1. Introduction
Silicones are important elastomeric materials for industry as they cover a large range of properties and application fields, from building, medical, cosmetic or aeronautical domains. Whereas most elastomers available on the market are filled and chemically crosslinked networks, more and more works describe the generation of a new type of thermoplastic silicone elastomers (TPS) [
1]. They present two main advantages compared to their crosslinked competitors: they are transparent and can be easily reprocessed by thermal curing.
One way of generating thermoplastic elastomer is through the generation and self-assembly of block copolymers. Those made of silicones are however neither conventional nor commercial. This fact mostly relies on the inherent chemistry of silicones, that is generally very specific and molecular (e.g. hydrosilylation [
2] or Piers-Rubinsztajn [
3] reactions). We have published some time ago a review on silicone copolymers made by radical means [
4], descriptions of other chemistries are also available here [
5].
Since commercial silicones are most often telechelic polymers, it is also possible to grow multiblock copolymers quite easily. This is the principle of silicone-urea family, mostly studied by Yilgör et al. [
6], and that were later converted into an industrial grade (Geniomer© from Wacker Chemie). They are constituted of high molecular weight segmented copolymers of polydimethylsiloxane (PDMS) soft sequences alternated with model hard segments capable of forming hydrogen bonding (e.g. urea, N-methyl urea and urethane). Thanks to the important differences in solubility parameters between hard and soft segments, all copolymers show efficient microphase separation. It has been demonstrated that thermal and mechanical properties are directly linked to the strength of the hydrogen bonds. With milder interacting block, i.e. bisamide [
7], silicone copolymers are much softer but exhibit self-healing propensity.
Another way of doing is to attach strongly-interacting stickers onto the polymer chains by grafting reactions. Advanced materials based on hydrogen, ionic and dative interactions have for instance recently emerged, as reviewed here [
8]. In particular, the strong bonds generated by metal ions/counterions generally allow building interesting mechanical properties while maintaining a very large elasticity not attainable by conventional crosslinked elastomers. The main general drawbacks of all these elastomers nevertheless are their limited thermal stability and lack of solvent resistance.
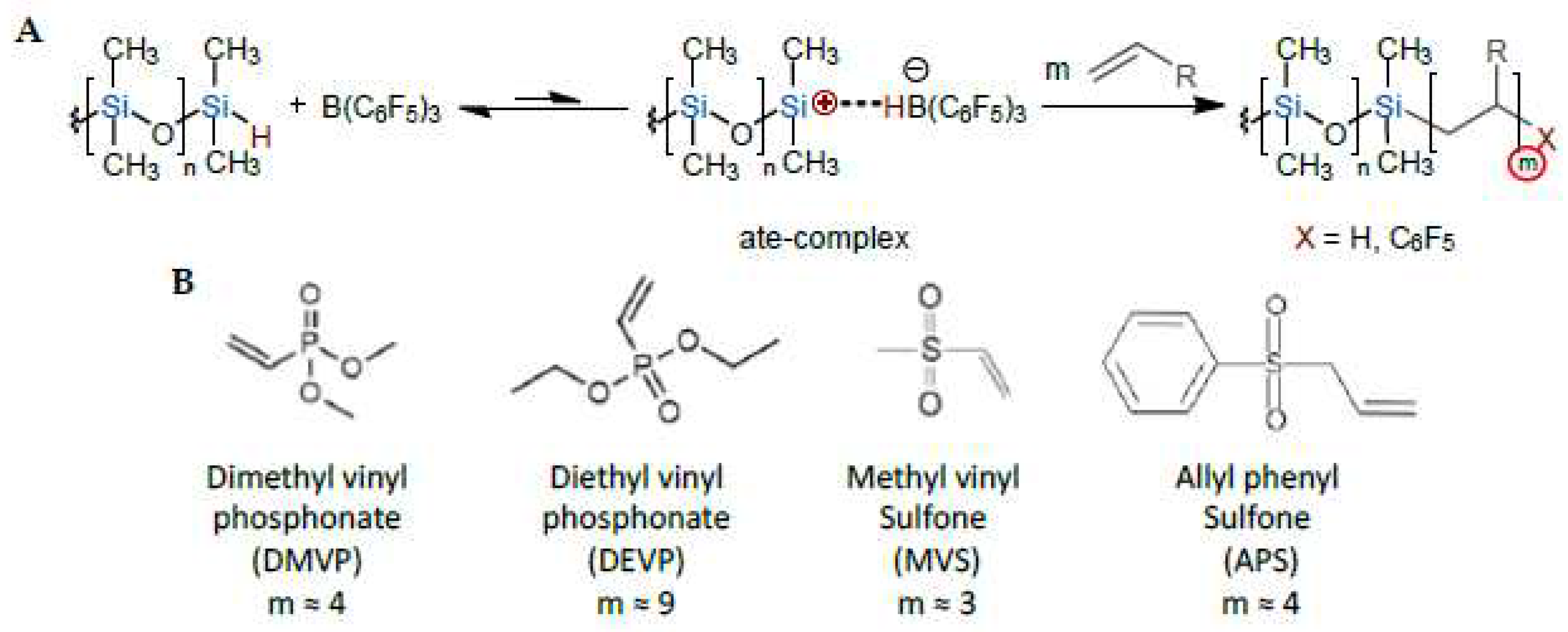
The objective of the present work was to generate new silicone elastomers from simple PDMS functionalized with typically few percent of strong interacting groups using a new functionalization technology. This paper is a follow-up of a previous article [
9] where we described the ate-complex-driven oligomerization of monomers with strong electron withdrawing groups on SiH moieties (
Scheme 1A). We previously described the mechanism of this new reaction between model silane or siloxane molecules and electron-withdrawing monomers, namely phosphonated and sulfoned monomers (see structures
Scheme 1B). In the present article, we proceeded to oligomerization of these functional monomers onto longer silicone polymer chains to generate original materials out of these block and grafted copolymers. This is very different from e.g. aza-Michael or thiol-ene additions in which only one monomer is added per reactive function (see a brief state of the art on the single functionalization of silicones by phosphonate and sulfone groups below).
In the following, we first summarize the different conditions of syntheses and chemical characterization of the phosphonated polymers prepared here. We also show briefly how these polymers exhibit interesting thermal properties and can lead to amphiphilic macromolecules. Then we describe the specific case of sulfoned polymers. Since strong and infusible elastomers are formed in most cases, we have characterized these thermoplastic elastomers mostly by physical techniques, namely rheology and electron microscopy. In a final discussion, we discuss on the strong interactions of sulfone moieties with the microstructure of the elastomers.
2. Rapid survey of phosphonate- and sulfone-modified silicone polymers
This part is not exhaustive but rather summarizes the different approaches and current interest in these functionalized copolymers.
2.1. Phosphonated silicone copolymers
It is known for long that phosphorous atoms and phosphorous-based groups implement strong thermal and fire resistances to materials. Knowing that silicones are themselves fire-proofing elastomers, the combination of these two chemistries is particularly relevant and was patented in 1996 [
10].
One simple approach to synthesize phosphorous-containing silicones is to first prepare functional silanes and then polymerize them into silicones. Hydrosilylation does not proceed using conventional platinum catalyst [
11]. Instead, the phosphorous_based groups can be introduced by different means: reaction between alkylphosphites and chloroalkyl-[
12] or amino-[
13] functional alkoxysilanes; thiol-ene reaction of an allyl or vinyl phosphonate monomer onto sulphide-based silane or silicone [
11]; Michael addition of diethyl vinyl phosphonate (DEVP, see
Scheme 1B) on aminofunctional silane [
14]. A less common approach reports the radical copolymerization of vinyl phosphonate monomers with vinyl trialkoxysilane catalyzed with peroxide initiators at high temperature [
15].
After partial or full hydrolysis of the ester groups of the phosphonate moiety, the functional silicones were blended with PEG for biomedical foamy implant applications [
16], or grafted to alumina particles as fire-resistant fillers in PMMA composites [
17]. It was observed in most cases a strong shift of the TGA curve towards higher temperature when introducing alkylphosphonate groups, and then a shift back when ester groups were hydrolyzed.
2.2. Sulfoned silicone copolymers
The modification of silicone chains by sulfone groups has long been reported in few patents, in a view to improve the resistance to polar solvents and high temperatures. Berger has first proposed the exothermic Michael addition between the aminopropyltriethoxysilane and the divinylsulfone [
18], to be used as primers between plastic resins and glass sheets. Curtis [
19] and Kanner [
20] synthesized a series of cyclosiloxanes bearing sulfolanyloxyalkyl groups, by hydrosilylation of methyl vinyl sulfone and other molecules using chloroplatinic acid as a catalyst at less than 120°C. Kantor [
21] has made use of a thiol-ene reaction between 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane (D
4V) and an alkyl thiol followed by an oxidation reaction; these have then been used in ring opening copolymerization between functional and non-functional cycles [
22]. The properties of the obtained polymers are not described, it is only noticed that such polymers would not swell in solvents of aeronautics interests, such as kerosene.
In the open literature, reports of sulfone-functionalized silicones are scarce. One can notice the significant contribution of the group of Feng that published a series of papers on the synthesis of different types of polymers with sulfone- and siloxane-based backbones (see e.g. [
23,
24]). These are prepared by thiol-ene addition, followed by oxone-based oxidation, then depolymerization into hybrid cycles and finally anionic polymerization. Both sulfone and silicone blocks are sensitive to mild acids and regenerate the starting cyclic monomer. The team of Opris functionalized some polyvinylmethylsiloxane with different sulfone and sulfolane groups to prepare polymers that showed low conductivity and high permittivity [
25].
A more significant literature has described the generation of grafted and block copolymers made of poly(olefin-sulfone) (POS) and PDMS. These date back to 1991 with the work of McGrath et al. on polybutylsulfone-
g-PDMS as prepared by anionic polymerization [
26], then Bercea et al. in 2004 [
27] through polycondensation of telechelic oligomers to generate multiblocks, and Swager et al in 2010 [
28] who performed click chemistry to produce crosslinked POS-
co-PDMS networks. The group of Feng also prepared multiblock copolymers of mixed-sulfone-
co-siloxane polymers with PDMS [29, 30]. In most of these studies, the nanostructuration of these copolymers through phase separation of the different blocks was systematically looked at, as well as their degradation by irradiation or bases with lithographic applications as a systematic target.
2.3. Why and how studying sulfoned silicone polymers
This study takes its infancy in the useful remark of Prof. Boutevin concerning the role of sulfone groups as a structuring group in pendant alkyl or fluoroalkyl-functionalized polymers. Corpart et al in 2001 [
31] showed that introducing a SO
2 rotula in the side groups of a perfluorinated homo-polyacrylate permitted ordering and crystallizing C
6F
13 groups, that would not occur without this group. Later on, Lee et al. [
32] prepared long alkyl chains-functionalized polysiloxanes. Again, a sulfone rotula allowed generating perfectly defined smectic liquid-like crystalline phases in copolymers from C
10 to C
16 side chains; whereas, only the C
16 SO
2-free equivalent polymer was able to partly crystallize into large spherulitic patterns. Clearly, the association of close-enough sulfone groups can induce interesting associations to silicone polymers.
Another important starting point that allowed us carrying out the present study is the discovery and use of a chaotropic salt first applied to carry out silanol polycondensation of PDMSs in bulk [
33]. We found out previously that this complex allows dissolving elastomers strongly crosslinked by physical interactions, e.g. non-soluble perfluorinated hybrid oligocarboxysiloxanes [
34]. This salt is prepared by making a 3/1 molar mixture between trifluoroacetic acid and tetramethylguanidine at 5% in THF. With this tool in hand, we can now manipulate some (but not all) elastomers after drying and/or purification by precipitation (
vide infra).
3. Results
3.1. Phosphonated silicone copolymers
Tables S1 and S2 gives an overview of the syntheses carried out with dimethyl- and diethyl-vinyl phosphonate monomers (DMVP and DEVP, respectively). Experiments were done with different Si-H functional polymers, one telechelic (PDMS A: M
n = 23.5 gk/mol) and two others with SiH side groups (PDMS B: 5 SiH/chain, M
n = 5.1 kg/mol; PDMS C: 12 SiH/chain, M
n = 10.3 kg/mol). All experiments were done in toluene, whereas the solvent of NMR and SEC analyses varied according to the solubility of the final polymers.
3.1.1. The case of dimethyl vinyl phosphonate (DMVP)
Several preliminary experiments on the grafting of DMVP onto polysiloxane backbone were performed. As shown in
Table S1, the grafting reaction of DMVP onto different kinds of polysiloxane proceeds smoothly. However, the obtained polymers (especially when using poly(dimethylsiloxane)s with 5 and 12 Si–H groups per chains, respectively) were found insoluble in most solvents after precipitation and drying. We did not pursue in characterizing further these new polymers to concentrate on DEVP-functionalized polymers.
3.1.2. The case of diethyl vinyl phosphonate (DEVP)
The reaction of DEVP with different PDMSs is characterized by a slower rate and starts at higher temperatures (typically between 75–85
oC) than previously shown with model hydridosiloxane components (PMDS, MD’M) [
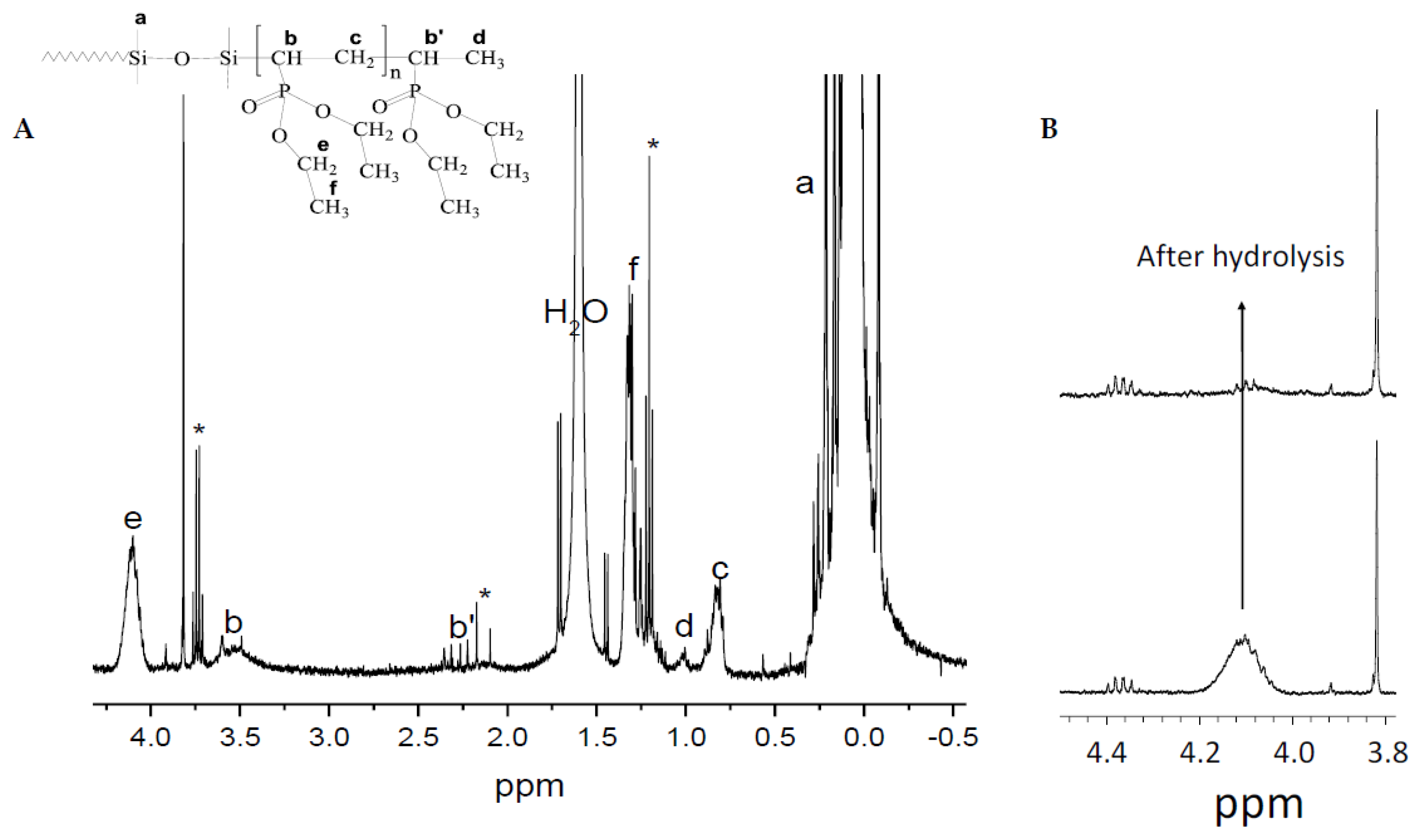
9]. Consequently, it proceeds in a controlled manner, giving modified silicones with high conversion of DEVP (see Table S2). The products were typically recovered under a very viscous liquid form or a gel (the latter when PDMS C with the larger number of Si–H groups was used). The final polymers prepared with DEVP were characterized by NMR (
Figure 1) and SEC (see traces in
Figure S1).
Organophosphoric fragments introduced in polymer chains are known to considerably increase the flame-resistance and thermal stability of the resulting material [
35]. Indeed, we checked that PDMS functionalized by oligoDEVP shows considerably higher thermal stability in comparison with original PDMS with larger residues at high temperature. Typically, the main peak of degradation shifts from basically 280°C to 410°C while adding phosphorus units in the silicone (
Figure 2). The thermal resistance of the present polymers is higher than the previously-reported phosphonated silicone copolymers [
11] (10 wt.% loss at about 400°C against 450°C here), obviously thanks to the oligomeric nature of grafted chains here.
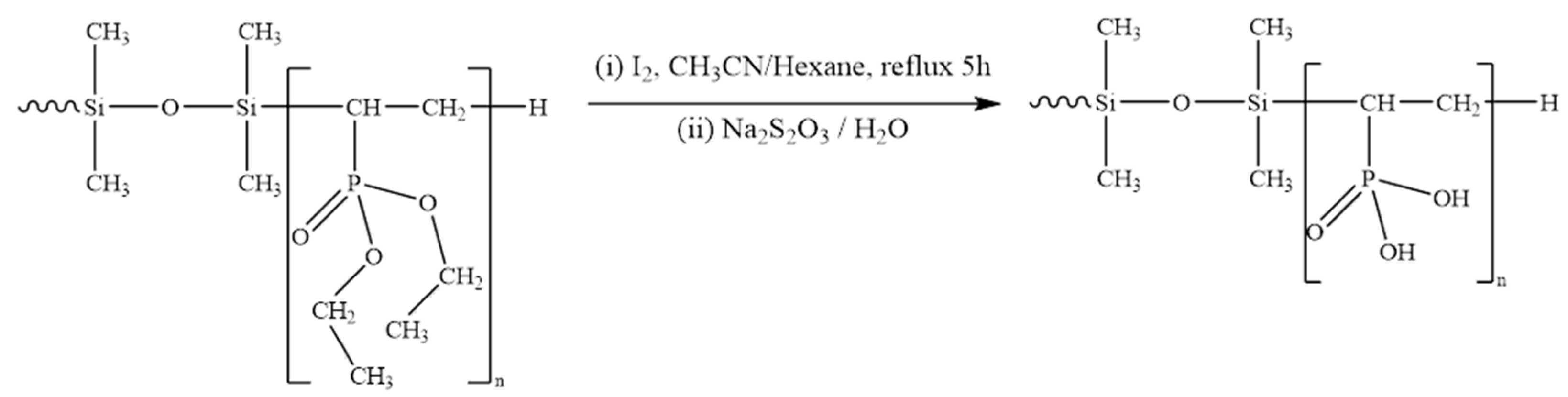
Other possible application of DEVP-modified siloxanes could be their use as surfactants. For this purpose, a hydrolysis of the DEVP-grafted polysiloxane was performed (see
Scheme 2 for conditions).
Figure 1B shows a zoom of the
1H NMR spectrum of pristine and hydrolyzed products around the peak corresponding to the phosphonate groups of oligoDEVP fragment centered around 4.1 ppm. The protons of the ethylene peak fully disappears, confirming that the ester hydrolysis procedure proceeded completely. Note that the thermogravimetric analysis of hydrolyzed samples showed noticeable drop in thermal stability with onset of decomposition at 200°C (not shown). This agrees with what was observed previously for monomolecular phosphonated side groups [
11].
In a patent, we have described the use of DEVP-grafted copolymers as a surface modifier of silica to prepare composite membranes by simple casting. The films were hydrolyzed in concentrated HCl solution (12 M) to be tentatively used in fuel cell applications [
36].
3.2. Sulfoned silicone copolymers
We completed a large variety of experiments with different silicone polymers and methyl vinyl sulfone (MVS) or allyl phenyl sulfone (APS), see structures
Scheme 1. We report here only the experiments that led to characterization (other examples are available in a patent that we published on the topic [
37]). The experiments are summarized in
Tables S3 and S4, respectively. Here, we used mainly three silicone samples: the same telechelic polymer as described before of about 23,500 g/mol and a polydispersity index of 1.5 (PDMS A); a copolymer of much larger molar mass, 55,000 g/mol, with an average of 2.3 pending Si-H groups per chains (PDMS D); a copolymer of 103,000 g/mol with an average number of 16 SiH groups per chain (PDMS E).
3.2.1. Synthesis and visual aspect of the copolymers
All conditions for the synthesis of copolymers presented here are summarized in
Table S3 and S4 for MVS and APS, respectively. Experiments were done in a solvent, toluene, in sealed tubes, typically in excess of monomer and at fair content of catalyst (see footnotes of Tables in supporting information). After a short inhibition period, polymerization took place rapidly with no gas released. Contrary to phosphonated monomers, the oligomerization of sulfone monomers proceeded readily at room temperature, generating a slight exotherm. Using PDMS A, the medium becomes viscous with time whereas with PDMS D, we observed a clear gelling of the medium, for both used monomers.
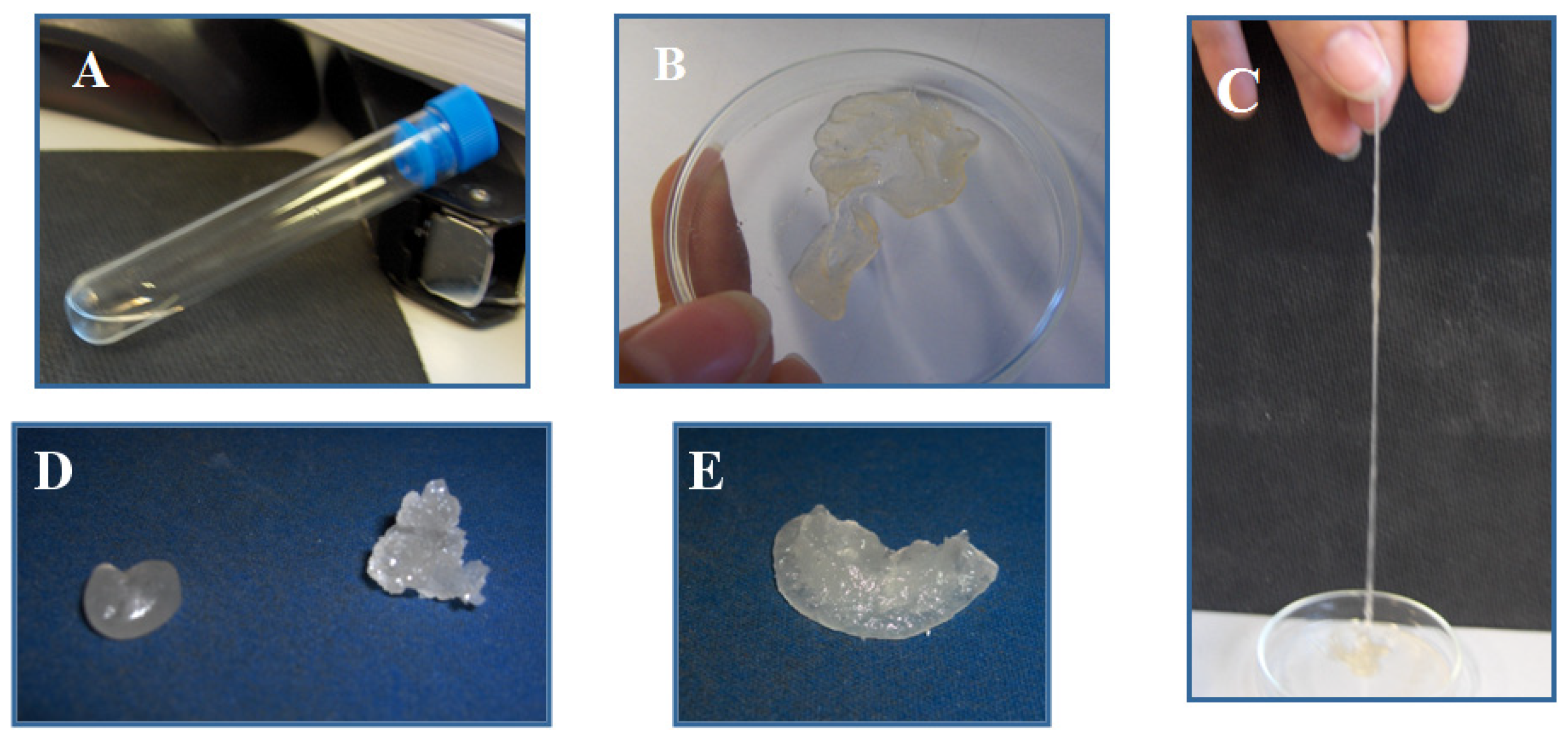
Figure 3 shows some samples obtained in this study after precipitating the polymers and removing the solvent. From the original oily PDMS A (Figure 3A), one generates gums (Figure 3B) that are visually highly elastic (Figure 3C). Gums obtained from MVS and PDMS A are easily hand-processable (Figure 3D-left). APS-based polymers, whether from telechelic PDMS A (Figure 3D-right) or PDMS-co-PHMS D (Figure 3E) are non-deformable elastomers that cannot be redissolved once dried, even by adding large contents of chaotropic salt in THF.
Figure 4.
(B, C, D-left) Materials obtained after functionalization by methyl vinyl sulfone of PDMS A (oil shown in A) and (D-right) PDMS D. Strong elastomer in E was obtained with PDMS D and APS.
Figure 4.
(B, C, D-left) Materials obtained after functionalization by methyl vinyl sulfone of PDMS A (oil shown in A) and (D-right) PDMS D. Strong elastomer in E was obtained with PDMS D and APS.
We did not succeed throughout this study in carrying out NMR measurements, because the chaotropic salt is not efficient in deuterated solvents. HR-MAS analysis of the copolymers were not conclusive either (see an example extracted from our patent in
Figure S2). We could thus neither determine the conversions in monomer, nor could we analyze the grafted oligomers’ DP. We infer from the previous model study [
9] that oligomers of typically 3 to 4 units are attached to the SiH groups for MVS and APS, respectively (see
Scheme 1).
3.2.2. SEC analyses of PDMS-co-o(MVS) triblock copolymer
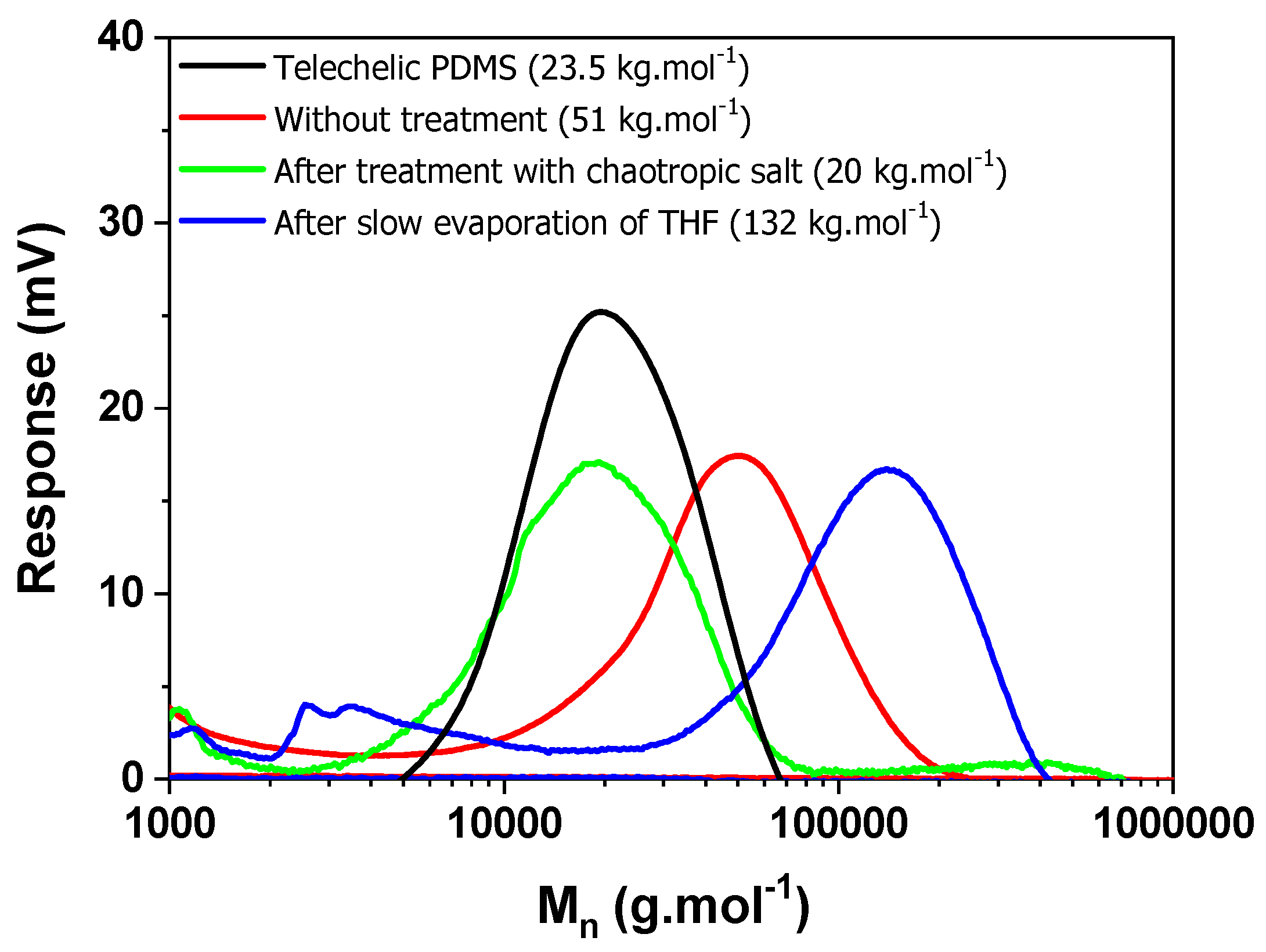
We confirmed by SEC the behavior of the telechelic polymer functionalized with oligo(MVS), which is soluble in THF (run 9,
Table S3). Results are given in
Figure 4. Clearly, the way the polymer is processed has a strong influence on the supramolecular associations and thus the molar mass as given by SEC. The polymer solubilized in THF in presence of chaotropic salt gives a molar mass very close from the starting PDMS-
co-PHMS, as expected from the few sulfone molecules attached to the backbone. Whereas, after precipitation in methanol, the apparent molar mass has doubled. By gently evaporating the sample and taking it back then in THF, even larger molar masses are obtained (about 5 times the actual value). This demonstrates that the supramolecular associations of sulfones work even in presence of solvent and depend strongly on the history of the sample.
3.2.3. Thermal analyses
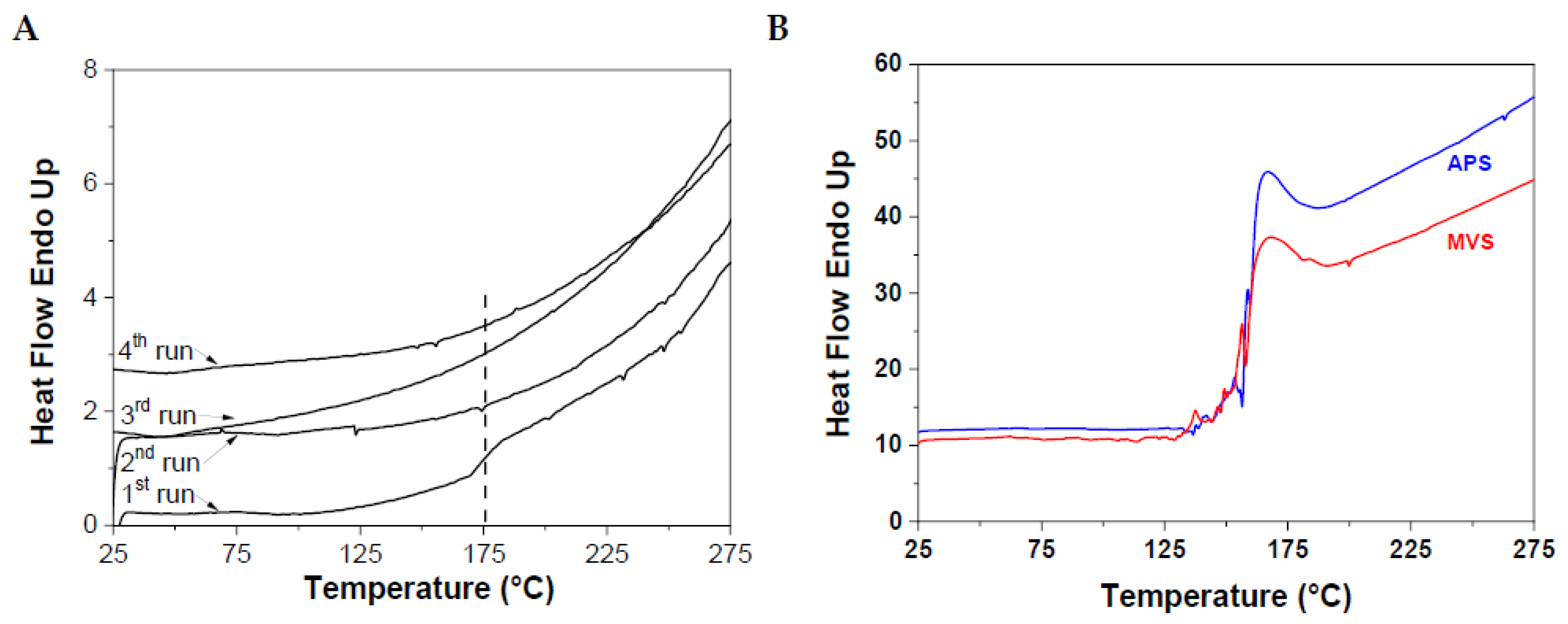
To remove any trace of monomer, we first solubilized the polymers, treated them with chaotropic salt and reprecipitated them few times to achieve a complete purification. Thermal analyses were done using a modulated DSC operating at 20°C per minute, making 4 ramps of temperature, two rising and two decreasing ramps.
Figure 5 shows two thermograms of functional polymers, namely one with PDMS A and APS (run 11
Table S4), which the full analysis is reported (
Figure 5A), and polymer D functionalized with MVS and APS (runs 10 and 12,
Tables S3 and S4, respectively,
Figure 5B). All copolymers show a clear transition at 170°C. This transition appears only during the first ramp, a signature of non-specific interactions; indeed, these do not have time to form back to show up on the second rising ramp and further ramps. It is thus assigned to the debonding of the sulfone complexes.
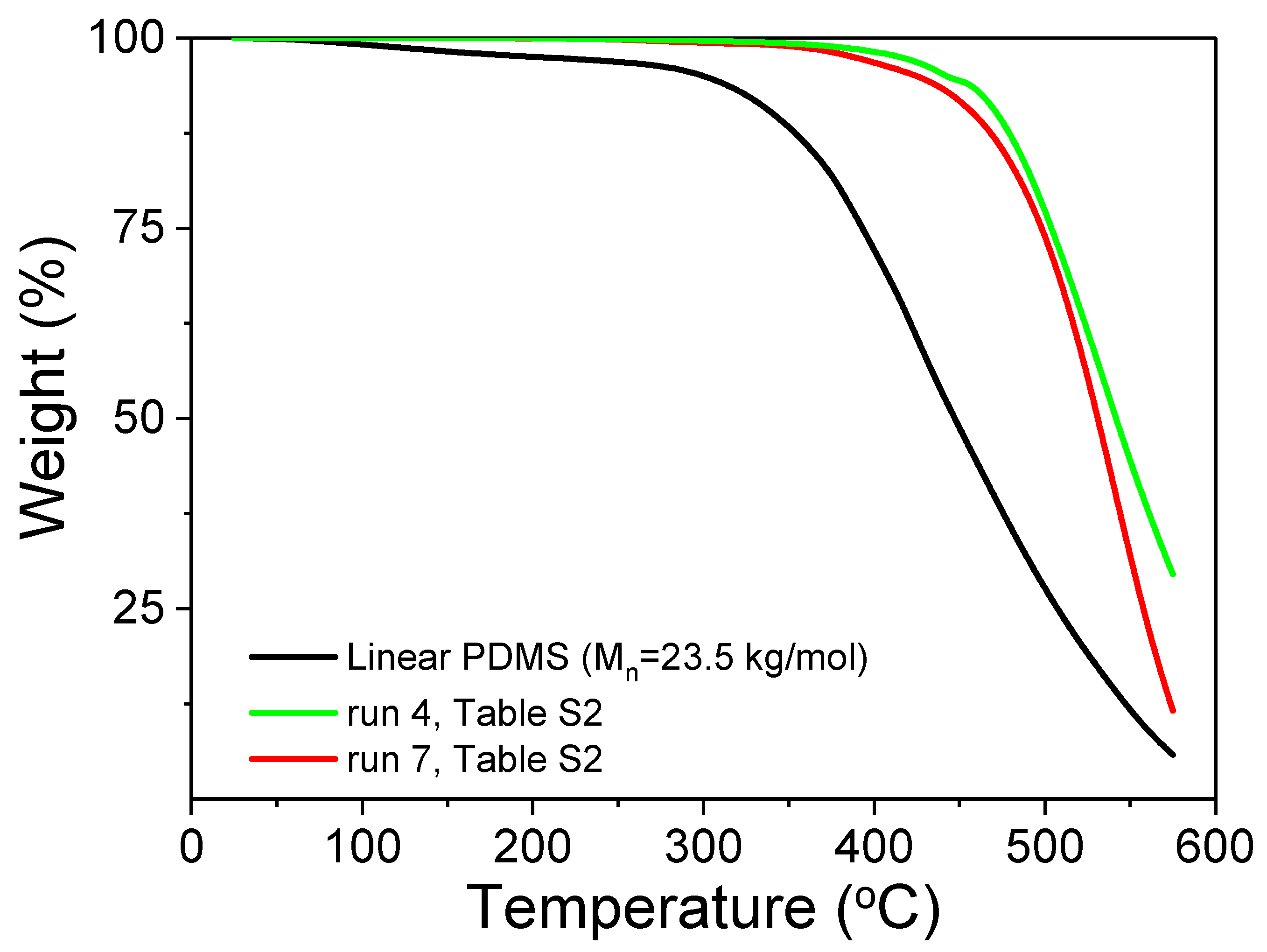
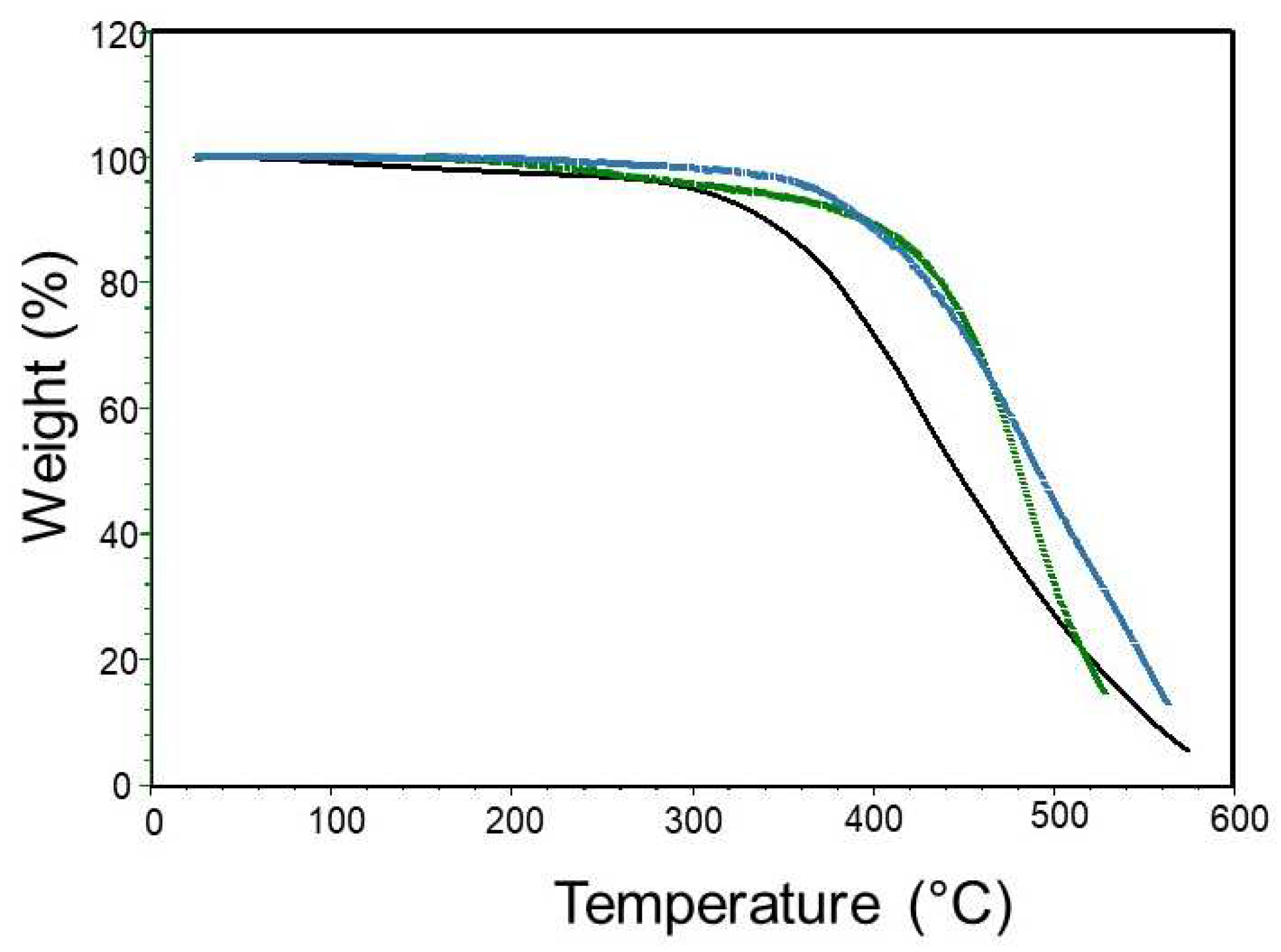
We had also a look on the TGA of the raw sulfoned silicone (PDMS A with MVS and APS,
Figure 6). The sulfone polymers are less sensitive to temperature degradation than the original PDMS, with no residue at 600°C.
3.2.4. Microscopy observations
We wanted here to confirm that a phase separation in the copolymer was responsible for the gelling of the materials. We analyzed both MVS- (run 8,
Table S3) and APS- (run 11,
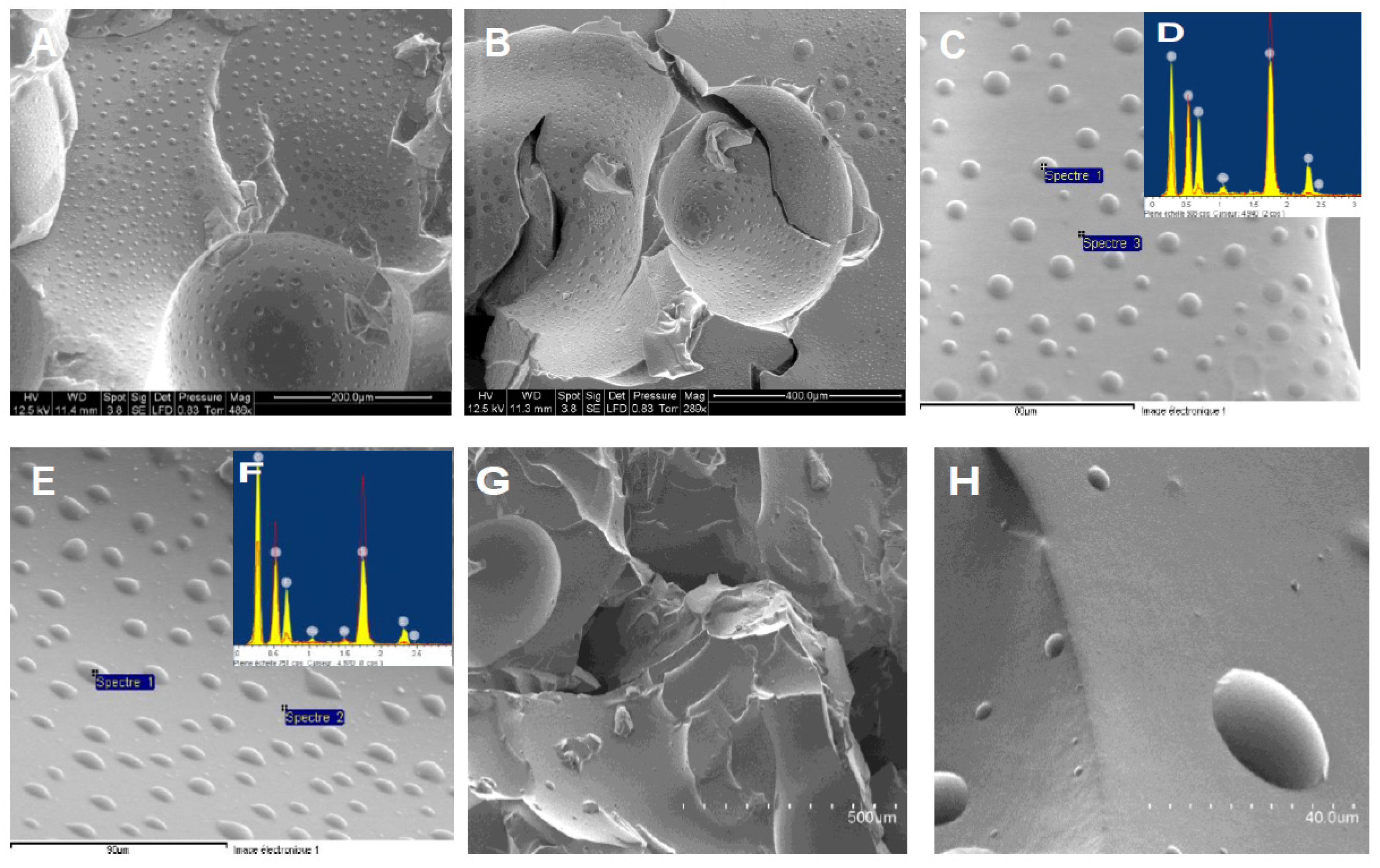
Table S4) grafted copolymers first on an environmental SEM/EDX apparatus (ESEM), that allows working at low vacuum and does not require that the sample be metallized; the APS-based sample was also characterized by a more conventional but very powerful SEM, which requires metallization by platinum and operates at very low vacuum (then removing any traces of solvents from the sample). A compilation of these different photos is given in Figure 7.
The ESEM gives similar photos for MVS and APS functionalized materials, namely a silicone matrix on which small water droplets are regularly distributed on the surface of the material. These are located on the polar phase of the copolymer, i.e. most likely onto clusters of oligosulfones. Droplets on APS-based copolymer seem to be smoother, but are basically of similar sizes (from 1 to 10 μm) and distribution (droplets are separated by spaces of about 20 to 50 μm). By EDX analysis on the matrix and the location of the droplets, we observed that the clusters topped by water effectively contain the sulfone oligomers with some fluorine inside (provided by the catalyst, see
Scheme 1), whereas the rest of the matrix is only made of silicone (
Figure 7, insets D and F). It is remarkable that so many clusters appear on TEM with so few contents of sulfone molecules (less than 1% in most cases).
To confirm the fact that water aggregates on the polar clusters, we performed a conventional SEM analysis (
Figure 7, G and H). In this case, the vacuum is so high that all traces of volatile molecules, including solvents, are prohibitive. We observed quite different pictures in this case, with smooth surfaces. Note finally that the MVS block copolymer only gave neat films in ESEM without particular structuration (
Figure S3).
Figure 8.
SEM analyses of PDMS D silicone oil functionalized with MVS (top line) and APS (bottom line). (A,B,C,E) photos from ESEM apparatus; (D, F) EDX analyses on spots in photos (C) and (E); (G, H) photos obtained with a conventional SEM after metallization. Scales are given on the photos.
Figure 8.
SEM analyses of PDMS D silicone oil functionalized with MVS (top line) and APS (bottom line). (A,B,C,E) photos from ESEM apparatus; (D, F) EDX analyses on spots in photos (C) and (E); (G, H) photos obtained with a conventional SEM after metallization. Scales are given on the photos.
3.2.5. Rheology of copolymers
Samples of gums functionalized by MVS were placed into two plates on a rheometer to measure the different moduli of the samples at different temperatures.
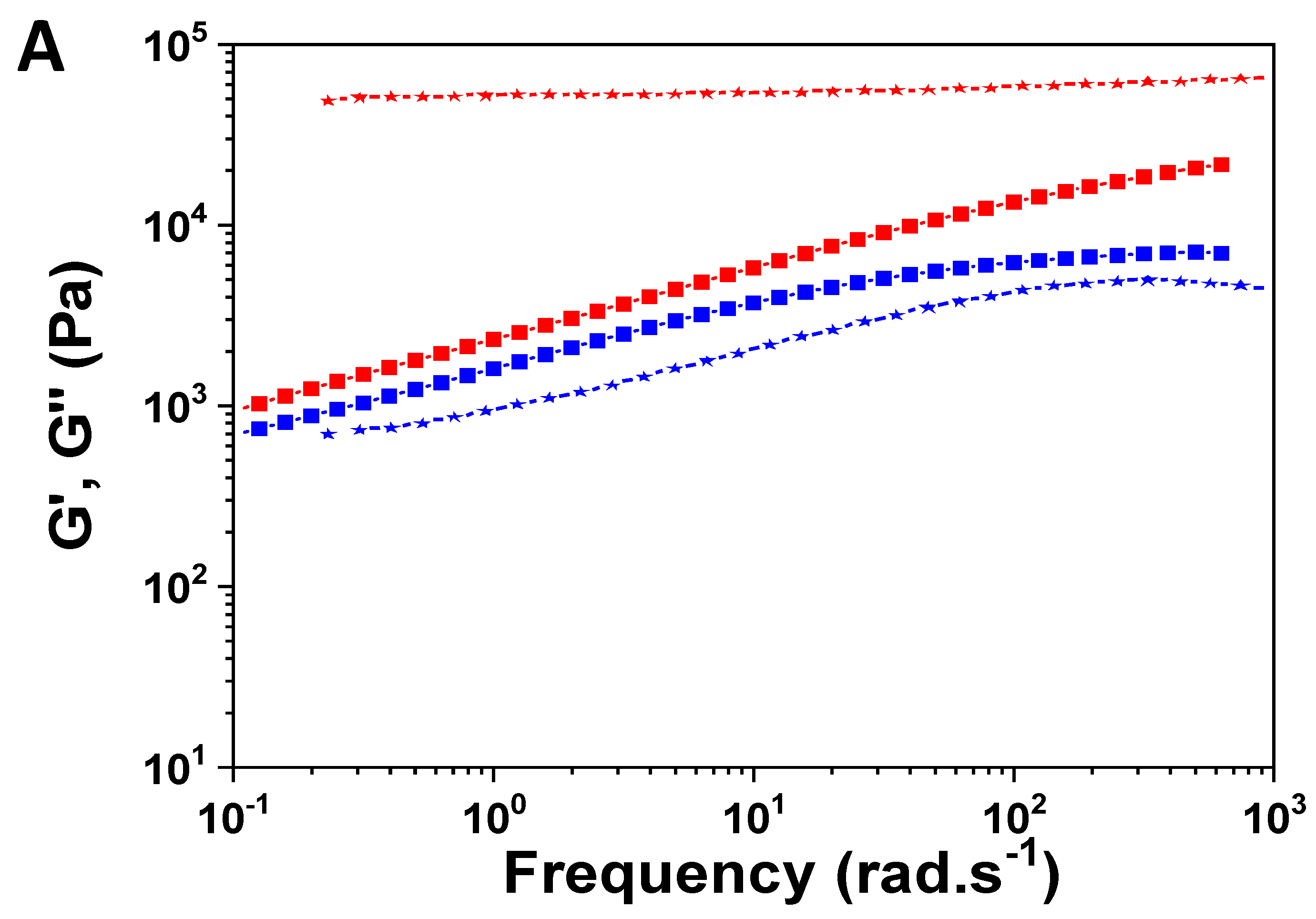
Figure 8A shows the resulting curves for the triblock copolymer (PDSM A, run 8,
Table S3) and the grafted copolymer (PDMS E, run 10,
Table S3) functionalized with MVS. Both copolymers are solid, with G’ systematically above G’’ on a large range of temperature.
On the other hand, the content of functional groups and location of the oligomer moieties has a strong effect both on the intrinsic values of the moduli and their variation with frequency. The triblock copolymer ‘melts’ at high temperature, typically above 175°C (
Figure 8B). Note that this relaxation is not accompanied by a homogenization of the material, because even when sulfones do not interact physically anymore, the corresponding grafted oligomers are still not compatible with PDMS and remain clusterized. However, above this temperature, it is easy to process the material by extrusion (not shown).
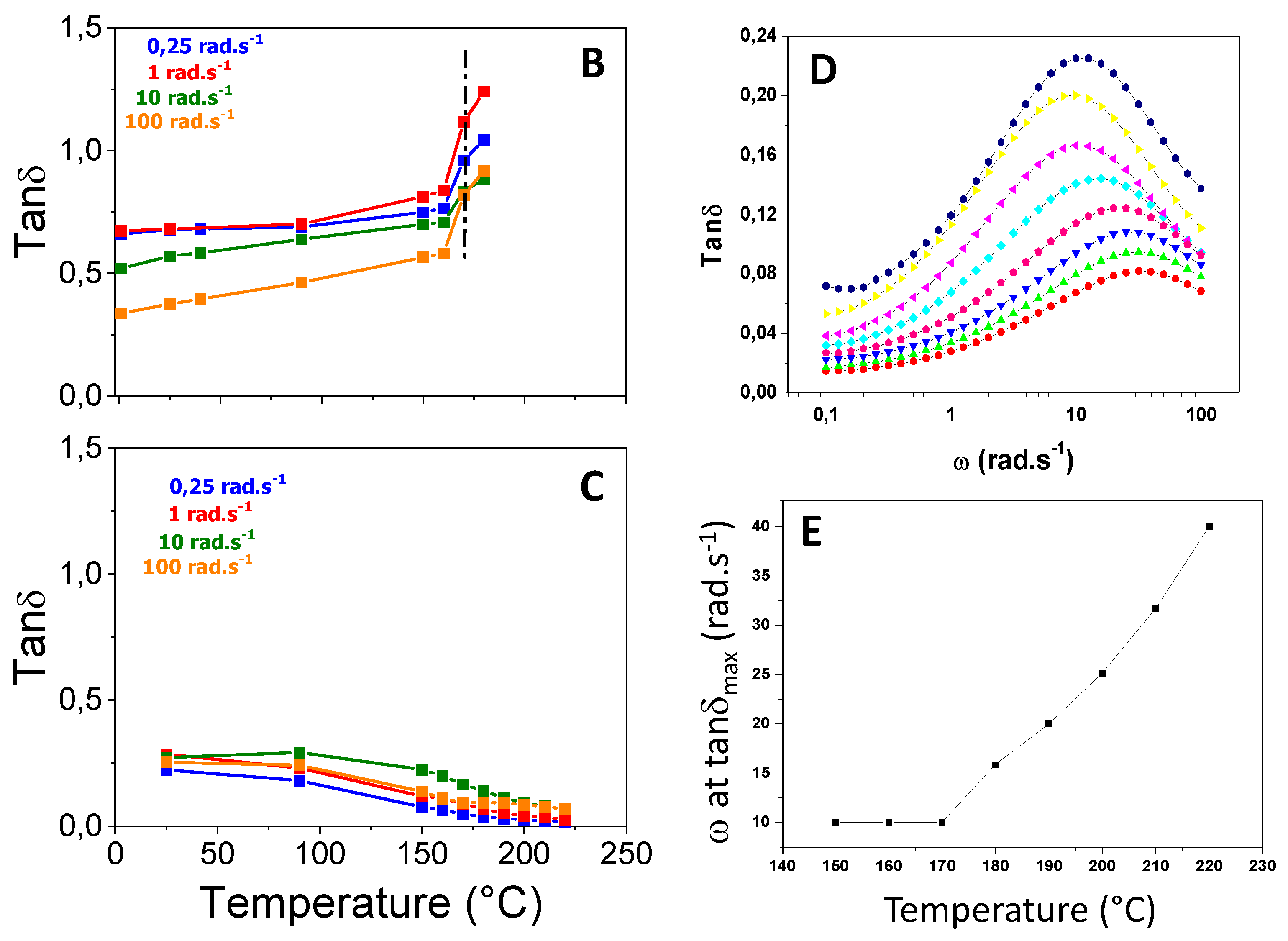
The situation is different for the grafted copolymer that strengthens with temperature (
Figure 8C). However, when plotting the tanδ as a function of frequency for increasing temperatures, a bell curve is obtained (
Figure 8D); the frequency at which the maximum tanδ is picked shifts above 175°C (
Figure 8E). This is an indication that the strongly functionalized material also phase-separates and restructures at high temperature.
4. Discussion
In this part, we discuss the very specific supramolecular associations of sulfone groups and how these allow the generation of a new class of thermoplastic silicone materials. We also propose an alternative way of generating perfectly-controlled sulfone double-functionalization.
4.1. Specific interactions of sulfones
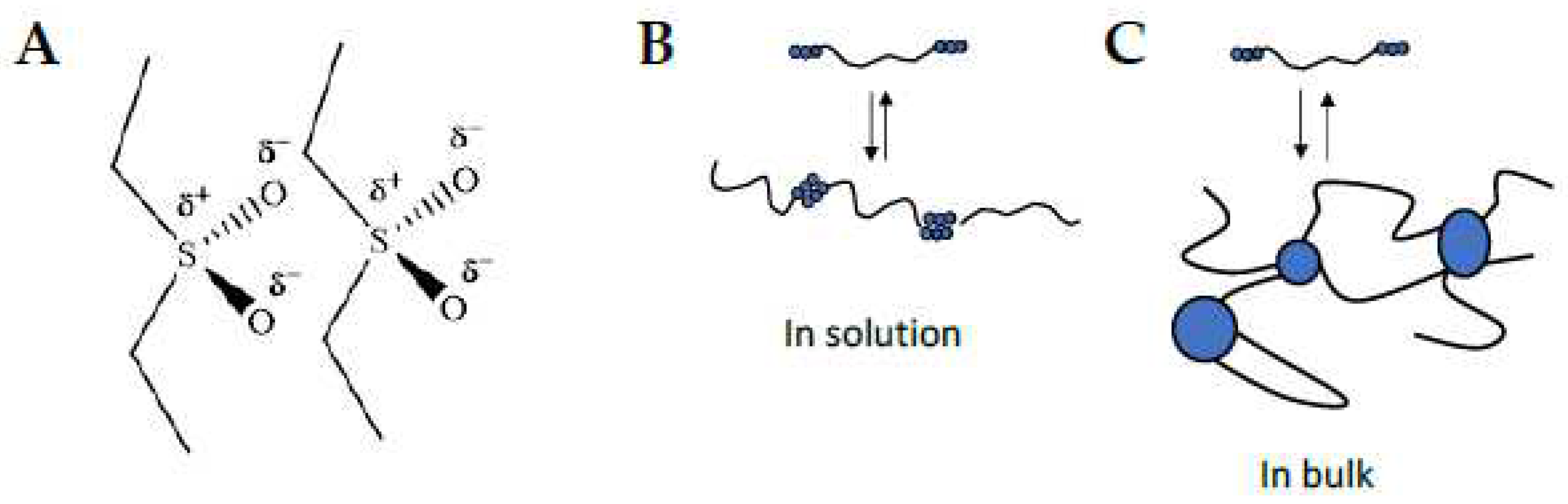
Strong dipole-dipole interactions between the sulfone groups are known and ascribed to their high dipole moment (4,49 D) [
38]. Evans et al. [
39] have shown also that the strong planar dipole moment (1.69 D) and the spatial arrangement of sulfone groups (O=S=O angle of ≈ 109°) induce a preferred orientation on neighboring molecules with pseudo-ionic interactions (
Scheme 3A).
As we have shown in our previous study, the ate-complex reaction of sulfone monomers onto SiH moieties proceeds through typically 3 to 4 additions. Among other popular techniques of functionalization, namely Michael addition of methylvinylsulfone and divinylsulfone, as well as thiol-ene reaction and oxidation, none of these were reported to give gels, as also verified by us [
40]. We also performed hydrosilylation of MVS using large excess of B(C
6F
5)
3 on PDMS A and did not observe any gelation (not shown). All these reactions proceed through a single addition. The supramolecular associations observed here thus involves a cooperative association of the oligomeric sulfones.
Some authors have proposed that sulfone complexes would crystallize after pairing (e.g. [
41]). We have performed SAXS experiments on the MVS-grafted copolymer and did not observe any transitions, before and after rheology analysis at high temperature (see
Figure S4).
4.2. Microstructuration into materials
In our experiments, we can distinguish different categories of materials, according to the location of the sulfone oligomers (at chain-ends or grafted to the chains) and the nature of the monomers (MVS and APS). Telechelic SiH silicones lead to triblock copolymers after modification with short oligosulfone external moieties that can be further characterized. Copolymers with pendent side chains were found neither soluble nor manipulatable, even under heat, so we do not consider these in the following discussion.
Block copolymers made out of MVS are soluble in THF. Their measured molar masses are directly related to their history. The polymer chains are elongating by pairing of oligo(MVS)s that however do not clusterize (
Scheme 3B). When precipitating and drying the polymer, the polar phase demixes and forms clusters in the material that are hardly broken without the help of the chaotropic salt in THF, coming back to block copolymer ‘unimers’ (
Scheme 3C).
By DSC and rheology, the temperature at which sulfone pairs are debonding has been estimated at 175°C. Such value is in line with literature values of closely related works where sulfone groups associate. For instance, Duprez et al. [
42] have prepared polysulfide polymers using an AB monomer thiol-ene polyaddition. After oxidation into sulfone, the new polymer shows a transition at 175°C ascribed to a melting point. In previously related sulfone-rotula work [
31], in addition to a crystallization of perfluorinated groups, a second transition was observed at 179°C. We have also showed that telechelic PEG polymers terminated by sulfone-
co-phosphonic acid exhibit a transition by DSC at 169°C [
43].
4.3. How many sulfone groups are needed in the row for polymer structuration?
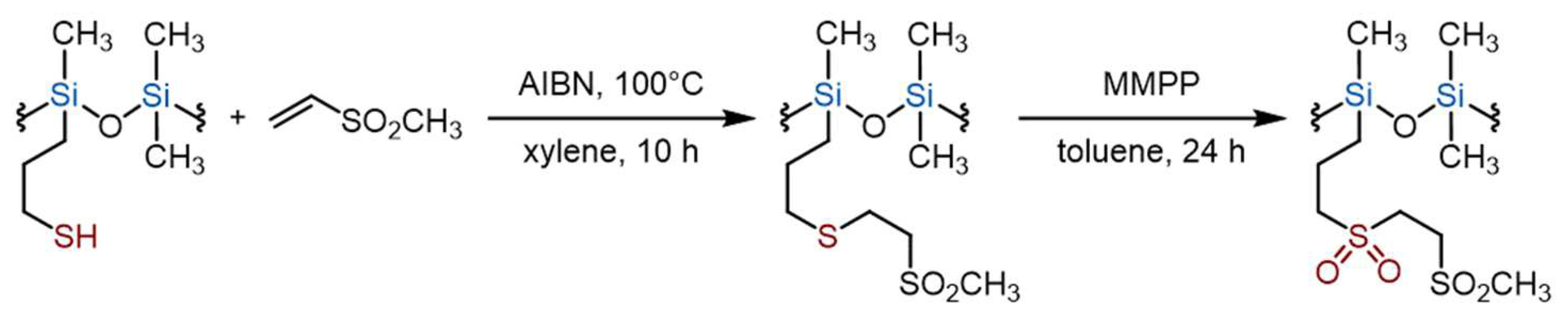
In the course of this study, we carried out a different chemistry to prepare a silicone copolymer grafted with bis-sulfone groups. We first performed the radical monoaddition of MVS to a PDMS grafted with thiol moieties, i.e. 4-6% mercaptopropylmethyl siloxane-dimethylsiloxane copolymer with M
n=11 kg/mol (Ð=1.81). Oxidation of the sulfide group using bis(monoperoxyphthalate) magnesium hexahydrate (MMPP) as oxidizing agent then followed to produce the bis-sulfone groups, see
Scheme 4 [
43]. The use of equimolar ratio of MVS to –S–H group produced the expected initial monoadduct, with some low molecular weight by-products, that are attributed to low molecular weight oligomers (see SEC traces
Figure S5A). The final product after purification by precipitation in methanol is a wax.
We then proceeded to the full oxidation of the sulfide atom by MMPP which generated a second sulfone moiety on each functional group (see
Scheme 4 and NMR spectrum in
Figure S5B). After drying the solvent, the obtained material is a gel, very close to those prepared using the ate-complex-mediated process. This 2-step molecular reactions allows generating a silicone elastomeric material, surely thanks to the facility of phase-separation in a such polymer matrix. It has also two extra advantages compared to the ate-complex reaction: i) it is not exothermic; ii) it generates structurally-controlled bis-oligomers.
5. Materials and Methods
5.1. Materials
B(C6F5)3 (Alfa Aesar, 97%), dimethyl vinyl phosphonate (DMVP, Aldrich, >95%), methyl vinyl sulfone (MVS, Aldrich; 98%) were used as received. Diethyl vinyl phosphonate (DEVP, Aldrich; 97%) was distilled from CaH2 under reduced pressure (bp 47°C/10-1 mbar). Toluene was refluxed with sodium during 6-8 h and then distilled under inert atmosphere. CH2Cl2, cyclohexane, tetrahydrofuran were dried and distilled from CaH2 under inert atmosphere. All polydimethylsiloxanes were purchased from Gelest and used as received, except for the thiol-functionalized polymer that was obtained from ABCR.
5.2. Polymerization procedures
Polymerization reactions were carried out under inert atmosphere and at different temperatures, according to the monomer involved. Reactions were carried out in special glass reactors equipped with a Teflon® coated magnetic stirrer bar. Before charging the reactants, reactors were vacuumed three times and filled by argon. During the first vacuum step, they were heated up to 200°C. A required amount of B(C6F5)3 was first poured into the glass reactor which was vacuumed without heating during 15-20 min and filled by argon; then, the toluene and the monomer were poured into the reactor. Reaction mixture was stirred a few minutes and then the polysiloxane was added through a syringe at room temperature. After that, the reactor was immediately placed into the temperature-controlled bath (T=25°C or 85 °C depending on the monomer, see tables’ footnotes for details) and reaction started to occur. After a given time, the reaction mixture was poured into a flask and dried by rotary evaporator and then in vacuum until reaching a constant weight.
5.3. Re-precipitation procedure
Telechelic siloxanes grafted by DEVP (0.2 g) were dissolved in CH2Cl2 (0.5 ml), and precipitated by excess ethanol (6-8 ml). Solid product was recovered by centrifugation followed by careful decantation. Finally, the reprecipitated polymer was dried to a constant weight in vacuum. Similar procedure was done for sulfone-modified copolymers.
5.4. Polymer characterization
Size exclusion chromatography (SEC) was performed on a Spectra Physics apparatus with two columns (PL gel, 5 mm, 300 mm, 500 and 100A°) and one pre-column (PL gel 5 mm guard) thermostatted at 30 °C. The detection was achieved by a SP8430 differential refractometer and tetrahydrofuran (THF) was eluted at a flow rate of 1.0 mL/min. The calculation of molar mass and polydispersity was based on polystyrene standards (Polymer Labs, Germany).
1H NMR spectra were recorded in CDCl3 at 25°C on a Bruker AC-400 spectrometer calibrated relative to the solvent peak in reference to tetramethylsilane standard.
Thermogravimetric (TG) measurements were performed on a TGA51 TA Instrument apparatus operating between 25 and 580 °C under nitrogen at a heating rate of 20 ºC min-1.
Perkin Elmer Pyris Diamond equipment was used to performed Differential Scanning Calorimetry (DSC) measurements. Sample masses were of 12.0 ± 0.5 mg and were heated from 25 °C to 275 °C at a rate of 10 °C.min-1 under nitrogen flow (20 mL.min-1), followed by an isotherm at 275°C during 2 min before a cooling ramp at 10 °C.min-1 from 275 to 25 °C. This full procedure was repeated a second time for one sample.
Environmental Scanning Electron Microscopy was performed on a FEI Quanta 200 SEM. All images were obtained under low vacuum at a voltage of 15.0 kV with a spot size of 2.8 mm and a working distance of 8.2 mm. Energy-dispersive X-ray (EDX) measurements were conducted as an integrated tool to determine the elemental composition of the residue on micrographs with a magnitude of 300x. Quantitative analyses of element content in plate residues were measured on ESEM micrographs using the XT Docu program. Conventional SEM was performed on a Hitachi 4500S apparatus on samples that were dried and metallized with platinum using a Balzers SCD 020 apparatus before observation.
Rheology measurements were carried out on an AR 1000 rheometer from TA Instruments operating in oscillation mode with a deformation of 1%, using two 25 mm plane plates. Temperature was swept from 25°C to 220°C for all samples using the oven adapted to the rheometer.
Small Angle X-ray scattering (SAXS) experiments were carried out in a transmission configuration on solid powders. A copper rotating anode X-ray source coupled with a multilayer focusing ‘Osmic’ monochromator giving high flux (108 photons/s) and punctual collimation was used as source (λ = 1.5418 Å). An ‘Image plate’ was used as 2D detector. Diffracted intensity was corrected by exposition time, transmission and intensity background.
6. Conclusions
The ate-complex mechanism allows the preparation of new types of functional triblock or grafted silicone copolymers with enhanced thermoplastic properties brought by the multiple functional groups. The described materials are thermally stable, generally an initial requirement asked to silicone materials for further applications. Supramolecular materials grafted by oligosulfones are solids on a full range of temperature up to 220°C. Finally, the possibility to structure the chains simply by introducing bis-sulfone groups opens new areas for physically crosslinking other conventional polymers.
7. Patents
See refs. [
36] and [
37] below.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, 4 Tables, additional SEC curves, HR-MAS NMR of sulfone polymer, DRX spectra, rheology results and full characterization of PDMS-g-bis-sulfone.
Author Contributions
Conceptualization, F.G.; methodology, E.P., E. H. G., S. V. K.; validation, C. L., P. L., I. V. V., C. J. D.; formal analysis, E. P.; S. V. K.; F.G.; writing—original draft preparation, E.P., S. V. K.; writing—review and editing, F.G..; supervision, F.G.; project administration, F.G.; funding acquisition, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wacker Chemie AG.
Data Availability Statement
Data available on demand.
Acknowledgments
This work has been done in the framework of the IAM lab under the supervision of Prof. Bernard Boutevin, who followed with great attention this work. The chemistry proposed here combines 3 atoms that were and still are the signature of the lab, i.e. P, S and Si. FG wants also to thank Dr Jurgen Pfeiffer and Dr Richard Weidner for their strong interest in the topic, Ecole des Mines d’Alès for helping with DSC and ESEM measurements, and Pr. E. Fleury and Dr. D. Portinha for helpful comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mark, J. E. Some Interesting Things about Polysiloxanes. Acc. Chem. Res. 2004, 37, 946–53. [Google Scholar] [CrossRef]
- A recent review: Hofmann, R. J.; Vlatković, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, Non-Thermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534. [Google Scholar] [CrossRef]
- Brook, M. A.; Grande, J. B.; Ganachaud, F. New Synthetic Strategies for Structured Silicones Using B(C6F5)3. In Silicon Polymers; Muzafarov, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 161–83.
- Pouget, E.; Tonnar, J.; Lucas, P.; Lacroix-Desmazes, P.; Ganachaud, F.; Boutevin, B. Well-Architectured Poly(dimethylsiloxane)-Containing Copolymers Obtained by Radical Chemistry. Chem Rev. 2010, 110, 1233–77. [Google Scholar] [CrossRef] [PubMed]
- See e.g. : Yilgor, I.; McGrath, J. E. Advances in Silicon Science, Polysiloxane-Containing Copolymers: a Survey of Recent Developments. Adv. Polym. Sci. 1988, 86, 1–86. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone Containing Copolymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2014, 39, 1165–95. [Google Scholar] [CrossRef]
- Fauvre, L.; Fleury, E.; Ganachaud, F.; Portinha, D. Extra-Soft Self-Healable Supramolecular Silicone Elastomers from Bis-Amide-PDMS Multiblock Copolymers Prepared by Aza-Michael Reaction. ACS Appl. Polym. Mater. 2023, 5, 1229–40. [Google Scholar] [CrossRef]
- Fauvre, L; Portinha, D; Fleury, E; Ganachaud, F. Thermoplastic Silicone Elastomers as Materials Exhibiting High Mechanical Properties and/or Self-Healing Propensity. J. Adhes. Sci. Technol. 2021, 35, 2723–35. [Google Scholar] [CrossRef]
- Pouget, E., Holgado-Garcia, E., Vasilenko, I. V., Kostjuk, S. V., Campagne, J. M.; Ganachaud, F. Oligomerization of Electron-Deficient Vinyl Monomers Through an Ate-Complex Mechanism: A New Role for B(C6F5)3 Lewis Acid. Macromol. Rapid Commun. 2009, 30, 1128–32. [Google Scholar] [CrossRef] [PubMed]
- Blount, D.H. Production of Silicon-Phosphorous Containing Compositions. U.S. Patent 5,563,285, 8 October 1996. [Google Scholar]
- Boulos, Y.; About-Jaudet, E.; Bunel, C. Free-Radical Synthesis of New Phosphorus-Containing Silane Monomers and Polysiloxanes. Eur. Polym. J. 1998, 34, 1649–55. [Google Scholar] [CrossRef]
- Gallagher, S. Synthesis and Characterization of Phosphonate-Containing Polysiloxanes. J. Polym. Sci. A: Polym. Chem. 2003, 41, 48–59. [Google Scholar] [CrossRef]
- Milenin, S. A.; Khairova, R. R.; Myakushev, V.D.; Buzin, A. I.; Vasiliev, V. G.; Stoikov, Ivan I.; Muzafarov, A. M. Synthesis of New Organoelement Copolymers Based on Polydimethylsiloxanes and Aminophosphonates. J. Organometal. Chem. 2018, 870, 110–15. [Google Scholar] [CrossRef]
- Schmider, M.; Müh, E.; Klee, J. E.; Mülhaupt, R. A Versatile Synthetic Route to Phosphonate-Functional Monomers, Oligomers, Silanes, and Hybrid Nanoparticles. Macromolecules 2005, 38, 9548–55. [Google Scholar] [CrossRef]
- Sato, T.; Hasegawa, M.; Seno, M.; Hirano, T. Radical Polymerization Behavior of Dimethyl Vinylphosphonate: Homopolymerization and Copolymerization with Trimethoxyvinylsilane. J. Appl. Polym. Sci. 2008, 109, 3746–52. [Google Scholar] [CrossRef]
- Frassica, M. T.; Jones, S. K.; Suriboot, J.; Arabiyat, A. S.; Ramirez, E. M.; Culibrk, R. A.; Hahn, M. S.; Grunlan, M. A. Enhanced Osteogenic Potential of Phosphonated-Siloxane Hydrogel Scaffolds. Biomacromolecules 2020, 21, 5189–99. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, N.; Azema, N.; Cochez, M.; Ferriol, M.; Essahli, M.; Ganachaud, F.; Lopez-Cuesta, J.-M. Influence of the Surface Modification of Alumina Nanoparticles on the Thermal Stability and Fire Reaction of PMMA Composites. Polym. Adv. Technol. 2008, 19, 701–09. [Google Scholar] [CrossRef]
- Berger, A. Amino-Functional Silicone Compounds. U.S. Patent 3,801,572, 2 April 1974. [Google Scholar]
- Curtis, L. S. Jr. Sulfolanyloxyalkyl Cyclic Polysiloxanes. U.S. Patent 4,049,616, 20 September 1977. [Google Scholar]
- Kanner, B.; Prokai, B. Sulfolanyl-Bearing Organosilicone Polymers. U.S. Patent 4,049,674, 20 September 1977. [Google Scholar]
- Kantor, S.W. Sulfone-Containing Organopolysiloxanes. U.S. Patent 2,997,457, 22 August 1961. [Google Scholar]
- Wu, T.C. Sulfone-Containing Organocyclotrisiloxanes. U.S. Patent 3,487,098, 30 December 1969. [Google Scholar]
- Xu, Y.; Guo, M.; Lu, S.; Wei, Z.; Feng, S. Synthesis and Characterization of Novel Poly(sulfone siloxane)s with Good Solubility and Recyclability based on Siloxane Units. New J. Chem. 2022, 46, 10237–45. [Google Scholar] [CrossRef]
- Guo, M.; Huang, Y.; Cao, J.; Sun, G.; Zhao, X.; Zhang, J.; Feng, S. Recyclable sulfone-containing polymers via ring-opening polymerization of macroheterocyclic siloxane monomers: synthesis, properties and recyclability, Polym. Chem. 2019, 10, 6166–6173. [Google Scholar] [CrossRef]
- Dünki, S. J.; Cuervo-Reyes, E.; Opris, D. M. A Facile Synthetic Strategy to Polysiloxanes Containing Sulfonyl Side Groups with High Dielectric Permittivity. Polym. Chem. 2017, 8, 715–24. [Google Scholar] [CrossRef]
- DeSimone, J. M; . York, G. A.; McGrath, J. E.; Gozdz, A. S.; Bowden, M. J. Synthesis, Bulk, Surface and Microlithographic Characterization of Poly(1-butene sulfone)-g-Poly(dimethylsiloxane). Macromolecules 1991, 24, 5330–39. [Google Scholar] [CrossRef]
- Bercea, M.; Airinei, A.; Hamciuc, V.; Ioanid, A. Conformations of Polysulfone-block-Polydimethylsiloxane Chains in Solution and in the Solid State. Polym. Int. 2004, 53, 1860–65. [Google Scholar] [CrossRef]
- Lobez, J.M.; Swager, T. M. Disassembly of Elastomers: Poly(olefin sulfone)−Silicones with Switchable Mechanical Properties. Macromolecules 2010, 43, 10422–26. [Google Scholar] [CrossRef]
- Guo, M.; Huang, Y.; Chen, Z.; Zhang, Y.; Zhang, Y.; Zhu, M.; Zhang, J.; Feng, S. Preparation and Properties of Benzylsulfonyl-Containing Silicone Copolymers via Ring-opening Copolymerization of Macroheterocyclosiloxane and Cyclosiloxane. Chem. Eur. J. 2021, 27, 7897. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Huang, Y.; Cao, J.; Feng, S. Synthesis, Properties, and Degradation Behaviors of Novel Polysulfone-Polysiloxane Multi-Block Copolymers. Polymer 2021, 212, 123134. [Google Scholar] [CrossRef]
- Corpart, J. M.; Girault, S.; Juhué, D. Structure and Surface Properties of Liquid Crystalline Fluoroalkyl Polyacrylates: Role of the Spacer. Langmuir 2001, 17, 7237–44. [Google Scholar] [CrossRef]
- Kim, B. G.; Moon, J. K.; Sohn, E. H.; Lee, S. J.; Yeo, J. K. Polysiloxanes Containing Alkyl Side Groups: Synthesis and Mesomorphic Behavior. Macromol. Res. 2008, 16, 36–44. [Google Scholar] [CrossRef]
- Pierce, O.R.; Kim, Y.K. Fluorosilicones as High Temperature Elastomers. Rubber Chem. Technol. 1971, 44, 1350–62. [Google Scholar] [CrossRef]
- Longuet, C.; Ratsimihety, A.; André, S.; Boutevin, G.; Guida-Pietrasanta, F.; Decamps, B.; Ramonda, M.; Joly-Duhamel, C.; Boutevin, B.; Ganachaud, F. Physically Crosslinked Fluorosilicone Elastomers obtained by Self-Assembly and Template Polycondensation of Tailored Building Blocks. J. Mater. Chem. 2010, 20, 10269–76. [Google Scholar] [CrossRef]
- Schulyndin, S. V.; Levin, Y. A.; Ivanov, B. E. Copolymerisation of Unsaturated Organophosphorus Monomers. Russ. Chem. Rev. 1981, 50, 865–78. [Google Scholar] [CrossRef]
- Buvat, P.; Boucheteau, T.; David, G.; Ganachaud, F.; Kostjuk, S. V. Specific Phosphonated Copolymers and Inorganic Particles Grafted by Said Copolymers, European Patent 2,697,857, 10 April 2012. [Google Scholar]
- Ganachaud, F.; Pouget, E.; Boutevin, B.; Loubat, C. Sulfoned Silicones Generating Elastomers by Self Assembly, Synthesis Process and use of such Silicones Materials, French Patent 2912410, 12 February 2007. [Google Scholar]
- David R. Lide, CRC Handbook of Chemistry and Physics, CRC Press, Cleveland, OH, 1979.
- Evans, S.D.; Goppert-Berarducci, K.E.; Urankar, E.; Gerenser, L.J.; Ulman, A.; Snyder, R.G. Monolayers Having Large in-Plane Dipole Moments: Characterization of Sulfone-Containing Self-Assembled Monolayers of Alkanethiols on Gold by Fourier Transform Infrared Spectroscopy, X-Ray Photoelectron Spectroscopy and Wetting. Langmuir 1991, 7, 2700–09. [Google Scholar] [CrossRef]
- Essahli, M. Synthesis of new oligomers functionalized by phosphonic acids, Ph. D. Thesis, Montpellier University, 2006.
- Dass, N. N.; Date, R. W.; Fawcett, A. H.; McLaughlin, J. D.; Sosanwo, O. A. Recognition of a new type of main chain liquid-crystalline polymer: poly(1-olefin sulfones). Macromolecules 1993, 26, 4192–95. [Google Scholar] [CrossRef]
- van den Berg, O.; Dispinar, T.; Hommez, B.; Du Prez, F. E. Renewable Sulfur-Containing Thermoplastics via AB-Type Thiol-Ene Polyaddition. Eur. Polym. J. 2013, 49, 804–12. [Google Scholar] [CrossRef]
- Essahli, M.; Ganachaud, F.; In, M.; Boutevin, B. Phosphonic Acid Functionalized Polyethylene Glycol and Derivatives. J Appl Polym Sci 2008, 108, 484–90. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).