1. Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a serious condition predominantly linked to drugs like bisphosphonates and denosumab, mainly used for treating osteoporosis and some cancers [
1]. It manifests as exposed, non-healing bone in the maxillofacial region, usually within eight weeks in the absence of radiation therapy [
2], significantly affecting patients’ quality of life by causing pain, infections, and impacting eating and speaking [
3,
4]. The risk of MRONJ increases with prolonged medication use, dental procedures, and comorbid conditions, highlighting the importance of early detection and prevention [
5].
In managing MRONJ, drug holidays, which involve a temporary cessation of medication, are considered a strategic but controversial approach [
6]. This method aims to reduce risks by balancing the benefits of primary treatment against potential negative effects on jaw health [
7]. It requires careful evaluation of patient-specific factors, timing, and drug properties, but its efficacy in MRONJ management is still a subject of debate within the medical community.
Treatment strategies for MRONJ are diverse, ranging from conservative symptom management and infection control to more advanced surgical interventions, depending on the condition’s severity and the patient’s individual needs [
1,
2]. The potential of emerging therapies like autologous platelet concentrates (APCs), mesenchymal stem cells (MSCs), and laser therapy is recognized [
8,
9]. However, a detailed discussion of these therapies is reserved for subsequent sections to avoid redundancy and ensure a focused discourse [
10].
The primary objective of this systematic review is to meticulously assess the latest advancements in the management of MRONJ, encapsulating not only the emerging treatment modalities but also providing a comprehensive synthesis of the current understanding of its etiology and the strategic implementation of drug holidays. This review aspires to critically analyze the efficacy of novel therapeutic approaches, spotlight potential gaps in existing knowledge, and identify avenues for future research. It aims to offer an exhaustive analysis of innovative treatments, particularly those leveraging the prowess of regenerative medicine and minimally invasive technologies, thereby contributing to the ongoing discourse on MRONJ management, steering clinical practice with evidence-based insights, and advocating for more precise, effective, and patient-centric treatment methodologies.
2. Materials and Methods
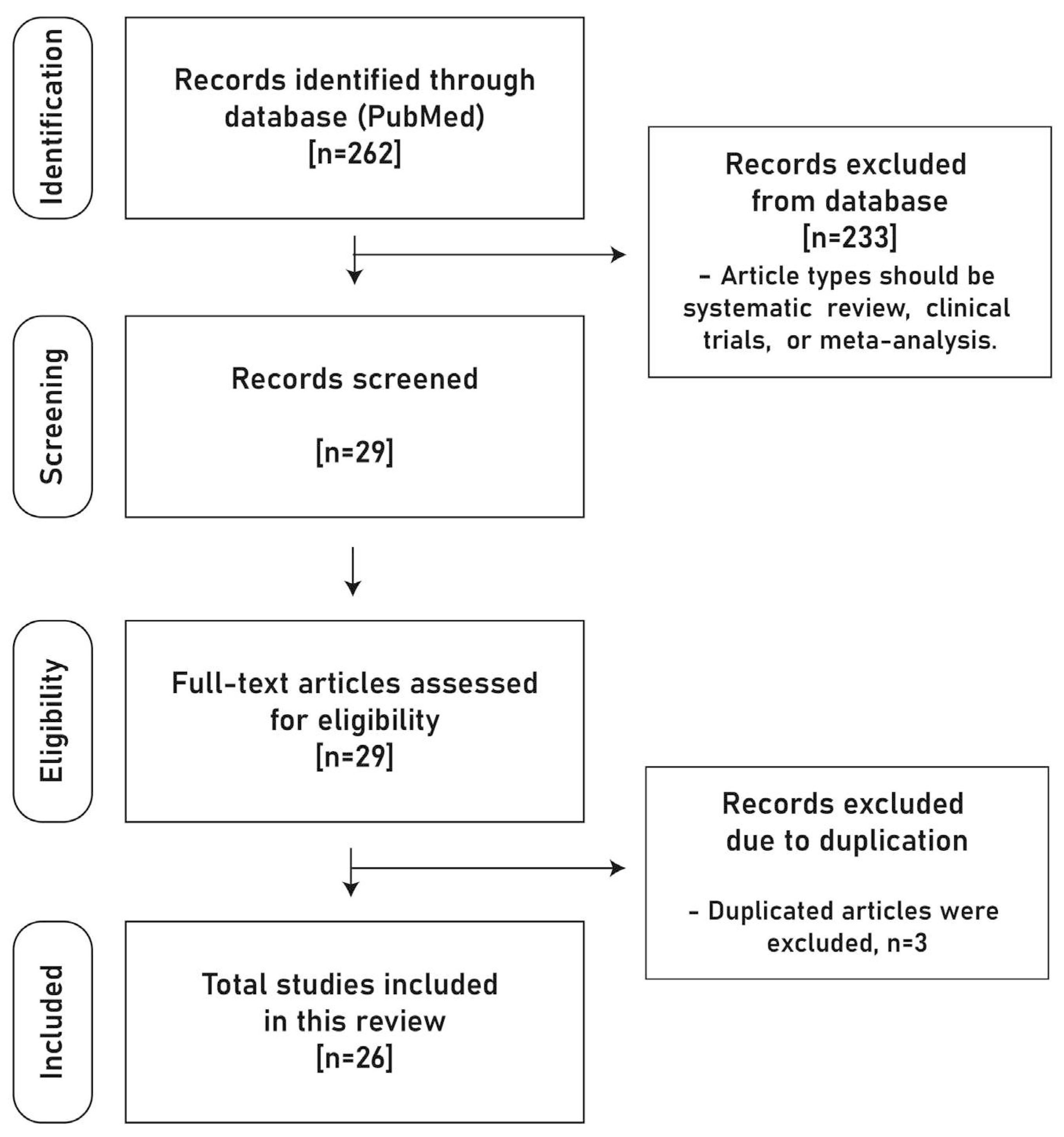
In this narrative review, a specific article selection protocol prioritized the most recent and pertinent literature on the treatment of MRONJ, specifically from 2022 to 2023. The search, conducted via PubMed (MEDLINE), targeted systematic reviews, clinical trials, and meta-analyses, using tailored keywords to ensure relevance and currency (
Figure 1).
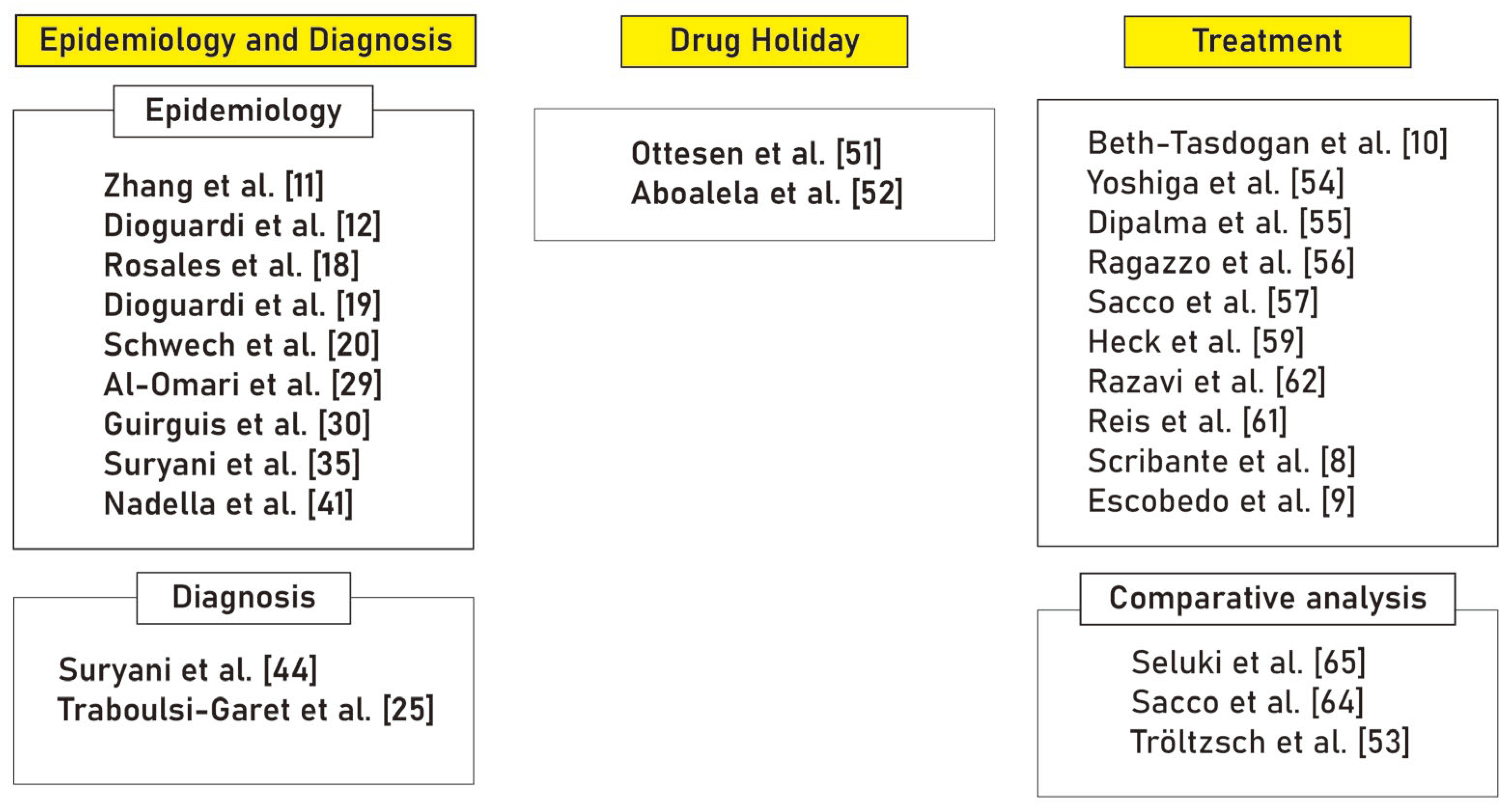
From an initial pool of 262 articles, 26 were meticulously chosen based on relevance and uniqueness. These were then carefully analyzed and categorized thematically into ‘Epidemiology and Diagnosis’ (11 articles), ‘Drug Holiday’ (2 articles), and ‘Treatment’ (13 articles), ensuring a comprehensive and up-to-date review (
Figure 2).
3. Epidemiology and Diagnosis
3.1. Incidence associated with Age and Sex
The relationship between sex and the incidence of MRONJ is multifaceted and subject to ongoing investigation, as highlighted by recent studies. A study found that using exposed necrotic bone as a diagnostic criterion did not reveal significant differences in MRONJ incidence between sexes [
1]. However, it did report subdued pain responses in male mice compared to female mice in cases of stage 0 MRONJ diagnosed histologically in murine models, suggesting that males might experience under-diagnosis or delayed diagnosis due to less severe pain symptoms, potentially leading to later stage identification of MRONJ in this group [
11].
Contrastingly, another study indicated a different trend, showing that MRONJ related to dentoalveolar surgical procedures disproportionately affected males, particularly in the posterior mandibular sectors [
12]. This suggests a possible higher susceptibility of males to MRONJ post-surgical procedures in specific jaw locations, though the study also emphasized the need for further research to confirm this trend [
12].
These divergent findings underscore the complexity of the sex-MRONJ incidence relationship. The first study [
11] highlights the risk of under-diagnosis in males due to less pronounced pain symptoms, whereas the second study [
12] points to a potentially higher incidence of MRONJ in males, especially in particular areas of the jaw after surgical interventions. Both studies stress the necessity of acknowledging sex-based differences in the presentation, diagnosis, and progression of MRONJ.
Moreover, antiresorptive drugs like bisphosphonates (BP) and denosumab (DS), primarily used in adults for managing cancer and osteoporosis, have recently seen increased usage among children and young individuals [
13,
14]. These medications are used for conditions such as osteogenesis imperfecta (OI), glucocorticoid-induced osteoporosis, McCune-Albright syndrome (MAS), and malignant hypercalcemia [
15,
16,
17]. A systematic review encompassing 29 articles published between 2007 and 2022 included 1192 patients, predominantly treated for OI [
18]. This study revealed a relatively low presence of MRONJ in the child and youth population undergoing antiresorptive drug therapy [
18]. However, it also highlighted significant challenges in data collection, with unclear therapy details and deficiencies in protocols and pharmacological characterizations in many reviewed articles.
3.2. Incidence associated with Surgical Intervention
3.2.1. Tooth extraction
Tooth extraction in patients undergoing antiresorptive medication therapy, such as BPs and DS, has been closely scrutinized due to the associated risk of MRONJ. Recent systematic reviews shed light on the incidence rates and risk factors tied to MRONJ following tooth extractions, underscoring the complex interplay between medication use, dental procedures, and patient characteristics [
19,
20].
A review delving into the impact of oral BP therapy on tooth extraction procedures highlighted an increased risk of developing MRONJ as a notable complication following such dental procedures [
19]. The study identified the duration of BP treatment as a pivotal factor in the incidence of MRONJ, particularly emphasizing a higher prevalence in the posterior region of the mandible [
19]. Patients with a history of BP treatment exceeding three years warrant careful monitoring and management due to the elevated risk [
21]. This heightened risk can be attributed to the significantly higher bone turnover rate in the mandible compared to other skeletal areas like the tibia [
22]; a factor that results in a greater accumulation of bisphosphonates in the mandibular bone [
23]. The review further underscored the importance of a comprehensive understanding of procedural details, including the selection of grafting materials, adherence to meticulous surgical protocols, and an awareness of expected success rates, as key components in mitigating the risk of MRONJ following tooth extraction procedures [
19].
Complementing these findings, another review specifically focused on cancer patients treated with high-dose BP and DS [
20]. This review reported a varying MRONJ incidence rate of 11% to 50% after tooth extractions, emphasizing the significant heterogeneity in the collected data [
20]. Key risk factors identified included teeth affected by pre-extraction inflammation [
24] and additional osteotomy during the surgical procedure [
20]. The study also highlighted the lack of statistically significant associations between MRONJ incidence and factors such as age, gender, tobacco, and alcohol use, emphasizing the need for a nuanced understanding of individual patient profiles when assessing MRONJ risk [
20].
Both reviews converge on the critical insight that while tooth extraction is a pivotal trigger for MRONJ, the condition’s manifestation is significantly influenced by the presence of pre-existing inflammation, the specifics of the surgical procedure, and the duration of antiresorptive medication use [
19,
20,
25]. Clinicians are thus urged to approach tooth extractions in patients on antiresorptive therapy with heightened vigilance, tailoring their strategies to the nuanced risk profiles presented by individual patients. The reviews collectively stress the importance of an integrated approach—combining precise surgical techniques, thorough patient education, and vigilant postoperative monitoring—to effectively manage and mitigate the risks associated with MRONJ in this sensitive patient cohort.
3.2.2. Dental implant
A previous study has identified the presence of dental implants as a potential risk factor for MRONJ, with implant treatment reported to trigger MRONJ in certain cases [
26,
27]. However, contrasting evidence from another systematic review suggests relatively high implant survival rates, reporting 97%, 99%, and 91% for groups not using antiresorptive drugs, those taking oral BP, and those receiving intravenous BP, respectively [
28]. Despite these high survival rates, recent reviews caution that surgical interventions, including dental implants and regenerative therapies, carry inherent risks, particularly for patients on antiresorptive medications like BP and denosumab [
29]. These interventions are known triggers for MRONJ, with an increased risk in scenarios involving bone manipulation or stress [
29]. Compounding this concern is the significant lack of detailed information on regenerative therapies, including the types of grafting materials used, the surgical protocols followed, and the metrics of success and failure rates [
29]. Details specific to implant treatments, such as the timing of insertion, implant dimensions, and the loading protocol, are also inadequately documented [
29]. This paucity of information hampers the ability of clinicians to fully assess and effectively mitigate the risks associated with these surgical interventions for each patient.
3.3. Incidence associated with Medication
Antiresorptive and antiangiogenic medications, primarily used in the treatment of osteometabolic diseases and cancer, have been associated with the onset of MRONJ, a condition notably characterized by its unique localization to the maxillofacial region [
1,
2]. A comprehensive review of the existing in vitro literature has shed light on the extensive research conducted on these medications, particularly nitrogen-containing BPs (N-BPs) like zoledronate (ZA) [
30]. ZA, owing to its high potency, prolonged duration of action, and role as a first-line therapy for osteoporosis and adjunct therapy in cancer treatment, has been at the forefront of these investigations [
31]. The review emphasizes the need for a nuanced understanding of these drugs’ cellular impact, especially given their potent antiresorptive properties compared to non-nitrogen BPs, and the variations in potency among different N-BPs due to structural differences in their R2 side chains [
30].
The cytotoxic effects of these medications are multifaceted, affecting cellular functions such as proliferation, viability, migration, and inducing apoptosis in oral tissues in a dose- and time-dependent manner. Drugs like ZA not only impede cell proliferation and viability starting at 5 μM [
32] but also interfere with key cellular pathways such as the mevalonate pathway, thereby affecting cell signaling and function [
33]. Interestingly, the review reveals that antiresorptive and antiangiogenic drugs do not solely impact the bone tissue; they also exhibit genotoxic and cytotoxic effects on oral soft tissues, impairing cell proliferation, metabolism, and migration, thereby altering cell morphology and inducing apoptosis and inflammation [
30]. This complex interplay of drug effects underscores the need for clinicians to approach treatment with these medications cautiously, especially considering the significant role of factors like transforming growth factor-β in wound healing and the implications of drug-induced reactive oxygen species production on cell growth and signaling pathways [
34].
Aside from antiresorptive (AR) therapy, various other medications have been identified as potential inducers of osteonecrosis of the jaw. These include angiogenesis inhibitors, immunosuppressants, anti-cancer drugs, and inhibitors of the mammalian target of rapamycin [
35]. Notably, patients undergoing treatment with anti-cancer drugs or steroids have been found to exhibit a higher association with MRONJ [
35]. Specifically, anti-cancer drugs like gemcitabine can contribute to the development of MRONJ due to their anti-angiogenic effects, which involve the inhibition of vascular endothelial growth factor [
36]. Similarly, long-term use of corticosteroids may lead to MRONJ through a mechanism akin to that of gemcitabine, highlighting the complex interplay between various drug therapies and the risk of MRONJ [
37].
3.4. Incidence associated with Disease
MRONJ has traditionally been linked to the treatment of osteoporosis and cancers, as the medications used for these conditions are often associated with the development of MRONJ. Besides these, other factors such as diabetes, immunosuppressive conditions, and anemia have been identified as potential risk contributors to MRONJ [
38]. However, the relationship between MRONJ and certain conditions like diabetes remains controversial, with some studies reporting no direct connection [
39,
40].
Recently, attention has shifted towards exploring the incidence of spontaneous MRONJ in individuals with fibrous dysplasia (FD) and McCune-Albright syndrome (MAS). A detailed systematic review was undertaken to assess the risk of MRONJ among patients diagnosed with FD and MAS, particularly focusing on those receiving bisphosphonates (BPs) and other antiresorptive treatments [
41]. This review analyzed data from a total of 312 patients with FD and FD/MAS and identified 12 reported cases of MRONJ, resulting in an incidence rate of 3.85% [
41]. Although the review suggests that the risk of MRONJ in patients with FD or FD/MAS is relatively low, it is noteworthy that this incidence rate is somewhat higher compared to those observed in patients with osteoporosis and other malignancies, underscoring a nuanced risk profile for MRONJ in this specific patient group [
35,
41].
3.5. Diagnostic Test for MRONJ
Several serum-based tests have been explored to assess the risk of MRONJ, including markers such as osteocalcin, alkaline phosphatase, N-terminal telopeptide, and C-terminal telopeptide cross-link of type I collagen (CTX) [
25]. CTX is released during the bone remodeling process and has been considered a potential diagnostic indicator for MRONJ [
42]. However, the serum level of CTX has its limitations, failing to predict MRONJ development in more than 40% of patients [
25]. In contrast, recent advancements in technology have led to the development of a machine learning model demonstrating a 100% sensitivity and 83.3% specificity in MRONJ risk assessment [
43]. For a test to be considered effective in diagnosing MRONJ, it is generally expected to meet a minimum specificity of 95% and sensitivity of 75% [
25]. However, CTX falls short of these benchmarks, with a sensitivity of 56.8% and specificity of 72%, indicating the need for more accurate and reliable diagnostic methods [
25].
The medication-induced salivary changes are also considered for the assessment of MRONJ risk [
44]. The study highlights those various pharmacological agents, including BPs, DS, corticosteroids, and chemotherapy, are known to alter salivary composition, secretion levels, and function [
44]. The review underscores the protective and functional properties of saliva in maintaining oral homeostasis, including teeth remineralization, buffering of acids, inhibition of harmful microorganisms, prevention of xerostomia, and facilitation of speech and swallowing. Any disruption in salivary function could lead to a range of complications, impacting the quality of life.
The review illuminates the potential role of salivary alterations as risk factors in the development of MRONJ [
44]. It identifies a decrease in salivary flow and changes in salivary protein levels as key indicators that may predispose individuals to MRONJ [
44]. The intricate relationship between salivary changes and MRONJ development is further explored, with particular emphasis on how bone-modifying agents affect the microstructure of saliva [
45]. These agents, by altering saliva’s microbiome profiles [
46], cytokine profiles [
47], interleukins [
48], hypotaurine [
49], RNA-binding protein such as RMBS3 [
50], have been associated with an increased risk of MRONJ due to their influence on salivary composition and function.
Despite these insights, the review does not present specific sensitivity and specificity values for using salivary changes as diagnostic markers for MRONJ [
44]. Therefore, while the review highlights a promising avenue for early detection and risk assessment, the actual diagnostic utility of salivary alterations in MRONJ remains to be validated through further research. This calls for more in-depth studies to substantiate the diagnostic value of these salivary changes, ideally through standardized, large-scale, prospective research efforts.
4. Drug Holiday
4.1. Definition and Rationale
A ‘drug holiday’ refers to the deliberate, temporary discontinuation of a medication regimen, in this case, high-dose AR therapy, typically administered to patients with cancer or osteoporosis [
6,
7]. The concept of a drug holiday has been proposed in the context of MRONJ as a potential preventive strategy. The rationale behind a drug holiday is to mitigate the risk of developing MRONJ, particularly in patients undergoing dentoalveolar surgery or tooth extractions, by pausing the AR therapy during the perioperative period.
4.2. The Effectiveness of Drug Holiday
The feasibility and effectiveness of this approach, however, have been subject to investigation in recent studies. One study aimed to evaluate the impact of a high-dose AR drug holiday, from 1 month before to 3 months after surgical tooth extraction, on the development of MRONJ and patient-reported health state [
51]. However, the results indicated that a high-dose AR drug holiday did not prevent the development of MRONJ post-surgical tooth extraction and was associated with a decline in the patient-reported health state compared to continuous medication [
51].
Complementing these findings, another study systematically reviewed the literature, including both observational and interventional studies, to assess the effect of an AR drug holiday on the development of MRONJ. This extensive analysis encompassed 6808 subjects, comparing cases from the drug holiday group with controls from the non-drug holiday group [
52]. The results revealed no significant difference between the groups in the development of MRONJ, leading to the conclusion that AR drug holidays do not minimize the risk of MRONJ, and their recommendation as a preventive measure is not substantiated by the current evidence [
52].
In summary, while the notion of a drug holiday for AR therapy in the context of MRONJ is driven by the intent to reduce the risk of this serious complication, recent studies suggest that this approach may not be effective [
53]. Given the findings of these studies, existing guidelines, and consensus statements on drug holidays for patients at risk of or suffering from MRONJ may need to be re-evaluated. The studies underscore the importance of relying on a robust evidence base when formulating guidelines and highlight the need for further research, including large-scale prospective studies and randomized trials, to clarify the role and efficacy of drug holidays in the context of MRONJ [
10].
5. Treatment
The treatment of MRONJ can be non-surgical conservative method and surgical method. Conservative treatment for early-stage MRONJ (stages 0-2) primarily focuses on symptom management, infection control, and preventing the progression of the disease [
10]. It includes practices like maintaining good oral hygiene, using chlorhexidine mouthwash, and taking antibiotics. This approach is particularly recommended for patients who are not eligible for surgery or prefer non-surgical interventions. Surgical intervention, on the other hand, was identified as a more effective approach, especially for patients with advanced stages of MRONJ. The review also highlighted the potential benefits of adjuvant treatments like hyperbaric oxygen, ozone therapy, and low-intensity laser therapy when combined with conservative treatment, suggesting that these might lead to higher success rates and positive outcomes [
10]. Additionally, the use of APCs like platelet rich fibrin and concentrated growth factor, derived from the patient’s blood, showed significant improvements in mucosal healing, down-staging, and low relapse rates due to their ability to release high quantities of growth factors that stimulate bone regeneration and tissue healing [
8,
9].
5.1. Conservative Management
Non-surgical management strategies for MRONJ typically encompass antiseptic rinsing and various pharmacological treatments. Although no systematic reviews on conservative management were identified in the studied period, a clinical case series study shed light on the potential of teriparatide (TPTD) as an adjunctive treatment. This study focused on patients with osteoporosis suffering from chronic, refractory MRONJ, who had not seen improvement with conservative treatment alone for at least two months [
54].
The study meticulously divided 27 patients into three distinct groups: one receiving daily TPTD treatment (20 μg), another undergoing weekly TPTD treatment (56.5 μg), and a third group serving as a non-TPTD control. Remarkably, the groups treated with TPTD exhibited a significantly shortened average healing time of 3.3 months, compared to the prolonged 11.9 months observed in the control group [
54]. Moreover, the cure rate in the TPTD-treated groups reached 100%, a notable increase from the 67% cure rate in the non-TPTD group [
54]. Despite these promising outcomes, it is worth noting that some patients receiving daily TPTD reported mild adverse effects, including palpitations, sickness, and headaches, leading to discontinuation of the treatment [
54].
In summary, the study highlights that when combined with standard conservative therapy, adjunctive TPTD therapy significantly reduces the healing time and enhances the cure rate for chronic, refractory MRONJ, offering a substantial improvement over conservative treatment alone [
54]. However, it also underscores the necessity for more comprehensive research and collaborative efforts to confirm these results and further elucidate the role of TPTD in the treatment of MRONJ.
5.2. Surgical Interventions
Surgical management of MRONJ often involves the resection of necrotic bone followed by reconstruction. Precision in resecting the affected area is crucial and necessitates advanced visualization techniques. A study conducted a rigorous evaluation of 17 articles, selected from a comprehensive pool of 320, to assess the clinical efficacy of autofluorescence-guided laser therapy in this context [
55]. This innovative technology leverages autofluorescence to enhance the visibility of bone structures impacted by MRONJ, significantly improving the accuracy and safety of surgical interventions [
55].
The review notes that surgeries guided by autofluorescence offer success rates like those achieved by tetracycline fluorescence-guided surgeries, especially in ensuring mucosal integrity, reducing infection, and alleviating pain [
55]. However, it also points out a notable gap in the literature concerning the standardization and broader clinical adoption of autofluorescence technology for MRONJ treatment. This indicates a pressing need for more research and development to fully integrate and standardize autofluorescence-guided procedures in clinical practice, ensuring a consistent and reliable approach to MRONJ surgical management [
55].
In the realm of surgical intervention for MRONJ, the use of human amniotic membrane (hAM) is emerging as a promising reconstructive material. While one study highlighted hAM’s potential in reducing post-surgical pain [
56], a review encompassing five studies with 91 patients revealed a high success rate (91.2%) for hAM in surgical therapy [
57]. Although these findings indicate hAM’s efficacy and potential for improving patient comfort, they also underline the need for more extensive research to fully grasp its long-term benefits and recurrence rates after MRONJ treatment [
56,
57].
5.3. Adjuvant Treatments
5.3.1. Hyperbaric Oxygen Therapy (HBO)
HBO is recognized for its role as an adjuvant treatment, particularly in managing anaerobic bacterial infections, and is noted for its potential to enhance wound healing through improved oxygenation [
58]. A recent systematic review sought to assess the efficacy of HBO in treating conditions like osteoradionecrosis and MRONJ [
59]. Out of the fourteen studies included in the review, only one specifically addressed the use of HBO in MRONJ treatment [
59]. While this study hinted at HBO’s positive impact on wound healing, the observed differences in outcomes were not statistically significant, which makes the conclusions less robust [
59,
60]. Given that the review incorporated just a single study focusing on MRONJ and HBO, it is difficult to definitively ascertain the benefits of HBO for MRONJ [
59]. Nevertheless, the review did recognize HBO as potentially beneficial in preventing osteoradionecrosis, although its effectiveness as a treatment for MRONJ remains to be conclusively proven and warrants further investigation [
59].
5.3.2. Photobiomodulation
The review provides a detailed analysis of the roles of autologous fibrin and photobiomodulation therapy (PBMT) in the prevention and treatment of MRONJ [
61]. It scrutinizes the effectiveness of these approaches in promoting healing and diminishing the recurrence of MRONJ, referencing data from 19 diverse studies, encompassing controlled interventions, before-after studies without control groups, and observational cohorts [
61]. Despite the positive findings, the review underscores the necessity for additional research to fully comprehend the long-term impacts and recurrence rates tied to these treatment modalities.
Complementing this, another comprehensive review involving 759 patients reinforces the efficacy of PBMT as a beneficial adjunct therapy in MRONJ treatment [
62]. It reveals that PBMT, when employed as the sole or primary treatment, did not result in complete wound healing [
63]. However, when PBMT was combined with surgical resection as an adjunct therapy, patients experienced complete wound healing, highlighting the synergistic potential of combining PBMT with surgical interventions [
62]. These findings advocate for the integration of PBMT as a supplementary treatment to traditional surgical methods, providing a more holistic approach to MRONJ management.
5.3.3. APCs
APCs have emerged as a notable advancement in the management of MRONJ, offering promising results in mitigating the onset of MRONJ and fostering rapid epithelialization [
8,
9]. These concentrates, enriched with growth factors, are instrumental in modulating inflammation and expediting the immune response, crucial factors in the healing process for patients undergoing bisphosphonate treatment.
A systematic review scrutinizing 19 studies, including controlled interventions, observational studies, and cohorts, delves into the efficacy of APCs, alongside laser therapy, in the prevention and treatment of MRONJ [
8]. The review accentuates the therapeutic potential of APCs in enhancing the healing of hard and soft tissues, thereby reducing the recurrence rates of MRONJ. Although initial, non-invasive treatments typically involve antibiotics and antiseptics, surgical intervention, such as the removal of necrotic bone, is sometimes indispensable. The review corroborates those surgical approaches, particularly when combined with APCs like “platelet-rich plasma” and “leucocyte- and platelet-rich fibrin”, yield superior clinical outcomes and comprehensive soft tissue healing, underscoring the synergistic effect of combining surgical debridement with APCs for improved bone tissue maintenance and overall healing [
8].
Complementing this, another review rigorously evaluates the application of APCs and MSCs as adjunct therapies for MRONJ, analyzing data from 29 articles encompassing 306 patients [
9]. This review acknowledges the role of APCs in advancing tissue healing by introducing growth factors that facilitate cell chemotaxis, proliferation, differentiation, angiogenesis, and the formation of a new extracellular matrix. However, it also recognizes the necessity for more extensive research to substantiate these preliminary findings and develop standardized clinical protocols for the effective application of APCs and MSCs in MRONJ management [
9].
In summary, both reviews highlight the potential of APCs in enhancing the recovery process for patients with MRONJ, suggesting a beneficial role in both prevention and treatment strategies. Nonetheless, they also underscore the need for further empirical studies to solidify the scientific evidence supporting the use of APCs and MSCs and to establish robust, standardized treatment protocols in clinical practice.
5.4. Comparison among Treatments
The systematic literature search, adhering to PRISMA guidelines, was performed across several databases for studies published from 2016 to 2021, focusing on diagnostic methods, prospective management trials, and innovative studies on MRONJ’s pathogenesis [
53]. The review emphasizes the importance of early and surgical intervention in MRONJ treatment, citing high long-term success rates exceeding 85% [
53]. The review indicates that while non-surgical treatment options like antibiotic therapy, antiseptic rinses, and eliminating causative factors can be attempted for limited disease stages, complete healing of MRONJ lesions under conservative treatment is extremely unlikely [
53,
64]. In contrast, surgical treatments, including fluorescence-guided surgery, are reported to have high success rates of up to 90% for complete healing [
53].
Another comparative review concludes that while conservative treatment can yield better outcomes for asymptomatic patients in early stages of MRONJ, surgical treatment is superior in patients with advanced stages of the disease [
65]. It emphasizes the importance of individualized treatment decisions, regular communication, and collaboration between dental and medical professionals to ensure optimal care, tailored to the severity of the patient’s condition, and achieve the best possible clinical outcomes while minimizing potential complications [
65]. However, it also acknowledges the limitations of the review, including the limited number of studies on conservative treatment and the small sample sizes, calling for further research to strengthen the evidence base and improve the significance of the findings [
65].
6. Conclusion
In conclusion, this comprehensive review critically evaluates the advancements and therapeutic innovations in managing MRONJ. It synthesizes current literature on emerging treatments, assessing their efficacy, and highlighting gaps in knowledge and future research areas. As MRONJ continues to challenge clinicians due to its complex pathophysiology and variable treatment efficacy, exploring and validating novel therapeutic strategies remains paramount. This review’s significance lies in its in-depth analysis of cutting-edge treatments, offering insights into their therapeutic value, limitations, and prospects, and guiding clinical practice with evidence-based recommendations. It emphasizes the need for a targeted, efficacious, and patient-centered approach in MRONJ management, reflecting the condition’s evolving landscape and the ongoing quest for improved patient outcomes.
Supplementary Materials
none.
Author Contributions
Conceptualization, O.J.H. and K.S.G.; methodology, K.S.G.; validation, K.S.G.; formal analysis, O.J.H.; investigation, K.S.G.; resources, K.S.G.; data curation, K.S.G.; writing—original draft preparation, K.S.G.; writing—review and editing, O.J.H.; visualization, K.S.G.; supervision, K.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2022-00166535).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data were shown in the paper and there was no additional dataset.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, H.J.; Kim, M.J.; Ahn, K.M. Associated systemic diseases and etiologies of medication-related osteonecrosis of the jaw: a retrospective study of 265 surgical cases. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 12. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Jo, H.; Kim, J.E.; Ohe, J.Y. A clinical retrospective study of implant as a risk factor for medication-related osteonecrosis of the jaw: surgery vs loading? Maxillofac. Plast. Reconstr. Surg. 2023, 45, 31. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- de Cassia Tornier, S.; Macedo, F.J.; Sassi, L.M.; Schussel, J.L. Quality of life in cancer patients with or without medication-related osteonecrosis of the jaw. Support Care Cancer. 2021, 29, 6713–6719. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Morishita, K.; Soutome, S.; Otsuru, M.; Hayashida, S.; Murata, M.; Sasaki, M.; Takagi, Y.; Sumi, M.; Umeda, M. Relationship between drug holiday of the antiresorptive agents and surgical outcome of medication-related osteonecrosis of the jaw in osteoporosis patients. Sci. Rep. 2022, 12, 11545. [Google Scholar] [CrossRef]
- Scribante, A.; Ghizzoni, M.; Pellegrini, M.; Pulicari, F.; Spadari, F. Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review. Medicina (Kaunas). 2023, 59, 972. [Google Scholar] [CrossRef]
- Escobedo, M.F.; Junquera, S.; Gonzalez, C.; Vasatyuk, S.; Gallego, L.; Barbeito, E.; Junquera, L.M. Efficacy of complementary treatment with autologous platelet concentrates and/or mesenchymal stem cells in chemical osteonecrosis of the jaw. Systematic review of the literature. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 51–58. [Google Scholar]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, CD012432. [Google Scholar]
- Zhang, W.; Gao, R.; Cui, Y.; Ding, F.; Zhu, S.; Luo, S.; Liu, H.; Li, M. Sex difference in the morbidity and pain response with stage 0 of medication-related osteonecrosis of the jaws. J. Oral Biosci. 2023, 65, 324–333. [Google Scholar] [CrossRef]
- Dioguardi, M.; Spirito, F.; Alovisi, M.; Aiuto, R.; Garcovich, D.; Crincoli, V.; Ballini, A.; Caloro, G.A.; Lo Muzio, L. Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis. J. Clin. Med. 2023, 12, 3299. [Google Scholar] [CrossRef]
- Baroncelli, G.; Bertelloni, S. The use of bisphosphonates in pediatrics. Horm. Res. Paediatr. 2014, 82, 290–302. [Google Scholar] [CrossRef]
- Boyce, A.M. Denosumab: An emerging therapy in pediatric bone disorders. Curr. Osteoporos. Rep. 2017, 15, 283–292. [Google Scholar] [CrossRef]
- Brown, J.J.; Ramalingam, L.; Zacharin, M.R. Bisphosphonate-associated osteonecrosis of the jaw: Does it occur in children? Clin. Endocrinol. 2008, 68, 863–867. [Google Scholar] [CrossRef]
- Chahine, C.; Cheung, M.S.; Head, T.W.; Schwartz, S.; Glorieux, F.H.; Rauch, F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J. Pediatr. 2008, 153, 719–720. [Google Scholar] [CrossRef]
- Feehan, A.; Zacharin, M.R.; Lim, A.S.; Simm, P.J. A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone 2018, 113, 137–143. [Google Scholar] [CrossRef]
- Rosales, H.D.; Garcia Guevara, H.; Requejo, S.; Jensen, M.D.; Acero, J.; Olate, S. Medication-Related Osteonecrosis of the Jaws (MRONJ) in Children and Young Patients—A Systematic Review. J. Clin. Med. 2023, 12, 1416. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Cosola, M.; Copelli, C.; Cantore, S.; Quarta, C.; Nitsch, G.; Sovereto, D.; Spirito, F.; Caloro, G.A.; Cazzolla, A.P.; Aiuto, R.; Cascardi, E.; Greco Lucchina, A.; Lo Muzio, L.; Ballini, A.; Mastrangelo, F. Oral bisphosphonate-induced osteonecrosis complications in patients undergoing tooth extraction: a systematic review and literature updates. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6359–6373. [Google Scholar]
- Schwech, N.; Nilsson, J.; Gabre, P. Incidence and risk factors for medication-related osteonecrosis after tooth extraction in cancer patients—A systematic review. Clin. Exp. Dent. Res. 2023, 9, 55–65. [Google Scholar] [CrossRef]
- Jeong, H.G.; Hwang, J.J.; Lee, J.H.; Kim, Y.H.; Na, J.Y.; Han, S.S. Risk factors of osteonecrosis of the jaw after tooth extraction in osteoporotic patients on oral bisphosphonates. Imaging Sci. Dent. 2017, 47, 45–50. [Google Scholar] [CrossRef]
- Pisacane, A.; Cascardi, E.; Berrino, E.; Polidori, A.; Sarotto, I.; Casorzo, L.; Panero, M.; Boccaccio, C.; Verginelli, F.; Benvenuti, S.; Dellino, M.; Comoglio, P.; Montemurro, F.; Geuna, E.; Marchiò, C.; Sapino, A. Real-world histopathological approach to malignancy of undefined primary origin (MUO) to diagnose cancers of unknown primary (CUPs). Virchows Arch. 2023, 482, 463–475. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Troiano, G.; Caponio, V.C.A.; Sabatucci, A.; Balercia, A.; Rubini, C.; Lo Muzio, L.; Santarelli, A. Odontogenic tumours: a 25-year epidemiological study in the Marche region of Italy. Eur. Arch. Otorhinolaryngol. 2020, 277, 527–538. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hayashida, S.; Kondo, E.; Takeda, Y.; Miyamoto, H.; Kawaoka, Y.; Ueda, N.; Iwata, E.; Nakahara, H.; Kobayashi, M.; Soutome, S.; Yamada, S.; Tojyo, I.; Kojima, Y.; Umeda, M.; Fujita, S.; Kurita, H.; Shibuya, Y.; Kirita, T.; Komori, T. Japanese Study Group of Co-operative Dentistry with Medicine (JCDM). Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: A multicenter retrospective study. Osteoporos. Int. 2019, 30, 231–239. [Google Scholar]
- Traboulsi-Garet, B.; Jorba-García, A.; Camps-Font, O.; Alves, F.A.; Figueiredo, R.; Valmaseda-Castellón, E. Is serum C-terminal telopeptide cross-link of type 1 collagen a reliable parameter for predicting the risk of medication-related osteonecrosis of the jaws? A systematic review and meta-analysis of diagnostic test accuracy. Clin. Oral Investig. 2022, 26, 2371–2382. [Google Scholar]
- Escobedo, M.F.; Cobo, J.L.; Junquera, S.; Milla, J.; Olay, S.; Junquera, L.M. Medication-related osteonecrosis of the jaw. Implant presence-triggered osteonecrosis: Case series and literature review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 40–48. [Google Scholar]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Gelazius, R.; Poskevicius, L.; Sakavicius, D.; Grimuta, V.; Juodzbalys, G. Dental Implant Placement in Patients on Bisphosphonate Therapy: a Systematic Review. J. Oral Maxillofac. Res. 2018, 9, e2. [Google Scholar] [CrossRef]
- Al-Omari, F.A.; Kuroshima, S.; Sawase, T. Medication-related Osteonecrosis of the Jaw Induced by Regenerative Therapy in Implant Dentistry: A Scoping Review. J. Dent. 2023, 138, 104682. [Google Scholar] [CrossRef]
- Guirguis, R.H.; Tan, L.P.; Hicks, R.M.; Hasan, A.; Duong, T.D.; Hu, X.; Hng, J.Y.S.; Hadi, M.H.; Owuama, H.C.; Matthyssen, T.; McCullough, M.; Canfora, F.; Paolini, R.; Celentano, A. In Vitro Cytotoxicity of Antiresorptive and Antiangiogenic Compounds on Oral Tissues Contributing to MRONJ: Systematic Review. Biomolecules. 2023, 13, 973. [Google Scholar] [CrossRef]
- Reid, I.R.; Green, J.R.; Lyles, K.W.; Reid, D.M.; Trechsel, U.; Hosking, D.J.; Black, D.M.; Cummings, S.R.; Russell, R.G.G.; Eriksen, E.F. Zoledronate. Bone 2020, 137, 115390. [Google Scholar] [CrossRef]
- Pabst, A.M.; Ziebart, T.; Koch, F.P.; Taylor, K.Y.; Al-Nawas, B.; Walter, C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin. Oral Investig. 2012, 16, 87–93. [Google Scholar] [CrossRef]
- Kumar, V.; Shahi, A.K. Nitrogen containing bisphosphonates associated osteonecrosis of the jaws: A review for past 10-year literature. Dent. Res. J. 2014, 11, 147–153. [Google Scholar]
- Ziebart, T.; Koch, F.; Klein, M.O.; Guth, J.; Adler, J.; Pabst, A.; Al-Nawas, B.; Walter, C. Geranylgeraniol—a new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011, 47, 195–201. [Google Scholar] [CrossRef]
- Suryani, I.R.; Ahmadzai, I.; Shujaat, S.; Ma, H.; Jacobs, R. Non-antiresorptive drugs associated with the development of medication-related osteonecrosis of the jaw: a systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 2269–2279. [Google Scholar] [CrossRef]
- DeSesa, C.R.; Appugounder, S.; Haberland, C.; Johnson, M.P. Osteonecrosis of the Jaw in Association With Chemotherapy in the Setting of Cutaneous T-Cell Lymphoma. J. Oral Maxillofac. Surg. 2016, 74, 292–301. [Google Scholar] [CrossRef]
- Tsao, C.; Darby, I.; Ebeling, P.R.; Walsh, K.; O’Brien-Simpson, N.; Reynolds, E.; Borromeo, G. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J. Oral Maxillofac. Surg. 2013, 71, 1360–6. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’ryan, F.; American Association of Oral and Maxillofacial, S. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Soutome, S.; Hayashida, S.; Funahara, M.; Sakamoto, Y.; Kojima, Y.; Yanamoto, S.; Umeda, M. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: Is tooth extraction a risk factor? PLoS One. 2018, 13, e0201343. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef]
- Nadella, S.; Mupparapu, M.; Akintoye, S.O. Risk of developing spontaneous MRONJ in fibrous dysplasia patients treated with bisphosphonates: a systematic review of the literature. Quintessence Int. 2022, 53, 616–623. [Google Scholar]
- Marx, R.E.; Cillo, J.E. Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 2007, 65, 2397–410. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, H.; Nam, W.; Kim, H.J.; Cha, I.H. Machine learning to predict the occurrence of bisphosphonate-related osteonecrosis of the jaw associated with dental extraction: A preliminary report. Bone. 2018, 116, 207–214. [Google Scholar] [CrossRef]
- Suryani, I.R.; Ahmadzai, I.; That, M.T.; Shujaat, S.; Jacobs, R. Are medication-induced salivary changes the culprit of osteonecrosis of the jaw? A systematic review. Front Med (Lausanne). 2023, 10, 1164051. [Google Scholar] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; Watts, N.B.; Brandi, M.L.; Peters, E.; Guise, T.; Eastell, R.; Cheung, A.M.; Morin, S.N.; Masri, B.; Cooper, C.; Morgan, S.L.; Obermayer-Pietsch, B.; Langdahl, B.L.; Al Dabagh, R.; Davison, K.S.; Kendler, D.L.; Sándor, G.K.; Josse, R.G.; Bhandari, M.; El Rabbany, M.; Pierroz, D.D.; Sulimani, R.; Saunders, D.P.; Brown, J.P.; Compston, J. ; International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar]
- Badros, A.Z.; Meddeb, M.; Weikel, D.; Philip, S.; Milliron, T.; Lapidus, R.; Hester, L.; Goloubeva, O.; Meiller, T.F.; Mongodin, E.F. Prospective observational study of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma: microbiota profiling and cytokine expression. Front Oncol. 2021, 11, 704722. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Bravo, S.B.; Carballo, J.; Chantada-Vázquez, M.D.P.; Bagán, J.; Bagán, L.; Chamorro-Petronacci, C.M.; Conde-Amboage, M.; López-López, R.; García-García, A.; Pérez-Sayáns, M. Quantitative proteomics in medication-related osteonecrosis of the jaw: A proof-of-concept study. Oral Dis. 2022, 29, 2117–29. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Sheth, C.C.; Soria, J.M.; Margaix, M.; Bagan, L. Bisphosphonates-related osteonecrosis of the jaws: A preliminary study of salivary interleukins. J. Oral Pathol. Med. 2013, 42, 405–8. [Google Scholar] [CrossRef]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Uezono, Y.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Sugimoto, M.; Ushijima, T. Metabolomic profiling reveals salivary hypotaurine as a potential early detection marker for medication-related osteonecrosis of the jaw. PLoS ONE. 2019, 14, e0220712. [Google Scholar] [CrossRef]
- Nicoletti, P.; Cartsos, V.M.; Palaska, P.K.; Shen, Y.; Floratos, A.; Zavras, A.I. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: the role of RBMS3. Oncologist. 2012, 17, 279–87. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Jensen, S.S.; Kofod, T.; Gotfredsen, K. Tooth extractions in patients with cancer receiving high-dose antiresorptive medication: a randomized clinical feasibility trial of drug holiday versus drug continuation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 165–173. [Google Scholar] [CrossRef]
- Aboalela, A.A.; Farook, F.F.; Alqahtani, A.S.; Almousa, M.A.; Alanazi, R.T.; Almohammadi, D.S. The Effect of Antiresorptive Drug Holidays on Medication-Related Osteonecrosis of the Jaw: A Systematic Review and Meta-Analysis. Cureus. 2022, 14, e30485. [Google Scholar] [CrossRef]
- Tröltzsch, M.; Tröltzsch, M.; Pautke, C.; Otto, S. Management of medication-related osteonecrosis of the jaw—a review of recent study results in comparison to established strategies. HNO. 2022, 70, 499–507. [Google Scholar] [CrossRef]
- Yoshiga, D.; Yoshioka, I.; Habu, M.; Sasaguri, M.; Tominaga, K. Effective ancillary role and long-term course of daily or weekly teriparatide treatment on refractory medication-related osteonecrosis of the jaw: a clinical case series. Br. J. Oral Maxillofac. Surg. 2022, 60, 604–609. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Piras, F.; Palmieri, G.; Pede, C.D.; Ciocia, A.M.; Siciliani, R.A.; Olio, F.D.; Inchingolo, A.M.; Palermo, A.; Inchingolo, F.; Favia, G.; Limongelli, L. Efficacy of guided autofluorescence laser therapy in MRONJ: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11817–11831. [Google Scholar]
- Ragazzo, M.; Val, M.; Montagner, G.; Trojan, D.; Fusetti, S.; Guarda Nardini, L. Human amniotic membrane: an improvement in the treatment of Medication-related osteonecrosis of the jaw (MRONJ)? A case-control study. Cell Tissue Bank. 2022, 23, 129–141. [Google Scholar] [CrossRef]
- Sacco, R.; Akintola, O.; Sacco, N.; Acocella, A.; Calasans-Maia, M.D.; Maranzano, M.; Olate, S. The Use of Human Amniotic Membrane (hAM) as a Treatment Strategy of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review and Meta-Analysis of the Literature. Medicina (Kaunas). 2023, 59, 968. [Google Scholar] [CrossRef]
- Wijesooriya, L.I.; Waidyathilake, D. Antimicrobial Properties of Nonantibiotic Agents for Effective Treatment of Localized Wound Infections: A Minireview. Int. J. Low Extrem. Wounds. 2022, 21, 207–218. [Google Scholar] [CrossRef]
- Heck, T.; Lohana, D.; Mallela, D.; Mandil, O.; Sun, L.; Saxena, P.; Decker, A.M.; Wang, H.L. Hyperbaric oxygen therapy as an adjunct treatment of periodontitis, MRONJ, and ONJ: a systematic literature review. Clin. Oral Investig. 2024, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, J.J.; Padilla-Burgos, R.; McGraw, T.; Suliman, H.B.; Kraft, K.H.; Stolp, B.W.; Moon, R.E.; Piantadosi, C.A. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J. Oral Maxillofac. Surg. 2012, 70, 1573–83. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.B.; Pereira, E.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers (Basel). 2022, 14, 3150. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Jafari, A.; Vescovi, P.; Fekrazad, R. Efficacy of Adjunctive Photobiomodulation in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 777–791. [Google Scholar] [CrossRef]
- Scoletta, M.; Arduino, P.G.; Reggio, L.; Dalmasso, P.; Mozzati, M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed. Laser Surg. 2010, 28, 179–84. [Google Scholar] [CrossRef]
- Sacco, R.; Woolley, J.; Patel, G.; Calasans-Maia, M.D.; Yates, J. Systematic review of medication related osteonecrosis of the jaw (MRONJ) in patients undergoing only antiangiogenic drug therapy: surgery or conservative therapy? Br. J. Oral Maxillofac. Surg. 2022, 60, e216–e230. [Google Scholar] [CrossRef]
- Seluki, R.; Seluki, M.; Vaitkeviciene, I.; Jagelaviciene, E. Comparison of the Effectiveness of Conservative and Surgical Treatment of Medication-Related Osteonecrosis of the Jaw: a Systematic Review. J. Oral Maxillofac. Res. 2023, 14, e1. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).