1. Introduction
Lumpy skin disease (LSD) is a viral disease of cattle and water buffalo characterized by multifocal cutaneous nodules, biphasic fever, oral and nasal discharge, accompanied by lymphadenitis. Clinical infections range from sub-clinical through mild to acute, and are often influenced by the age, breed, immune status and production period of animals [
1]. LSD is associated with moderate-to-high morbidity and low mortality. Despite that, the disease has a high socio-economic impact, resulting from the decrease in milk production, decreased weight gain, permanent damage to hides, reduced reproduction due to increased infertility and abortion, costs related to surveillance activity and serious trade restrictions [
2,
3,
4]. For these reasons, the Animal Health Law (Regulation (EU) 2016/429), classified the LSD in the list of diseases belonging to Category A. LSDV is subject to early notification and reporting throughout the European Union Countries, and its prevention and control measures are governed by the Regulation (EU) 2016/429 and supplemented by Delegated Regulation 2020/687 and Implementing Regulation 2021/1070.
The disease is caused by the lumpy skin disease virus (LSDV) which belongs to the family
Poxviridae, genus
Capripoxvirus, together with sheeppox virus (SPPV) and goatpoxvirus (GTPV). Transmission of LSDV occurs mainly mechanically via blood-feeding insects and ticks. In addition, direct and indirect transmission via common use of feeders or drinking troughs by infected cattle [
5] as well as seminal fluid [
6] has been reported. LSD diagnosis is primarily based on the clinical diagnosis of LSD, confirmed by the PCR analysis of lesion crusts or biopsies of the nodules [
7,
8,
9]. However, LSDV infection is not always apparent, and mild and subclinical disease occurs, also confirmed by cattle experimentally infected, when up to 50% of animals remain uninfected or sub-clinically infected [
10,
11]. The incubation period in field conditions varies from 2 to 4 weeks, while in experimental disease from 4 to 14 days [
12,
13]. The first skin lesions appear at the inoculation site after 4-20 days. In the acute form, animals develop a biphasic febrile reaction that may exceed 41°C and lasts from 4 to 14 days. This is accompanied by depression, reluctance to move, inappetence, and sialorrhea, nasal discharge from mucous to purulent and tearing. Lymph nodes are enlarged, especially pre-scapular and pre-crurarl [
12,
13,
14].
LSD is endemic in most of the African continent and in recent years has spread throughout the Middle East, including Turkey. In 2015 it arrived in Europe, in particular in Greece where more than 100 outbreaks were reported [
15]. In 2016, cases were also reported in Bulgaria, Serbia, the Former Yugoslav Republic of Macedonia (FYROM) and Albania [
16]. Thanks to mass vaccination campaigns with homologous LSD vaccines in the infected countries of south-eastern Europe as well as in neighbouring countries (Bosnia and Herzegovina, Croatia), the spread of the disease was contained, and no LSD cases have been reported since 2017. LSD outbreaks were reported in Armenia in 2015, Georgia, and Kazakhstan in 2016, later in 2019 and 2020 in Israel, Russia, Saudi Arabia and Syria. Between July and August 2019 LSDV was introduced in Asia affecting Bangladesh, China and India and in 2020 it was reported in Bhutan, Hong Kong, Myanmar, Nepal, Sri Lanka, Taiwan and Vietnam [
17]. To date, no LSDV outbreaks have been reported in Italy.
In Italy the number of buffalo breedings and animals are constantly increasing, resulting the country with the largest number of buffaloes reared in the EU. The buffalo breeding represents an important economic reality, with increasing potential. Due to the triple aptitude of the buffalo, for milk, meat and work, in the last 10 years its breeding increased almost around 50%. In particular in the central and south regions of Italy, such as Campania, Lazio and Molise, it is mainly raised for the production of milk, PDO mozzarella (EEC 2081/92) and ricotta. The demand for buffalo meat has also increased in recent years thanks to the increasingly appreciated organoleptic properties.
Under field condition, LSDV infection in water buffalo is a controversial matter. Some studies described isolation of LSDV from skin lesions in buffalo [
18,
19]. Previously, Davies (1991) [
20] reported that African buffalo (
Syncerus caffer) and Asian water buffalo (
Bubalus bubalis) did not show lesions in the field during LSD outbreaks though they had seroconverted. A recent field study reported that blood and skin biopsy samples collected from buffaloes in outbreaks in Egypt were negative for the presence of LSDV [
9].
To date, there are some descriptive articles on buffaloes that confirm their sensitivity to LSD [
9,
21,
22] but, there is only a field study on buffaloes naturally infected by LSDV [
23]. No experimental trials are reported. The susceptibility of water buffalo to lumpy skin disease virus (LSDV) and their role in spreading the disease are unclear, so further studies are needed to fill these gaps
The aims of this study were to determine the susceptibility of buffaloes to LSDV infection and to describe the clinical, virological and serological responses of water buffaloes after the LSDV infection.
2. Materials and Methods
2.1. Animals
A group of eight Mediterranean buffaloes (Bubalus bubalis), 5 males and 3 females, and two cattle used as positive control, were included in the study. All animals were between seven to eleven months old. The animals were consecutively numbered from 1 to 10 and maintained in the high-containment animal facilities (Insect proof-establishment) of the Institute Zooprofilattico Sperimentale G. Caporale, Teramo, Italy, and housed with a 12-hourly light-dark cycle, temperature between 10°C and 25°C, relative humidity of 40% to 70%. Animals were fed concentrated rations twice daily and given ad libitum access to hay and water. Environmental enrichment was provided, including rubber toys and a hollow ball stuffed with hay.
The animals, sourced from a commercial herd, were confirmed as negative for Bovine Viral Diarrhea Virus, Parainfluenza type 3 virus, Bovine Adenovirus, Bovine herpesvirus-4, Bovine herpesvirus-1, Bluetongue, Chlamydia psittaci and Coxiella burnetii prior to study commencement.
To detect any possible dipteran presence, indoor blood-feeding insect UV light traps and sticky traps were mounted at regular intervals on the walls of the high-containment animal facilities.
The respective experimental protocols were reviewed by the state ethics commission (OPBA) and approved by Italian Ministry of Health. The experimental procedures were conducted according to the Law decree n. 26, art. 31, 2014, and they were approved by the Minister of Health (n.722 of 13/07/2020).
2.2. Experimental infection
LSDV inoculation stock was obtained at “Istituto Zooprofilattico Sperimentaledell’Abruzzo e del Molise G. Caporale” by five consecutive passages on MDBK cells of the LSDV field strain named 7416/5, isolated from a symptomatic calf skin nodule, as described by Babiuk et al. (2008) [
7]. The sample was collected during an outbreak occurred in Albania in 2017 and was kindly provided by Dr. LediPite and Dr. Liljana Cara working at the Food Safety and Veterinary Institute (FSVI) in Tirana. Six of the 8 buffaloes (nos 3 to 8) were randomly assigned to inoculated group, the remaining 2 buffaloes (nos 1-2) were used as control group, while the two cattle (nos 9-10) were used as positive control. The six buffaloes, from number 3 to 8, were inoculated intravenously into the jugular vein with 5 ml of LSDV field strain suspension with titre of 10
5.8 TCID
50/ml, and with 1 ml injected intra dermally in 2 sites on each side of the neck (0.25 ml in each site). The same procedure and the same LSDV suspension were used to inoculate two calves used as positive control. The remaining two buffaloes were mock inoculated with the same amount of supernatant of LSDV negative cell culture using the same procedure. All animals were examined daily for clinical signs, in particular fever, anorexia, depression, lesions, including cutaneous nodules, and lymphadenopathy, for 42 days.
2.3. Clinical observation
Buffaloes and calves were clinically monitored daily during the entire trial. Body temperature was registered each day and scored as fever if ≥ 39.5°C for at least 2 days. Others observations were collected (
Table 1) and used to calculate a cumulative clinical scores [
11,
24].
2.4. Samples
For molecular analysis, EDTA blood samples were collected from all animals from day 2 post- inoculation (p.i.) to day 22 p.i., and oral, nasal, ocular swabs were collected daily from day 2 p.i.to day 14 p.i. and then every 3-4 days until the end of the experiment. For serological analysis, serum samples were collected every 3-4 days from day 3p.i. until the end of the study.
Skin biopsies were carried out on three buffaloes (nos 3, 6 and 8) and cattle n.10 at different time. On day 13 a skin biopsy was collected from buffalo 6 from a nodule at the inoculation site. On buffalo no. 3, six biopsies were taken on day 16, 22, 23, and 25 from nodules present in different parts of the body (neck, thorax, dewlap, inoculum site, and intermandibolar zone). On buffalo no. 8, skin biopsies were taken from one nodule at inoculation site on day 16 and another one at the tip of the right shoulder on day 30. From cattle no. 10, skin biopsy was collected from a nodule on the neck on day 7. Hair was removed from the biopsy sites with electric clippers and cleaned with skin wipes containing 2% chlorhexidine in 70% alcohol (Clinell, GAMA Healthcare); 2.5 ml of lignocaine (Lidocaine Hydrochloride injection 2%, Hameln Pharmaceuticals) was injected subcutaneously, and after 10 minutes, a 0.8 cm punch biopsy was taken using a disposable biopsy punch (Integra Miltex). Half of the biopsy tissue was placed into 10% sterile buffered formalin (Merck) for a minimum of 48 hours. The remaining tissue was tested in real time PCR [
8]. Animals were sedated with guaifenesin 80 mg/Kg, rompum 50 mg/Kg (xylazine hydrochloride 23,32 mg/ml, Bayer AG 51368 Leverkusen, Germany), were anesthetized with pentothal sodium 7-13 mg/kg (tiopentalesodico MSD Animal Health S.r.l.) and then euthanized using Tanax T-61 (Mebezonium iodide 50,00 mg/ml, Embutramide 200,00 mg/ml, Tetracaine 5,00 mg/ml, MSD Animal Health S.r.l.). During necropsy, a panel of organs and tissues (spleen, liver, kidney, tonsils, skin, lymph nodes, heart, rumen, abomasum, ileum, testicles, ovaries, nasal mucosa, and tongue) was collected and analyzed using the pan-capripox real-time qPCR [
8].
2.5. Serologicalexamination
Serological analysis was carried out by commercially available ID Screen Capripox Double Antigen ELISA (ID.vet, Montepellier, France) and SN test. The ID Screen Capripox ELISA was performed according to the manufacturer’s instructions. Samples with an S/P% ratio of ≥ 30% were considered positive.
For the detection of neutralizing antibodies, an LSDV specific SN test based on modified protocol of WOAH was performed [
25,
26]. For this purpose, test serum samples were inactivated at 56°C for 30 minutes, and log2 dilution series in serum-free minimal essential medium from 1:5 to 1:640 were prepared in duplicate using a 96-well format (50µl of each serum dilution/well), in order to be titrated against a constant titre of 100 TCID
50 in 50µl of LSDV Neethling strain. Microtiter plates with serum-virus suspension were incubated for 2 hours at 37°C in 5% CO2 and after incubation 100μL of MDBK cell suspension was added to each well. Plates were observed daily using an inverted microscope (20-40X Leica DFC425 C, Leica Microsystem Ltd.) to evaluate the presence of virus–specific cytopathic effect (CPE), and after 4 days at 37°C in 5% CO
2 the titre was determined. Wells were scored as positive for neutralisation of virus if 100% of the cell monolayer is intact. The highest dilution of serum resulting in complete neutralisation of virus (no CPE) in half of the test wells is the 50% end-point titre of that serum. A titre of 1:10 or greater was considered to be positive.
2.6. DNA extraction and molecular analysis
Organ samples and skin biopsies were homogenized in PBS plus antibiotics (10
6IU/L penicillin, 10 g/L streptomycin, 5 × 10
6 IU/L nystatin, and 125 mg/L gentamicin, IZSAM ) using a TissueLyser II tissue homogenizer (QIAGEN, Hilden, Germany). Nasal, oral, ocular and rectal swabs were frozen at -80°C and thawed 3 times before being tested. DNA was extracted from homogenized organ and biopsy samples, EDTA blood and swab samples using BioSprint 96 One-For-All Vet Kit (Indical Bioscience, Leipzig, Germany) following the manufacturer’s instruction. Subsequently, all samples were tested using the pan-capripox real-time qPCR [
8].
3. Results
3.1. Clinical observation
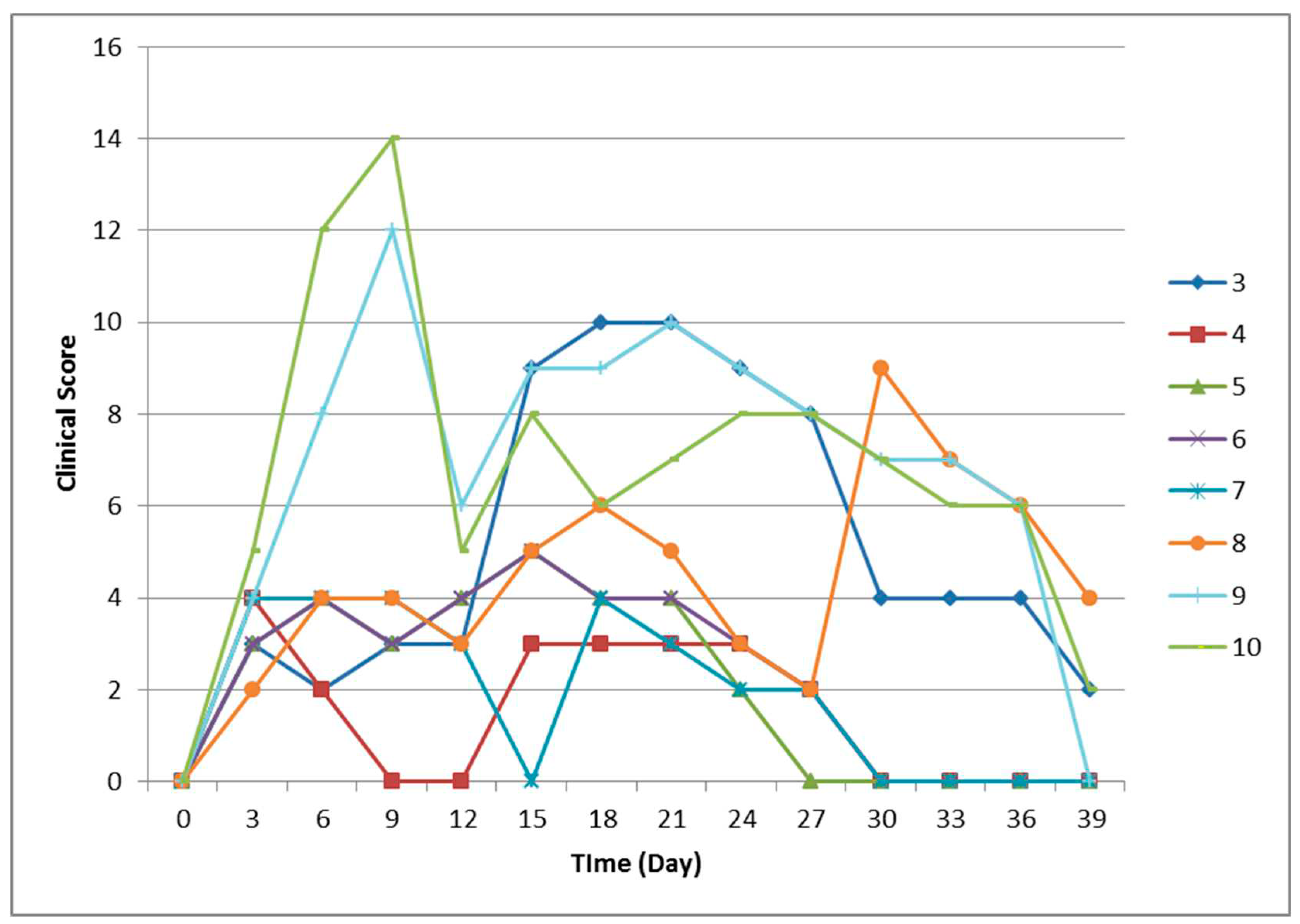
Six water buffaloes and two cattle were inoculated with an LSDV field strain while two buffaloes were inoculated with placebo. All the animals were monitored for clinical signs (
Table 2;
Figure 1) and viraemia over 42 days. Two mock inoculated animals did not show any clinical signs. Instead, buffaloes nos 4, 5, 6 had enlarged prescapular and prefemoral lymph nodes from day 5 to day 8, but they did not developed any other clinical sign apart from nodules at inoculation sites. Buffaloes nos 3 and 8 developed fever on day 14 and on day 27, respectively, associated with a decrease of food intake, and generalized lymphadenomegaly. After 24 hours from inoculation, small nodules appeared on both sides of their neck and spread to the whole body (neck, legs, back and flanks) on the following days. Buffalo no. 3 developed well-circumscribed cutaneous nodules which reduced in size and disappeared by day 30 onward. Nodules in buffalo no. 8 were well-circumscribed, and some nodules start to reduce in size by day 37.
In the challenged control group, typical clinical signs of LSD were observed from day 4 p.i.: calves showed pyrexia, enlargement of superficial lymph nodes, appearance of skin nodules at inoculation site, inappetence (
Figure 1;
Table 2). Calve no. 9 developed fever on day 7 and, while calve no. 10 had a fever from day 4 (39.7 °C) to day 8 (39.5°C) and then increased again until day 10 (40.4°C) when the animal was humanely euthanized due to severe clinical signs and to avoid unnecessary suffering.
Clinical score was recorded daily after the infection to measure the severity of LSDV infection (
Figure 1). All the animals started from score 0 on the day of the inoculation. The difference in clinical scores between the two buffaloes with clinical signs and the other 4 animals without was evident from day 14 to day 30, when the score increased in buffaloes nos 3 and 8 up to 10 and then decreased. The other buffaloes had a clinical score ranging between 0 and 5, while calves of positive control group had a clinical score between 9 and 14 (from day 6 to day 9 and then start to decrease).
3.2. Serological analysis
Serological analysis were conducted using ELISA and the SN test to assess the seroconversion. On the day of the inoculation (D0), all animals were sero-negative. Buffaloes inoculated with placebo remained serologically negative during the 42 days of the experiment. The two buffaloes with characteristic clinical signs resulted positive in ELISA on day 39 (no. 3, S/P 38%), 25 days after the onset of fever, and on day 42 (no. 8, S/P 30%), 15 days after feverish rise, and remained positive to the end of the study. Buffalo no. 6 seroconverted on day 42 (S/P 32%). The other inoculated buffaloes did not show any serological response during the whole study. None of the infected buffaloes developed neutralizing antibodies against LSDV. Calf no.9, of the positive control group, seroconverted from day 31p.i., and remained ELISA positive until the end of the study. The calf developed neutralizing antibodies from day 14 (1:80) till the end of the trial. Neutralization titres peaked on day 22p.i.(1:320) and remained strongly positive until the time of euthanasia, confirming the development of neutralizing antibodies against LSDV.
3.3. Molecular analysis
No viraemia was detectable in any buffalo, at any time during the trial, even in febrile animals. Viral DNA was not found in the skin biopsies, swabs and organs of the challenged animals (
Table 3). On the contrary, viral DNA was found in blood sample of the cattle no. 9 from day 9 to day 14 with a Ct value between 28.54 and 38.4, while in cattle no. 10 viraemia was detected from day 5 (Ct 35.57) to day 9 (Ct 32.97). Real-time PCR were performed on nasal, oral and conjunctival swabs at different times (
Table 4).After 42 days viral DNA was found in prescapular lymph nodes of calf no. 9 and in all organs and in the skin biopsy collected from calf no.10, that was euthanized on day 10, by real time PCR (
Table 3).
4. Discussion
There is little evidence of the susceptibility of water buffaloes to the LSDV infection as well as their epidemiological role in course of outbreaks. In order to fill these gaps 6 Mediterranean buffaloes were experimentally inoculated with LSDV isolated from an Albanian calf in 2016 to monitor the clinical, serological and virological response evoked by the infection.
As previously described in other experimentally infected cattle, the intravenous inoculation route was used because current data indicate this route to be the most effective to produce severe generalized disease [
4,
13]. In addition, intradermal inoculation was also selected in order to reproduce the natural route of infection.
The total clinical score of the LSD clinically diseased buffaloes reached 9-10, while the score for animals without clinical signs did not surpass 5, which is a clinical evolution comparable to the patterns described previously [
42,
43]. It is important to note that the same inoculum evoked an important clinical response when used to infect cattle [
27].
The susceptibility of buffaloes to develop the typical clinical symptoms following LSDV infection is controversial. Ahmed and Dessouki (2013) [
35] reported LSD in cattle while water buffaloes from the same affected area appeared clinically healthy. On the contrary, other papers reported the evidence of natural infection with LSDV in Egyptian buffaloes [
36]. In recent years, anobservational study conducted on buffaloes during an outbreak in Egypt, confirm their low susceptibility to this virus [
37]. Researchers hypothesized that the reason is that buffalo have thick skin, and the mouthparts of blood sucking insects such as mosquitoes, flies and ticks cannot easily pass through the skin of buffalo, so the transmission rate and susceptibility of this disease are low [
23,
38]. In the current experiment the virus was injected in the skin and blood of the buffaloes, nevertheless only two out of six animals developed a mild generalized disease with the appearance of small skin nodules resembling the “Neethling disease” often observed after the use of attenuated vaccines [
39].
The diagnosis of LSD is mainly based on clinical surveillance, confirmed by laboratory test on the biopsies of nodules or blood samples [
33,
40]. Identification of infected subclinical animals remains difficult as nodules are absent and viraemia is short or intermittent making it difficult to identify the virus in the blood [
41]. The assumption is particularly true in buffaloes which develops LSD mainly in the subclinical form [
33] with mild or absent clinical signs as confirmed in our trial.
One of the most interesting outcomes of the trial is the lack of detection of LSDV in any sample collected from the infected buffaloes regardless the development of clinical signs. The presence of the virus in nasal and ocular discharges in infected animals and the amount of virus shed by infected animals is key to virus spread. Clinical signs and molecular results obtained in this trial, suggest that buffalo did not shed the virus making us assume they do not have a preminent role in the spread of the disease.
This data agrees with what was recently reported in a work by Elhaig et al. (2021) [
28], during Egyptian outbreaks between 2016 and 2018, in which all collected blood and biopsy samples were negative in real time PCR, but the presence of antibodies confirmed LSDV infection in the animals. Such findings strengthen the hypothesis of the buffalo’s resistance to LSD and their role as an accidental non-adapted host [
33,
34].
LSDV DNA was detected neither in the blood nor in the skin nodules collected from the buffalos with generalized lesions or in the animals with local skin reactions in the inoculation sites. It suggests the development of a weak viraemia and a scarce distribution of the virus in the peripheral sites once inoculated in this species. Whether the virus is promptly cleared from the blood circulation and/or not capable to replicate efficiently to evoke the severe generalized form of the disease is hard to say and deserve further studies to investigate LDSV pathogenicity in buffaloes.
The limited susceptibility of water buffalo against LSD was also confirmed by the different evolution of the infection in the challenged vs the positive control group. In the challenged group only two buffaloes showed mild clinical signs (fever, skin nodules) that have totally regressed at the end of the trial. On the contrary, the same inoculum evoked a severe disease leading to the euthanasia of cattle n.10 to avoid the suffering of the animal. Both animals developed a detectable viraemia, eliminated LSDV through nasal and ocular discharge and displayed a peripheral distribution of the virus in the skin nodules detected by PCR.
The cattle infected with the field LSDV strain developed severe clinical signs proving the efficacy of the experimental infection procedure.
Seroconversion was detected in 3 out of 6 challenged buffaloes between 39 and 42 days p.i. only by ELISA test. The short duration of the trial and the late development of the antibody response have probably prevented to record the seroconversion of the remaining animals. However, the immune response to LSDV infection is predominantly cell-mediated [
35] and the scarcity of humoral immune response in buffaloes following capripoxvirus infection has been described also in field condition. A study conducted in Turkey [
29] reported a low seroprevalence (7.6%) in buffaloes vaccinated against LSDV with heterologous sheep-goat pox vaccine, using a commercial ELISA.
Nevertheless, the ELISA test confirmed its sensitivity in the early detection of anti-capripoxvirus antibodies [
7,
9,
13] compared to the neutralization assay. In fact, virus neutralization test is the most specific serological method, but lacks sensitivity [
30,
31,
32].None of our buffaloes developed neutralizing antibodies during the experimental trial. Whether the lack of circulating neutralising antibodies is attributable to the short duration of the trial or to the immunological response of buffaloes to LSDV infection is difficult to say.
However, the poor neutralizing response to LSDV in this species is not a novelty being reported by several authors. Elhaig et al. (2017) [
33] did not detect neutralizing antibodies in naturally infected buffaloes with clinical LSD lesions. In a previous work of Fagbo and colleagues conducted on buffalo sera collected (2014) [
21] during an inter-epidemic period, the SN test results confirm only partially the results obtained by the ELISA and withlow neutralizing titres were limited to two buffaloes (1:20).
On the contrary, a different scenario occurred for the surviving bovine, with the detection of neutralizing antibodies from day 14 p.i. followed by positive ELISA results seventeen days later (day 31 p.i.).
We should probably consider that the sensitivity of the ELISA may differ between animal species, as already reported [
7].In recent experimental study on bulls, the authors reported seroconvertion after 42 days [
10], confirming what observed in our trial.
If Water buffaloes’ limited susceptibility to LSD infection, could be ascribed to specific genetic variants [
48] as for tuberculosis, mastitis or foot-and-mouth disease [
44,
45,
46,
47], is difficult to say but the hypothesis deserves to be further explained in future studies.
5. Conclusions
LSD is a cross-border disease characterised by severe economic losses that continues to spread worldwide. Real time PCR is the method of choice for a rapid routine diagnosis in cases of suspicion, however, in species not particularly susceptible to infection such as buffalo it may also be useful to combine genome detection with serological tests to detect any previous contact with the virus. The present study addresses the hypothesis that water buffaloes are less susceptible to the LSDV infection under experimental conditions compared to cattle and that diseased buffaloes may develop mild clinical signs of the disease.
Author Contributions
Conceptualization, F.M. and D.M.; methodology, F.M..; software, E.D.F., C.P.; validation, M.D.V., F.M. and E. R.; formal analysis, G.D.D..; investigation, E.D.F., C.P., F.I., A.T., A.G., E.M., G.D.T.; resources, F.M.; data curation, C.P., E.D.F., G.D.D.; writing—original draft preparation, E.D.F. G.D.D.; writing—review and editing, E.D.F., G.D.D., F.M., G.F.R, M.T.M.; visualization, E.D.F.; supervision, F.M., D.M.; project administration, D.M.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, grant number IZSAM 07/15 RC.
Acknowledgments
The authors would like to thank to professor David Wallace (Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, P/Bag X4, Pretoria 0110, South Africa) for his support in the drafting of the experimental protocol and to all the technicians that with their work have allowed the realization of this experimental study taking care of the animals used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. TransboundEmerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Abera, Z.; Box, P.O.; Abera, Z.; Degefu, H.; Gari, G.; Ayana, Z. Review on Epidemiology and Economic Importance of Lumpy Skin Disease 2015, 4, 8. [CrossRef]
- Saegerman, C.; Bertagnoli, S.; Meyer, G.; Ganiere, J.; Caufour, P.; De Clercq, K.; Jacquiet, P.; Fournie, G.; Hautefeuille, C.; Etore, F.; et al. Risk of Introduction of Lumpy Skin Disease in France by the Import of Vectors in Animal Trucks. PLoS One 2018, 13, e0198506. [Google Scholar] [CrossRef] [PubMed]
- TuppurainenEeva S.M. 2005. The Detection of Lumpy Skin Disease Virus in Samples of Experimentally Infected Cattle using Different Diagnostic Techniques.
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of Lumpy Skin Disease Virus: A Short Review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Irons, P.C.; Tuppurainen, E.S.M.; Venter, E.H. Excretion of Lumpy Skin Disease Virus in Bull Semen. Theriogenology 2005, 63, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Manning, L.; Neufeld, J.; Embury-Hyatt, C.; Copps, J.; Boyle, D.B. Quantification of Lumpy Skin Disease Virus Following Experimental Infection in Cattle. TransboundEmerg. Dis. 2008, 55, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.R.; Babiuk, S.L.; Parkyn, G.R.; Copps, J.S.; Boyle, D.B. Capripoxvirus Tissue Tropism and Shedding: A Quantitative Study in Experimentally Infected Sheep and Goats. Virology 2008, 371, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; Eltarabilli, M.M.A.; Shahein, M.A.; Fawzy, M. Lumpy Skin Disease Outbreaks Investigation in Egyptian Cattle and Buffaloes: Serological Evidence and Molecular Characterization of Genome Termini 2021, 76, 101639. [CrossRef]
- Kononov A., Olga B, David WB, Pavel P, Yana P, Svetlana K, Alexander N, Vladimir R, Dmitriy L, Alexander S. Non-vector-borne transmission of lumpy skin disease virus. Sci Rep. 2020 May 4;10(1):7436. [CrossRef] [PubMed]
- Sohier, C.; Haegeman, A.; Mostin, L.; De Leeuw, I.; Campe, W.V.; De Vleeschauwer, A.; Tuppurainen, E.S.M.; van den Berg, T.; De Regge, N.; De Clercq, K. Experimental Evidence of Mechanical Lumpy Skin Disease Virus Transmission by StomoxysCalcitrans Biting Flies and Haematopota Spp. Horseflies 2019, 9, 20076-10. [Google Scholar] [CrossRef]
- Prozesky, L.; Barnard, B.J. A Study of the Pathology of Lumpy Skin Disease in Cattle. Onderstepoort J. Vet. Res. 1982, 49, 167–175. [Google Scholar] [PubMed]
- Carn, V.M.; Kitching, R.P. The Clinical Response of Cattle Experimentally Infected with Lumpy Skin Disease (Neethling) Virus. Arch. Virol. 1995, 140, 503–513. [Google Scholar] [CrossRef]
- Barnard, B.J. Antibodies Against some Viruses of Domestic Animals in Southern African Wild Animals. Onderstepoort J. Vet. Res. 1997, 64, 95–110. [Google Scholar]
- Agianniotaki, E.I.; Tasioudi, K.E.; Chaintoutis, S.C.; Iliadou, P.; Mangana-Vougiouka, O.; Kirtzalidou, A.; Alexandropoulos, T.; Sachpatzidis, A.; Plevraki, E.; Dovas, C.I.; et al. Lumpy Skin Disease Outbreaks in Greece during 2015-16, Implementation of Emergency Immunization and Genetic Differentiation between Field Isolates and Vaccine Virus Strains. Vet. Microbiol. 2017, 201, 78–84. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority, (. Lumpy Skin Disease: I. Data Collection and Analysis. EFSA J. 2017, 15, e04773.
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; et al. Assessment of the Control Measures for Category A Diseases of Animal Health Law: Lumpy Skin Disease. EFSA J. 2022, 20, e07121. [Google Scholar] [CrossRef] [PubMed]
- El-Nahas, E.M.; El-Habbaa, A.S.; El-bagoury, G.F.; Radwan, M.E.I. Isolation and Identification of Lumpy Skin Disease Virus from Naturally Infected Buffaloes at Kaluobia, Egypt. Global Vet. 2011, 7, 234–237. [Google Scholar]
- Sharawi, S.S.A.; Abd El-Rahim, I.H.A. The Utility of Polymerase Chain Reaction for Diagnosis of Lumpy Skin Disease in Cattle and Water Buffaloes in Egypt. Rev. Sci. Tech. 2011, 30, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G. Lumpy Skin Disease, an African Capripox Virus Disease of Cattle. Br. Vet. J. 1991, 147, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Fagbo, S.; Coetzer, J.A.W.; Venter, E.H. Seroprevalence of Rift Valley Fever and Lumpy Skin Disease in African Buffalo (SyncerusCaffer) in the Kruger National Park and Hluhluwe-iMfolozi Park, South Africa. J. S. Afr. Vet. Assoc. 2014, 85, e1–e7. [Google Scholar] [CrossRef]
- Pandey, N.; Hopker, A.; Prajapati, G.; Rahangdale, N.; Gore, K.; Sargison, N. Observations on Presumptive Lumpy Skin Disease in Native Cattle and Asian Water Buffaloes Around the Tiger Reserves of the Central Indian Highlands. N. Z. Vet. J. 2022, 70, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Neamat-Allah, A.N.F.; Mahmoud, E.A. Assessing the possible causes of hemolyticanemia associated with lumpy skin disease naturally infected buffaloes. Comp Clin Pathol 2019, 28, 747–753. [Google Scholar] [CrossRef]
- Aerts, L.; Haegeman, A.; De Leeuw, I.; Philips, W.; Van Campe, W.; Behaeghel, I.; Mostin, L.; De Clercq, K. Detection of Clinical and Subclinical Lumpy Skin Disease using Ear Notch Testing and Skin Biopsies 2021, 9, 2171. [CrossRef]
- World Organization for Animal Health (WOAH) Terrestrial manual Chapter 3.4.12. Lumpy skin disease.
- Samojlović, M.; Polaček, V.; Gurjanov, V.; Lupulović, D.; Lazić, G.; Petrović, T.; Lazić, S. Detection of Antibodies Against Lumpy Skin Disease Virus by Virus Neutralization Test and ELISA Methods 2019, 69, 47-60. [CrossRef]
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, RP. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. TransboundEmerg Dis 2008, 55(7), 263-72. [Google Scholar] [CrossRef]
- Elhaig, M.M.; Almeer, R.; Abdel-Daim, M.M. Lumpy Skin Disease in Cattle in Sharkia, Egypt: Epidemiological and Genetic Characterization of the Virus. Trop. Anim. Health Prod. 2021, 53, 287–5. [Google Scholar] [CrossRef] [PubMed]
- Okur-Gumusova S.;Tamer, C.; Ozan, E.; A.; Kadi, H.; B.; Elhag, E.; A.; Yazici, Z.; et al.An Investigation of the Seroprevalence of Crimean- Congo Hemorrhagic Fever and Lumpy Skin Disease in Domesticated Water Buffaloes in Northern Turkey 2020, 37, 165.
- European Food Safety Authority Panel on Animal Health and Welfare). Scientific Opinion on lumpy skin disease. EFSA Journal, 2015; 13, 1, 398673 pp.
- European Food Safety Authority, European Food Safety Authority; Calistri, P.; DeClercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; CortinasAbrahantes, J.; Antoniou, S.; Broglia, A.; Gogin, A. Lumpy Skin Disease: III. Data Collection and Analysis. EFSA J. 2019, 17, e05638. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH)2021Terrestrial Animal Health Code, Chapter 11.9: Infection with Lumpy Skin Disease. 2021. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_lsd.pdf.
- Elhaig, M.M.; Selim, A.; Mahmoud, M.; Elhaig, M. Onderstepoort Journal of Veterinary Research Authors 2017.
- House, J.A.; Wilson, T.M.; el Nakashly, S.; Karim, I.A.; Ismail, I.; el Danaf, N.; Moussa, A.M.; Ayoub, N.N. The Isolation of Lumpy Skin Disease Virus and Bovine Herpesvirus-4 from Cattle in Egypt. J. Vet. Diagn. Invest. 1990, 2, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Dessouki, A.A. Abattoir-Based Survey and Histopathological Findings of Lumpy Skin Disease in Cattle at Ismailia Abattoir 2013, 3, 372. [CrossRef]
- El-Tholoth, M.; El-Kenawy, A.A. G-Protein-Coupled Chemokine Receptor Gene in Lumpy Skin Disease Virus Isolates from Cattle and Water Buffalo (BubalusBubalis) in Egypt. TransboundEmerg. Dis. 2016, 63, e288–e295. [Google Scholar] [CrossRef] [PubMed]
- Sharawi, S.S.A.; Abd El-Rahim, I.H.A. The Utility of Polymerase Chain Reaction for Diagnosis of Lumpy Skin Disease in Cattle and Water Buffaloes in Egypt. Rev. Sci. Tech. 2011, 30, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Chihota, C.M.; Rennie, L.F.; Kitching, R.P.; Mellor, P.S. Attempted Mechanical Transmission of Lumpy Skin Disease Virus by Biting Insects. Med. Vet. Entomol. 2003, 17, 294–300. [Google Scholar] [CrossRef] [PubMed]
- J.Ben-Gera, E.;Klement, E.; nKhinich, Y.;Stram, N.Y. Shpigel. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease–The results of a randomized controlled field study. Vaccine 2015, 33(38), 4837–4842. [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; et al. Scientific Opinion on the Assessment of the Control Measures for Category A Diseases of Animal Health Law: Foot and Mouth Disease. EFSA J. 2021, 19, e06632. [Google Scholar] [CrossRef]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Basu, S.; Larner, W.; Diaz, A.V.; Langlands, Z.; Denison, E.; Stoner, J.; White, M.; et al. Quantifying and Modeling the Acquisition and Retention of Lumpy Skin Disease Virus by Hematophagus Insects Reveals Clinically but Not Subclinically Affected Cattle are Promoters of Viral Transmission and Key Targets for Control of Disease Outbreaks. J. Virol. 2021, 95, e02239-20, Print 2021 Apr 12. [Google Scholar] [CrossRef]
- Haegeman, A.; De Leeuw, I.; Mostin, L.; Van Campe, W.; Aerts, L.; Vastag, M.; De Clercq, K. An Immunoperoxidase Monolayer Assay (IPMA) for the Detection of Lumpy Skin Disease Antibodies 2020, 277, 113800. [CrossRef]
- Tuppurainen, E., Alexandrov, T.;Beltrán-Alcrudo, D. 2017. Lumpy skin disease field manual – A manual for veterinarians. FAO Animal Production and Health Manual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 60 pages.
- Iannaccone, M.; Cosenza, G.; Pauciullo, A.; Martino, G.; Capparelli, R. The SNP g.4667G>A at 3'-UTR of IFNG gene is associated with susceptibility to bovine tuberculosis in Mediterranean water buffalo (Bubalusbubalis). Animal genetics 2018, 49(5), 496–497. [Google Scholar] [CrossRef]
- El-Halawany, N.; Abd-El-Monsif, S.A.; Al-Tohamy Ahmed, F.M.; Hegazy, L.; Abdel-Shafy, H.; Abdel-Latif, M.A.; Ghazi, Y.A.; Neuhoff, C.; Salilew-Wondim, D.; Schellander, K. Complement component 3: characterization and association with mastitis resistance in Egyptian water buffalo and cattle. J Genet 2017, 96(1), 65–72. [Google Scholar] [CrossRef] [PubMed]
- El-Halawany, N.; Shawky, A.A.; Al-Tohamy, A.F.M.; Abdel-Latif, M.A.; Abdel-Shafy, H.; Ghazi, Y.A.; Neuhoff, C.; Schellander, K. Effect of complement component 5 polymorphisms on mastitis resistance in Egyptian buffalo and cattle. Research in veterinary science 2018, 119, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Othman, O.E.; Khodary, M.G.; El-Deeb, A.H.; Hussein, H.A. Five BoLA-DRB3 genotypes detected in Egyptian buffalo infected with Foot and Mouth disease virus serotype O. Journal, genetic engineering & biotechnology 2018, 16(2), 513–518. [Google Scholar] [CrossRef]
- Rehman, S.U.; Hassan, F.U.; Luo, X.; Li, Z.; Liu, Q. Whole-Genome Sequencing and Characterization of Buffalo Genetic Resources: Recent Advances and Future Challenges. Animals: an open access journal from MDPI 2021, 11(3), 904. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).