Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
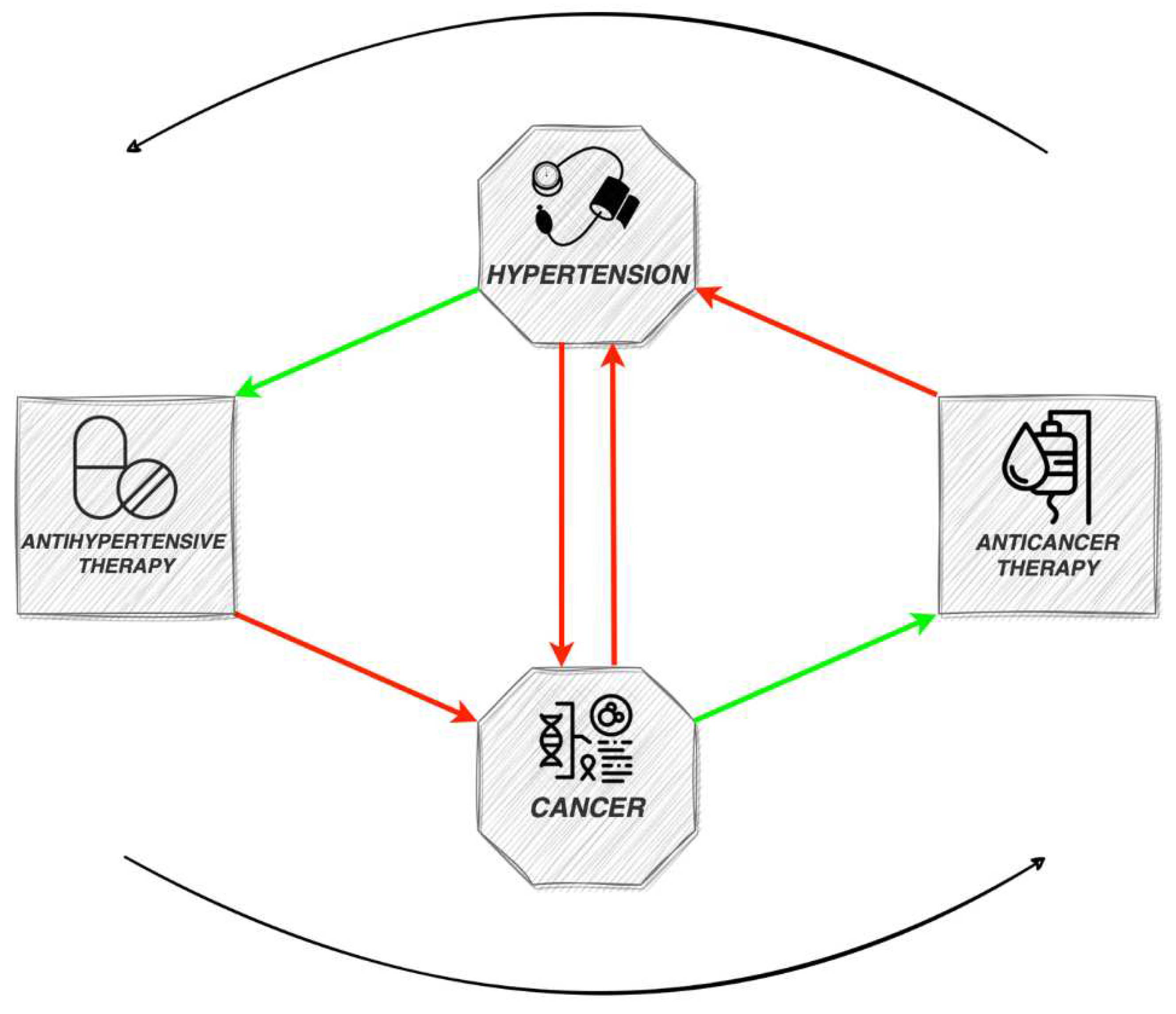
1. Introduction: Cancer and Hypertension
2. Anticancer therapy and hypertension
3. Hypertension as a possible risk factor for cancer development
4. Management of hypertension related to anticancer drugs
5. Antihypertensive drugs and carcinogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dorst, D.C.H.; Dobbin, S.J.H.; Neves, K.B.; Herrmann, J.; Herrmann, S.M.; Versmissen, J.; Mathijssen, R.H.J.; Danser, A.H.J.; Lang, N.N. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ Res 2021, 128, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Angel-Korman, A.; Rapoport, V.; Leiba, A. The Relationship between Hypertension and Cancer. Isr Med Assoc J 2022, 24, 165–169. [Google Scholar] [PubMed]
- Cohen, J.B.; Brown, N.J.; Brown, S.A.; Dent, S.; Van Dorst, D.C.H.; Herrmann, S.M.; Lang, N.N.; Oudit, G.Y.; Touyz, R.M. Cancer Therapy-Related Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2023, 80, E46–E57. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernánde, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klei, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Tocci, G.; Battistoni, A.; Sarocchi, M.; Pietrantoni, C.; Russo, D.; Musumeci, B.; Savoia, C.; Volpe, M.; Spallarossa, P. Role of Arterial Hypertension and Hypertension-Mediated Organ Damage in Cardiotoxicity of Anticancer Therapies. Curr Heart Fail Rep 2023, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Mincu, R.I.; Mrotzek, S.; Schadendorf, D.; Rassaf, T. Cardiovascular Diseases in Patients Receiving Small Molecules with Anti-Vascular Endothelial Growth Factor Activity: A Meta-Analysis of Approximately 29,000 Cancer Patients. Eur J Prev Cardiol 2018, 25, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Singh, M.; Tiu, J.G.; Kim, A.S. Etiology and Management of Hypertension in Patients with Cancer. Cardio-Oncology, 2021; 7. [Google Scholar] [CrossRef]
- Sagstuen, H.; Aass, N.; Fosså, S.D.; Dahl, O.; Klepp, O.; Wist, E.A.; Wilsgaard, T.; Bremnes, R.M. Blood Pressure and Body Mass Index in Long-Term Survivors of Testicular Cancer. Journal of Clinical Oncology 2005, 23, 4980–4990. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or Bortezomib in Relapsed or Refractory Multiple Myeloma (ENDEAVOR): An Interim Overall Survival Analysis of an Open-Label, Randomised, Phase 3 Trial. Lancet Oncol 2017, 18, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. New England Journal of Medicine 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Friedlander, M.; Lee, Y.C.; Tew, W.P. Managing Adverse Effects Associated With Poly (ADP-Ribose) Polymerase Inhibitors in Ovarian Cancer: A Synthesis of Clinical Trial and Real-World Data. American Society of Clinical Oncology Educational Book 2023. [Google Scholar] [CrossRef]
- Fleming, M.R.; Xiao, L.; Jackson, K.D.; Beckman, J.A.; Barac, A.; Moslehi, J.J. Vascular Impact of Cancer Therapies: The Case of BTK (Bruton Tyrosine Kinase) Inhibitors. Circ Res 2021, 128, 1973–1987. [Google Scholar] [CrossRef]
- Cardiovascular Adverse Effects of Novel Bruton Tyrosine Kinase Inhibitors: What All Cardiologists Should Know - American College of Cardiology. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2023/08/15/16/45/CV-Adverse-Effects-of-Novel-Bruton-Tyrosine-Kinase-Inhibitors (accessed on 22 January 2024).
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; Nuri Yenerel, M.; Ilí es, A.; Kay, N.; Garcia-Marco, J.A.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial; 2021; Vol. 39; [CrossRef]
- Tam, C.S.; Opat, S.; D’sa, S.; Jurczak, W.; Lee, H.-P.; Cull, G.; Owen, R.G.; Marlton, P.; Bj¨, B.; Wahlin, B.E.; et al. A Randomized Phase 3 Trial of Zanubrutinib vs Ibrutinib in Symptomatic Waldenström Macroglobulinemia: The ASPEN Study; Vol. 17; [CrossRef]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; Lamanna, N.; O, S.M.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; SimkovičSimkoviˇSimkovič, M.; et al. Zanubrutinib Versus Ibrutinib in Relapsed/ Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial. J Clin Oncol 2022, 41, 1035–1045. [Google Scholar] [CrossRef]
- Chuquin, D.; Abbate, A.; Bottinor, W. Hypertension in Cancer Survivors: A Review of the Literature and Suggested Approach to Diagnosis and Treatment. J Cardiovasc Pharmacol 2022, 80, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Weikert, S.; Boeing, H.; Pischon, T.; Weikert, C.; Olsen, A.; Tjonneland, A.; Overvad, K.; Becker, N.; Linseisen, J.; Trichopoulou, A.; et al. Blood Pressure and Risk of Renal Cell Carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol 2008, 167, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Han, K. Do; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Hypertension and Blood Pressure with Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension 2020, 75, 1439–1446. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur Heart J 2021, 42, 2439–2454. [CrossRef] [PubMed]
- SCORE2-OP working group and ESC Cardiovascular risk collaboration SCORE2-OP Risk Prediction Algorithms: Estimating Incident Cardiovascular Event Risk in Older Persons in Four Geographical Risk Regions. Eur Heart J 2021, 42, 2455–2467. [CrossRef]
- Small, H.Y.; Montezano, A.C.; Rios, F.J.; Savoia, C.; Touyz, R.M. Hypertension Due to Antiangiogenic Cancer Therapy with Vascular Endothelial Growth Factor Inhibitors: Understanding and Managing a New Syndrome. Canadian Journal of Cardiology 2014, 30, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, S.J.H.; Cameron, A.C.; Petrie, M.C.; Jones, R.J.; Touyz, R.M.; Lang, N.N. Toxicity of Cancer Therapy: What the Cardiologist Needs to Know about Angiogenesis Inhibitors. Heart 2018, 104, 1995–2002. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement of Arterial Hypertension. Eur Heart J 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol 2019, 1, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Https://Www.Fda.Gov/. Available online: Https://Www.Fda.Gov/News-Events/Press-Announcements/Fda-Statement-Fdas-Ongoing-Investigation-Valsartan-and-Arb-Class-Impurities-and-Agencys-Steps (Accessed on 15 January 2024).
- Flaherty, K.T.; Fuchs, C.S.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Willett, W.C.; Curhan, G.C. A Prospective Study of Body Mass Index, Hypertension, and Smoking and the Risk of Renal Cell Carcinoma (United States). Cancer Causes and Control 2005, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, J.K.; Chow, W.H.; Mandel, J.; Mellemgaard, A.; Mccredie, M.; Lindblad, P.; Schlehofer, B.; Pommer, W.; I W A, S.N.; Ada, H.-O.M. International Renal-Cell Cancer Study. VIII. Role of Diuretics, Other Anti-Hypertensive Medications and Hypertension. Int J Cancer 1995, 63, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, E.S.; Kim, J.; Guerra, L.; Naik, D.; Prida, X. Association Between the Use of Thiazide Diuretics and the Risk of Skin Cancers: A Meta-Analysis of Observational Studies. J Clin Med Res 2019, 11, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Nakatani, E.; Sasaki, H.; Miyachi, Y. Hydrochlorothiazide Increases Risk of Nonmelanoma Skin Cancer in an Elderly Japanese Cohort with Hypertension: The Shizuoka Study. JAAD Int 2023, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Pedersen, S.A.; Schmidt, S.A.J.; Hölmich, L.R.; Friis, S.; Gaist, D. Association of Hydrochlorothiazide Use and Risk of Malignant Melanoma. JAMA Intern Med 2018, 178, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Nochaiwong, S.; Chuamanochan, M.; Ruengorn, C.; Noppakun, K.; Awiphan, R.; Phosuya, C.; Tovanabutra, N.; Chiewchanvit, S.; Sood, M.M.; Hutton, B.; et al. Use of Thiazide Diuretics and Risk of All Types of Skin Cancers: An Updated Systematic Review and Meta-Analysis. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Shao, S.C.; Lai, C.C.; Chen, Y.H.; Lai, E.C.C.; Hung, M.J.; Chi, C.C. Associations of Thiazide Use with Skin Cancers: A Systematic Review and Meta-Analysis. BMC Med 2022, 20. [Google Scholar] [CrossRef]
- Walther, T.; Menrad, A.; Orzechowski, H.-D.; Siemeister, G.; Paul, M.; Schirner, M. Differential Regulation of in Vivo Angiogenesis by Angiotensin II Receptors. FASEB J 2003, 17, 2061–2067. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Togias, A.; Proud, D. Airway Neural Responses to Kinins Tachyphylaxis and Role of Receptor Subtypes. Am J Respir Crit Care Med 1999, 159, 431–438. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of Substance P and the NK-1 Receptor in Human Pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Almutlaq, M.; Alamro, A.A.; Alamri, H.S.; Alghamdi, A.A.; Barhoumi, T. The Effect of Local Renin Angiotensin System in the Common Types of Cancer. Front Endocrinol (Lausanne) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Hicks, B.M.; Filion, K.B.; Yin, H.; Sakr, L.; Udell, J.A.; Azoulay, L. Angiotensin Converting Enzyme Inhibitors and Risk of Lung Cancer: Population Based Cohort Study. BMJ 2018, 363, k4209. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I.; Chou, J.; Mishra, P.; Debanne, S.M.; Simon, D.I.; Fang, J.C. Meta-Analysis of Randomized Controlled Trials on Effect of Angiotensin-Converting Enzyme Inhibitors on Cancer Risk. American Journal of Cardiology 2011, 108, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Copland, E.; Canoy, D.; Nazarzadeh, M.; Bidel, Z.; Ramakrishnan, R.; Woodward, M.; Chalmers, J.; Teo, K.K.; Pepine, C.J.; Davis, B.R.; et al. Antihypertensive Treatment and Risk of Cancer: An Individual Participant Data Meta-Analysis. Lancet Oncol 2021, 22, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I.; Simon, D.I.; Fang, J.C.; of Epidemiology, D.; M Debanne, B.S.; Rowland, D.Y.; Western, C.; Sipahi, I.; Debanne, S.M.; Rowland, D.Y.; et al. Angiotensin-Receptor Blockade and Risk of Cancer: Meta-Analysis of Randomised Controlled Trials. Lancet Oncology 2010, 11, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I. Risk of Cancer with Angiotensin-Receptor Blockers Increases with Increasing Cumulative Exposure: Meta-Regression Analysis of Randomized Trials. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- ARB Trialists Collaboration Effects of Telmisartan, Irbesartan, Valsartan, Candesartan, and Losartan on Cancers in 15 Trials Enrolling 138 769 Individuals. J Hypertens 2011, 29, 623–635. [CrossRef] [PubMed]
- Azoulay, L.; Soldera, S.; Yin, H.; Bouganim, N. Use of Calcium Channel Blockers and Risk of Breast Cancer: A Population-Based Cohort Study. Epidemiology 2016, 27, 594–601. [Google Scholar] [CrossRef]
- Wilson, L.E.; D’Aloisio, A.A.; Sandler, D.P.; Taylor, J.A. Long-Term Use of Calcium Channel Blocking Drugs and Breast Cancer Risk in a Prospective Cohort of US and Puerto Rican Women. Breast Cancer Research 2016, 18. [Google Scholar] [CrossRef]
- Minegishi, S. Use of Calcium Channel Blockers and Risk of Breast Cancer among Women Aged 55 Years and Older: A Nationwide Population-Based Cohort Study. Hypertension Research 2023, 46, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



