1. Introduction
The use of nanotechnology in medicine has provided an opportunity for unparalleled development of the treatment of various severe diseases. The unique properties of nanoparticles(NPs), such as small size and higher surface-to-volume ratio, the ability to encapsulate different drugs and tunable surface chemistry, offer many advantages over already existing technologies: efficient navigation of the nanoparticle complex in vivo, multivalent surface modification for greater accuracy, increased intracellular trafficking, addition of charged particles to increase target selectivity or sustained drug release. Nanoparticles can thus be an ideal candidate in medical applications for the most widespread and challenging health problems, such as cancer. Nanotechnology offers a promising strategy in enhancing antitumor immunity by enhancing immunogenicity and presentation of tumor autoantigens for cancer immunotherapy.
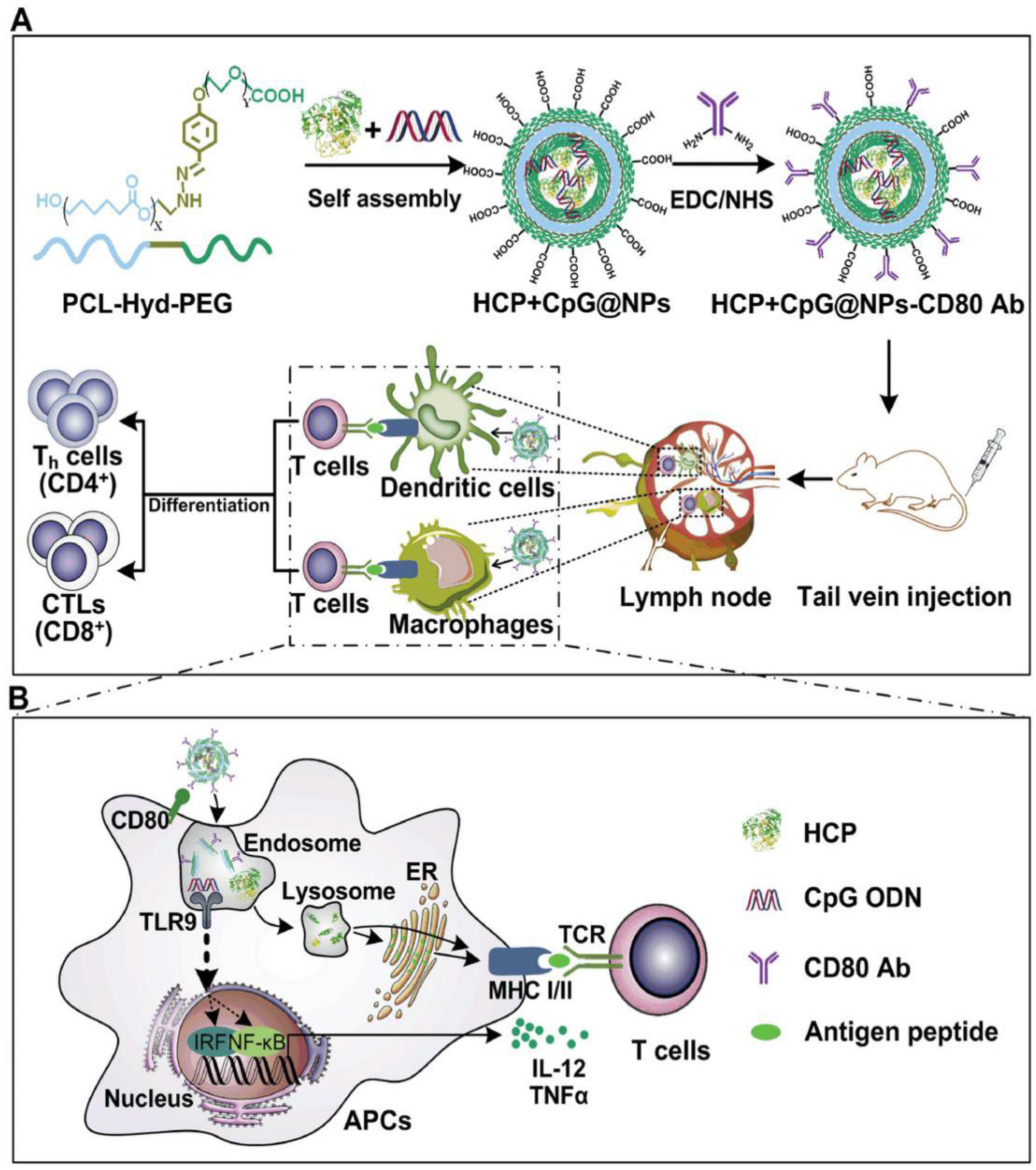
Nanoparticles may be useful as a new platform for vaccine development [
1]. Most vaccines used contain live, attenuated, killed or fragmented pathogens; and due to their complex nature, the quality of such vaccinations varies significantly from batch to batch and adverse effects such as those associated with polio or rubella vaccine may occur. The use of nanoparticles in the development of new vaccines can offer important advantages, such as a more efficient targeting of antigen-presenting cells [
2,
3]. Functionalized nanoparticles can be obtained by coating biodegradable polymer nanoparticles with cancer cell membranes [
4]. These carrier nanoparticles can function as an effective antigen delivery system, increasing or facilitating antigen uptake by antigen-presenting cells, such as dendritic cells and macrophages. These cancer cell membrane-coated NPs display multiple membrane antigens in common with cancer cell membranes that enable them to have immunogenic or surface drug delivery functionalities. For cancer immunotherapy, endogenous antigen-carrying nanoparticles (EAC-NPs) can have an enhanced immunogenic effect by incorporating immunological adjuvants such as mono phosphoryl lipid A [
5], leading to enhanced antigen uptake for immune presentation and activation (
Figure 1).
2. Types of immunostimulating nanoparticles
Nanoparticles with immunostimulatory effects are widely used in cancer immunotherapy, are summarized in
Table 1 and discussed in the following sections.
Dendrimers have shown promise in stimulating the immune system. Xu et al used guanidinobenzoic acid (DGBA) modified polyamidoamine dendrimers to co-deliver OVA and CpG-ODN, which were efficiently taken up by dendritic cells (DCs) and promoted their maturation and antigen presentation [
6] . This nanovaccine induced CD8+ T cell immune responses and demonstrated prophylactic efficacy against B16-OVA melanoma. In combination with immune checkpoint blockade therapy (ICBT) , this vaccine showed synergistic effects on T cell antitumor response. Chen et al developed methoxy polyethylene glycol decorated dendrimer-entrapped gold nanoparticles (PEG-Au DENPs) for CpG-ODN delivery to DCs, promoting DC maturation and activating T cells for an adaptive antitumor immune response [
7] .
Liposomal nanoparticles are very useful vehicles in pharmacology, but also as a means of delivering antigens to antigen-presenting cells (APCs) or with an adjuvant role in mediating the specific immune response. Liposomes modified with pH-sensitive dextran, loaded with ovalbumin, were efficiently absorbed by dendritic cells, causing an effective antitumor immune response in E.G7-OVA tumor-bearing mice [
8]. Also, liposomes can be used to deliver long synthetic peptides to dendritic cells, activating antigen presentation and the immune response mediated by CD8+ cytotoxic T cells [
9].
Superparamagnetic iron oxide nanoparticles are useful in oncological diagnosis, but they are capable of therapeutic effects, being useful in cancer theranostic applications [
10]. Luo L and colleagues proposed the loading of iron oxide nanocomposites with OVA in order to efficiently mature dendritic cells and activate T cells. They showed that these nanocomposites induced a competent antitumor immune response through the simultaneous activation of macrophages. Applied to murine models, these biofunctionalized nanocomposites had significant antitumor effects for B16-OVA tumors [
11].
Micelles have shown promise as effective nanocarriers to enhance the efficacy of cancer vaccines by delivering antigens and adjuvants. Zeng et al used polymer hybrid micelles (PHMs) to encapsulate melanoma antigen peptides and TLR-9 agonists, observing successful lymph node targeting and payload internalization by dendritic cells [
12]. This co-delivery system stimulated antigen-specific CD8+ T cell immune responses and demonstrated potent antitumor effects in a lung metastatic melanoma model. In another study, Li et al developed PHMs using specific polymers and observed that these micelles effectively induced stronger antigen-specific CD8+ T cell immune responses and antitumor efficacy compared to mixtures of free antigen and adjuvant [
13]. In addition, carboxylated polymer mixed micelles were used to co-deliver antigens and TLR-7 agonist, resulting in enhanced dendritic cell maturation, cytokine secretion, and antigen cross-presentation, ultimately leading to a potent antigen-specific immune response. Furthermore, immunization with these nanovaccines significantly inhibited tumor growth in experimental mice.
Silicon nanoparticles are used due to their acceptable biocompatibility and characteristic porosity in various fields such as bioimaging, tumor localization, or transport of vaccine or drug molecules [
14]. Furthermore, because of their low cytotoxicity and easily adaptable morphology, gold nanoparticles are used in macrophage activation and T cell response triggering. Ong et al showed in 2019 that mesoporous silicon nanoparticles (MSN) decorated with gold nanoparticles can be loaded and can deliver large amounts of CpG-ODNs to the tumor. Thus, a specific antigen was generated at the tumor level, which was processed and presented by tumor infiltrated dendritic cells, activating and triggering a specific immune response [
15].
Liu et al fabricated a mesoporous silicon nanoparticle (MSN) engraved with polyethyleneimine, then used it as a nucleotide delivery agent in an experimental model. Using the tyrosinase-related protein-2 molecule as an antigen, they obtained an increased cellular absorption of the antigen and dendritic cell maturation, confirmed by high levels of pro-inflammatory cytokines and the costimulatory molecules of the immune response, CD86 and CD83 [
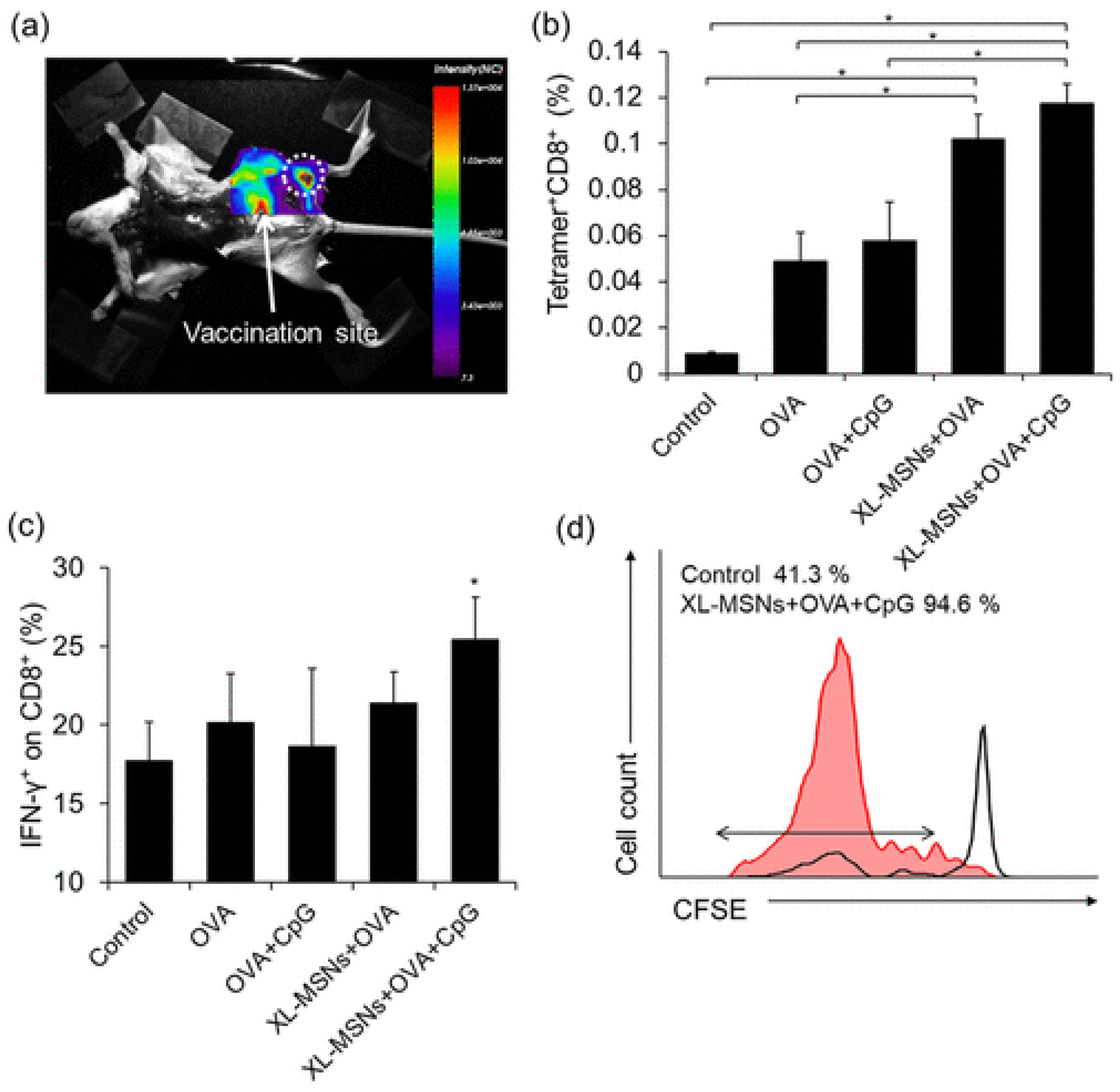
16]. Cha and colleagues prepared MSNs with a particle size of 100-200 nm, used OVA as an antigen and unmethylated CpG as a TLR9 agonist. They showed that, compared to free OVA, this nanoparticulate complex increased the expression of CD86, necessary for the priming of cytotoxic T lymphocytes, together with the major histocompatibility complex I, and produced the highest level of antigen presenting cells (APCs) [
17].
Figure 2.
(a) Fluorescent images of mouse injected with RITC-labeled XL-MSNs subcutaneously on abdomen region, showing targeting of XL-MSNs to the draining lymph node (white dotted circle). (b) OVA-specific and (c) intracellular cytokine secreting CTLs in the spleens of vaccinated mice measured in flow cytometry (n = 6). Error bars, mean ± s.d. *P < 0.05. (d) Proliferation of CFSE-labeled OVA-specific CD8+ T cells in the lymph node (red line: XL-MSN + OVA + CpG, black line: control), Reproduced with permission from [
17].
Figure 2.
(a) Fluorescent images of mouse injected with RITC-labeled XL-MSNs subcutaneously on abdomen region, showing targeting of XL-MSNs to the draining lymph node (white dotted circle). (b) OVA-specific and (c) intracellular cytokine secreting CTLs in the spleens of vaccinated mice measured in flow cytometry (n = 6). Error bars, mean ± s.d. *P < 0.05. (d) Proliferation of CFSE-labeled OVA-specific CD8+ T cells in the lymph node (red line: XL-MSN + OVA + CpG, black line: control), Reproduced with permission from [
17].
Carbon nanotubes have also been used to induce immunostimulatory effects both in vitro and in vivo. Dong et al assembled an antigen delivery system using mannose modified multi-walled carbon nanotubes, that are able to bind specifically to the mannose receptor on the dendritic cell membrane [
18]. Loading the nanotubes thus prepared with an antigen, in the model the antigen used was ovalbumin (OVA), they showed that this system has a low cytotoxicity and demonstrated the efficient incorporation of the nanotube-antigen complex at the level of dendritic cells and their maturation in order to release cytokines in vitro. Xia et al developed a nano-delivery system for Cytosine -Guanine Oligodeoxynucleotides (CpG ODNs) based on multi-walled carbon nanotubes (MWCNTs) conjugated with H3R6 polypeptide for prostate cancer immunotherapy. The in vivo anticancer efficacy study on RM-1 tumor-bearing mice demonstrated that this nanotube system could deliver immunotherapeutics to the tumor site and could suppress tumor growth [
19].
Nanoemulsions (NEs) are used as adjuvants or antigen delivery vehicles to enhance antitumor immune responses. Tailored NEs functionalized with the C-type lecitin receptor (Clec9A) have been developed for antigen-specific immunotherapy by efficiently targeting and activating DCs and inducing antigen-specific T cell responses [
20] . Encapsulation of tumor antigens such as HPV16 E6/E7 in Clec9A nanoemulsions inhibited tumor growth and stimulated strong immune responses. NEs loaded with Toll-like receptor 7/8 (TLR7/8) agonists and tumor antigens activated DCs and T cells, while modulating the immunosuppressive tumor microenvironment. Combining NE treatment with immune checkpoint blockade therapy (ICBT) synergistically induced antitumor immune responses [
21] .
Nanogels have emerged as effective antigen or protein delivery systems in cancer immunotherapy. Wang et al developed pH-sensitive galactosyl dextran-retinal (GDR) nanogels for targeted delivery of OVA into dendritic cells (DCs), enhancing DC maturation and antigen uptake [
22] . Additionally, cationic dextran nanogels conjugated with OVA enabled intracellular release in a reductive environment, promoting DC maturation and generating strong antitumor responses when combined with the adjuvant poly (I:C) [
23] . Carboxyl group modified cholesterol-bearing pullulan self-assembly nanogels have also been developed for OVA delivery into DCs, inducing significant adaptive immune responses [
24] .
Polymeric nanoparticles are among the most used due to their biocompatibility, biodegradability, loading capacity, stable chemical properties and water solubility. Of these, PLGA (poly-lactic co-glycolic acid), PGA (poly glutamic acid), PLG (poly lactide co-glycolic), PEG (polyethylene glycol), PEI (polyethyleneimine) or chitosan are among the most used as adjuvants of anticancer vaccines due to the immunostimulatory effect. In association with the toll-like receptor 7/8(TLR 7/8), PLGA nanoparticles lead to a significant increase in the expression of the TLR agonist and to a more intense stimulation of dendritic cells [
25]. Subcutaneous administration leads to a concentration of nanoparticles in the lymph nodes, where they activate the immune response mediated by dendritic cells and cytotoxic CD8+ cells. In combination with TLR agonists, PLGA NPs lead to a significant increase in the antitumor immune response of anticancer vaccines in the murine experimental models of da Silva et al [
26].
Protein nanoparticles have demonstrated their effectiveness as vaccine platforms for delivering tumor antigens and adjuvants, thereby eliciting a potent anti-tumor immune response. In a study by Molino et al , biomimetic protein nanoparticles were successfully utilized to co-deliver peptide epitopes and CpG-ODN activators to dendritic cells (DCs) [
27]. This approach led to heightened and sustained activation of CD8+ T cells, along with improved antigen cross-presentation. Another study revealed that the concurrent administration of melanoma-associated gp100 epitope and CpG-ODN, utilizing viral mimicking protein nanoparticles, substantially enhanced CD8+ T cell proliferation and secretion of IFN-γ [
28].
Virus-like particles (VLPs) emerged as a promising nanovaccine platform for enhancing cancer immunotherapy. Lizotte et al has shown that self-assembled VLPs derived from cowpea mosaic virus (CPMV) can significantly reduce lung melanomas and induce a potent systemic antitumor immune response in mice [
29]. Also, encapsulation of CpG-ODNs into VLPs derived from Cowpea chlorotic mottle virus (CCMV) has enabled targeted delivery to tumor-associated macrophages (TAMs) in the tumor microenvironment, enhancing their phagocytic activity and promoting more effective antitumor responses both in vitro and in vivo [
30].
3. Types of Vaccines against cancer
Depending on the mechanism of action, anticancer vaccines can be divided into three large groups: cellular vaccines, protein or peptide vaccines and genetic vaccines (RNA, DNA or viral particles) [
31].
Cell vaccines can use autologous tumor cells. These are inactivated by irradiation and are usually used in combination with adjuvant molecules. The mechanism of action consists in activating the specific and unique immune response to the type of cancer of the patient, these immune cells being exposed to the entire spectrum of antigens associated with the respective tumor. The disadvantage of this approach specific to the tumor in question is that it requires a sufficient tumor volume for the sampling and preparation of tumor cell specimens for vaccine production [
32].
From this point of view, the use of predetermined cell lines allows the creation of allogenic cell vaccines, which can be produced for specific types of cancer.
Another approach is represented by the use of autologous dendritic cells activated by exposure to tumor-associated antigens and the augmentation of the cellular response by exposure to adjuvant molecules [
33,
34]. Dendritic cells, with the role of antigen presenters, are autologously inoculated, activated, as is the case with Sipuleucel-T, approved in the treatment of metastatic prostate cancer [
35].
Protein or peptide vaccines use tumor-associated antigens to elicit an immune response to these antigens, which are overexpressed in tumor tissue, but too little in the rest of normal tissue types. The association of these antigens with adjuvant immunomodulatory factors increases the effectiveness of this type of vaccine [
36].
Genetic vaccines use DNA, RNA or viral particles, whose genetic information is presented to antigen-presenting cells, to be later translated into tumor-specific antigens or antigenic fragments [
37]. RNA vaccines, especially mRNA vaccines, are associated with fewer side effects than DNA vaccines, being more rapidly degraded in the target organism (
Figure 3).
Cancer immunotherapy and immunoprophylaxis represent an exciting field of application for synthetic RNA technology. It’s shortcomings are mainly caused by the instability in vivo and the rapid natural clearance in the body of the synthetic RNA. Nanoparticles can be functionalized to provide a delivery platform for mRNA-based vaccines. Dendritic cells can be activated with tumor RNA, generating a systemic immune antitumor response. The main disadvantage is the rapid degradation of RNA molecules in the target organism. The encapsulation of tumor RNA molecules inside liposomal nanoparticles can provide the necessary protection to significantly improve the effectiveness of an RNA vaccine. The size of these nanoparticles must be able to condense the mRNA molecules, protect them from degradation and rapid clearance from the body, and also avoid unwanted delivery destinations. These characteristics, which can be designed in certain nanoparticles, can be useful in mediating the interaction of some adjuvant molecules, capable of modulating and potentiating the specific immune response induced by the mRNA vaccine [
38].
Dai et al used a nanoliposome - tumor RNA complex, with RNA extracted from colorectal cancer tumor cells (CT-26) and developed it into a vaccine with significant antitumor immunological efficiency demonstrated in murine experimental models [
39]. The antitumor effects of oxaliplatin in association with this RNA vaccine were also improved.
Mohammad Ariful Islam and collaborators demonstrate a concept consisting of a nanoparticle associated with an mRNA-based vaccine, using an experimental murine model with tumor allografts of prostate cancer and colorectal cancer expressing Ovalbumin [
40]. Thus, an mRNA encoded for ovalbumin (OVA) and an agonist of toll-like receptors 7 and 8, Requisimob (R848), were embeded into a lipidic nanoparticle and coated with polyethylene glycol (LNP-PEG). This NP-mRNA vaccine formulation preserved the adjuvant activity of encapsulated R848 and significantly improved mRNA transfection efficiency (>95%) and subsequent major histocompatibility complex (MHC) class I presentation of OVA mRNA-derived antigen in antigen-presenting cells. This approach with the lipid-NP and R848 adjuvated pulsed mRNA vaccine induced an effective adaptive immune response by significantly enhancing the expansion of anti-OVA-specific CD8 + T cells and infiltration of these cells into the tumor bed in vivo, relative to unadjuvated NP mRNA vaccine. In the murine model, effective antitumor immunity was achieved against OVA, expressed in the syngeneic allograft of lymphoma and prostate cancer, resulting in significant prevention of tumor growth when the vaccine was administered prior to tumor engraftment (84% reduction vs. control) and suppression of tumor growth when administered after engraftment (60% reduction vs. control).
Lipid nanoparticles (LNPs) can also be used in the targeted delivery of therapeutic molecules up to the level of lymph nodes [
41]. These LNPs can be loaded with nucleic acids in significant quantities, with their release in the cytoplasm of the target cells. Onpattro is one such commercially available LNP that can transport small RNA molecules of the siRNA type. The addition of some helper lipid molecules, such as distearolyphosphatidylcholine and cholesterol provides stability to the charged LNP molecules [
42,
43].
Vaccines based on viral particles use the ability of these viruses, such as HSV or those from the poxviridae family, to determine the production of specific viral peptides at the level of infected cells [
44].
Photodynamic therapy has a generally low and insufficient antitumor effect, but it aggravates the hypoxia of tumor cells, possibly even favoring the survival and metastasis of cancer cells.
Cai et al built a model of nanoparticles based on a metal-organic framework with the aim of combining photodynamic therapy, antihypoxic signaling and a CpG type adjuvant in order to obtain an in situ antitumor vaccine. These NPs, self-assembled from meso-Tetra(4-carboxyphenyl)porphine (H2TCPP) ions and zirconia with hypoxia-inducible factor signaling inhibitor and loaded with immunological adjuvant (CpG ODNs) and hyaluronic acid (HA) coating on the surface, specifically target cancer cells overexpressing the CD44 receptor. Photodynamic therapy generates multiple tumor antigens at the tumor level through cell destruction, without determining the hypoxic signaling effects suppressed by the presence of signaling inhibitor, and the presence of the CpG ODN adjuvants generates a strong antitumor immune response, which eliminates residual tumor cells [
45].
4. Vaccines in digestive tract cancers
In 2003, Liang Wei et al published a study describing the use of an autologous vaccine using tumor cells combined with the Newcastle disease virus in patients with malignant tumors of the digestive tract who underwent surgical and radiochemotherapy treatment, obtaining an increase in the average survival period in the long term with one year [
46].
Different types of vaccine have been tested in colorectal cancer. from peptides such as carcino-embryonic antigen (CEA), melanoma-associated antigen (MAGE) or mutant neoantigens such as mutant KRAS peptide to combinations of peptide molecules of tumor-associated antigens and amputated BCG vaccine [
47]. Even if in experimental studies they had antitumor effects and also determined an immune response, these vaccines did not develop a significant antitumor response in phase I and II clinical rials.
In 2013, Kimura et al presented a clinical study conducted in patients diagnosed with advanced adenomas of the colon, who were administered a vaccine containing a tumor-associated antigen, MUC1 [
48]. This is a glycoprotein identified as tumor- associated antigen. After administration, 44% of subjects developed anti-MUC1 antibodies. In the patients who did not present an adequate immune response, a peculiarity was discovered: they had a significantly higher pre-vaccination concentration of suppressive myeloid cells in the peripheral blood mononuclear population of leucocytes. This type of cells has an intense suppressive role of the immune response mediated by T lymphocytes [
49].
Wang et al presented an experimental model of polydopamine nanoparticles carrying tumor cell lysate as a potential vaccine for colorectal cancer immunotherapy [
50]. Polydopamine nanoparticles (NP-PDA) were prepared by self-polymerization of dopamine, on the surface of which the product obtained by tumor cell lysis (TCL) was attached. The loading capacity was 0.96mg TCL at 2mg NP-PDA, and the resulting loaded nanoparticles had a size of 241.9 nm, perfect storage stability and negligible cytotoxicity against dendritic cells. Tumor-bearing mice vaccinated with tumor lysate-loaded nanoparticles showed significant delays in tumor progression due to the sufficient amount of TCLs and M1 tumor-associated macrophages, as well as the deficient number of immunosuppression related cells in the tumor tissues. Moreover, hollow PDA NPs demonstrated an ability to modulate dendritic cell maturation and delayed tumor development by facilitating the production of activated T cells and decreasing the subpopulation of myeloid-derived suppressor cells in the tumor microenvironment [
51]. Nanoparticles show the ability to protect the antigen against natural degradation mechanisms inside the body, until delivery to the target cells [
52]. They can serve as a reservoir for the controlled release of antigen, increasing its availability for immune cells and implicitly the intensity and quality of the immune response. At the same time, they can modulate the type of immune responses induced when used alone or in combination with other immunostimulatory compounds [
53].
Tumor-associated antigens (TAA) and activated dendritic cells have been incorporated into experimental vaccines against gastric cancer, but with limited effectiveness. Even though phase I clinical studies showed safety in administration, the antitumor efficiency was insignificant [
54]. Ajani et al conducted a multicenter study testing a peptide vaccine, G17DT(Gastrimmune), which neutralizes gastrin 17, in combination with chemotherapy in patients with metastatic gastroesophageal cancer. Out of 94 patients tested, 65 produced an immune response, and they had a longer period to disease progression and a longer survival period than those who did not develop an immune response [
55]. Fujiwara tested a vaccine cocktail based on the HLA-24 antigen ligand peptide containing the new testicular cancer antigen and a multitude of antiangiogenic peptides derived from DEPC1, URLC10, FOXM1, KIF20A and VEGFR1 in patients with metastatic gastric cancer refractory to chemotherapy. The study was limited and only of the first phase, but it demonstrated safety and the patients developed a significant immune response mediated through cytotoxic T lymphocytes [
56]. However, clinical efficacy was not observed.
The modern therapeutic vision in gastric cancer combines antitumor therapy with the use of nanoparticles with the role of modulating the tumor microenvironment, the degradation of the extracellular matrix and inhibition of tumor angiogenesis, through processes of lipid peroxidation, apoptosis or autophagocytosis. Preliminary studies suggest that this approach is effective in reducing the rate of resistance to cytostatic drugs and overcoming the therapeutic barriers associated with their toxicity [
57].
In the case of hepatocellular cancer, TAA-based vaccines have not proven clear clinical benefits. Alpha-fetoprotein (AFP) is expressed in 80% of hepatocarcinomas, so Butterfield et al included the AFP vaccine in a phase I and II trial. Even if it determined the appearance of an immune reaction of T cells, the antitumor effect of the vaccine could not be demonstrated [
58]. Peptide vaccines have been tried in combination with chemotherapy in limited groups of patients, with inconsistent results from the perspective of the correlation of the immune response with the clinical antitumor response. Optimizing the composition and physico-chemical characteristics of the nanoparticles allowed safe delivery to the liver tumor, eradicating up to 70% of the hepatocellular tumor in an experimental murine model [
59].
CA 19-9 is a tumor-associated antigen intensively expressed in pancreatic cancer, therefore it was used as a target antigen for the production of anti-CA 19-9 antibodies, and these in turn represented a protective factor against the progression of pancreatic cancer within murine experimental models. That is why this antigen can be a candidate for the development of an antitumor vaccine against pancreatic cancer [
60].
Antigens specific to pancreatic cancer tumor cells show an important genetic variability, due to their genetic instability. These antigens can be used to sensitize dendritic cells, which subsequently cause an immune response from CD4+ lymphocytes [
61]. MUC-1 peptide is also able to determine an antitumor immune response mediated by dendritic cells in patients with metastatic pancreatic cancer [
62].
5. The tumor microenvironment
Understanding the microenvironment of tumor cells has resulted in the formulation of strategies aimed at augmenting the antitumor efficacy of diverse cytotoxic agents, while concurrently enhancing their targeted delivery at the tumor level. This involves the incorporation of inhibitory factors of the inflammatory response to cytotoxic agents within nanoparticulate complexes.
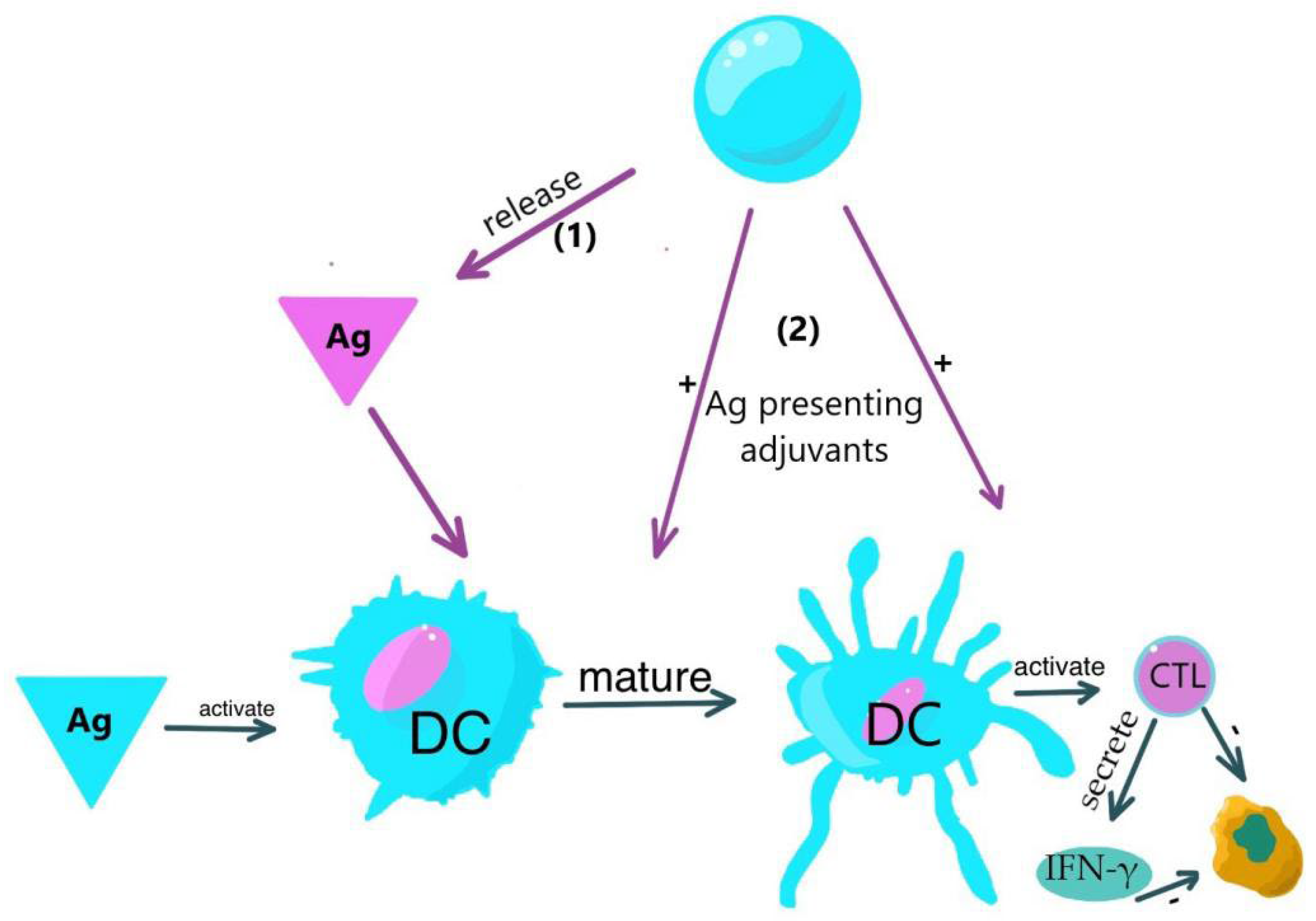
Although dendritic cells (DCs) are typically not abundant at tumor sites, investigations have established a correlation between higher densities of DC populations and improved clinical outcomes, thereby indicating their potential role in regulating cancer progression. Certain subsets of these dendritic cells possess the ability to activate CD4+ helper T cells and CD8+ cytotoxic T cells, both of which are immune cells capable of restraining tumor growth. Experimental models have demonstrated the contribution of dendritic cells to the efficacy of cancer immunotherapies, including the utilization of immune checkpoint blockers such as anti-PD-1. (
Figure 4).
Tumor-associated macrophages (TAMs) have recently garnered significant attention due to their crucial roles in tumor metastasis and therapeutic resistance. TAMs are derived from circulating lymphocyte antigen 6C + (Ly6C) and monocytes originating from the bone marrow as well as from reservoirs located in the spleen [
63]. Upon infiltration into tumors, monocyte-derived macrophages receive inflammatory signals mediated by cytokines released locally from stressed and dying tumor cells [
64]. M1 macrophages are involved in proficient antigen presentation and pathogen eradication; they secrete substantial amounts of pro-inflammatory cytokines and promote TH1 cell responses. On the other hand, M2 macrophages exhibit heightened phagocytic activity but produce lower levels of pro-inflammatory cytokines and higher levels of anti-inflammatory cytokines such as interleukin (IL)-10; these macrophages aid in the resolution of inflammation. Different phenotypes of TAMs can either expedite tumor growth (M2-like cells) or possess tumoricidal roles (M1-like cells) [
51]. Rodell et al. demonstrated that R848, an agonist of the toll-like receptors TLR7 and TLR8 that was identified in a morphometry-based screen, is a potent inducer of the M1 phenotype in vitro. Furthermore, they found that R848-loaded β-cyclodextrin nanoparticles (CDNP-R848) lead to drug efficacy in tumor-associated macrophages in vivo [
65].
Although antiapoptotic protein 1 therapy holds promise as an anticancer treatment, it is plagued by a high incidence of adaptive resistance characterized by the up-regulation of alternative immune checkpoints [
66]. However, a dual glutathione/pH-based nanoplatform has emerged as a potential solution. This nanoplatform sequentially releases 5-aminolevulinic acid and induces immunological responses with doxorubicin molecules, thereby stimulating acidity and reversing the tumor microenvironment. Through its action, this nanoplatform effectively stimulates the immune system by promoting the maturation of dendritic cells and reducing the population of immune-suppressive cells. This augmentation appears as an enhanced adjuvant effect of anti-apoptosis protein 1 therapy [
67].
There exist numerous immune processes of varied antitumor leukocytes, and tumor cells employ various strategies to evade the immune response. The tumor microenvironment plays a role in determining the activated immunosuppressive pathways that suppress antitumor immunity [
68]. These pathways involve immune checkpoint receptors on effector T cells and myeloid cells, as well as the release of inhibitory cytokines and metabolites. Therapeutic approaches that target these pathways, particularly immune checkpoint receptors, have the potential to induce durable antitumor responses in patients with advanced cancer, such as melanoma. Feng et al have developed a prodrug in the form of a nanoparticle that is based on oxaliplatin (OXA) and the activatable homodimer NLG919 (Navoximod) [
69]. This activatable binary cooperative complex enhances the efficacy of immunotherapy through synergistic immune modulation of the tumor microenvironment. The intratumoral activation of the binary complex (OXA-NLG919) determines the accumulation of cytotoxic T lymphocytes within the tumor and reduces the activity of immunosuppressive factors and regulatory T cells in the tumor microenvironment. The binary nature of this complex exhibits significantly greater efficacy compared to free oxaliplatin or the combination of free oxaliplatin and separate administration of NLG919 in terms of tumor growth regression and prevention of metastasis in murine models of breast and colorectal cancer [
70].
Xia Dong et al have developed a nanovaccine for cancer immunotherapy that is composed of self-crosslinked nanoparticles of ovalbumin (NP-OVA) serving as transport and antigen, along with adjuvant molecules (CpG ODNs) [
71]. NP-OVAs not only act as antigens but also serve as natural carriers of CpG ODNs, thereby allowing for continuous immune response stimulation [
72]. As a result, a robust immune response was achieved in both in vitro and in vivo settings, including dendritic cell maturation, T cell activation, and production of IFN-gamma. The NPOVA-CpGODN vaccine induces potent specific antitumor immunity and demonstrates remarkable immunomediated antitumor effects in an experimental mouse model of lymphoma. Additionally, this vaccine can be easily tracked through dual fluorescence, providing a reliable platform for cancer immunoprophylaxis and immunotherapy, as well as a visible system for monitoring the antigen-adjuvant complex.
6. Functionalized nanoparticles
For a generalized clinical approach in individual cancer therapy, it is necessary to find specific tumor peptides adapted individually to the patient. This peptide complex can be obtained by lysing tumor cells [
73]. Following this design, CaP nanoparticles can be functionalized using an entire peptide complex derived from primary tumor cell lysis.
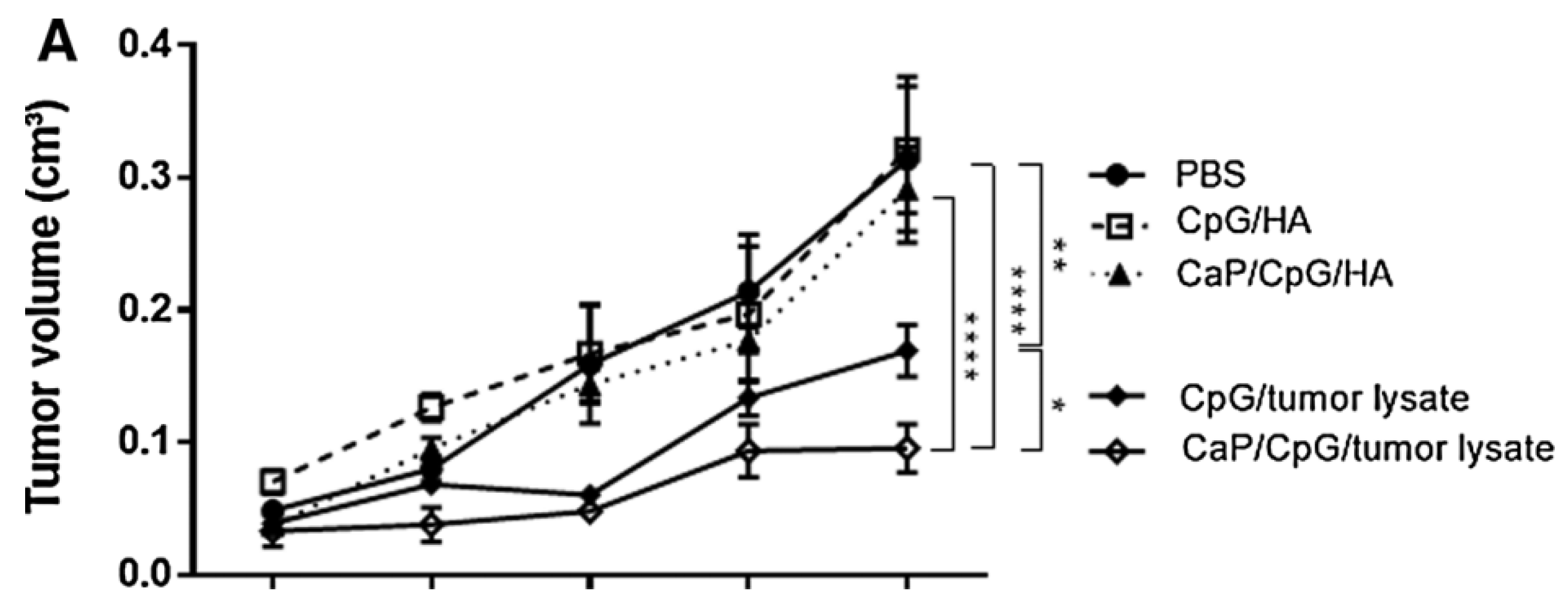
A group led by Carolin Hesse from Essen, Germany, conducted a study using the murine experimental model, in which a Ca phosphate nanoparticle (CaP) was biofunctionalized with tumor antigens from tumor cell lysate and CpG adjuvant molecules. These functionalized NPs showed in vivo a strong suppressive effect of tumor cell mass growth, by activating immunity mediated by specific anti-tumor CD8+ T cells [
74]. Using the murine model with a xenograft tumor expressing the viral antigen hemagglutinin (HA), it was shown that the administration of CaP nanoparticles functionalized with this peptide (HA) and the adjuvant molecule CpG were highly effective in enhancing the antitumor T cell response and suppressing the progression of the tumors. Mice were subcutaneously transplanted with CT26 tumor cells and therapeutically vaccinated with CaP nanoparticles containing CpG and HA peptides or a whole tumor peptide from a cell lysate. Therapeutic vaccination of tumor-bearing mice with CpG and CaP nanoparticle-delivered tumor lysate significantly suppressed tumor growth. Vaccination with soluble and lysed CpG also had a statistically significant effect (P < 0.01), although it induced only a 1.9-fold decrease in tumor volume compared to the 3.2-fold decrease after vaccination with CaP nanoparticles (
Figure 5). In contrast, immunization of CT26 tumor-bearing mice with nanoparticles functionalized with CpG and HA, or with soluble CpG and HA, did not significantly alter tumor growth, underscoring the need for tumor-associated antigens in the vaccine.
Long Chen et al. developed a nanoparticulate delivery system for antigen and an adjuvant based on the cytoplasmic membrane of E. Coli and the membranes of tumor cells, with the aim of activating a sufficiently robust specific antitumor response, without notable side effects. The introduction of this E.Coli membrane adjuvant in the nanoparticle formula of the antitumor vaccine succeeded in increasing the immunogenicity, through the maturation of dendritic cells and the activation of T lymphocytes from the splenic level. This hybrid vaccine based on a nanoparticle loaded with antigens from the tumor cell membrane and with the immunogenic adjuvant of the cytoplasmic membrane of E.Coli proved its effectiveness in the murine experimental model with CT26 colon tumor and 4T1 breast tumor. It also caused a prolonged specific antitumor immune response in CT26 tumors, mediated especially by CD8+ T cells and NK cells [
75].
Nanoparticles of poly-lactic-co-glycolic acid, a polymer known for its ability to protect the antigen encapsulated in it from enzymatic proteolytic action, can be used as delivery vehicles of the antigen from the tumor lysate to the target dendritic cells. Thus, using an experimental murine model of gastric cancer immunotherapy, an important increase in antitumor immunological stimulation mediated by dendritic cells was demonstrated by the presentation of tumor antigen encapsulated in polymeric nanoparticles [
76].
Cholesterol introduced into the hydrophobic polysaccharide pullulan (ChP) forms spontaneously aggregated nanoparticles that can act as a vector for a vaccine based on tumor antigens. This system leads to the activation of CD4+ and CD8+ T lymphocytes. The NY-ESO-1 antigen (New York esophageal squamous cell carcinoma 1) is expressed exclusively in testicular, placental or tumor tissue, which makes it an ideal candidate for use in cancer immunotherapy [
64]. Likewise, the HER2 protein has been used repeatedly in anticancer vaccines, without causing any significant side effects. Both antigens led to the activation of antigen-specific cellular immunity mediated by CD4+ and CD8+ T lymphocytes [
77].
ChP-NY-ESO-1 complexes proved ineffective as an anticancer vaccine in clinical trials, due to interactions with the immunoinhibitory tumor microenvironment. The addition of molecules with an adjuvant role, such as TLR stimulants (OK-432, CpG or poly-ICLC), can make this vaccine more effective in triggering an adequate immune response, without increasing the rate of side effects.
Ishihara et al conducted a phase 1 clinical study in which they used the ChP-NY-ESO-1 protein vaccine and an adjuvant derived from Propionibacterium Acnes, MIS416, with a role in activating the immune response mediated by toll-like receptors 9 and NOD 2 [
78]. Although the safety and tolerability profile was satisfactory, no complete or partial response to this immunotherapy was observed. In general, tumor antigens used only in combination with molecules with an adjuvant role did not demonstrate a satisfactory antitumor effect. The same results were obtained using another adjuvant, polyinosinic-polycytidylic acid (poly I/C) mixed with the stabilizers carboxymethylcellulose and polylysine (Poly ICLC) which is a ligand for the toll-3 receptor [
79]. In the phase 1 clinical study in patients with advanced and recurrent esophageal cancer, the side effects were relatively minor, but no significant tumor response was demonstrated in the patients in the two groups, study and control. However, both groups, both those vaccinated with ChP-NY-ESO-1 alone and those with the combination with Poly ICLC, developed comparable antibody titers, but higher in the group with the combined vaccine. In the murine model, the addition of anti-PD-1 monoclonal antibodies, Nivolumab, in the nanoparticulate vaccine combination led to the suppression of tumor growth in tumors expressing the NY-ESO-1 antigen. The association of an immune checkpoint inhibitor could lead to significantly improved antitumor results in clinical trial scenarios [
80].
Advanced cancer immunotherapy involves systemic administration of drugs. One strategy for improving the antitumor efficiency is to identify the potential for the transport and delivery of compounds with immunological functions directly at the level of the lymph nodes. Thus, interest in the role of biofunctionalized nanoparticles in cancer immunotherapy has increased. Nanostructured lipid carriers are a pharmaceutical vector with significant pharmacodynamic advantages, reduced toxicity and offer increased bioavailability [
81]. Topical immunotherapy has been shown to be effective in skin cancer when using a Toll-Like receptor (TRL) 7 and 8 agonist, but systemic administration is burdened with increased toxicity at therapeutic doses. Widmer et al propose a targeted approach using polymeric nanoparticles in which Requisimob (TRL7 ligand) was encapsulated [
39]. As these nanoparticles are largely captured in macrophages and dendritic cells, which allows specific targeting of lymph nodes, the primary antitumor immune response of these cells is activated [
82].
As a transport vector for cisplatin, this nanoparticle model proved more effective in murine experimental models, in the treatment of ovarian cancer, when it was used in a lipid-polymer hybrid state, with antitumor advantages compared to lipid or polymer transport nanoparticles [
83].
7. Delivery of nanoparticle-based cancer vaccines
Compared to conventional vaccines, cancer vaccines delivered via nanomaterials can be adjusted to desired immune profiles by optimizing the physicochemical properties of the nanomaterial carriers, modifying the nanomaterials with targeting molecules, or co-encapsulating them with immunostimulators. To develop vaccines with desired immunogenicity, a comprehensive understanding of the factors that influence immune responses is necessary. Recently, there has been a focus on the potential of nanoparticles (NPs) as delivery systems for vaccines. The vaccine antigen is either enclosed within or attached to the surface of the NP. By encapsulating the antigenic material, NPs offer a means of delivering antigens that may otherwise degrade rapidly upon injection or induce a short-lived, localized immune response. The conjugation of antigens onto NPs enables the presentation of the immunogen to the immune systems in a manner similar to that of the pathogen, thereby stimulating a comparable response. Furthermore, NPs made from certain composites not only facilitate targeted delivery of antigens, but also allow for the sustained release of antigens to maximize exposure to the immune system. Additionally, researchers are investigating the potential of NPs to deliver vaccines through unconventional methods such as topical, inhalation, or optical delivery, as well as the combination of multiple antigens within a single particle to provide protection against multiple diseases [
87,
88,
89]
Only a small number of nucleic vaccine delivery systems based on nanomaterials have achieved success in their progression to clinical trials, and none have received approval for usage thus far. In human trials, strategies involving nucleic acid vaccines have demonstrated efficacy in inducing specific humoral and cell-mediated immune responses. However, there remain certain limitations when considering therapeutic applications, primarily due to the significantly higher dosage requirements in humans compared to animals. One example of a nanomaterial-based adjuvant that has been investigated in a clinical setting is Vaxfectin, a cationic liposome capable of ionically attaching to DNA and augmenting the immune response against H5N1 influenza-associated proteins, including HA, nucleoproteins, and viroporins.
8. Conclusions
Cancer vaccines must be safe, effective and affordable. We have seen many examples of vaccines effective in developing an anti-tumor immune response. The unique properties of nanoparticles in the development of these vaccines is substantial, due to their safety, controlled release, targeting to DC and improved antigen uptake, as well as enhanced immunogenicity. However, most tumors that become clinically evident have developed a tumor microenvironment that protects them from autoimmunity and inflammation, so previous failures of cancer vaccines are primarily due to the inability of the generated immune response to reach its full potential, the immunoregulatory mechanisms developed in the tumor tissue being extremely effective in counteracting the immune system. Therefore, the combination of cancer vaccines together with treatments that restrict the cancer microenvironment interaction would further improve the immunotherapeutic outcome and have been extensively evaluated both preclinically and clinically [
84].
One of the newest and most efficient methods is the blocking of checkpoint inhibitors, which leads to the amplification of the immune response mediated by T cells, the latter being considered the main effector of cancer vaccines [
85].
Several researches in the literature and clinical trials have suggested that more than 70% of cancers (especially solid tumors) are poorly infiltrated by CD8+ T cells, which are the main causes of therapeutic failure, not only for cancer vaccines but also for point blockade control and CAR-T cell therapy [
86]. Thus, modulators of the tumor environment such as small kinase inhibitors, antibodies or RNAi that modulate the suppressive immune environment (ie siRNA against STAT3, small molecules against CXCR4, tyrosine kinase inhibitor against vascular endothelial growth factor) are very useful additions to the cancer vaccine complex [
90]
Combining the vaccine with chemotherapeutic agents is another option for improving the antitumor efficiency. Their immunomodulatory properties can improve the vaccine-mediated antitumor immune response. Adapted to specific chemotherapeutic treatment schemes, nanoparticles, through their versatility, can help combined therapy reach its full potential.
NPs are good candidates for the delivery of cancer vaccines due to their safety and versatility. The design considerations discussed in the review provide guidance for improving cancer vaccine potency. However, due to the extensive suppressive immune microenvironment, cancer vaccines have dificulties preventing disease recurrence, which requires further regulation of the suppressive tumor microenvironment to enhance in situ T cell penetration and activation. Therefore, we envision that the combination therapy together with intelligent NP design can overcome many of these obstacles.
In conclusion, we consider that nanoparticles, due to their versatility and safety, are very suitable candidates as platforms for the design of vaccines against cancer. However, the interaction with the tumor microenvironment can have an immunosuppressive effect and the solitary effectiveness of vaccines decreases significantly. This fact expresses the need for a favorable modulation of the immunosuppressive tumor microenvironment to improve the penetration and activation of T lymphocytes in situ. Combined therapy, and the complex design of biofunctionalized nanoparticles by associating immunomodulatory molecules should be the way forward in the development of future vaccines against cancer.
Supplementary Materials
Not Applicable.
Author Contributions
Conceptualization, L.M.; methodology, R.Z., C.D. and L.M.; software, R.Z.; validation, R.Z., C.D. and L.M.; formal analysis, R.Z., C.D. and L.M.; investigation, R.Z. , C.D. and L.M.; resources, R.Z, C.D. and L.M.; data curation, R.Z., C.D. and L.M.; writing—original draft preparation, R.Z. and L.M.; writing—review and editing, R.Z., C.D. and L.M. ; visualization, R.Z., C.D. and L.M.; supervision, R.Z. and L.M.; project administration, R.Z. and L.M.; funding acquisition, R.Z. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
List of abbreviations
| Abbreviations |
Expanded terms |
| AFP |
Alpha-fetoprotein |
| APC |
Antigen-presenting cell |
| CaP |
Calcium Phosphate |
| CAR-T cells |
T-cells with chimeric antigen receptors (CARs) on their surface. |
| CEA |
Carcino-embryonic antigen |
| ChP |
Cholesterol - Pullulan |
| CpG |
Cytosine -phosphate - Guanine dinucleotide |
| CpG ODN |
Single stranded synthetic DNA molecule that contain a cytosine triphosphate deoxynycleotide followed by a guanine triphosphate deoxynucleotide |
| CXCR4 |
C-X-C chemokine receptor type 4 |
| DC |
Dendritic cell |
| DEPC1 |
Diethyl pyrocarbonate 1 |
| FOXM1 |
Forkhead box protein |
| G17DT |
Gastrimmune |
| GNP |
Gold nanoparticle |
| HA |
Hemagglutinin |
| KIF20A |
Kinesin-like protein |
| MAGE |
melanoma-associated antigen |
| MSN |
Mesoporous silicon nanoparticle |
| MUC-1 |
Mucin-1 |
| MWCNT |
Multi-walled carbon nanotube |
| NLG919 |
Navoximod |
| NP |
Nanoparticle |
| NP-PDA |
Polydopamine nanoparticle |
| NY-ESO-1 |
New York esophageal squamous cell carcinoma 1 |
| ODN |
Oligodeoxynucleotide |
| OVA |
Ovalbumin |
| OXA |
Oxaliplatin |
| PBS |
Phosphate-buffered saline |
| PEG |
Poly-ethylene glycol |
| PEI |
Poly-ethyleneimine |
| PGA |
Poly-glutamic acid |
| PLGA |
Poly-lactic co-glycolic acid |
| Poly I:C: |
Polyinosinic:polycytidylic acid |
| RNAi |
RNA interference |
| siRNA |
small interfering RNA |
| STAT3 |
Signal transducer and activator of transcription 3 |
| TAA |
Tumor-associated antigen |
| TAM |
Tumor-associated macrophage |
| TCL |
Tumor cell lysis/lysate |
| TLR |
Toll-like receptor |
| URLC10 |
Up-regulated in lung cancer 10 |
| VEGFR1 |
Vascular endothelial growth factor receptor 1 |
References
- Liu J, Miao L, Sui J, Hao Y, Huang G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J Pharm Sci [Internet]. 2020;15(5):576–90. [CrossRef]
- Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release [Internet]. 2006;112(1):26–34. [CrossRef]
- Walter E, Dreher D, Kok M, Thiele L, Kiama SG, Gehr P, et al. Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. J Control Release [Internet]. 2001;76(1–2):149–68. [CrossRef]
- Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett [Internet]. 2014;14(4):2181–8. [CrossRef]
- Chang H-C, Zou Z-Z, Wang Q-H, Li J, Jin H, Yin Q-X, et al. Targeting and specific activation of antigen-presenting cells by endogenous antigen-loaded nanoparticles elicits tumor-specific immunity. Adv Sci (Weinh) [Internet]. 2020;7(1):1900069. [CrossRef]
- Xu J., Wang H., Xu L., Chao Y., Wang C., Han X., et al.. (2019). Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials 207, 1–9. [CrossRef]
- Chen H., Fan Y., Hao X., Yang C., Peng Y., Guo R., et al.. (2020). Adoptive cellular immunotherapy of tumors via effective CpG delivery to dendritic cells using dendrimer-entrapped gold nanoparticles as a gene vector. J. Mater. Chem. B 8, 5052–5063. [CrossRef]
- Yu A, Dai X, Wang Z, Chen H, Guo B, Huang L. Recent advances of mesoporous silica as a platform for cancer immunotherapy. Biosensors (Basel) [Internet]. 2022;12(2):109. [CrossRef]
- Ong C, Cha BG, Kim J. Mesoporous silica nanoparticles doped with gold nanoparticles for combined cancer immunotherapy and photothermal therapy. ACS Appl Bio Mater [Internet]. 2019;2(8):3630–8. [CrossRef]
- Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials [Internet]. 2014;35(9):3091–101. [CrossRef]
- Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine [Internet]. 2014;32(48):6377–89. [CrossRef]
- Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic nanoparticles in cancer theranostics. Theranostics [Internet]. 2015;5(11):1249–63. [CrossRef]
- Luo L, Iqbal MZ, Liu C, Xing J, Akakuru OU, Fang Q, et al. Engineered nano-immunopotentiators efficiently promote cancer immunotherapy for inhibiting and preventing lung metastasis of melanoma. Biomaterials [Internet]. 2019;223(119464):119464. [CrossRef]
- Zeng Q., Li H., Jiang H., Yu J., Wang Y., Ke H., et al.. (2017). Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 122, 105–113. [CrossRef]
- Li H., Li Y., Wang X., Hou Y., Hong X., Gong T., et al.. (2017). Rational design of polymeric hybrid micelles to overcome lymphatic and intracellular delivery barriers in cancer immunotherapy. Theranostics 7:4383. [CrossRef]
- Liu Q, Zhou Y, Li M, Zhao L, Ren J, Li D, et al. Polyethylenimine hybrid thin-shell hollow mesoporous silica nanoparticles as vaccine self-adjuvants for cancer immunotherapy. ACS Appl Mater Interfaces [Internet]. 2019;11(51):47798–809. [CrossRef]
- Cha BG, Jeong JH, Kim J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent Sci [Internet]. 2018;4(4):484–92. [CrossRef]
- Dong Z, Wang Q, Huo M, Zhang N, Li B, Li H, et al. Mannose-modified multi-walled carbon nanotubes as a delivery nanovector optimizing the antigen presentation of dendritic cells. ChemistryOpen [Internet]. 2019;8(7):915–21. [CrossRef]
- Xia Q, Gong C, Gu F, Wang Z, Hu C, Zhang L, et al. Functionalized Multi-walled carbon nanotubes for targeting delivery of immunostimulatory CpG oligonucleotides against prostate cancer. J Biomed Nanotechnol [Internet]. 2018;14(9):1613–26. [CrossRef]
- Zeng B., Middelberg A. P. J., Gemiarto A., MacDonald K., Baxter A. G., Talekar M., et al.. (2018). Self-adjuvanting nanoemulsion targeting dendritic cell receptor Clec9A enables antigen-specific immunotherapy. J. Clin. Invest. 128, 1971–1984. [CrossRef]
- Kim S.-Y., Kim S., Kim J.-E., Lee S. N., Shin I. W., Shin H. S., et al.. (2019). Lyophilizable and multifaceted toll-like receptor 7/8 agonist-loaded nanoemulsion for the reprogramming of tumor microenvironments and enhanced cancer immunotherapy. ACS Nano 13, 12671–12686. [CrossRef]
- Wang C., Li P., Liu L., Pan H., Li H., Cai L., et al.. (2016). Self-adjuvanted nanovaccine for cancer immunotherapy: role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 79, 88–100. [CrossRef]
- Li D., Sun F., Bourajjaj M., Chen Y., Pieters E. H., Chen J., et al.. (2016). Strong in vivo antitumor responses induced by an antigen immobilized in nanogels via reducible bonds. Nanoscale 8, 19592–19604. [CrossRef]
- Miura R., Sawada S., Mukai S., Sasaki Y., Akiyoshi K. (2019). Antigen delivery to antigen-presenting cells for adaptive immune response by self-assembled anionic polysaccharide nanogel vaccines. Biomacromolecules 21, 621–629. [CrossRef]
- Feng X, Xu W, Li Z, Song W, Ding J, Chen X. Immunomodulatory nanosystems. Adv Sci (Weinh) [Internet]. 2019;6(17):1900101. [CrossRef]
- Da Silva CG, Camps MGM, Li TMWY, Chan AB, Ossendorp F, Cruz LJ. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials [Internet]. 2019;220(119417):119417. [CrossRef]
- Molino N. M., Anderson A. K. L., Nelson E. L., Wang S.-W. (2013). Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 7, 9743–9752. [CrossRef]
- Molino N. M., Neek M., Tucker J. A., Nelson E. L., Wang S.-W. (2016). Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials 86, 83–91. [CrossRef]
- Lizotte P. H., Wen A. M., Sheen M. R., Fields J., Rojanasopondist P., Steinmetz N. F., et al.. (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 11, 295–303. [CrossRef]
- Cai H., Shukla S., Steinmetz N. F. (2020). The antitumor efficacy of CpG Oligonucleotides is improved by encapsulation in plant virus-like particles. Adv. Funct. Mater. 30:1908743. [CrossRef]
- Sobhani N, Scaggiante B, Morris R, Chai D, Catalano M, Tardiel-Cyril DR, et al. Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials. Cancer Treat Rev [Internet]. 2022;109(102429):102429. [CrossRef]
- Elizondo CR, Bright JD, Bright RK. Vaccination with a shared oncogenic tumor-self antigen elicits a population of CD8+ T cells with a regulatory phenotype. Hum Vaccin Immunother [Internet]. 2022;2108656. [CrossRef]
- Caro AA, Deschoemaeker S, Allonsius L, Coosemans A, Laoui D. Dendritic cell vaccines: A promising approach in the fight against ovarian cancer. Cancers (Basel) [Internet]. 2022;14(16):4037. [CrossRef]
- Pancisi E, Granato AM, Scarpi E, Ridolfi L, Carloni S, Moretti C, et al. Stability program in dendritic cell vaccines: A “real-world” experience in the immuno-gene therapy factory of Romagna cancer center. Vaccines (Basel) [Internet]. 2022;10(7):999. [CrossRef]
- Hannan R, Dohopolski MJ, Pop LM, Mannala S, Watumull L, Mathews D, et al. Phase II trial of sipuleucel-T and stereotactic ablative body radiation for patients with metastatic castrate-resistant prostate cancer. Biomedicines [Internet]. 2022;10(6):1419. [CrossRef]
- Du Y, Liu Y, Wang D, Bai H, Wang Z, He X, et al. Peptidic microarchitecture-trapped tumor vaccine combined with immune checkpoint inhibitor or PI3Kγ inhibitor can enhance immunogenicity and eradicate tumors. J Immunother Cancer [Internet]. 2022;10(2):e003564. [CrossRef]
- Bordoloi D, Xiao P, Choi H, Ho M, Perales-Puchalt A, Khoshnejad M, et al. Immunotherapy of prostate cancer using novel synthetic DNA vaccines targeting multiple tumor antigens. Genes Cancer [Internet]. 2021;12:51–64. [CrossRef]
- Gamat-Huber M, Jeon D, Johnson LE, Moseman JE, Muralidhar A, Potluri HK, et al. Treatment combinations with DNA vaccines for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Cancers (Basel) [Internet]. 2020;12(10):2831. [CrossRef]
- Dai D, Yin Y, Hu Y, Lu Y, Zou H, Lu G, et al. Tumor RNA-loaded nanoliposomes increases the anti-tumor immune response in colorectal cancer. Drug Deliv [Internet]. 2021;28(1):1548–61. [CrossRef]
- Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials [Internet]. 2021;266(120431):120431. [CrossRef]
- Ickenstein LM, Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv [Internet]. 2019;16(11):1205–26. [CrossRef]
- Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale [Internet]. 2019;11(45):21733–9. [CrossRef]
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov [Internet]. 2018;17(4):261–79. [CrossRef]
- Dailey GP, Crosby EJ, Hartman ZC. Cancer vaccine strategies using self-replicating RNA viral platforms. Cancer Gene Ther [Internet]. 2022. [CrossRef]
- Cai Z, Xin F, Wei Z, Wu M, Lin X, Du X, et al. Photodynamic therapy combined with antihypoxic signaling and CpG adjuvant as an in situ tumor vaccine based on metal-organic framework nanoparticles to boost cancer immunotherapy. Adv Healthc Mater [Internet]. 2020;9(1):e1900996. [CrossRef]
- Liang W. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive traet. World J Gastroenterol [Internet]. 2003;9(3):495. [CrossRef]
- Dailey GP, Crosby EJ, Hartman ZC. Cancer vaccine strategies using self-replicating RNA viral platforms. Cancer Gene Ther [Internet]. 2022. [CrossRef]
- Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) [Internet]. 2013;6(1):18–26. [CrossRef]
- Shaib W, Goldstein D, El-Rayes BF. Peptide vaccines for treatment of colon cancer: Have we made progress? Curr Colorectal Cancer Rep [Internet]. 2014;10(4):477–86. [CrossRef]
- Wang X, Wang N, Yang Y, Wang X, Liang J, Tian X, et al. Polydopamine nanoparticles carrying tumor cell lysate as a potential vaccine for colorectal cancer immunotherapy. Biomater Sci [Internet]. 2019;7(7):3062–75. [CrossRef]
- Dysthe M, Parihar R. Myeloid-derived suppressor cells in the tumor microenvironment. Adv Exp Med Biol [Internet]. 2020;1224:117–40. [CrossRef]
- Taleuzzaman M, Sartaj A, Vijay N, Alam MJ. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. In: Nanotherapeutics in Cancer Vaccination and Challenges. Elsevier; 2022. p. 129–48.
- Thakur N, Thakur S, Chatterjee S, Das J, Sil PC. Nanoparticles as smart carriers for enhanced cancer immunotherapy. Front Chem [Internet]. 2020;8:597806. [CrossRef]
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An update. Bioeng Transl Med [Internet]. 2019;4(3):e10143. [CrossRef]
- Ajani JA, Randolph Hecht J, Ho L, Baker J, Oortgiesen M, Eduljee A, et al. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer : The GC4 study. Cancer [Internet]. 2006;106(9):1908–16. [CrossRef]
- Fujiwara Y, Okada K, Omori T, Sugimura K, Miyata H, Ohue M, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol [Internet]. 2017;50(5):1655–62. [CrossRef]
- Avgustinovich AV, Bakina OV, Afanas’ev SG, Cheremisina OV, Spirina LV, Dobrodeev AY, et al. Nanoparticles in gastric cancer management. Curr Pharm Des [Internet]. 2021;27(21):2436–44. [CrossRef]
- Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother [Internet]. 2007;56(12):1931–43. [CrossRef]
- Kong F-H, Ye Q-F, Miao X-Y, Liu X, Huang S-Q, Xiong L, et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics [Internet]. 2021;11(11):5464–90. [CrossRef]
- Ziske C, Märten A, Schöttker B, Buttgereit P, Schakowski F, Gorschlüter M, et al. Resistance of pancreatic carcinoma cells is reversed by coculturing NK-like T cells with dendritic cells pulsed with tumor-derived RNA and CA 19-9. Mol Ther [Internet]. 2001;3(1):54–60. [CrossRef]
- Matsui H, Hazama S, Shindo Y, Nagano H. Combination treatment of advanced pancreatic cancer using novel vaccine and traditional therapies. Expert Rev Anticancer Ther [Internet]. 2018;18(12):1205–17. [CrossRef]
- Gong Y-F, Zhou Q-B, Liao Y-D, Mai C, Chen T-J, Tang Y-Q, et al. Optimized construction of MUC1-VNTRn DNA vaccine and its anti-pancreatic cancer efficacy. Oncol Lett [Internet]. 2017;13(4):2198–206. [CrossRef]
- Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater [Internet]. 2021;6(7):1973–87. [CrossRef]
- Bronte V, Murray PJ. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat Med [Internet]. 2015;21(2):117–9. [CrossRef]
- Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng [Internet]. 2018;2(8):578–88. [CrossRef]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun [Internet]. 2016;7(1):10501. [CrossRef]
- Bai S, Yang L-L, Wang Y, Zhang T, Fu L, Yang S, et al. Prodrug-based versatile nanomedicine for enhancing cancer immunotherapy by increasing immunogenic cell death. Small [Internet]. 2020;16(19):e2000214. [CrossRef]
- Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol [Internet]. 2016;13(3):143–58. [CrossRef]
- Feng B, Zhou F, Hou B, Wang D, Wang T, Fu Y, et al. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater [Internet]. 2018;30(38):1803001. [CrossRef]
- Shen F, Feng L, Zhu Y, Tao D, Xu J, Peng R, et al. Oxaliplatin-/NLG919 prodrugs-constructed liposomes for effective chemo-immunotherapy of colorectal cancer. Biomaterials [Internet]. 2020;255(120190):120190. [CrossRef]
- Dong X, Liang J, Yang A, Qian Z, Kong D, Lv F. A visible codelivery nanovaccine of antigen and adjuvant with self-carrier for cancer immunotherapy. ACS Appl Mater Interfaces [Internet]. 2019;11(5):4876–88. [CrossRef]
- Shirota H, Tross D, Klinman DM. CpG oligonucleotides as cancer vaccine adjuvants. Vaccines (Basel) [Internet]. 2015;3(2):390–407. [CrossRef]
- Berzofsky JA, Terabe M, Wood LV. Strategies to use immune modulators in therapeutic vaccines against cancer. Semin Oncol [Internet]. 2012;39(3):348–57. [CrossRef]
- Heße C, Kollenda S, Rotan O, Pastille E, Adamczyk A, Wenzek C, et al. A tumor-peptide-based nanoparticle vaccine elicits efficient tumor growth control in antitumor immunotherapy. Mol Cancer Ther [Internet]. 2019;18(6):1069–80. [CrossRef]
- Chen L, Qin H, Zhao R, Zhao X, Lin L, Chen Y, et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med [Internet]. 2021;13(601):eabc2816. [CrossRef]
- Kohnepoushi C, Nejati V, Delirezh N, Biparva P. Poly lactic-co-glycolic acid nanoparticles containing human gastric tumor lysates as antigen delivery vehicles for dendritic cell-based antitumor immunotherapy. Immunol Invest [Internet]. 2019;48(8):794–808. [CrossRef]
- Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, Naota H, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res [Internet]. 2006;12(24):7397–405. [CrossRef]
- Ishihara M, Tono Y, Miyahara Y, Muraoka D, Harada N, Kageyama S, et al. First-in-human phase I clinical trial of the NY-ESO-1 protein cancer vaccine with NOD2 and TLR9 stimulants in patients with NY-ESO-1-expressing refractory solid tumors. Cancer Immunol Immunother [Internet]. 2020;69(4):663–75. [CrossRef]
- Kawabata R, Wada H, Isobe M, Saika T, Sato S, Uenaka A, et al. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int J Cancer [Internet]. 2007;120(10):2178–84. [CrossRef]
- Ishikawa T, Kageyama S, Miyahara Y, Okayama T, Kokura S, Wang L, et al. Safety and antibody immune response of CHP-NY-ESO-1 vaccine combined with poly-ICLC in advanced or recurrent esophageal cancer patients. Cancer Immunol Immunother [Internet]. 2021;70(11):3081–91. [CrossRef]
- Nakamura T, Harashima H. Dawn of lipid nanoparticles in lymph node targeting: Potential in cancer immunotherapy. Adv Drug Deliv Rev [Internet]. 2020;167:78–88. [CrossRef]
- Widmer J, Thauvin C, Mottas I, Nguyen VN, Delie F, Allémann E, et al. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int J Pharm [Internet]. 2018;535(1–2):444–51. [CrossRef]
- Zhang Y, Zhang P, Zhu T. Ovarian carcinoma biological nanotherapy: Comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed Pharmacother [Internet]. 2019;109:475–83. [CrossRef]
- Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest [Internet]. 2015;125(9):3401–12. [CrossRef]
- Mougel A, Terme M, Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol [Internet]. 2019;10:467. [CrossRef]
- Geiger JL, Grandis JR, Bauman JE. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol [Internet]. 2016;56:84–92. [CrossRef]
- Chen, S.; Huang, X.; Xue, Y.; Álvarez-Benedicto, E.; Shi, Y.; Chen, W.; Koo, S.; Siegwart, D.J.; Dong, Y.; Tao, W. Nanotechnology-based mRNA vaccines. Nature Reviews Methods Primers 2023, 3, 63.
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nature Reviews Materials 2021, 6, 1078-1094.
- Li, B.; Jiang, A.Y.; Raji, I.; Atyeo, C.; Raimondo, T.M.; Gordon, A.G.; Rhym, L.H.; Samad, T.; MacIsaac, C.; Witten, J. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nature Biomedical Engineering 2023, 1-18.
- Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci [Internet]. 2018;109(3):523–30. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).