1. Introduction
Ovarian cancer, known as the “silent killer,” has the highest mortality rate of all gynecological tumors[
1], and over 90% of ovarian cancers are epithelial carcinomas[
2]. Over 60% of ovarian cancers are diagnosed at advanced stages due to ineffective early detection strategies, leading to a high mortality rate[
3]. The five-year survival rate is above 50%[
4,
5]. Ovarian cancer originates from the ovarian surface epithelial cells or the fallopian tube secretory epithelial cells, and malignant cells can spread directly to the peritoneum, resulting in metastasis and poor treatment outcomes[
6]. Improvements in ovarian cancer treatments are urgently needed. Current research is focused on accurately targeting cancer cells and enhancing the efficacy of therapeutic drugs while reducing side effects. Chemotherapy remains the most important treatment modality for ovarian cancer.
SN38 (7-ethyl-10-hydroxythectothecin) is an active metabolite of irinotecan (CPT-11) and a derivative of camptothecin obtained by chemical structure modification. It has strong anti-tumor effect and high anti-tumor activity[
7]. In vitro cytotoxicity test results showed that the anti-tumor activity of SN38 was 100-1000 times that of irinotecan for some tumor cell lines. However, due to the extremely poor physiological and pharmaceutical compatibility, and low tumour targeting ability, SN38 cannot be administered directly as a chemotherapeutic agent[
8]. Clinical use of SN-38 is limited by poor water solubility and instability at physiological pH. The lactone ring of SN-38 is hydrolyzed at pH > 6 resulting in the inactive carboxylate form[
9]. Recently, nanomedicines improved the pharmacological and therapeutic properties of SN-38. Nanosized delivery systems can improve anticancer efficacy, reduce side effects, and reverse drug resistance by targeting tumor tissues through enhanced permeability and retention and active targeting strategies[
10]. Many attempts have been made to develop intravenous or oral preparations of SN-38 with better antitumor effects. Liposomes are small spherical vesicles derived from naturally occurring non-toxic phospholipids and are biodegradable nanosystems. Liposomes have been extensively utilized as drug delivery systems. Onivyde (Irinotecan liposome injection), a pioneer in the development of liposomes loaded with camptothecin and its derivatives, is used to treat patients with tumors. Although SN38-based chemotherapy improved the efficacy of ovarian cancer treatment, drug resistance develops in cancer cells and serious side effects occur. To overcome the inherent limitations of single chemotherapy drugs, chemotherapy has been combined with phototherapy induced by photosensitizers as a practical clinical cancer treatment platform. Combining chemotherapy and phototherapy increases the sensitivity of cancer cells to chemotherapy and improves the uptake efficiency of chemotherapeutic drugs by increasing cancer cell membrane permeability[
11].
Photodynamic therapy (PDT) involves light activation of photosensitizers and the generation of short-lived reactive molecular species that induce spatially and temporally controlled photodamage to cellular organelles and biological targets[
12]. PDT induces subcellular toxicity, which sensitizes target cells to subsequent therapies, including chemotherapy and direct cell killing[
13]. PDT can be combined with chemotherapy, immunotherapy, gene therapy, or other treatment modalities to enhance antitumor efficacy[
14,
15]. By irradiating lesion sites at specific wavelengths, photosensitizers selectively accumulate and become activated in diseased tissue, triggering a photochemical reaction that destroys the lesion. Photosensitizers in PDT transfer energy to the surrounding oxygen, generating highly reactive singlet oxygen. Singlet oxygen can react with nearby biomolecules, producing cytotoxic effects that kill cancer cells[
16,
17]. Indocyanine green (IR820) is a clinical infrared fluorescent dye that efficiently absorbs laser light for photothermal and photodynamic therapy. IR820 is non-toxic until exposed to laser irradiation. The limitations of IR820 include rapid clearance (blood half-life of 2–4 min) and low cellular uptake. These limitations restrict the diagnostic and treatment applications of IR820. Enhancing the intracellular release of chemotherapeutic drugs during the PDT process is essential to amplifying the synergistic therapeutic effects of chemo-photodynamic therapy.
The follicle-stimulating hormone receptor (FSHR), a G protein-coupled receptor with seven transmembrane domains, is found on the surfaces of cells in the ovaries, testes, and other reproductive organs[
18,
19]. Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein composed of non-covalently bound α and β subunits[
20]. The α subunit is encoded by a single gene shared by FSH, luteinizing hormone (LH), human chorionic gonadotropin (hCG), and thyroid-stimulating hormone (TSH). The β subunit is specific to FSH. The association of FSH β with the FSHR in the cell membrane affects target cells, such as granulosa cells and Sertoli cells. FSHβ 33–53 and FSHβ 81–95 peptides promote paclitaxel-loaded NP entry into FSH receptor (FSHR)-positive ovarian tissue to achieve excellent antitumor effects[
21]. Thus, targeting therapy mediated by FSHR has a high potential for ovarian cancer treatment[
18,
20].
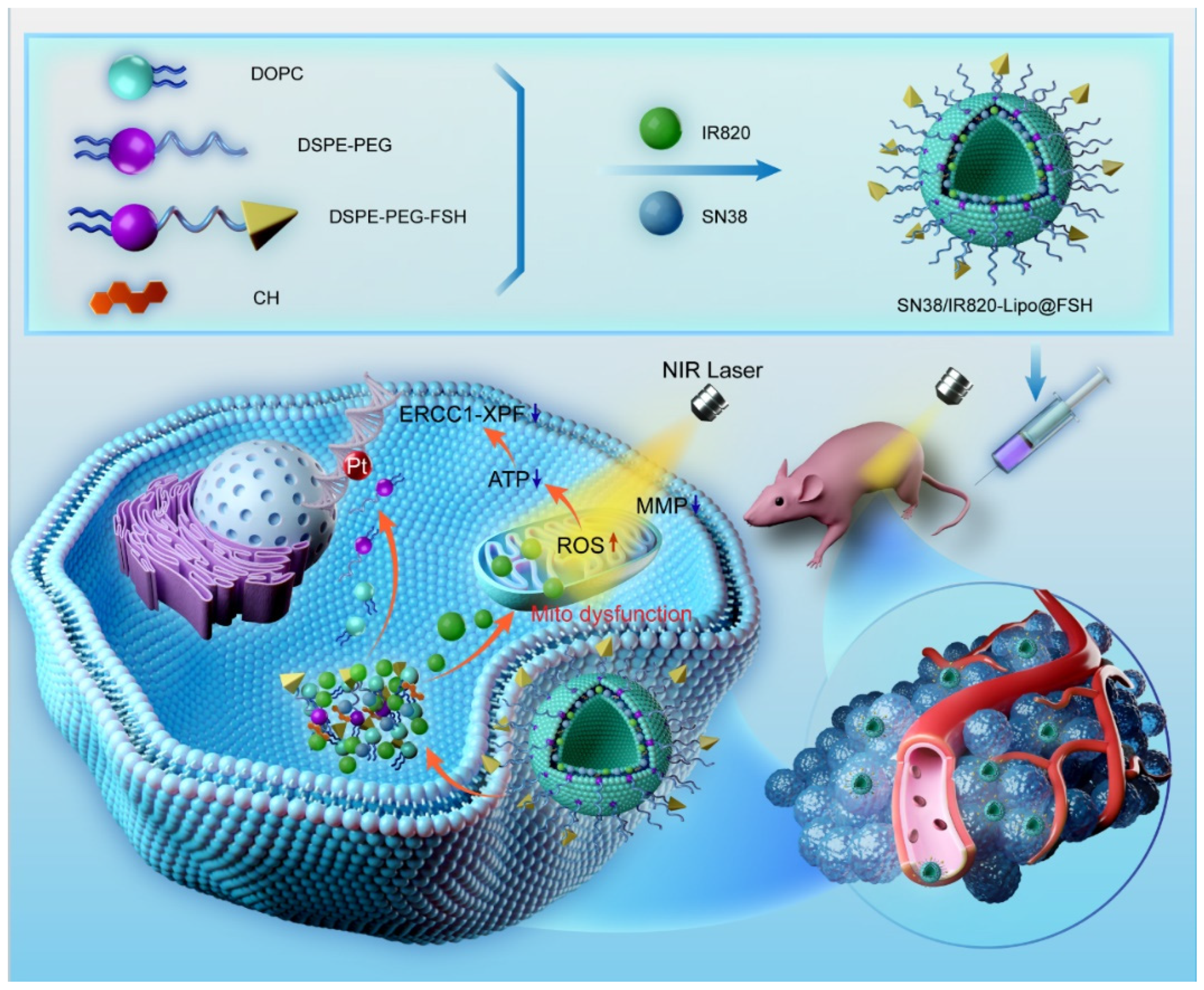
We developed an ovarian cancer-targeting drug delivery system based on the FSHβ (33–53) peptide. The lipophilic chemotherapeutic drug SN38 and the photosensitizer IR820 were loaded into the phospholipid bilayer of the liposomes. Near-infrared laser irradiation generates reactive oxygen species via IR820 to induce short-term reactive molecular species and spatial-temporal restricted photodamage in biological targets. These effects induce the release and uptake of chemotherapy drugs and increase sensitivity to chemotherapy drugs in ovarian cancer cells, as illustrated in
Figure 1. Chemotherapy and phototherapy act synergistically and this dual-delivery liposome may be a promising strategy for tumor treatment.
2. Materials and Methods
2.1. Materials
SN38 was purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, P. R. China). The FSH β (FSH) peptide (YTRDLVYKDPARPKIQKTCTF) was custom-synthesized by Nanjing TGpeptide Biotechnology Co., Ltd. (Nanjing, China). New indocyanine green (IR820) and Coumarin-6 (98%) were obtained from J&K Scientific Co., Ltd. (Beijing, China). DSPE-PEG2000, DSPE-PEG2000-Mal, 2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and cholesterol were purchased from AVT (Shanghai) Pharmaceutical Technology Co., Ltd. (Shanghai, China). The Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan). The Fluorescein Isothiocyanate (FITC)-Annexin V/Propidium Iodide (PI) apoptosis detection kit was acquired from Beyotime Biotechnology Co., Ltd. (Shanghai, China). The ROS Detection Kit was purchased from Enzo Life Sciences Co., Ltd. (Beijing, China). The Calcineurin-AM/PI Staining Kit and 4′, 6-diamidino-2-phenylindole (DAPI) were purchased from Solarbio Life Sciences Co., Ltd. (Beijing, China). The 1,1-dioctadecyltetramethyl indotricarbocyanine iodide (DIR) was obtained from Biotium Inc. (Hayward, CA, USA). Chloroform and methanol (HPLC grade) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Fetal bovine serum (FBS) was purchased from GIBCO LLC. (Grand Island, NY, USA). RPMI 1640 medium, DMEM medium, and phosphate-buffered saline (PBS) were obtained from Thermo Fisher Scientific Co., Ltd. (Beijing, China).
2.2. Cells and Animals
High FSHR-expressing mouse A2780 cells and low FSHR-expressing HepG2 and C26 cells were purchased from the Department of Pathology at Peking Union Medical College Institute of Medicinal Biotechnology. A2780 cells and C26 cells were cultured in RPMI-1640 medium with 10% FBS at 37°C and 5% CO2. HepG2 cells were cultured in DMEM media with 10% FBS at 37°C and 5% CO2 in a humidified atmosphere. All experiments were performed in cells at the logarithmic growth stage.
Female BALB/c Nude mice (initial weight 18-20 g) were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, PR China) and housed under standard conditions. All animal experiments were conducted following the guidelines prepared and approved by the Laboratory Animal Ethics Committee of the Institute of Materia Medica in Chinese Academy of Medical Sciences and Peking Union Medical College.
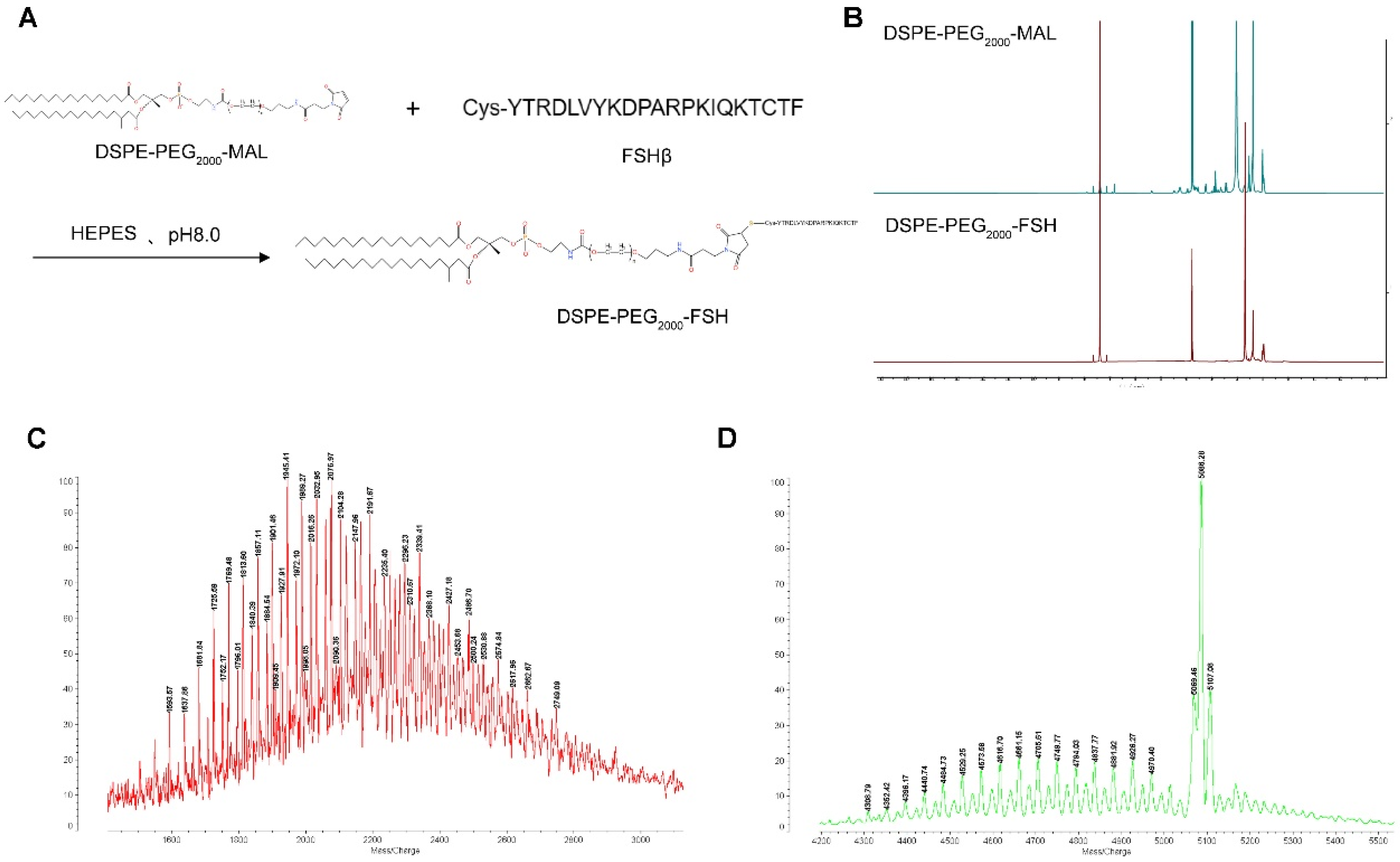
2.3. DSPE-PEG2000-FSH Synthesis and Characterization
The synthesis of DSPE-PEG
2000-FSH was based on the method by Hong et al.[
21], with slight modifications. The FSH-Cys cysteine residue was coupled to DSPE-PEG
2000-MAL to make DSPE-PEG
2000-FSH. FSH-Cys and DSPE-PEG
2000-MAL (molar ratio 1:1) were dissolved in HEPES buffer at pH 8.0, and nitrogen was stirred at room temperature for 48 h. The reaction was conducted in dialysis bags in water. The unreacted FSH peptide was removed by aeration in water through a dialysis bag (MWCO: 2500) for 48 h. The final product was obtained by freeze-drying. The DSPE-PEG
2000-FSH synthesis was confirmed by
1H nuclear magnetic resonance spectroscopy (400 MHz, Varian Medical Systems, Inc., Palo Alto, CA, USA) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (4800 Plus, Applied Biosystems Inc., Waltham, MA, USA).
2.4. Liposome Preparation with or without FSH Peptide Modification
SN38/IR820-Lipo@FSH liposomes were prepared using thin-film hydration. After dissolving DOPC, cholesterol, DSPE-PEG2000, DSPE-PEG2000-FSH, SN38, and IR820 in chloroform, the organic solvents were removed by rotary evaporation at 37°C. The lipid membranes were hydrated with deionized water under spinning for 30 min. The whole mixture was sonicated at 65 W for 10 min with 2 s of sonication and 2 s of a break in an ice bath using an ultrasonic cell pulverizer (Scientz 950E; Ningbo Scientz Biotechnology Co., Ltd., Zhejiang, P. R. China). The unbound SN38 and IR820 were removed by filtration through a 0.22 μm nitrocellulose membrane. Samples were stored at 4°C. Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, and SN38/IR820-Lipo (without FSH peptide) were prepared using the same method.
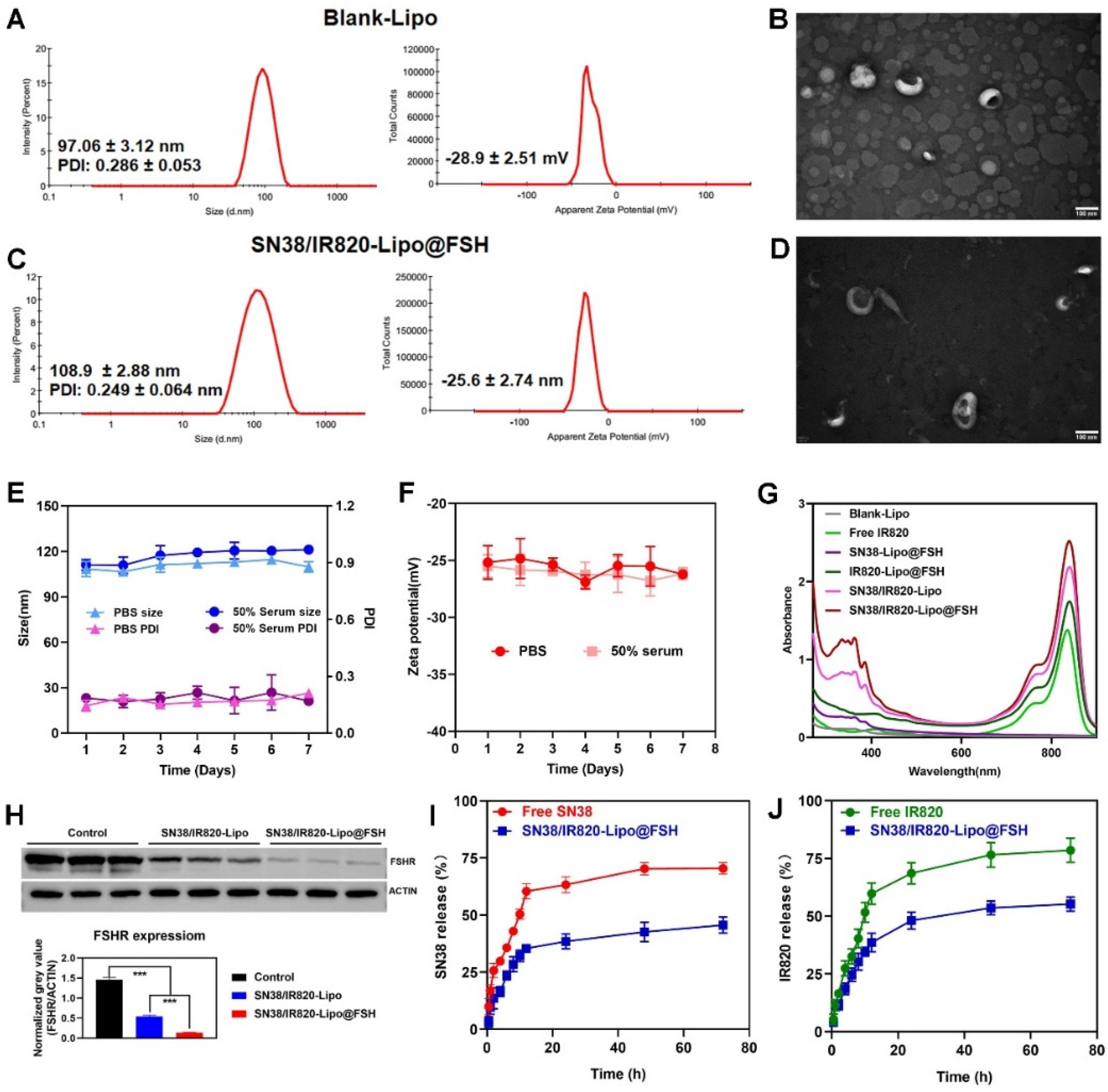
2.5. Characterization of the liposomes
The average particle size, polydispersity index, and zeta potential of SN38/IR820-Lipo@FSH were measured using dynamic light scattering (DLS) and electrophoretic light scattering (Zetasizer Nano ZS90, Malvern Instruments, Malvern, UK). The structure and morphology were examined using transmission electron microscopy (JEM-1400PLUS, JEOL Ltd., Tokyo, Japan). The SN38 and IR820 concentrations were determined using high-performance liquid chromatography (HPLC, Agilent 1200 infinity; Agilent Technologies, Santa Clara, CA, USA) and UV-VIS spectrophotometry (TU-1810, Pulse Analyzer General Instrument Ltd., Beijing, China), respectively. The detection wavelength of SN38 was 265 nm, and methanol:water (70:30) was used as the mobile phase. Free SN38 and IR820 were separated from the liposomes using minicolumn centrifugation[
22]. The entrapment efficiency (EE%) and drug loading capacity (DL%) were calculated as follows:
EE (%)=(weight of encapsulated drug)/(weight of total drug)× 100%
DL(%)=(weight of encapsulated drug)/(weight of total drug and lipid)× 100%
To determine the stability of SN38/IR820-Lipo@FSH, the prepared solution was stored at 4°C for 7 days in PBS or PBS containing 50% of serum. The color, transparency, particle size, zeta potential, and PDI of SN38/IR820-Lipo@FSH were recorded during the days.
The in vitro release of drugs from SN38/IR820-Lipo@FSH in a simulated physiological environment was evaluated using dialysis. SN38/IR820-Lipo@FSH solution (0.5 mL) was placed in a dialysis bag (MW: 10 kDa; MYM Biological Technology Co., Ltd., Hyderabad, India), and the dialysis bag was immersed in 10 mL of PBS (pH 7.4) containing 0.5% (v/v) Tween 80. The dialysis solution was stirred at 100 rpm for 12 h at 37°C, and 0.5 ml aliquots were removed at 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h. The aliquot volume was replaced with fresh medium. The SN38 and IR820 concentrations in the samples were determined using HPLC and UV-VIS spectrophotometry respectively. The cumulative release of SN38 and IR820 over time was calculated.
2.6. Interaction of SN38/IR820-Lipo@FSH with FSHR in vitro
SN38/IR820-Lipo and SN38/IR820-Lipo@FSH were added to A2780 cells and incubated at 37°C for 24 h to measure binding to the FSHR. Cells were washed twice with cold PBS and lysed (lysis buffer: 50 nM Tris-HCl pH = 6.8, 2% SDS, 6% Glycerol, 1% β-mercaptoethanol, 0.04% bromophenol blue). The cell lysates were incubated for 30 min at 4°C and centrifuged for 15 min at 12000 × g. The protein concentration was determined using a BCA Protein Assay Kit (Lot: PA115-01, Tiangen Biotech (Beijing) CO., LTD). The protein lysates were separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The membranes were incubated with ACTIN (Servicebio, GB12001) and FSHR antibodies (1:1000; Cell Signaling, 2808s) overnight at 4°C. After washing, the membranes were incubated with goat anti-rat IgG antibody for 1 h, and bands were detected using enhanced chemiluminescence (ImageQuant LAS 4000 mini, Fuji, Japan)[
23].
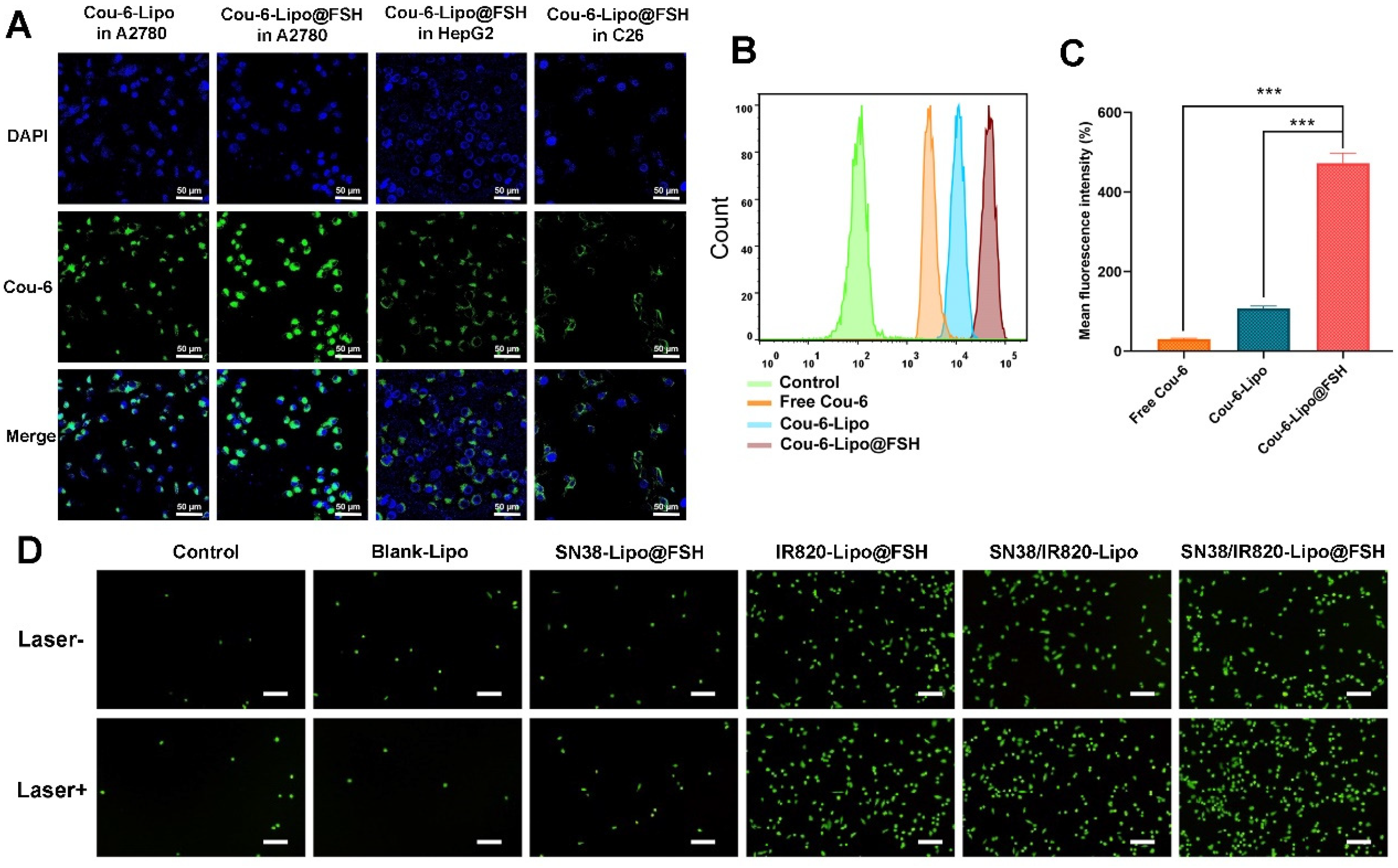
2.7. Cellular Uptake Analysis
As a drug delivery system, the efficiency of liposome internalization by target cells is crucial[
24]. Cou-6, a fluorescent probe and laser dye, was used to track liposome uptake[
25]. Cou-6-labeled liposomes with or without FSH modifications (Cou-6-Lipo and Cou-6-Lipo@FSH) were prepared. A2780 cells, HepG2 cells, and C26 cells (1.5 × 10
5 cells/well) were seeded on circular glass slides in 12-well plates. After incubation at 37°C for 24 h, the cells were treated with free Cou-6, Cou-6-Lipo, or Cou-6-Lipo@FSH (Cou-6, 5 µg/mL) for 4 h. After washing three times with cold PBS buffer, the cells were fixed with 4% paraformaldehyde. Cells were stained with DAPI to visualize the cell nuclei. Finally, cells were imaged with a confocal laser scanning microscope (Carl Zeiss LSM 710; Carl Zeiss Microscope, Jena, Germany).
Free Cou-6, Cou-6-Lipo, and Cou-6-Lipo@FSH uptake efficiencies in A2780 cells were quantified using a flow cytometer (Becton Dickinson, Franklin Lake, NJ, USA). A2780 cells were seeded in a 12-well plates (1.5 × 105 cells/well). After 24 h, the cells were treated with free Cou-6, Cou-6-Lipo, or Cou-6-Lipo@FSH (Cou-6, 1 µg/mL) in serum-free culture medium at 37°C for 4 h. Cells were washed three times with cold PBS, treated with 0.25% trypsin, centrifuged, and resuspended in 0.5 mL PBS. The fluorescence intensity of the cells was measured by flow cytometry.
2.8. ROS Generation
To measure ROS production, A2780 cells (1.5 × 105 cells/well) were seeded into 12-well plates and cultured for 24 h at 37°C and 5% CO2. After washing three times with cold PBS, the cells were treated with Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo, SN38/IR820-Lipo@FSH (SN38: 1 µg/mL; IR820: 1 µg/mL), or physiological saline (control) for 4 h. The cells were treated with 1 mL of the ROS detection probe DCFH-DA (25 μM) and incubated for 10 min. The photic group was exposed to 5 min of 808 nm laser irradiation (0.5 W/cm2). Cells were treated following the kit instructions, and the fluorescence resulting from ROS production was observed using an inverted fluorescence microscope (CKX41, Olympus, Japan).
2.9. Cell Apoptosis Assay
Cell apoptosis was qualitatively evaluated using Calcein-AM/PI staining. A2780 cells were seeded into 12-well plates and incubated for 24 h. Cells were treated with Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo, or SN38/IR820-Lipo@FSH (SN38 and IR820 both 1 μg/mL) diluted in fresh culture medium. After 4 h of treatment, the cells were irradiated with an 808 nm laser (0.5 W/cm2) for 3 min. After 24 h, Calcein-AM/PI live/dead cell staining was performed. Cell apoptosis was observed and photographed using an inverted fluorescence microscope.
The apoptosis rate of A2780 cells was quantified using an Annexin V-FITC/PI apoptosis detection kit. A2780 cells (1.5 × 105 cells/well) were inoculated in 6-well plates and cultured for 24 h. Cells were treated as previously described, and irradiated for 3 min using an 808 nm laser (0.5 W/cm2). After 24 h, the cells were treated with 0.25% EDTA-free trypsin, washed three times with cold PBS, centrifuged, and resuspended in binding buffer. Annexin V-FITC/PI apoptosis detection was performed according to the manufacturer's protocol. Cells were immediately analyzed using flow cytometry.
2.10. Wound healing Assay
The migratory ability of tumor cells after treatment with different formulations were evaluated. A2780 cells were plated in 12-well plates and cultured until they formed a monolayer. A scratch wound was created in the middle of the well using a sterile 200-μL pipette tip. The cells were washed with PBS to remove detached cells. The cells were treated with free Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH (Laser +), SN38/IR820-Lipo (Laser +) or SN38/IR820-Lipo@FSH (Laser +) (SN38 and IR820 both 0.2 μg/mL). Fresh medium was used as control. Migrating cells in the wound area were observed and imaged at 0 and 24 h using an inverted light microscope (Olympus, Hamburg, Germany).
2.11. Cell Viability Assay
The cytotoxic effects of the blank vectors on A2780 cells were measured using the CCK-8 assay. A2780 cells (4 × 103 cells/well) were seeded in a 96-well plates. After 24 h, the cells were treated with 0.001–50 μg/mL Blank-Lipo in fresh media for 24 or 48 h. After replacing the micelle with CCK-8 solution, the cells were incubated for 3 h at 37°C. The optical density was measured at 450 nm using a Synergy H1 Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). Cells treated with culture media only were used as the control. Each group consisted of three parallel samples.
The inhibitory effects of SN38/IR820-Lipo and SN38/IR820-Lipo@FSH on A2780 cell proliferation were determined using the CCK-8 assay. A2780 cells (4 × 103 cells/well) were seeded into 96-well plates. After 24 h, cells were treated with SN38/IR820-Lipo and SN38/IR820-Lipo@FSH at SN38 concentrations of 0.001, 0.01, 0.1, 1, 10, and 50 μg/mL for 2 h. Control and Blank groups were included in the analysis. Cells were irradiated with an 808 nm laser (0.5 W/cm2) for 3 min per well, and the cells were incubated at 37°C for another 24 or 48 h. CCK-8 reagent was added to each well, and the optical density was measured at 450 nm. Cell viability (%) was calculated as follows:
Cell viability (%) = [(Atest − Ablank) / (Acontrol − Ablank)] ×100%
2.12. In vivo Imaging
BALB/c Nude mice were injected in the right flank with 200 μL of A2780 cells (1 × 108 cells/mL). After tumor volumes reached 100 mm3, mice were randomly divided into three groups (n = 3/group). Free DIR, DIR-loaded liposomes (DIR-Lipo), or DIR-loaded liposomes with FSH (DIR-Lipo@FSH) were injected intravenously at a dose of 0.1 mg/kg of DIR. Mice were anesthetized and imaged using an in vivo imaging system (Caliper Life Sciences Inc., Mountain View, CA, USA) 1, 4, 8, and 24 h after injection. Hearts, livers, spleens, lungs, kidneys, and tumor tissues were collected from the mice 24 h after injection to assess the distribution of the different formulations in the major organs. Regions of interest were quantitatively analyzed using Living Image software (Version 4.3.1; Caliper Life Sciences Inc.).
2.13. In Vivo US-induced ROS generation
To evaluate US-induced ROS generation at the tumor sites, the tumor-bearing BALB/c nude mice (n = 3/group) were intravenously injected with PBS, Free IR820, SN38/IR820-Lipo, and SN38/IR820-Lipo@FSH (IR820 dose of 10 mg/kg). After 24 h, the mice were anesthetized with chloralic hydras and injected with 50 µg DCFH-DA intra-tumorally. After 10 min, the tumor sites of the mice were irradiated (808 nm, 0.5 W/cm2) for 10 min and then, the mice were sacrificed. The tumors were collected and flash frozen, conterstained with DAPI, and observed by CLSM.
2.14. Antitumor Efficacy and Safety Assessment
BALB/c nude mice were injected in the right flank with 200 μL of A2780 cell suspension (1 × 108 cells/mL). After the tumor volumes reached 100 mm3, mice were randomly divided into six groups (n = 4/group). Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo, SN38/IR820-Lipo@FSH (SN38, 10 mg/kg; IR820, 10 mg/kg), or saline (Negative Control) were injected intravenously. All the formulations were given to mice via the tail vein every 4 days for five times. Mice in the SN38/IR820-Lipo@FSH group were subjected to 808 nm laser irradiation (0.5 W/cm2) for 10 min on the second day after the injection. The irradiation treatment was repeated once every three days for a total of five treatments. Body weights and tumor dimensions (measured with a caliper) were recorded after each treatment.
Three days after the final treatment, mice were euthanized by cervical dislocation after blood collection from the orbital sinus. Hearts, livers, spleens, lungs, and kidneys were collected, fixed with 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin for histopathological analysis. Apoptotic cells were detected in the tumor tissues using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). Lung tissues were fixed in Bouin's solution for 24 h and immersed in 50% ethanol for 2 h to detect tumor lung metastases. Blood samples were centrifuged at 3000 rpm at 4°C for 10 min to collect serum for measuring blood urea nitrogen, creatinine, aspartate transaminase, and alanine transaminase levels.
2.15. Statistical Analysis
All data are presented as the means ± standard deviations. GraphPad Prism software was used for data analysis. Groups were compared using two-tailed Student’s t-tests or one-way analysis of variance. Differences between groups were considered statistically significant at *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 1.
Preparation of SN38/IR820-Lipo@FSH and synergistic effects of chemotherapy and phototherapy in ovarian cancer.
Figure 1.
Preparation of SN38/IR820-Lipo@FSH and synergistic effects of chemotherapy and phototherapy in ovarian cancer.
Figure 2.
(a) Synthesis route of DSPE-PEG2000-FSH; (b) 1H NMR spectra of DSPE-PEG2000-Mal and DSPE-PEG2000-FSH; (c). (d) MALDI-TOF-MS spectra of DSPE-PEG2000-FSH.
Figure 2.
(a) Synthesis route of DSPE-PEG2000-FSH; (b) 1H NMR spectra of DSPE-PEG2000-Mal and DSPE-PEG2000-FSH; (c). (d) MALDI-TOF-MS spectra of DSPE-PEG2000-FSH.
Figure 3.
Characterization of Blank Lipo and SN38/IR820-Lipo@FSH. (a). (b) The average size and zeta of Blank Lipo and TEM images of Blank Lipo (scale bar: 100 μm); (c). (d) The average size and zeta of SN38/IR820-Lipo@FSH and TEM images of SN38/IR820-Lipo@FSH (scale bar: 100 μm); (e). (f) Stability of SN38/IR820-Lipo@FSH in 4 ◦C within 7 days; (g) UV/VIS absorption spectra; (i). (j) The drug release behavior of SN38/IR820-Lipo@FSH within 12 h.
Figure 3.
Characterization of Blank Lipo and SN38/IR820-Lipo@FSH. (a). (b) The average size and zeta of Blank Lipo and TEM images of Blank Lipo (scale bar: 100 μm); (c). (d) The average size and zeta of SN38/IR820-Lipo@FSH and TEM images of SN38/IR820-Lipo@FSH (scale bar: 100 μm); (e). (f) Stability of SN38/IR820-Lipo@FSH in 4 ◦C within 7 days; (g) UV/VIS absorption spectra; (i). (j) The drug release behavior of SN38/IR820-Lipo@FSH within 12 h.
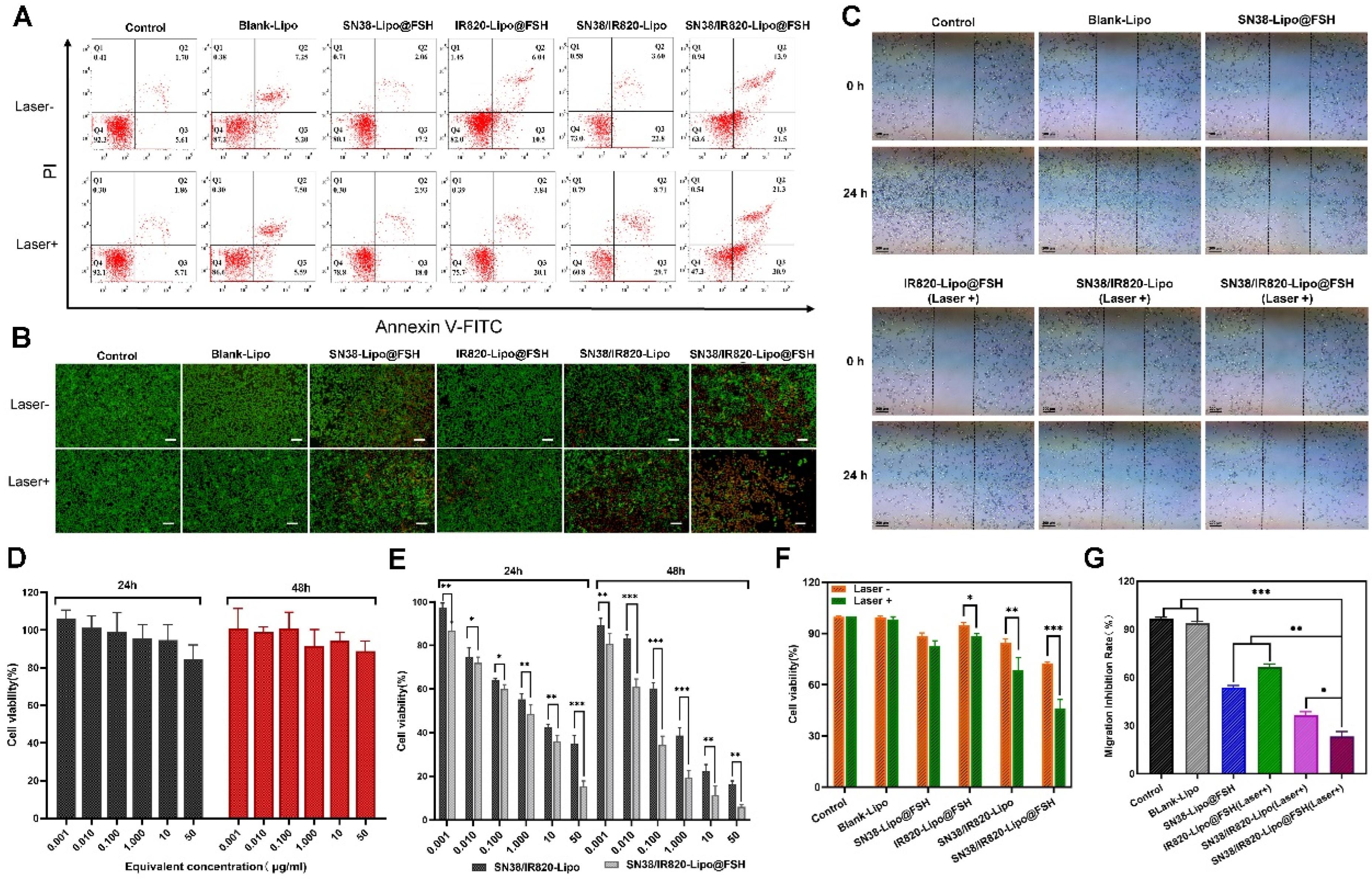
Figure 4.
Cellular uptake of SN38/IR820-Lipo@FSH. (a) Confocal images of cellular uptake in A2780, HepG2 and C26 cells (Scale bar: 50 μm); (b) Analysis of cellular uptake of the control, free Cou-6, Cou-6-Lipo and Cou-6-Lipo@FSH by flow cytometry and mean fluorescence intensity; (c). (d) Inverted fluorescence microscope image of ROS (Scale bar: 200 μm). Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
Cellular uptake of SN38/IR820-Lipo@FSH. (a) Confocal images of cellular uptake in A2780, HepG2 and C26 cells (Scale bar: 50 μm); (b) Analysis of cellular uptake of the control, free Cou-6, Cou-6-Lipo and Cou-6-Lipo@FSH by flow cytometry and mean fluorescence intensity; (c). (d) Inverted fluorescence microscope image of ROS (Scale bar: 200 μm). Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
(a). (f) The flow cytometry results of A2780 cell apoptosis and the percentage of early and late apoptosis after the 48 h treatment and near-infrared laser irradiation with Control, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN/IR-Lipo and SN/IR-Lipo@FSH; (b). (g) Live/dead examination of A2780 cells subjected to different treatments was conducted using calcein-acetoxymethyl ester/propidium iodide (Calcein-AM/PI) double staining and observing by inverted fluorescence microscope. (Scale bar: 200 μm); (c) Wound healing Assay; (c) Wound healing Assay; (d) In vitro cytotoxicity of Blank-Lipo on A2780 cells; (e) Inhibitory capacity of SN38/IR820-Lipo and SN38/IR820-Lipo@FSH against A2780 cells proliferation. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
(a). (f) The flow cytometry results of A2780 cell apoptosis and the percentage of early and late apoptosis after the 48 h treatment and near-infrared laser irradiation with Control, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN/IR-Lipo and SN/IR-Lipo@FSH; (b). (g) Live/dead examination of A2780 cells subjected to different treatments was conducted using calcein-acetoxymethyl ester/propidium iodide (Calcein-AM/PI) double staining and observing by inverted fluorescence microscope. (Scale bar: 200 μm); (c) Wound healing Assay; (c) Wound healing Assay; (d) In vitro cytotoxicity of Blank-Lipo on A2780 cells; (e) Inhibitory capacity of SN38/IR820-Lipo and SN38/IR820-Lipo@FSH against A2780 cells proliferation. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
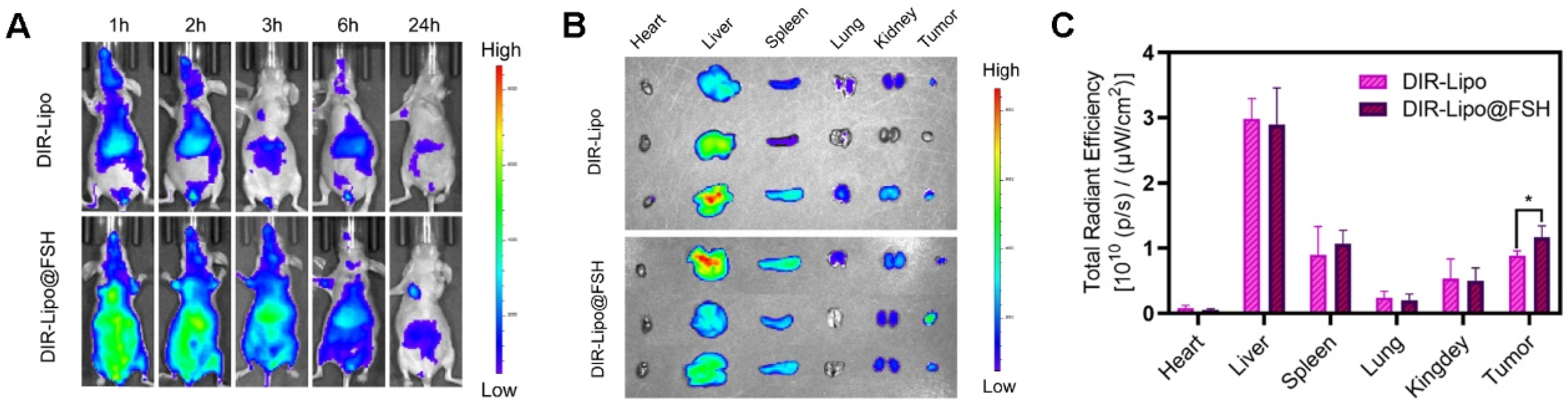
Figure 6.
Biodistribution of SN38/IR820-Lipo@FSH in vivo. (a) In vivo fluorescence images of A2780-bearing BALB/c Nude mice treated with DIR-Lipo and DiR-Lipo@FSH. Images were taken at 1, 2, 3, 6, and 24 h after injection; (b) The fluorescence images of the excised tumors and major organs at 24 h after injection; (c) The quantitative ROI analysis of the excised tumors and major organs at 24 h after injection. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6.
Biodistribution of SN38/IR820-Lipo@FSH in vivo. (a) In vivo fluorescence images of A2780-bearing BALB/c Nude mice treated with DIR-Lipo and DiR-Lipo@FSH. Images were taken at 1, 2, 3, 6, and 24 h after injection; (b) The fluorescence images of the excised tumors and major organs at 24 h after injection; (c) The quantitative ROI analysis of the excised tumors and major organs at 24 h after injection. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
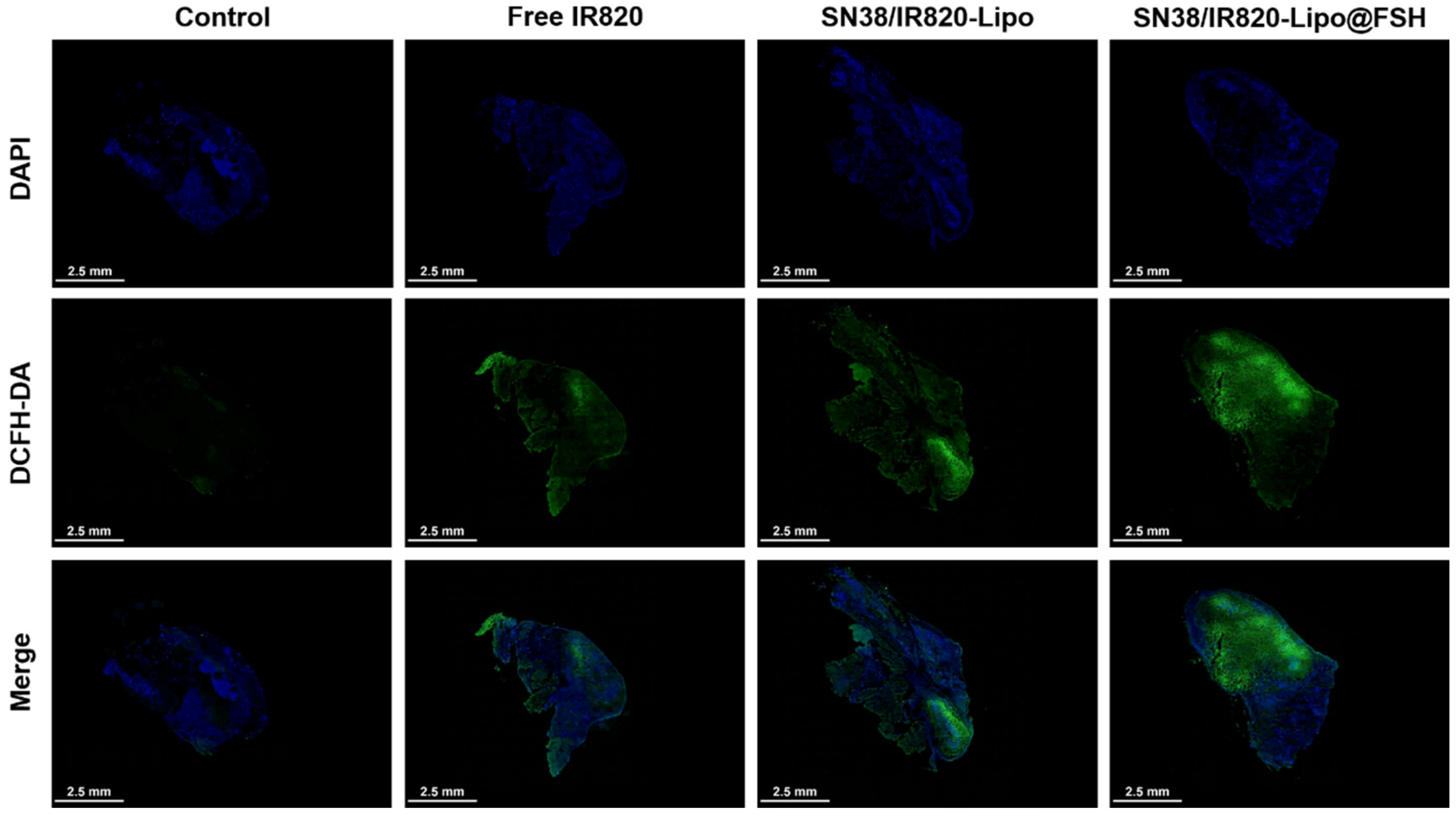
Figure 7.
In vivo ROS generation using CLSM (Scale bar: 2.5 mm).
Figure 7.
In vivo ROS generation using CLSM (Scale bar: 2.5 mm).
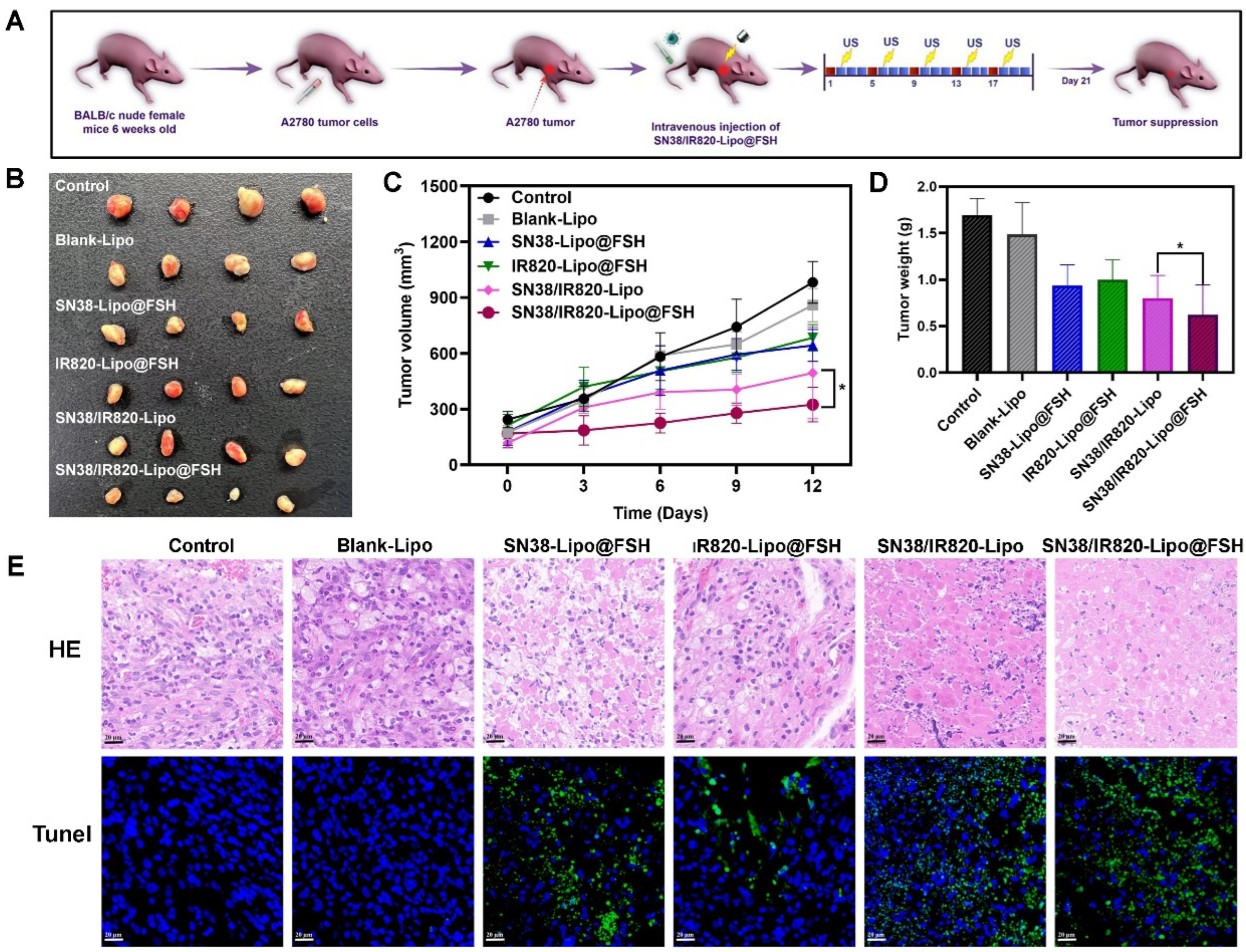
Figure 8.
Antitumor Evaluation of SN38/IR820-Lipo@FSH in vivo. The A2780-bearing BALB/c Nude mice were treated with Saline, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo and SN38/IR820-Lipo@FSH. (a) Experimental procedure; (b) Image of isolated tumor tissues of mice; (c) Tumor volume of mice; (d) Weight of isolated tumors; (e) H&E and TUNEL assay of tumor tissues isolated from mice (Scale bar: 50 μm). Data are shown as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 8.
Antitumor Evaluation of SN38/IR820-Lipo@FSH in vivo. The A2780-bearing BALB/c Nude mice were treated with Saline, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo and SN38/IR820-Lipo@FSH. (a) Experimental procedure; (b) Image of isolated tumor tissues of mice; (c) Tumor volume of mice; (d) Weight of isolated tumors; (e) H&E and TUNEL assay of tumor tissues isolated from mice (Scale bar: 50 μm). Data are shown as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
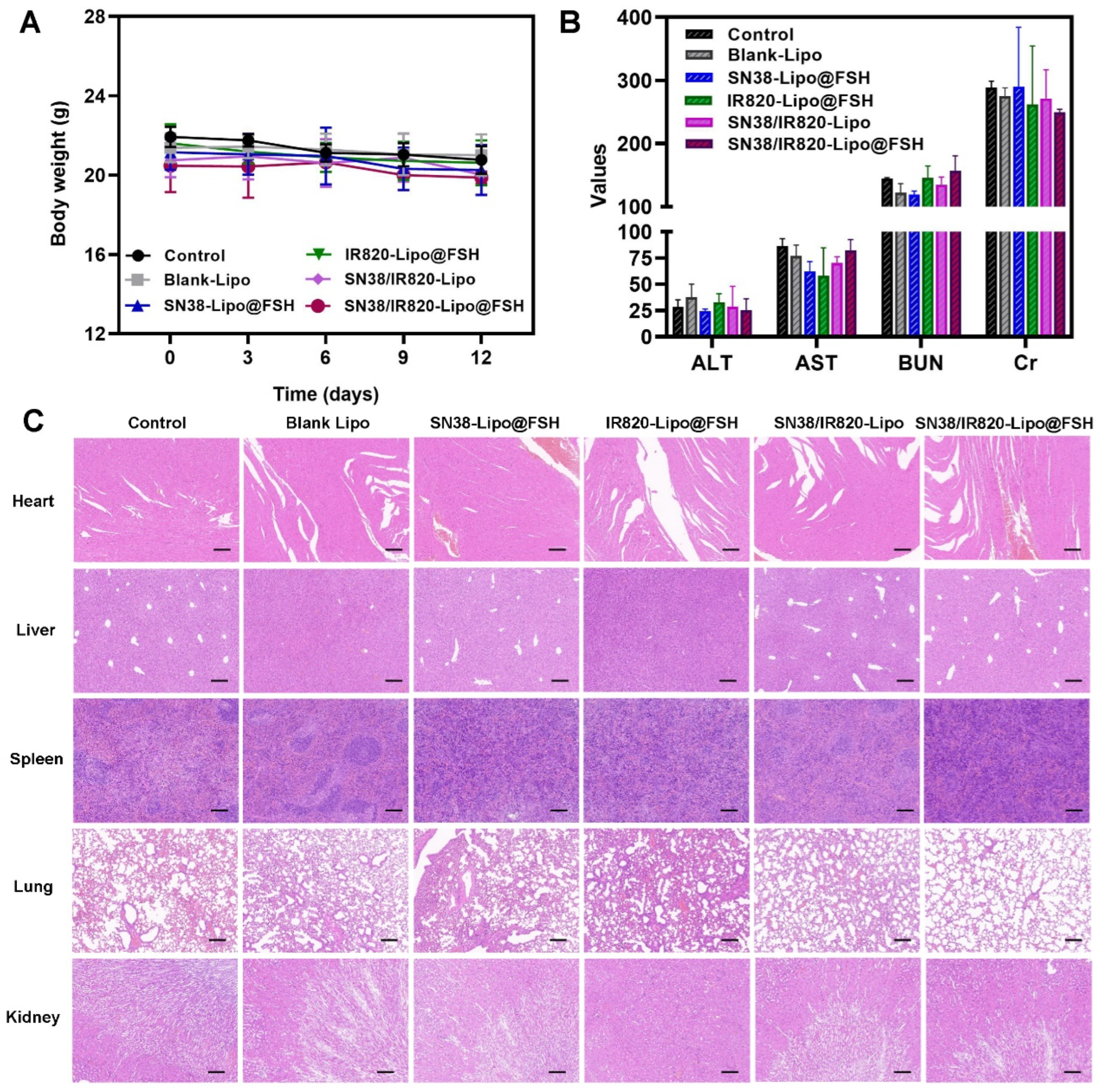
Figure 9.
Anti-metastasis evaluation of SN38/IR820-Lipo@FSH in vivo. The A2780-bearing BALB/c Nude mice were treated with Saline, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo and SN38/IR820-Lipo@FSH. (a) Body weight of mice; (b) ALT, AST, BUN and Cr from the bloodsamples isolated from mice; (c) H&E staining of tumor and organs isolated from mice (Scale bar: 200 μm). Data are shown as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 9.
Anti-metastasis evaluation of SN38/IR820-Lipo@FSH in vivo. The A2780-bearing BALB/c Nude mice were treated with Saline, Blank-Lipo, SN38-Lipo@FSH, IR820-Lipo@FSH, SN38/IR820-Lipo and SN38/IR820-Lipo@FSH. (a) Body weight of mice; (b) ALT, AST, BUN and Cr from the bloodsamples isolated from mice; (c) H&E staining of tumor and organs isolated from mice (Scale bar: 200 μm). Data are shown as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.