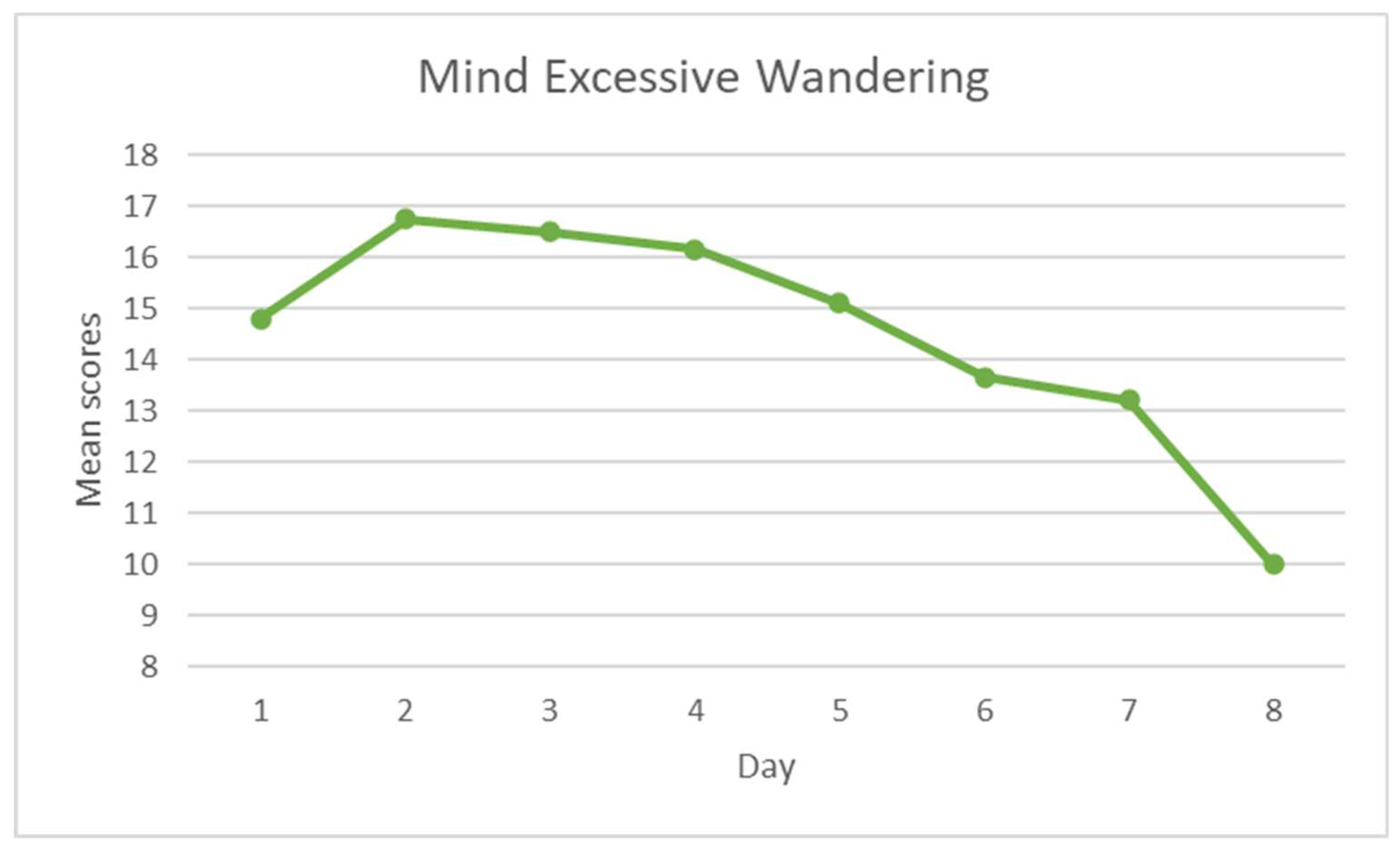
3.3. Training progression
3.3.1. Mood states
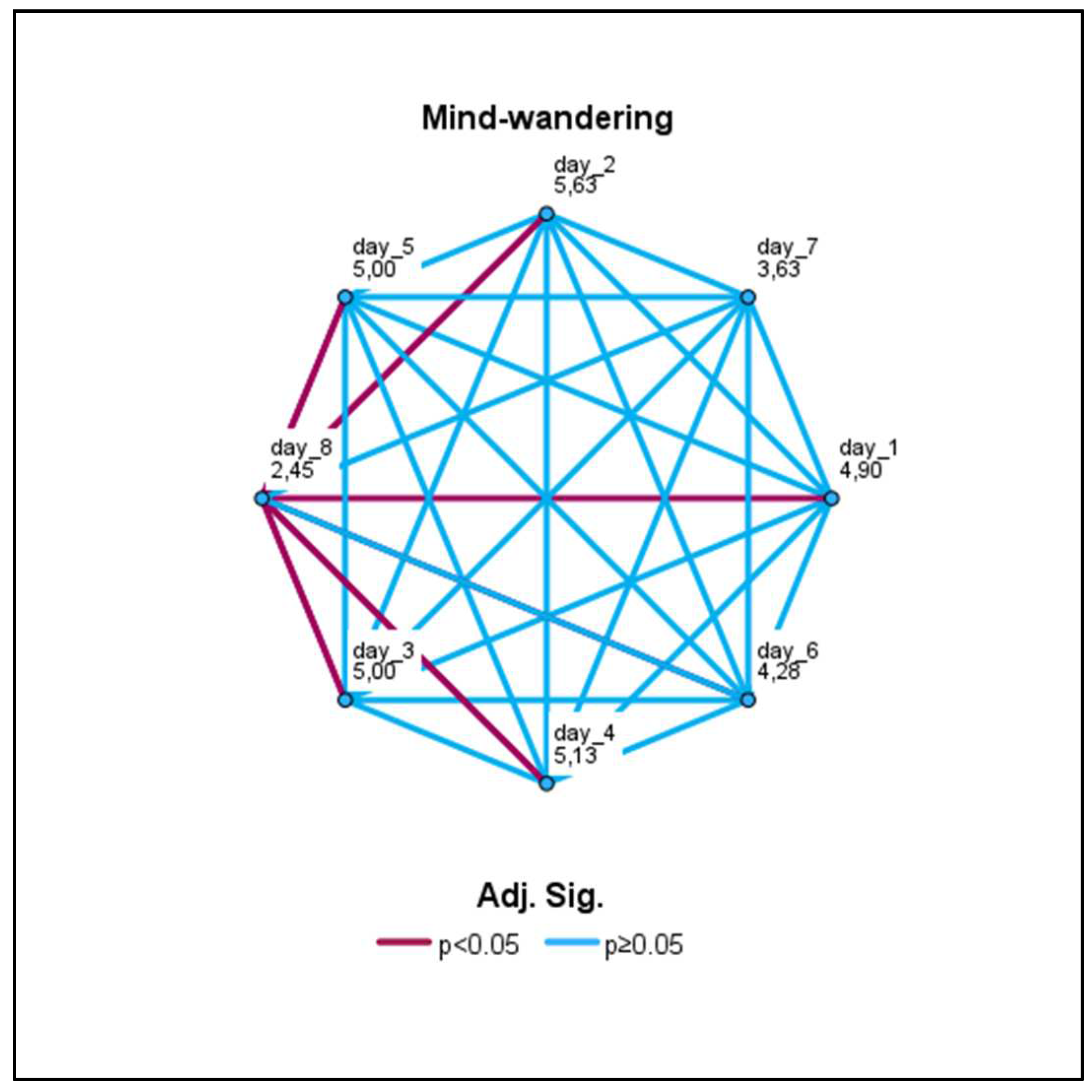
3.3.2. Mind-wandering
3.4. Comparison between left vs right nostril breathing
3.4.1. Mood scales
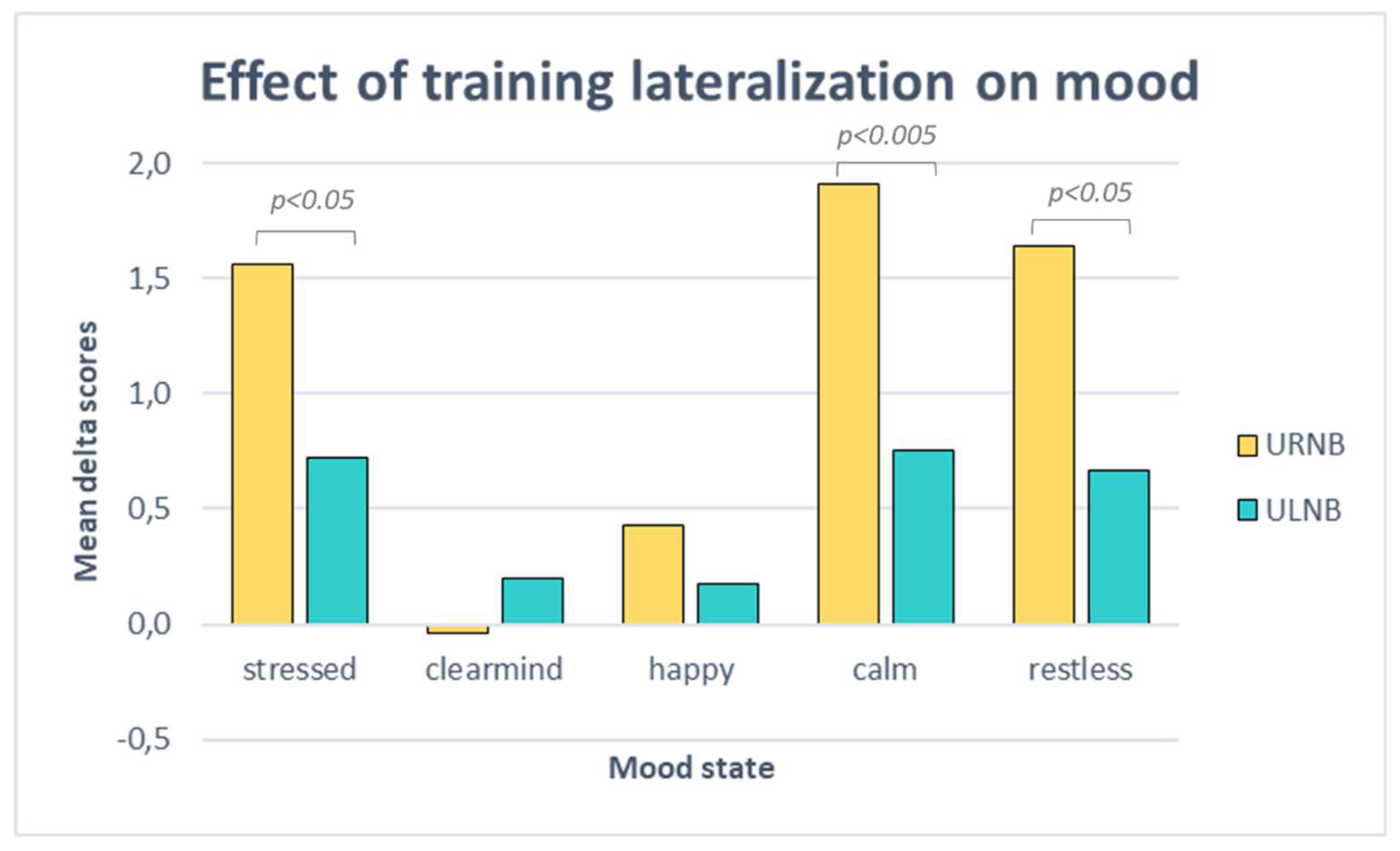
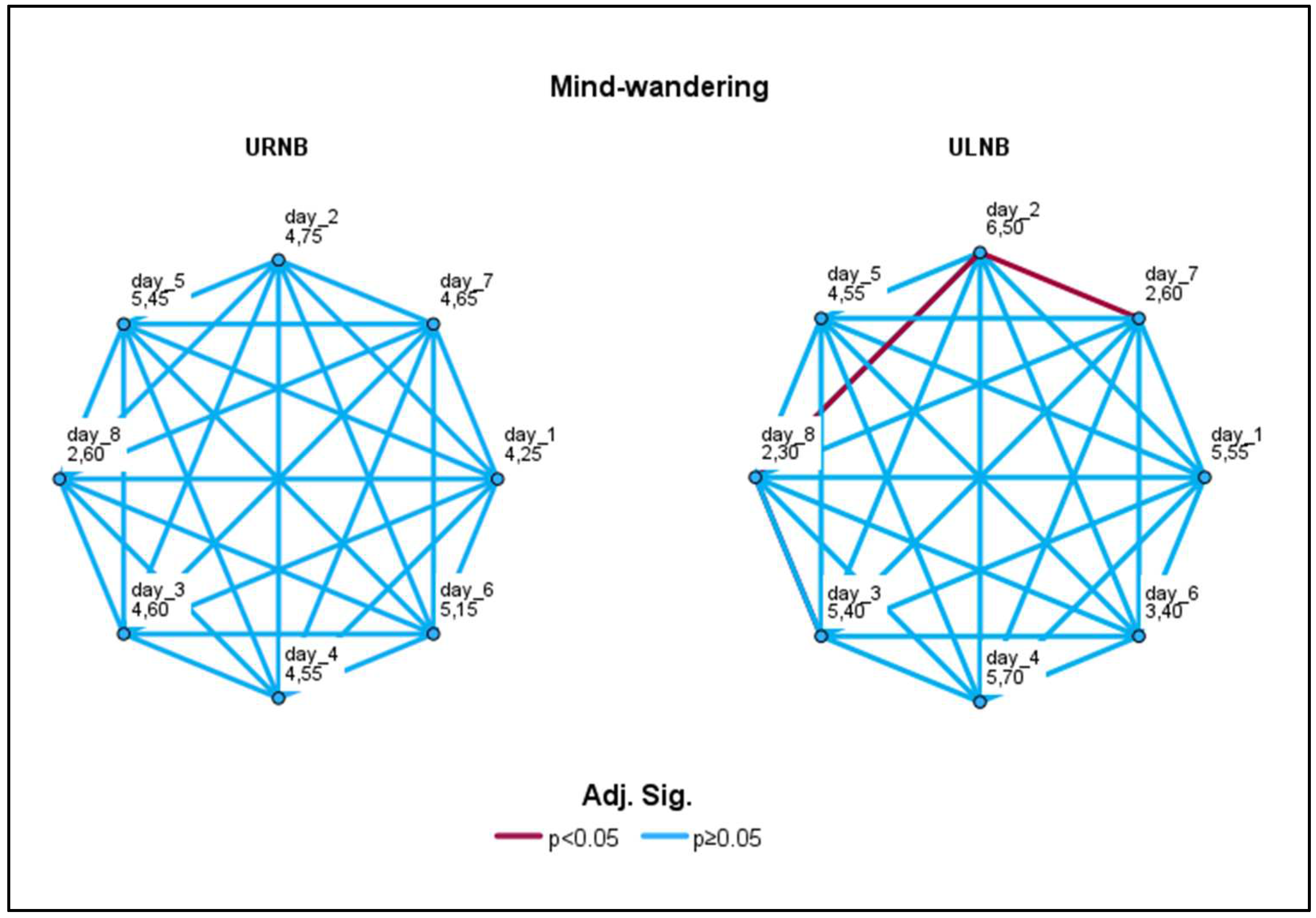
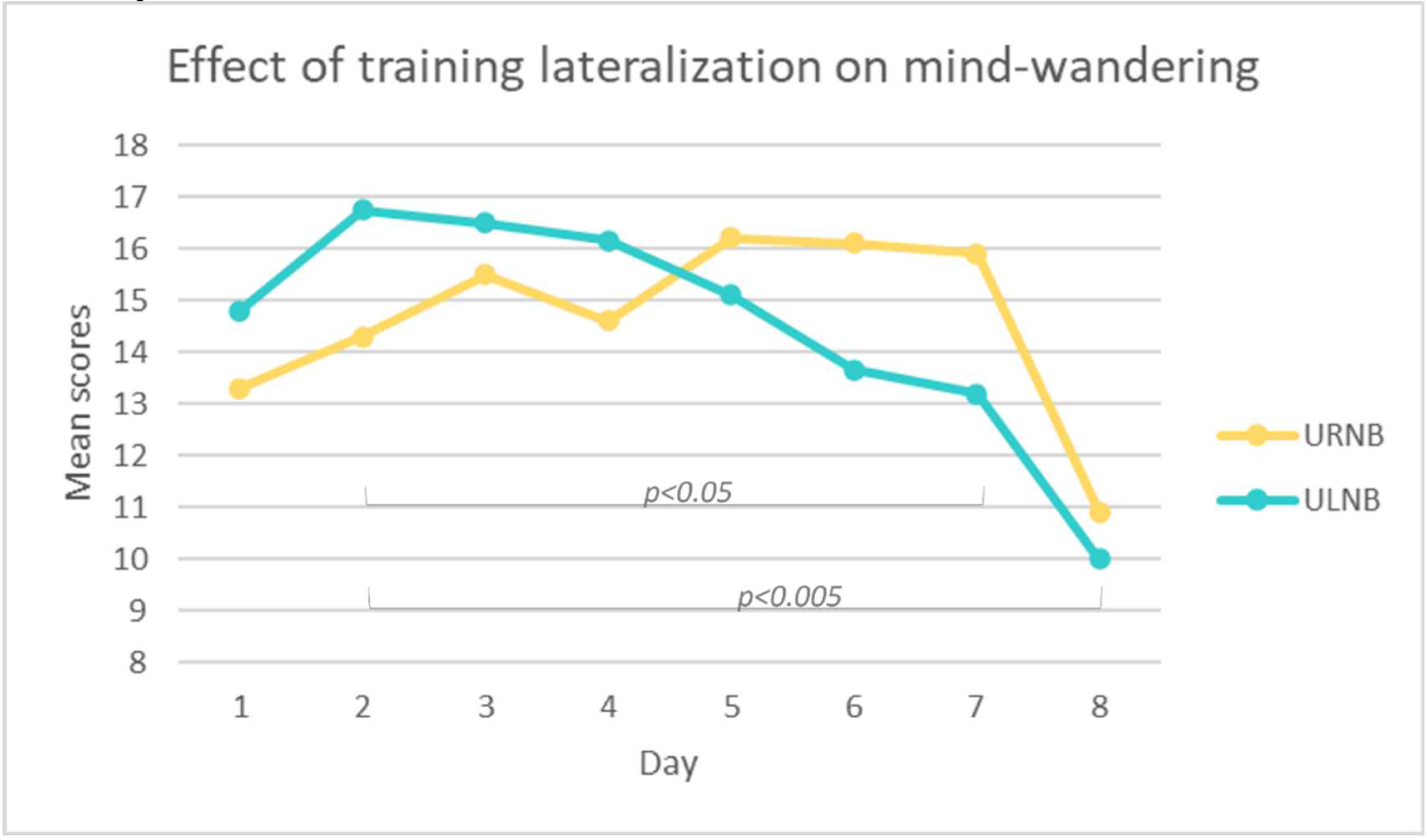
3.4.2. Mind-wandering
Discussion
Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ocklenburg, S.; Mundorf, A. Symmetry and Asymmetry in Biological Structures. Proc. Natl. Acad. Sci. 2022, 119, e2204881119. [CrossRef]
- Kong, X.; Postema, M.C.; Guadalupe, T.; de Kovel, C.; Boedhoe, P.S.W.; Hoogman, M.; Mathias, S.R.; Van Rooij, D.; Schijven, D.; Glahn, D.C. Mapping Brain Asymmetry in Health and Disease through the ENIGMA Consortium. Hum. Brain Mapp. 2022, 43, 167–181. [CrossRef]
- Wang, B.; Yang, L.; Yan, W.; An, W.; Xiang, J.; Li, D. Brain Asymmetry: A Novel Perspective on Hemispheric Network. Brain Sci. Adv. 2023, 9, 56–77. [CrossRef]
- Knecht, S.; Deppe, M.; Dräger, B.; Bobe, L.; Lohmann, H.; Ringelstein, E.-B.; Henningsen, H. Language Lateralization in Healthy Right-Handers. Brain 2000, 123, 74–81. [CrossRef]
- Martin, I.; McDonald, S. That Can’t Be Right! What Causes Pragmatic Language Impairment Following Right Hemisphere Damage? Brain Impair. 2006, 7, 202–211. [CrossRef]
- Rangarajan, V.; Hermes, D.; Foster, B.L.; Weiner, K.S.; Jacques, C.; Grill-Spector, K.; Parvizi, J. Electrical Stimulation of the Left and Right Human Fusiform Gyrus Causes Different Effects in Conscious Face Perception. J. Neurosci. 2014, 34, 12828–12836. [CrossRef]
- Davidson, R.J. Anterior Cerebral Asymmetry and the Nature of Emotion. Brain Cogn. 1992, 20, 125–151. [CrossRef]
- Balconi, M.; Grippa, E.; Vanutelli, M.E. Resting Lateralized Activity Predicts the Cortical Response and Appraisal of Emotions: An FNIRS Study. Soc. Cogn. Affect. Neurosci. 2015, 10, 1607–1614.
- Duboc, V.; Dufourcq, P.; Blader, P.; Roussigné, M. Asymmetry of the Brain: Development and Implications. Annu. Rev. Genet. 2015, 49, 647–672. [CrossRef]
- Vallortigara, G.; Rogers, L.J. A Function for the Bicameral Mind. Cortex 2020, 124, 274–285. [CrossRef]
- Niazi, I.K.; Navid, M.S.; Bartley, J.; Shepherd, D.; Pedersen, M.; Burns, G.; Taylor, D.; White, D.E. EEG Signatures Change during Unilateral Yogi Nasal Breathing. Sci. Rep. 2022, 12, 520. [CrossRef]
- Kahana-Zweig, R.; Geva-Sagiv, M.; Weissbrod, A.; Secundo, L.; Soroker, N.; Sobel, N. Measuring and Characterizing the Human Nasal Cycle. PLoS One 2016, 11, e0162918. [CrossRef]
- Mason, H.; Gerbarg, P.L.; Brown, R.P. Psychophysiology: Healing Effects of Voluntarily Regulated Breathing Practices. In Handbook of Research on Evidence-Based Perspectives on the Psychophysiology of Yoga and its Applications; IGI Global, 2021; pp. 24–48.
- Yau, K.K.-Y.; Loke, A.Y. Effects of Diaphragmatic Deep Breathing Exercises on Prehypertensive or Hypertensive Adults: A Literature Review. Complement. Ther. Clin. Pract. 2021, 43, 101315. [CrossRef]
- Shannahoff-Khalsa, D.S. Psychophysiological States: The Ultradian Dynamics of Mind-Body Interactions. Int. Rev. Neurobiol. 2008, 80, 249.
- Hopper, S.I.; Murray, S.L.; Ferrara, L.R.; Singleton, J.K. Effectiveness of Diaphragmatic Breathing for Reducing Physiological and Psychological Stress in Adults: A Quantitative Systematic Review. JBI Evid. Synth. 2019, 17. [CrossRef]
- Konrad, A.C.; Engert, V.; Albrecht, R.; Dobel, C.; Döring, N.; Haueisen, J.; Klimecki, O.; Sandbothe, M.; Kanske, P. A Multicenter Feasibility Study on Implementing a Brief Mindful Breathing Exercise into Regular University Courses. Sci. Rep. 2023, 13, 7908. [CrossRef]
- Cook, N.E.; Huebschmann, N.A.; Iverson, G.L. Safety and Tolerability of an Innovative Virtual Reality-Based Deep Breathing Exercise in Concussion Rehabilitation: A Pilot Study. Dev. Neurorehabil. 2021, 24, 222–229. [CrossRef]
- Price, A.; Eccles, R. Nasal Airflow and Brain Activity: Is There a Link? J. Laryngol. Otol. 2016, 130, 794–799. [CrossRef]
- Kala, N.; Chetry, D.; Telles, S. Psychophysiological Effects and the Applications of Yoga Breathing Practices. In Handbook of Research on Evidence-Based Perspectives on the Psychophysiology of Yoga and Its Applications; IGI Global, 2021; pp. 1–23.
- Telles, S.; Nagarathna, R.; Nagendra, H.R. Breathing through a Particular Nostril Can Alter Metabolism and Autonomic Activities. Indian J. Physiol. Pharmacol. 1994, 38, 133.
- Muktibodhananda, S. Swara Yoga The Tantric Science of Brain Breathing; Yoga Publications Trust: Munger, Bihar, India, 1984.
- Werntz, D.A.; Bickford, R.G.; Shannahoff-Khalsa, D. Selective Hemispheric Stimulation by Unilateral Forced Nostril Breathing. Hum. Neurobiol. 1987.
- Telles, S.; Nagarathna, R.; Nagendra, H.R. Physiological Measures of Right Nostril Breathing. J. Altern. Complement. Med. 1996, 2, 479–484. [CrossRef]
- Raghuraj, P.; Telles, S. Immediate Effect of Specific Nostril Manipulating Yoga Breathing Practices on Autonomic and Respiratory Variables. Appl. Psychophysiol. Biofeedback 2008, 33, 65–75. [CrossRef]
- Bhavanani, A.B.; Sanjay, Z. Immediate Effect of Chandra Nadi Pranayama (Left Unilateral Forced Nostril Breathing) on Cardiovascular Parameters in Hypertensive Patients. Int. J. Yoga 2012, 5, 108. [CrossRef]
- Telles, S.; Raghuraj, P.; Maharana, S.; Nagendra, H.R. Immediate Effect of Three Yoga Breathing Techniques on Performance on a Letter-Cancellation Task. Percept. Mot. Skills 2007, 104, 1289–1296. [CrossRef]
- Block, R.A.; Arnott, D.P.; Quigley, B.; Lynch, W.C. Unilateral Nostril Breathing Influences Lateralized Cognitive Performance. Brain Cogn. 1989, 9, 181–190. [CrossRef]
- Santhanam Kumar, S.S.; Kamath, A.; Poojary, S. Effect of Unilateral Left Nostril Breathing (Chandra Anga Pranayama) on Cognitive Function in Healthy Yoga-Naïve Individuals: A Randomized, Controlled, Pilot Study. Complement. Med. Res. 2020, 27, 319–327. [CrossRef]
- Sanders, B.; Lattimore, C.; Smith, K.; Dierker, L. Forced Single-Nostril Breathing and Cognition. Percept. Mot. Skills 1994, 79, 1499–1506. [CrossRef]
- Jella, S.A.; Shannahoff-khalsa, D.S. The Effects of Unilateral Forced Nostril Breathing on Cognitive Performance. Int. J. Neurosci. 1993, 73, 61–68. [CrossRef]
- Singh, K.; Bhargav, H.; Srinivasan, T.M. Effect of Uninostril Yoga Breathing on Brain Hemodynamics: A Functional near-Infrared Spectroscopy Study. Int. J. Yoga 2016, 9, 12. [CrossRef]
- Deng, Y.-Q.; Li, S.; Tang, Y.-Y. The Relationship between Wandering Mind, Depression and Mindfulness. Mindfulness (N. Y). 2014, 5, 124–128. [CrossRef]
- Mason, M.F.; Brown, K.; Mar, R.A.; Smallwood, J. Driver of Discontent or Escape Vehicle: The Affective Consequences of Mindwandering. Front. Psychol. 2013, 4, 477. [CrossRef]
- Seli, P.; Beaty, R.E.; Marty-Dugas, J.; Smilek, D. Depression, Anxiety, and Stress and the Distinction between Intentional and Unintentional Mind Wandering. Psychol. Conscious. Theory, Res. Pract. 2019, 6, 163. [CrossRef]
- Killingsworth, M.A.; Gilbert, D.T. A Wandering Mind Is an Unhappy Mind. Science (80-. ). 2010, 330, 932. [CrossRef]
- Kajimura, S.; Kochiyama, T.; Nakai, R.; Abe, N.; Nomura, M. Causal Relationship between Effective Connectivity within the Default Mode Network and Mind-Wandering Regulation and Facilitation. Neuroimage 2016, 133, 21–30. [CrossRef]
- Lucchiari, C.; Sala, P.M.; Vanutelli, M.E. Promoting Creativity Through Transcranial Direct Current Stimulation (TDCS). A Critical Review. Front. Behav. Neurosci. 2018, 12, 167.
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113.
- Veale, J.F. Edinburgh Handedness Inventory–Short Form: A Revised Version Based on Confirmatory Factor Analysis. Laterality Asymmetries Body, Brain Cogn. 2014, 19, 164–177. [CrossRef]
- Rammstedt, B. The 10-Item Big Five Inventory. Eur. J. Psychol. Assess. 2007, 23, 193–201. [CrossRef]
- Carver, C.S.; White, T.L. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [CrossRef]
- Leone, L.; Pierro, A.; Mannetti, L. Validità Della Versione Italiana Delle Scale Bis/Bas Di Carver e White (1994): Generalizzabilità Della Struttura e Relazioni Con Costrutti Affini. G. Ital. di Psicol. 2002, 29, 413–434. [CrossRef]
- Aron, E.N.; Aron, A. Sensory-Processing Sensitivity and Its Relation to Introversion and Emotionality. J. Pers. Soc. Psychol. 1997, 73, 345. [CrossRef]
- Gerstenberg, F.X.R. Sensory-Processing Sensitivity Predicts Performance on a Visual Search Task Followed by an Increase in Perceived Stress. Pers. Individ. Dif. 2012, 53, 496–500. [CrossRef]
- Jagiellowicz, J.; Xu, X.; Aron, A.; Aron, E.; Cao, G.; Feng, T.; Weng, X. The Trait of Sensory Processing Sensitivity and Neural Responses to Changes in Visual Scenes. Soc. Cogn. Affect. Neurosci. 2011, 6, 38–47. [CrossRef]
- Dias Da Silva, M., Gonçalves, Ó. F., & Postma, M. Assessing the Relationship between Trait and State Levels of Mind Wandering during a Tracing Task. In Proceedings of the Proceedings of the 42nd Annual Meeting of the Cognitive Science Society: Developing a Mind: Learning in Humans, Animals, and Machines .; S. Denison, M. Mack, Y. Xu, & B.C.A., Ed.; Cognitive Science Society, 2020; pp. 3289–3294.
- Menzies, V.; Taylor, A.G.; Bourguignon, C. Absorption: An Individual Difference to Consider in Mind–Body Interventions. J. Holist. Nurs. 2008, 26, 297–302. [CrossRef]
- Tellegen, A.; Atkinson, G. Openness to Absorbing and Self-Altering Experiences (“ Absorption”), a Trait Related to Hypnotic Susceptibility. J. Abnorm. Psychol. 1974, 83, 268. [CrossRef]
- Badran, B.W.; Short, E.B. Abstract# 11: TDCS-Enhanced Meditation (E-Meditation) Reduces Mind Wandering and Stress State: An Open-Label Pilot Trial. Brain Stimul. Basic, Transl. Clin. Res. Neuromodulation 2019, 12, e4. [CrossRef]
- Mowlem, F.D.; Skirrow, C.; Reid, P.; Maltezos, S.; Nijjar, S.K.; Merwood, A.; Barker, E.; Cooper, R.; Kuntsi, J.; Asherson, P. Validation of the Mind Excessively Wandering Scale and the Relationship of Mind Wandering to Impairment in Adult ADHD. J. Atten. Disord. 2019, 23, 624–634. [CrossRef]
- Perciavalle, V.; Blandini, M.; Fecarotta, P.; Buscemi, A.; Di Corrado, D.; Bertolo, L.; Fichera, F.; Coco, M. The Role of Deep Breathing on Stress. Neurol. Sci. 2017, 38, 451–458. [CrossRef]
- Zaccaro, A.; Piarulli, A.; Laurino, M.; Garbella, E.; Menicucci, D.; Neri, B.; Gemignani, A. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front. Hum. Neurosci. 2018, 353. [CrossRef]
- Tran, E.; Aly, M.; Shah, N.; Phu, V.; Malvankar-Mehta, M.S. Benefits of Meditation and Breathing Exercises in Vision Loss Patients. Br. J. Vis. Impair. 2023, 02646196231201773. [CrossRef]
- Ito, J.; Roy, S.; Liu, Y.; Cao, Y.; Fletcher, M.; Lu, L.; Boughter, J.D.; Grün, S.; Heck, D.H. Whisker Barrel Cortex Delta Oscillations and Gamma Power in the Awake Mouse Are Linked to Respiration. Nat. Commun. 2014, 5, 3572. [CrossRef]
- Posner, M.I.; Fan, J. Attention as an Organ System. In Topics in Integrative Neuroscience: From cells to Cognition; Pomerantz, J.R., Ed.; Cambridge University Press: Cambridge, 2001; pp. 31–61.
- Simonton, D.K. On Praising Convergent Thinking: Creativity as Blind Variation and Selective Retention. Creat. Res. J. 2015, 27, 262–270. [CrossRef]
- Homma, I.; Masaoka, Y. Breathing Rhythms and Emotions. Exp. Physiol. 2008, 93, 1011–1021. [CrossRef]
- Jafari, H.; Courtois, I.; Van den Bergh, O.; Vlaeyen, J.W.S.; Van Diest, I. Pain and Respiration: A Systematic Review. Pain 2017, 158, 995–1006. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





