Introduction
Acute peritonitis invariably elicits functional disruptions of the gastrointestinal tract, clinically manifesting as dynamic intestinal obstruction. The intricate mechanisms underlying the genesis of intestinal dysmotility are multifaceted. Research highlights the pivotal role of chemical stimulation, originating from bacterial sources, in activating peritoneal receptors, both parietal and visceral, even during the initial phases of peritonitis. This stimulation triggers visceromotor reflexes, relayed to higher brainstem centers via axon-reflex pathways, thereby initiating the inhibition of intestinal motility [
1].
Currently, it is considered that the intestinal insufficiency syndrome occurs in the acute surgical pathology of the abdominal cavity, in which there is a disturbance of the functions of the gastrointestinal tract, and the intestine becomes the main source of toxicity and the development of multiple organ failure [
2,
3].
The small intestine
The intestine was originally described as the "motor" of multiple organ failure (MOF), by Meakins and Marshall, in 1985, during a group debate of the Infections Society in Surgery. According to the majority of authors who study the function of the gastrointestinal tract and the antimicrobial protection system of the body, the intestine with "associated lymphoid tissue" is the largest immune organ in humans [
4].
The immune system of the small intestine forms a protective barrier that protects the body against pathogenic and conditionally pathogenic microorganisms, and it is proven that it interacts very closely with epithelial, nerve, muscle, and stromal cells. Local immunity of the small intestine is an integral part of the global antimicrobial defense and is due to the presence of the normal microflora of the intestinal lumen, the mechanical and biological barrier, the enzymatic systems of cells, which contribute to the destruction and elimination of external agents. This process takes place with the participation of specific blood proteins involved in defense reactions (α-1-antitrypsin, lipoproteins, complement, lysozyme, properdin, interferon, transferrin, and C-reactive protein), as well as through phagocytes, natural killer cells, blood clotting mechanisms and fibrinolysis [
3]. The immune system of the small intestine, taking into account the surface at the level of the mucous membranes, has more than 400 m² (compared to the skin ~ 1.8 m²). Approximately 80% of plasma cells produce IgA at the level of the mucous membranes. The density of the distribution of these cells depends on the portion of the immune system of the small intestine.
The small intestine has two zones that are conditionally designated [
3]: inductive (Peyer’s patches, regional lymph nodes) and effector (lamina propria). In the upper and middle segments of the small intestine, there is a small population, predominantly composed of aerobes and facultative anaerobes, yeasts, and fungi (up to 102-104 ufc/ml chim) [
3,
5]. In the distal portion, ileum and ileocecal region, the composition of the "microbial spectrum" is represented by facultative families of anaerobic microorganisms, coliforms, bifidobacteria, fusobacteria, and Bacteroides, at a concentration of 105-107 cfu/ml. The colon is the site of over 400 species of bacteria. Due to the absence of oxygen and the very low oxidation-reduction potential (-250 mV), it becomes the main habitat of anaerobes (10¹¹-10¹² cfu/g) [
3]. In the context of a peritoneal suppuration, an imbalance is created between the different types of microorganisms and their distribution in different segments of the intestine.
Intestinal Insufficiency Syndrome
Four degrees (phases) are distinguished, characteristic of the syndrome of intestinal insufficiency in peritonitis [
3]. In the first phase (initial), there is a sudden decrease in the amount of natural symbionts in their habitat. In the second phase, the species-to-microflora ratio is modified, increasing the number of certain bacteria (Escherichia, Klebsiella, Lactobacillus, enterococci). In the third stage, there is a change in the localization of the autochthonous microflora, by moving into intestinal segments in which it was not previously encountered, the so-called process of "proximal microbial contamination or colonization". In the fourth phase, from the presented microbial association, displaced proximally through the digestive tract, there are signs of pathogenicity [
3].
This process produces excessive microbial colonization of the small intestine, the average number of microorganisms in the jejunum reaching approximately 10⁹-10¹² cfu/ml, this level of concentration corresponding to the normal microbial invasion from the lumen of the colon in 1 g of feces [
3,
5]. The secretion of these pathogenic microorganisms provides the possibility of adhesion to the surface of enterocytes [
6,
7]. The presence of endotoxin disrupts electrolyte transport, leading to increased secretion into the lumen, water imbalance, and severe dehydration [
3].
According to the studies of AN Kosinets (1993), the vast majority of aerobic and anaerobic bacteria have β-lactamase activity: pathogenic staphylococcus - at 62.1%, E. coli - in 56.7%, Pseudomonas aeruginosa - 75%, Proteus and Bacteroides - 100% of cases. Inhibition of this β-lactam enzyme by antibiotics (penicillins and cephalosporins) drastically reduces the effectiveness of their use in the treatment of peritonitis.
The multidirectional impact of these numerous pathogens on the intestinal mucosa causes a sudden change in its properties (especially the barrier property) and the "discovery" of pathogenic microflora in the lymphatic canal, portal circulation, and in the free peritoneal cavity. This process is called "bacterial translocation" [
2,
3,
4,
8]. The mechanisms involved in bacterial translocation include loss of intestinal barrier function, host immune system failure, and alteration of the intestinal microbiota [
9]. While, according to some authors, the most likely site of bacterial translocation would be the colon, due to the large microbial load [
3], according to others, bacterial translocation occurs mainly in the small intestine [
10]. Currently, it is a pathological syndrome attached to endotoxemia and a major inductor of the systemic inflammatory response syndrome, abdominal sepsis, and multiple organ failure [
2,
4,
5].
Once purulent inflammation develops in the abdominal cavity, one of the pathogenic mechanisms underlying the change in the motor function of the gastrointestinal tract is a violation of the relationship between the sympathetic and parasympathetic nervous systems [
3]. Many authors consider the inhibition of the motor function of the gastrointestinal tract as a protective response associated with neuro-reflex inhibition, inhibition in the central nervous system, in response to strong afferent impulses from abdominal receptors [
3].
The hypertonia of the sympathetic nervous system extends not only to the smooth muscle of the intestinal wall, but also to the micro vascularization territory, which leads to increased arterial spasm and drastic reduction of regional blood flow. This leads to intestinal wall hypoxia and profound inhibition of intestinal motility [
3].
In the early onset of paralytic ileus in the context of abdominal sepsis, endocrine regulation mechanisms can be involved: release of catecholamines, activation of the kallikrein-kinin system through the entry of excess histamine, bradykinin, proteolytic enzymes and other biologically active substances into the bloodstream, reduction of the biological activity of Apud-system cells (serotonin [substance P] and motilin), disruption of the secretion of secretin, cholecystokinin and enteroglucagon [
3].
With the progression of the septic process in the abdominal cavity, a large number of acidic intermediate hydrolases of unfinished metabolism (aldehydes, ethanol, skatole, cadaverine, hydrogen sulfide, indole, phenol, ammonia) enter the systemic circulation. These products have a negative impact on nerve impulse transmission (alteration of their cholinergic transmission at the neuromuscular junction), causing ischemic lesions of the intestinal wall and death of the neurons of the intramuscular plexus [
3].
Subsequently, myocytes are unable to perceive nerve impulses due to the intracellular metabolic change and electrolyte imbalance. The mucosa of the small intestine has been shown to be more susceptible to ischemic revascularization injury, while the colon is more resistant to hypoperfusion, which provides a possible explanation for why patients with low intestinal ischemia tend to have less favorable outcomes than in cases of colon ischemia.
In the early stage, intestinal ischemia causes ileus, so that the proximal intestine becomes a reservoir for pathogens and toxins, which contribute to late sepsis and MOF. [
11]. Factors that contribute to the increased susceptibility of enterocytes to hypoxia include:
Low oxygen levels in the tissues of the villus tips, due to oxygen feedback perfusion,
The concentration of active oxidant (xanthine dehydrogenase) during the development of intestinal wall hypoxia, in the distal half of the villi,
Changes in the absorption of amino acids, glucose, and electrolytes from enterocytes.
Histological examination has shown that necrosis develops most rapidly in the ischemic mucosa of the intestine, at the tips of the villi. [
12,
13] As the ischemic changes progress in enterocytes, which are located in the distal part of the villi, the lamina propria is removed due to edema, then the necrotic enterocytes are exfoliated into the lumen, resulting in the underlying tissues being exposed to the intestinal content. Thus, following the disruption of the epithelial barrier, the very toxic content from the intestinal lumen is aspirated into the venous and lymphatic vascular bed.
In conditions of intestinal ischemia, there is a reduction in the oxygen content and nutrients in tissues (with increasing concentrations of active toxic oxidants), resulting in tissue acidosis, with the appearance of histamine, serotonin, bradykinin, nitric oxide, leukotrienes, thromboxanes, interleukins -1, 2, 4, 6, 8,10, complement, and thrombin. [
15]
The serotonin role
It is known that serotonin plays an important role in regulating the gastrointestinal tract and peripheral hemodynamics. The largest amount of serotonin in the body is in the gastrointestinal tract, representing more than 95% of the total body. Most of the serotonin is contained in the enterochromaffin cells of the intestinal epithelium, in which it is synthesized from L-tryptophan. [
16]
Based on clinical and experimental studies, it has been established that, under pathological conditions in the body, the number of ligands of serotonin receptors increases, which in turn are divided into agonists and antagonists. [
16,
17]
Serotonin antagonists, when they interact with serotonin receptors, cause paralysis of smooth muscle. Agonists, on the other hand, cause spasms of smooth muscle. According to this concept, smooth muscle disorders, which occur as a result of the interaction of serotonin with its receptors, lead to an endogenous vasomotor disturbance and microcirculation disturbance in hypoxia, with local and regional tissue necrosis lesions. Subsequently, myocytes are unable to perceive nerve impulses due to intracellular metabolic changes and electrolyte imbalance.
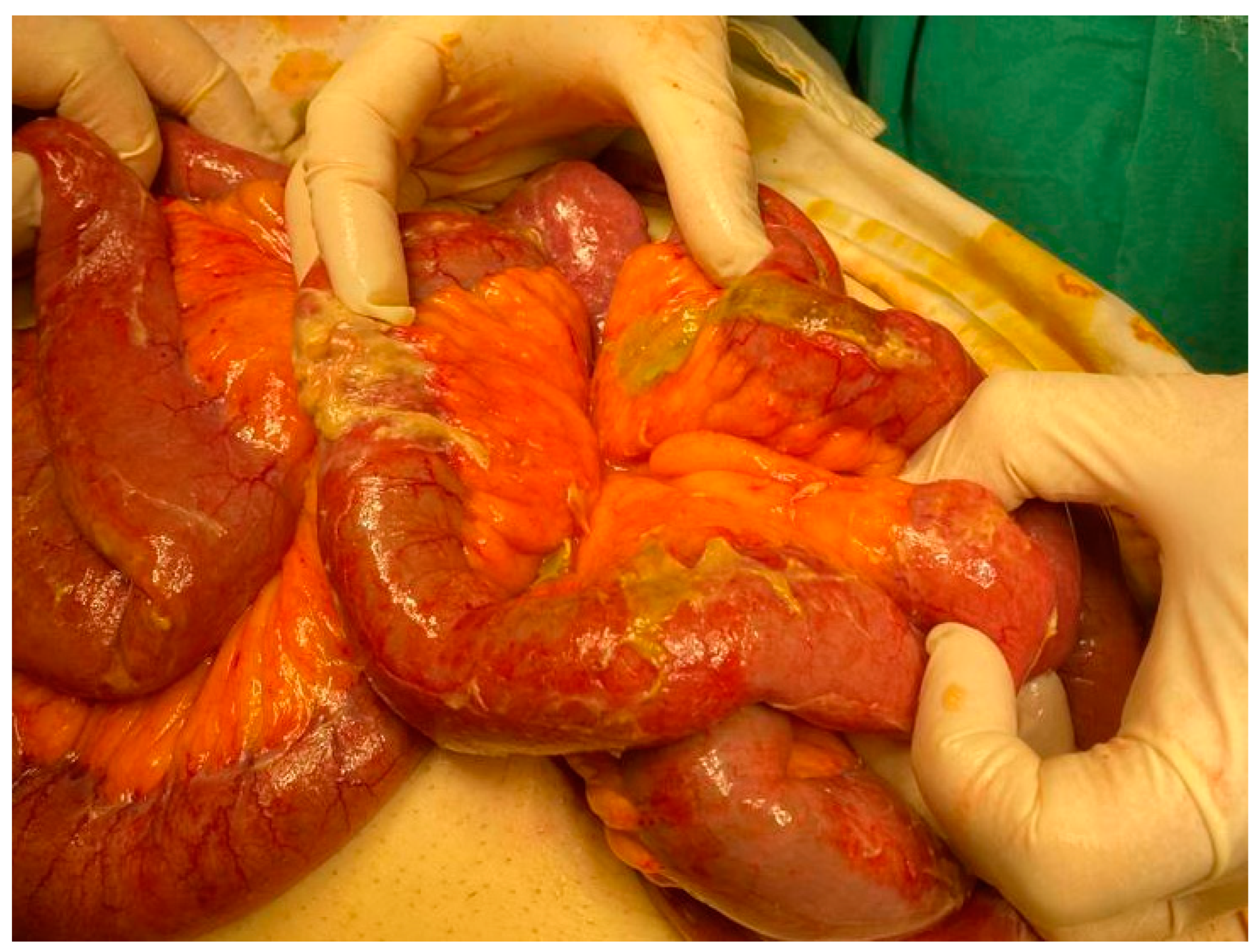
Figure 1.
Generalized fecaloid peritonitis due to neglected perforated sigmoid diverticulitis (intraoperative appearance personal collection).
Figure 1.
Generalized fecaloid peritonitis due to neglected perforated sigmoid diverticulitis (intraoperative appearance personal collection).
Pathophysiological changes
The main pathophysiological changes, in the course of the development of intestinal ischemia in peritonitis, are:
Release of cytokines and suppression of natural protective factors;
Disruption of the epithelial barrier of the small intestinal mucosa;
Formation of active oxidants with reduced antioxidant protection;
Chemotaxis of neutrophils and adhesion of neutrophils to endothelial cells;
Chemical depletion of intracellular energy reserves.
Furthermore, abdominal perfusion pressure, defined as the mean arterial pressure minus intra-abdominal pressure, plays a decisive role in the blood flow of the small intestine. Intra-abdominal hypertension greater than 15 mmHg causes abdominal ischemia and, therefore, early ischemic changes at the intestinal level, which lead to bacterial translocation. [
19,
20]
The characteristic properties of intestinal arterioles consist in the formation of free resistance to blood flow - the so-called "barrier of microcirculatory function", through the passage of blood flow through the intestinal tissue. Thus, there is a reduction in mean arterial pressure from 100-120 mmHg to 35-40 mmHg. [
12] It has been confirmed that in the onset of intestinal occlusion, the intestinal lumen pressure does not always reach high figures, but in the evolution of the pathological process, it is important not so much the pressure gradient, but the duration and persistence of this phenomenon. [
21]
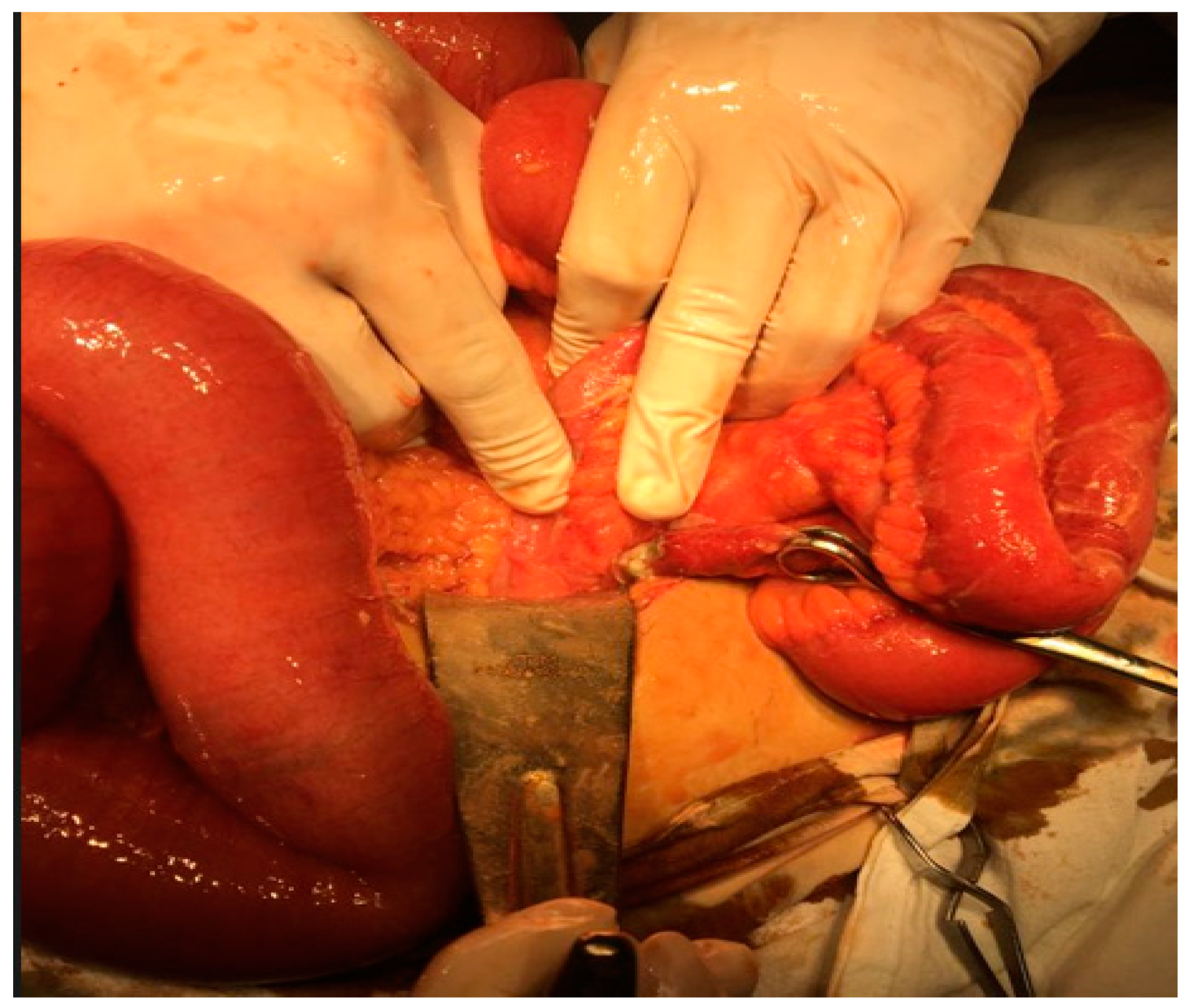
Figure 2.
Ileus generated by peritonitis from a perforated gangrenous appendicitis (intraoperative appearance personal collection).
Figure 2.
Ileus generated by peritonitis from a perforated gangrenous appendicitis (intraoperative appearance personal collection).
Ohman’s study (1975) confirmed the relationship between the degree of intestinal wall tension and the nature of the intramural blood flow. When the pressure level in the intestinal lumen is over 100 mm Hg, there is a profound alteration of the capillary filtration function, with a drastic limitation of tissue oxygen consumption and an increase in intestinal wall ischemia. The latter develops by reducing blood flow in the intestinal wall by 50% of its actual volume [
22,
23]. The signs and morphological changes correlate and are dependent on the stage of peritonitis, the degree of biological damage, the nature of the somatic condition and the degree of endotoxemia.
It is worth mentioning, when appreciating the pathogenic mechanisms of acute peritonitis, that intestinal insufficiency, in turn, even in the early stages of peritonitis, is included in the pathogenic vicious circles and aggravates its evolution, and that of multiple organ failure.
Future directions
To advance our understanding of intestinal ischemia in peritonitis and develop effective treatment strategies, future research should delve into the following areas:
Investigating the role of serotonin receptors:
Serotonin plays a crucial role in regulating intestinal function, but its involvement in intestinal ischemia is still poorly understood. Studies should focus on identifying specific serotonin receptors that are involved in mediating intestinal ischemia and its consequences. This knowledge could lead to the development of targeted therapies that modulate serotonin signaling to mitigate intestinal ischemia-induced damage.
Developing novel therapies:
The current treatment options for intestinal ischemia in peritonitis are limited and often ineffective. Research should focus on developing novel therapies that target the underlying pathophysiological mechanisms of intestinal ischemia, such as antioxidant therapy, anti-inflammatory drugs, and vasoactive agents. These therapies could potentially improve tissue perfusion, reduce inflammation, and prevent further damage to the intestinal wall.
Exploring stem cell therapy:
Stem cell therapy holds promise for the regeneration of injured intestinal tissue in patients with peritonitis. Studies should investigate the potential of stem cell-based therapies to promote repair of ischemic intestinal lesions and restore intestinal function. This could be a promising avenue for improving outcomes in patients with severe intestinal ischemia.
Establishing preventive strategies:
Preventing intestinal ischemia in the first place is crucial for improving patient outcomes. Research should focus on identifying effective strategies for preventing intestinal ischemia in patients with peritonitis. This could involve interventions such as early surgical intervention to address the underlying cause of peritonitis and optimizing hemodynamic parameters to maintain adequate blood flow to the intestine.
Conducting randomized controlled trials:
To evaluate the efficacy of new therapies and preventive strategies, randomized controlled trials are essential. These trials should compare the effectiveness of new interventions to standard care or placebo and provide robust evidence for their clinical utility.
Developing animal models:
Animal models that accurately mimic the human disease process are crucial for preclinical testing of new therapies and preventive strategies for intestinal ischemia. Researchers should develop animal models that exhibit the key pathological features of intestinal ischemia in peritonitis, such as intestinal wall damage, inflammation, and impaired blood flow.
Collaborating across disciplines:
Effective research on intestinal ischemia requires collaboration between researchers from various disciplines, including gastroenterology, surgery, pharmacology, and basic science. This multidisciplinary approach can bring together expertise from different areas to advance our understanding of the disease and develop innovative treatment strategies.
Raising awareness among healthcare providers:
Increasing awareness of intestinal ischemia among healthcare providers is essential to improve early diagnosis and treatment. This could involve educational programs, clinical guidelines, and disseminating research findings to healthcare professionals. Early diagnosis and timely intervention can significantly impact patient outcomes and reduce the risk of complications.
Conclusions
Intestinal ischemia is a complex and potentially life-threatening complication of peritonitis. It arises from a reduction in blood flow to the intestinal wall, leading to a cascade of detrimental effects on intestinal function and overall patient prognosis.
Several factors contribute to the development of intestinal ischemia in peritonitis. Increased intra-abdominal pressure, inflammation-mediated vasoconstriction, and mechanical obstruction all play a role in impairing intestinal perfusion.
The hallmarks of intestinal ischemia in peritonitis include abdominal distension, pain, fever, leukocytosis, hypotension, metabolic acidosis, edema of the intestinal wall, and necrosis of the intestinal wall. These clinical signs reflect the compromised physiology of the intestine and the potential for widespread organ dysfunction.
Intestinal insufficiency, the inability of the intestine to adequately perform its absorptive and digestive functions, is a profound consequence of intestinal ischemia in peritonitis. This dysfunction can lead to a spectrum of complications, including malnutrition, dehydration, electrolyte imbalances, septicemia, and multi-organ failure.
Treatment of intestinal ischemia in peritonitis is multifaceted and aims to optimize intestinal perfusion, address the underlying cause of peritonitis, and provide supportive care. Surgical interventions to remove the source of infection, abdominal drainage, pharmacological therapies to reduce inflammation and improve vascular tone, and nutritional support are all crucial components of management.
Early recognition and prompt intervention for intestinal ischemia in peritonitis are essential to improve patient outcomes and reduce the risk of complications. Early diagnosis allows for timely intervention and may minimize the extent of intestinal damage and its associated morbidities.
Author Contributions
Conceptualization, C.Ș. A.T. and R.M.; methodology, B.C..; writing—original draft preparation, C.Ș.; writing—review and editing, A.T.; visualization, P.C. and B.F.; supervision, V.D.. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of „Sf. Apostol Andrei” Emergency Clinical County Hospital Galați (protocol code 312 from 15 December 2023)”.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Çiğdem, Murat Kemal, et al. "The mechanical complications of colostomy in infants and children: analysis of 473 cases of a single center." Pediatric surgery international 22 (2006): 671-676. [CrossRef]
- Deitch E.A. Simple intestinal obstruction causes bacterial translocation in man / E.A.Deitch / / Arch Suig. - 1989. Vol. 124. №6. P. 699-701. [CrossRef]
- Timerbulatov, V. M., et al. „Sindromul de insuficiență intestinală în peritonită”. (2008).
- Saenko VF et al.The administration of peflocine (pefloxacin) in the treatment and prevention of surgical infections. Klin Khir. 1997;(7-8):13-4. Russian. PMID: 9518087. [PubMed]
- Golbach S. Intra-abdominal infections / S.Golbach / / Clin Infect Dis. — 1993. Vol. 17. P. 961-967.
- Jones, G.W. and Rutter, J.M., 1972. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infection and immunity, 6(6), pp.918-927. [CrossRef]
- Källenius, G., Svenson, S.B., Hultberg, H., Möllby, R., Helin, I., Cedergren, B. and Winberg, J., 1981. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. The Lancet, 318(8260-8261), pp.1369-1372. [CrossRef]
- Van Leeuwen PA, Boermeester MA, Houdijk AP, Ferwerda CC, Cuesta MA, Meyer S, Wesdorp RI. Clinical significance of translocation. Gut. 1994 Jan;35(1 Suppl):S28-34. doi: 10.1136/gut.35.1_suppl.s28. PMID: 8125386; PMCID: PMC1378143. [CrossRef]
- Zanoni, Fernando Luiz, et al. "Mesenteric microcirculatory dysfunctions and translocation of indigenous bacteria in a rat model of strangulated small bowel obstruction." Clinics 64 (2009): 911-919.
- Fritz, Vanessa, and Lluis Fajas. "Metabolism and proliferation share common regulatory pathways in cancer cells." Oncogene 29.31 (2010): 4369-4377. [CrossRef]
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001 Jan;15(1):1-10. PMID: 11198350. [CrossRef] [PubMed]
- Scott J. Boley, Lawrence J. Brandt, Frank J. Veith.Ischemic disorders of the intestines, Current Problems in Surgery,Volume 15, Issue 4,1978,Pages 1-85,ISSN 0011-3840. [CrossRef]
- Kurilov V. P., Struchkov Yu. V., Sotnikov D. N. Insuficiență enterală în obstrucția intestinală acută în perioada postoperatorie timpurie // Doctor postuniversitar. - 2010. - T. 41. - Nr. 4.1. - S. 117-122.
- Mironov A.V. Sindromul de insuficiență intestinală cu peritonită larg răspândită: diagnostic și metode de corecție enterală // Moscova. - 2011. - or. 18.
- Petukhov V. A., Magomedov M. S. Viziune modernă asupra problemei agresiunii endotoxinelor și a disfuncției endoteliale în chirurgie // Chirurgie. Supliment la Consilium Medicum. - 2008. - nr. 2. - S. 37-46.
- Kolunov A. V. Infuzia endolimfatică de adipat de serotonină în tratamentul complex al parezei intestinale postoperatorii: dis. – Sankt Petersburg, 2007.–20 p. Ediție educațională, 2007.
- Al’yanov AL Influența adipinatului de serotonină asupra modificărilor ischemice la nivelul intestinului subțire în obstrucția intestinală acută (studiu experimental): 2009.
- Gain, Yu. M., et al. „Problema sepsisului abdominal în chirurgie”. (2003).
- Sukhotnik I, Bejar J, Srugo I, Krausz MM, Bernshteyn A, Hirsh M, Mogilner JG. Adverse effects of increased intra-abdominal pressure on small bowel structure and bacterial translocation in the rat. J Laparoendosc Adv Surg Tech A. 2006 Aug;16(4):404-10. PMID: 16968194. [CrossRef] [PubMed]
- Kaussen, Torsten, et al. "Influence of two different levels of intra-abdominal hypertension on bacterial translocation in a porcine model." Annals of intensive care 2.1 (2012): 1-13. [CrossRef]
- Van Westerloo, David J., et al. "The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis." Journal of Infectious Diseases 191.12 (2005): 2138-2148. [CrossRef]
- Clavien, P-A., et al. "Gallstone ileus." British Journal of Surgery 77.7 (1990): 737-742. [CrossRef]
- Makedonskaia TP, Pakhomova GV, Popova TS, Selina IE, Skvortsova AV. Lechenie sindroma kishechnoĭ nedostatochnosti u bol’nykh s peritonitom [Treatment of intestinal insufficiency syndrome in patients with peritonitis]. Khirurgiia (Mosk). 2004;(10):31-3. Russian. PMID: 15477823. [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).