Submitted:
05 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction to Pediatric Oncology and Cardiotoxicity
1.1. Pharmaceutical Development of Natural Anti-Tumor Substances
1.2. Investigating Cardiotoxicity in Pediatric Oncology
2. General Aspects of Anthracyclines and Cardiotoxicity
2.1. Cardiotoxicity due to Irradiation
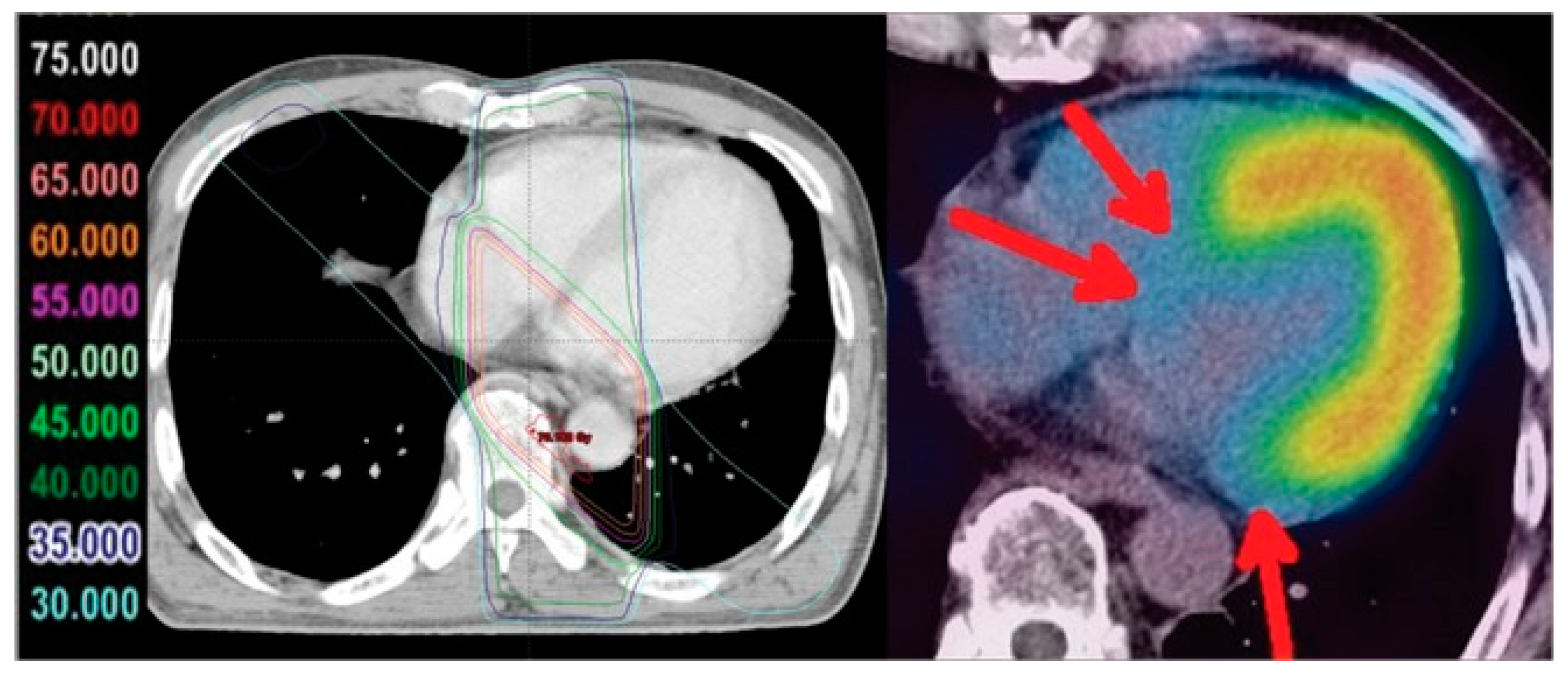
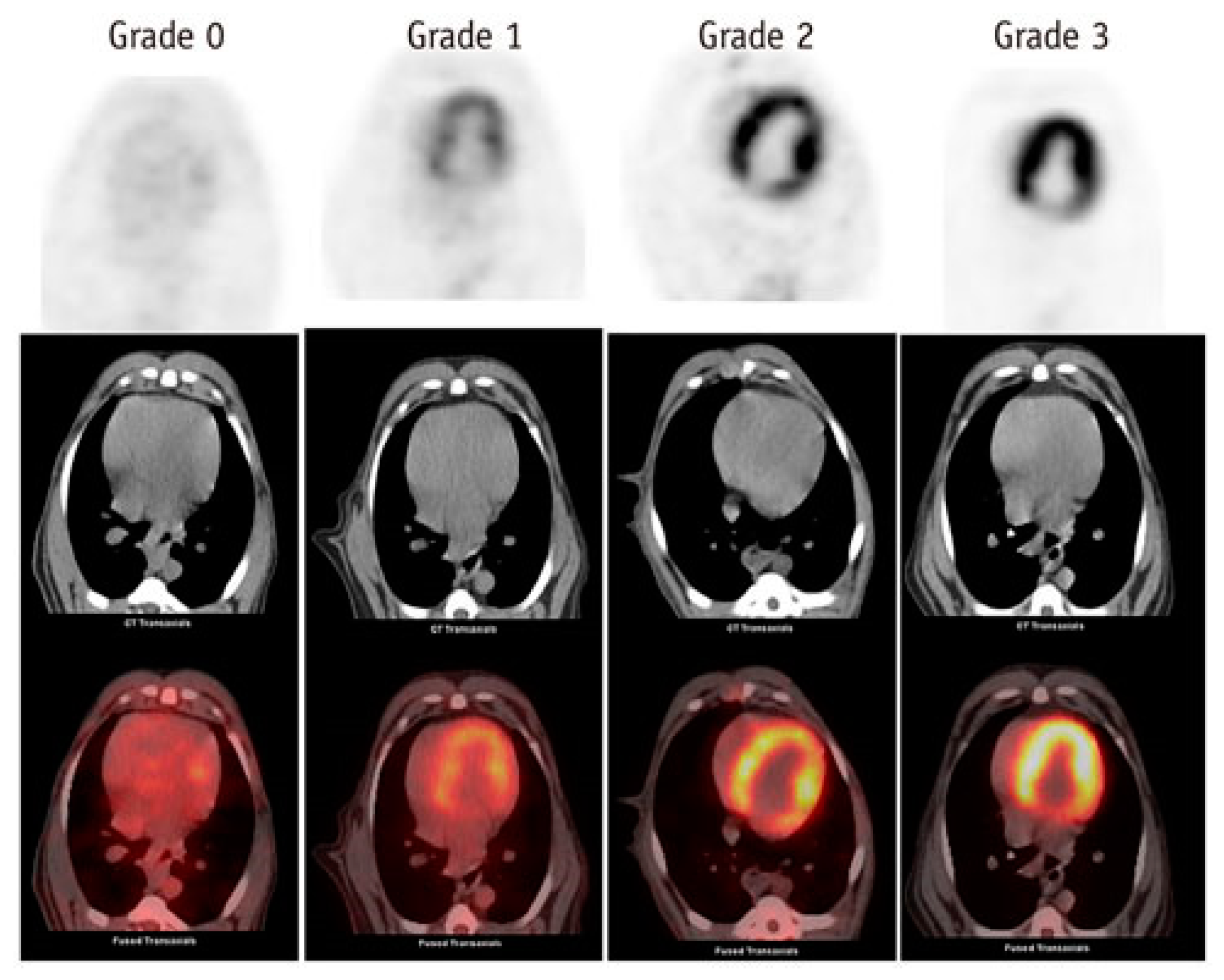
2.2. The Use of Nuclear Medicine Diagnostic Imaging to Visualize Cardiac Metabolism and Cardiotoxicity
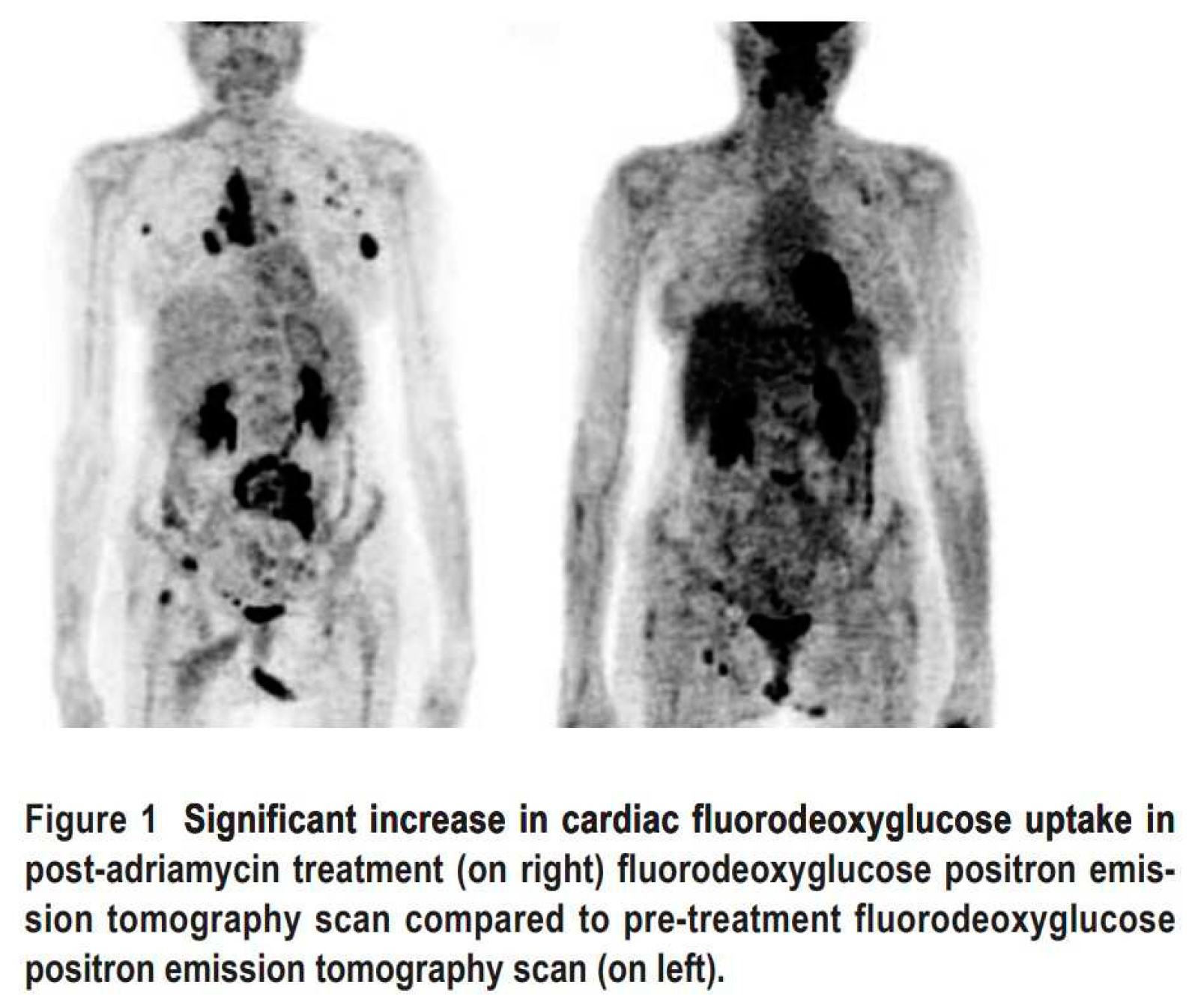
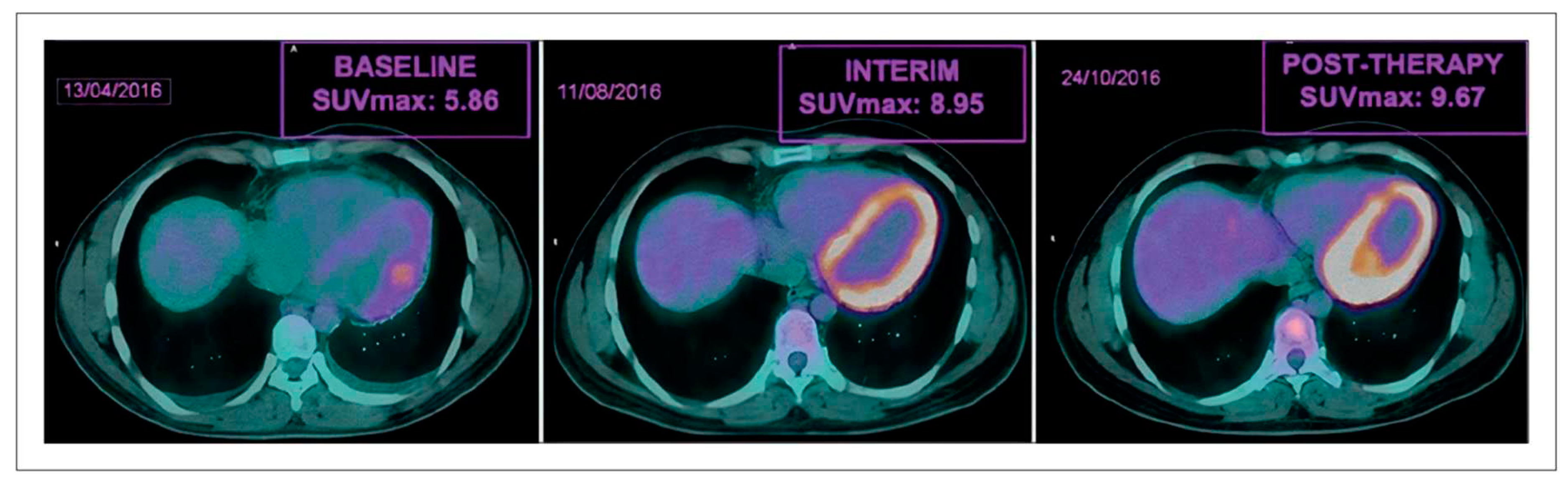
2.3. Nuclear Medicine Diagnostic Imaging of Cardiotoxicity Using 18F-FDG
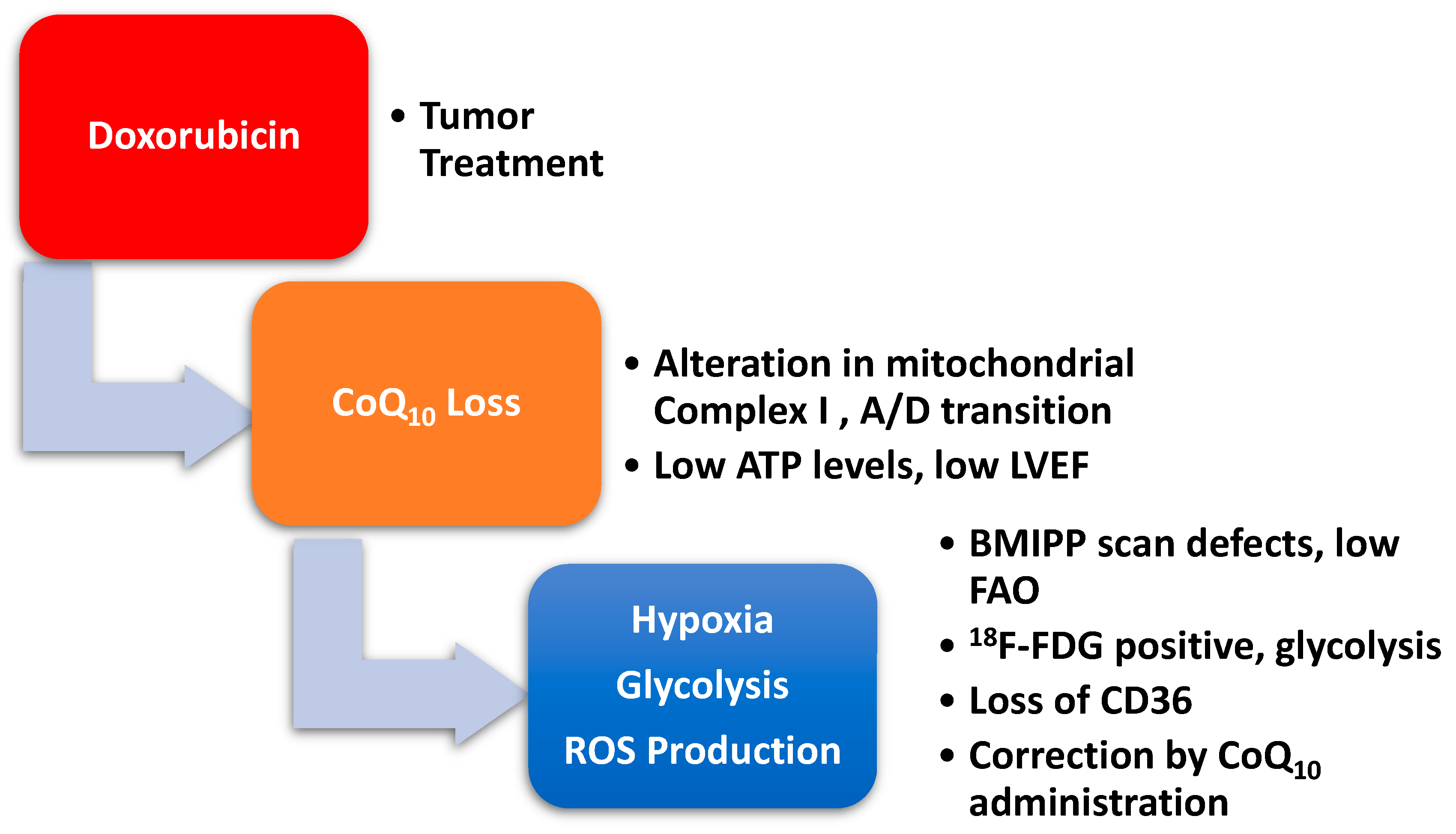
2.4. Doxorubicin-Induced Mitochondrial Damage and Reduced Cardiac Function
2.5. Looking beyond Oncology: Postpartum Cardiomyopathy and Cardiotoxicity. The Role of Selenium and BMIPP



2.6. Connecting Heat Stress to Cardiovascular Disease and PPCM
2.7. Gonadotoxicity
3. Discussion and Conclusions
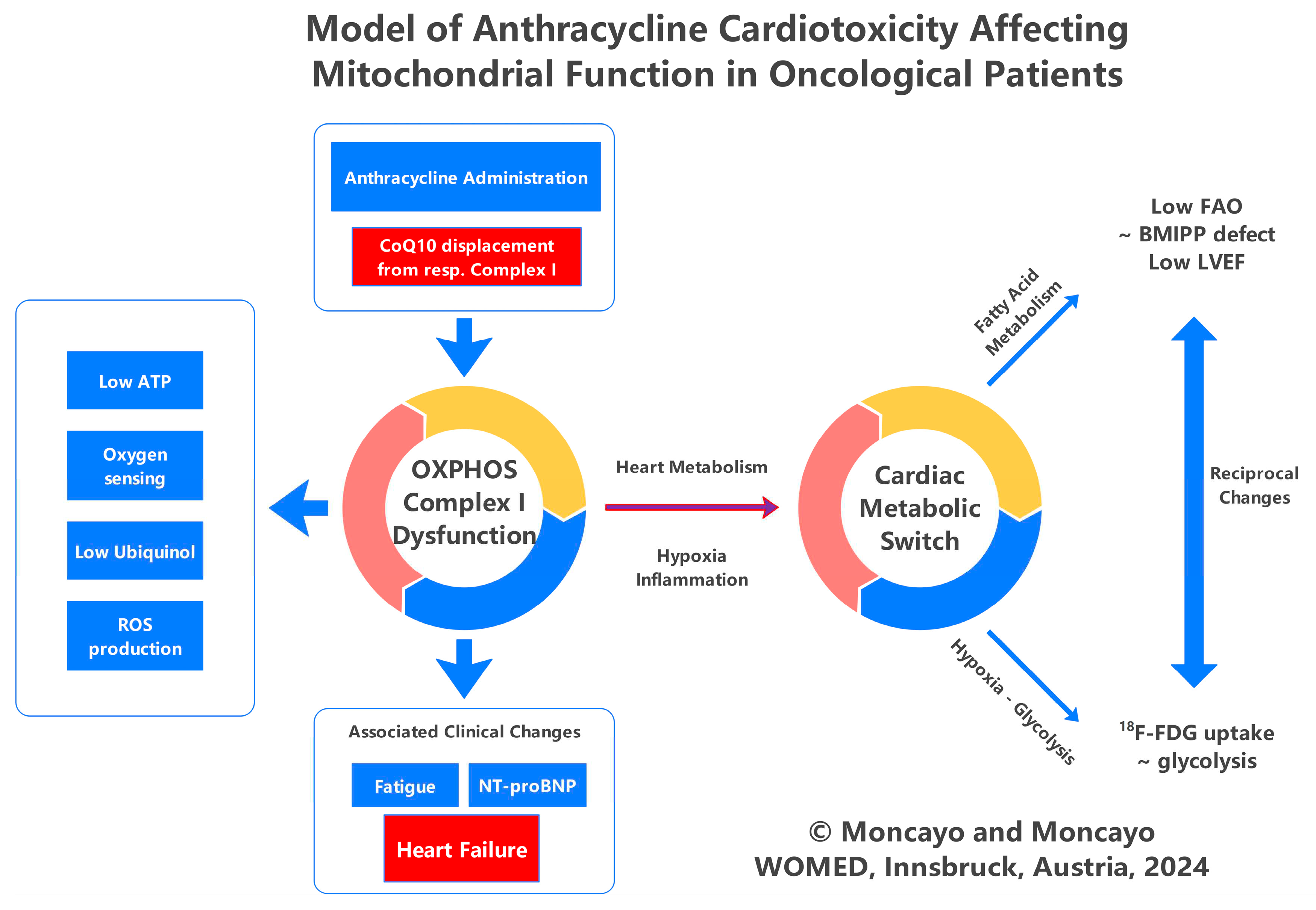
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearson, H.A. History of pediatric hematology oncology. Pediatr Res 2002, 52, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D. Una nuova frontiera: la cardioncologia. Cardiologia 1996, 41, 887–891. [Google Scholar] [PubMed]
- Beutner, R. The cardiac toxicity of injectable local anesthetics. Fed Proc 1946, 5, 166. [Google Scholar] [PubMed]
- Butany, J.; Ahn, E.; Luk, A. Drug-related cardiac pathology. J Clin Pathol 2009, 62, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L., 2nd; O'Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e32–69. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, M.T.; van der Graaf, W.T.; van Veldhuisen, D.J.; Gietema, J.A.; de Vries, E.G.; Sleijfer, D.T. Detection of anthracycline-induced cardiotoxicity. Cancer treatment reviews 1999, 25, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Altena, R.; Perik, P.J.; van Veldhuisen, D.J.; de Vries, E.G.; Gietema, J.A. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol 2009, 10, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Altena, R.; Hubbert, L.; Kiani, N.A.; Wengström, Y.; Bergh, J.; Hedayati, E. Evidence-based prediction and prevention of cardiovascular morbidity in adults treated for cancer. Cardiooncology 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Rodolico, A.; Galdieri, A.; Russo, M.; Campi, G.; Franco, R.; Bruno, D.; Aran, L.; Carannante, A.; Attanasio, U.; et al. Heart Failure and Cancer: Mechanisms of Old and New Cardiotoxic Drugs in Cancer Patients. Card Fail Rev 2019, 5, 112–118. [Google Scholar] [CrossRef]
- Thomas, S.A. Chemotherapy Agents That Cause Cardiotoxicity. US Pharm 2017, 42, HS24–HS33. [Google Scholar]
- Cardinale, D.; Mills, N.L.; Mueller, C. Cardiac biomarkers in the field of cardio-oncology. Eur Heart J Acute Cardiovasc Care 2022, 11, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, K.M.; Mitsis, A.; Grigoriadis, G.; Karanasiou, G.; Lakkas, L.; Mauri, D.; Toli, M.A.; Alexandraki, A.; Keramida, K.; Cardinale, D.; Fotiadis, D.I. Risk Stratification for Cardiotoxicity in Breast Cancer Patients: Predicting Early Decline of LVEF After Treatment(). Annu Int Conf IEEE Eng Med Biol Soc 2023, 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020, 41, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Gavila, J.; Seguí, M.; Calvo, L.; López, T.; Alonso, J.J.; Farto, M.; Sánchez-de la Rosa, R. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol 2017, 19, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Belger, C.; Abrahams, C.; Imamdin, A.; Lecour, S. Doxorubicin-induced cardiotoxicity and risk factors. Int J Cardiol Heart Vasc 2024, 50, 101332. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.R.; Beasley, G.S.; Goldberg, J.F.; Absi, M.; Ryan, K.A.; Guerrier, K.; Joshi, V.M.; Johnson, J.N.; Morin, C.E.; Hurley, C.; et al. Pediatric Cardio-Oncology Medicine: A New Approach in Cardiovascular Care. Children (Basel) 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L. Cancer Survivorship. N Engl J Med 2018, 379, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial function as a therapeutic target in heart failure. Nature reviews. Cardiology 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Littarru, G.P.; Ho, L.; Runge, T.M.; Havanonda, S.; Cooley, D. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int Z Vitaminforsch 1970, 40, 380–390. [Google Scholar] [PubMed]
- Cassinelli, G. The roots of modern oncology: from discovery of new antitumor anthracyclines to their clinical use. Tumori 2016, 2016, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dimarco, A.; Gaetani, M.; Orezzi, P.; Scarpinato, B.M.; Silvestrini, R.; Soldati, M.; Dasdia, T.; Valentini, L. 'Daunomycin', A new antibiotic of the rhodomycin group. Nature 1964, 201, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Dubost, M.; Ganter, P.; Maral, R.; Ninet, L.; Pinnert, S.; Preudhomme, J.; Werner, G.H. Rubidomycin: A New Antibiotic with Cytostatic Properties. Cancer Chemother Rep 1964, 41, 35–36. [Google Scholar]
- Di Marco, A. Attività biologica ed utilizzazione terapeutica dell'antibiotico daunomicina. Tumori 1967, 53, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; Monfardini, S.; De Lena, M.; Fossati-Bellani, F.; Beretta, G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res 1970, 30, 2572–2582. [Google Scholar] [PubMed]
- Arcamone, F.; Cassinelli, G.; di Marco, A.; Gaetani, M. Patent application Farmitalia Research Laboratories 251 NSA. 1967.
- Bonadonna, G.; Monfardini, S.; De Lena, M.; Fossati-Bellani, F. Clinical evaluation of adriamycin, a new antitumour antibiotic. British medical journal 1969, 3, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Tasaka, H.; Yu, K.P.; Murphy, M.L.; Karnofsky, D.A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Cassinelli, G.; Arcamone, F. The discovery of daunorubicin. Cancer Treat Rep 1981, 65 Suppl 4, 3–8. [Google Scholar]
- Waksman, S.A.; Woodruff, H.B. Actinomyces antibioticus, a New Soil Organism Antagonistic to Pathogenic and Non-pathogenic Bacteria. J Bacteriol 1941, 42, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Dalgliesh, C.E.; Todd, A.R. Actinomycin. Nature 1949, 164, 820. [Google Scholar] [CrossRef] [PubMed]
- Thalhimer, W.; Palmer, B. The Bactericidal Action of Quinone and Other Phenol Oxidation Products as Determined by the Rideal-Walker Method. J Infect Dis 1911, 9, 172–180. [Google Scholar] [CrossRef]
- Cooper, E.A. On the Relations of the Phenols and their Derivatives to Proteins. A contribution to our knowledge of the Mechanism of Disinfection: Part III. The Chemical Action of Quinone upon Proteins. The Biochemical journal 1913, 7, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.T.; Cooper, E.A. The Bactericidal Action of the Quinones and Allied Compounds. The Biochemical journal 1921, 15, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Ostenhof, O. Die Biochemie der Chinone. Experientia 1947, 3, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Franceschi, G.; Penco, S.; Pol, C.; Redaelli, S.; Selva, A. Structure and Physicochemical Properties of Adriamycin (Doxorubicin). Berlin, Heidelberg, 1972; pp. 9-22.
- Iwamoto, Y.; Hansen, I.L.; Porter, T.H.; Folkers, K. Inhibition of coenzyme Q10-enzymes, succinoxidase and NADH-oxidase, by adriamycin and other quinones having antitumor activity. Biochem Biophys Res Commun 1974, 58, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Watanabe, T.; Folkers, K. Bioenergetics in clinical medicine: prevention by forms of coenzyme Q of the inhibition by adriamycin of coenzyme Q10-enzymes in mitochondria of the myocardium. Proc Natl Acad Sci U S A 1976, 73, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Abdel-aleem, S.; el-Merzabani, M.M.; Sayed-Ahmed, M.; Taylor, D.A.; Lowe, J.E. Acute and chronic effects of adriamycin on fatty acid oxidation in isolated cardiac myocytes. J Mol Cell Cardiol 1997, 29, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.R.; Hong, Y.M.; Boriack, R.L.; Bennett, M.J. Effect of L-carnitine supplementation on cardiac carnitine palmitoyltransferase activities and plasma carnitine concentrations in adriamycin-treated rats. Pediatr Res 2003, 53, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Doroshow, J.H. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 1986, 261, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H.; Davies, K.J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 1986, 261, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, K.J.; Richel, D.J.; van den Brink, R.B.; Guchelaar, H.J. Cardiotoxicity of cytotoxic drugs. Cancer treatment reviews 2004, 30, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circulation research 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Choe, J.Y.; Combs, A.B. Rescue by coenzyme Q10 from electrocardiographic abnormalities caused by the toxicity of adriamycin in the rat. Proc Natl Acad Sci U S A 1978, 75, 5178–5180. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, E.; McGuire, W. Doxorubicin (adriamycin) cardiomyopathy. West J Med 1983, 139, 332–341. [Google Scholar] [PubMed]
- Ohhara, H.; Kanaide, H.; Nakamura, M. A protective effect of coenzyme Q10 on the adriamycin-induced cardiotoxicity in the isolated perfused rat heart. J Mol Cell Cardiol 1981, 13, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Lubawy, W.C.; Whaley, J.; Hurley, L.H. Coenzyme Q10 or alpha-tocopherol reduce the acute toxicity of anthramycin in mice. Res Commun Chem Pathol Pharmacol 1979, 24, 401–404. [Google Scholar] [PubMed]
- Takimoto, M.; Sakurai, T.; Kodama, K.; Yokoi, H.; Suzuki, Y.; Enomoto, K.; Okada, N. [Protective effect of CoQ 10 administration on cardial toxicity in FAC therapy]. Gan To Kagaku Ryoho 1982, 9, 116–121. [Google Scholar] [PubMed]
- Tsubaki, K.; Horiuchi, A.; Kitani, T.; Taniguchi, N.; Masaoka, T.; Shibata, H.; Yonezawa, T.; Tsubakio, T.; Kawagoe, H.; Shinohara, Y.; et al. [Investigation of the preventive effect of CoQ10 against the side-effects of anthracycline antineoplastic agents]. Gan To Kagaku Ryoho 1984, 11, 1420–1427. [Google Scholar] [PubMed]
- Sarvazyan, N. Visualization of doxorubicin-induced oxidative stress in isolated cardiac myocytes. Am J Physiol 1996, 271, H2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H. Effect of Anticancer Quinones on Reactive Oxygen Production by Adult Rat Heart Myocytes. Oxid Med Cell Longev 2020, 2020, 8877100. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Coenzyme Q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther 2005, 4, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Lempek, M.R.; Branco, S.; Nogueira, M.M.; de Almeida, M.E.; Costa, A.G.; Freitas, T.G.; Rocha, M.; Moreira, M.V.L.; Barreto, T.O.; et al. Coenzyme Q10 Cardioprotective Effects Against Doxorubicin-Induced Cardiotoxicity in Wistar Rat. Cardiovasc Toxicol 2020, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Hudson, M.M. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Caspi, O.; Aronson, D. Surviving Cancer without a Broken Heart. Rambam Maimonides Med J 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, L.M.; Liu, Q.; Yasui, Y.; Arnold, M.A.; Hammond, S.; Howell, R.M.; Smith, S.A.; Weathers, R.E.; Henderson, T.O.; Gibson, T.M.; et al. Temporal Trends in Treatment and Subsequent Neoplasm Risk Among 5-Year Survivors of Childhood Cancer, 1970-2015. JAMA 2017, 317, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Tichelli, A.; Bhatia, S.; Socié, G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. British journal of haematology 2008, 142, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Brice, P.; de Kerviler, E.; Friedberg, J.W. Classical Hodgkin lymphoma. Lancet 2021, 398, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, M.V.; Giusti, F.; Vogelius, I.R.; Lundemann, M.; van der Kaaij, M.A.; Ramadan, S.; Meulemans, B.; Henry-Amar, M.; Aleman, B.M.; Raemaekers, J.; et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2015, 2, e492–502. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Bradley, J.A.; Ng, A.K.; Samson, P.; Robinson, C.; Lopez-Mattei, J.; Mitchell, J.D. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncol 2021, 3, 343–359. [Google Scholar] [CrossRef]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Bristow, M.R.; Glatstein, E.; Mason, J.W.; Masek, M.A.; Daniels, J.R. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol 1977, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.E.; Ludwig, R.; Geiger, H. Assessment of adriamycin cardiotoxicity in children by systolic time intervals. Eur J Pediatr 1979, 131, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, W.; Robinson, E.; Peled, B.; Kaufman, S.; Carter, A. Early detection of doxorubicin cardiotoxicity by M-mode echocardiography. Cancer Chemother Pharmacol 1980, 5, 119–125. [Google Scholar] [CrossRef]
- Ritchie, J.L.; Singer, J.W.; Thorning, D.; Sorensen, S.G.; Hamilton, G.W. Anthracycline cardiotoxicity: clinical and pathologic outcomes assessed by radionuclide ejection fraction. Cancer 1980, 46, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, R.; Uusitupa, M.; Kuikka, J.; Länsimies, E. Non-invasive evaluation of anthracycline-induced cardiotoxicity in man. Acta Med Scand 1982, 212, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.B.; Crouse, V.L.; Evans, W.; Takahashi, M.; Siegel, S.E. Recovery of left ventricular function following discontinuation of anthracycline chemotherapy in children. Pediatrics 1981, 68, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Kumar, R.; Bhatnagar, V.; Bajpai, M.; Gupta, D.K.; Mitra, D.K. High incidence of adriamycin cardiotoxicity in children even at low cumulative doses: role of radionuclide cardiac angiography. J Pediatr Surg 2000, 35, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Leerink, J.M.; van der Pal, H.J.H.; Kremer, L.C.M.; Feijen, E.A.M.; Meregalli, P.G.; Pourier, M.S.; Merkx, R.; Bellersen, L.; van Dalen, E.C.; Loonen, J.; et al. Refining the 10-Year Prediction of Left Ventricular Systolic Dysfunction in Long-Term Survivors of Childhood Cancer. JACC CardioOncol 2021, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, B.L.; Mitchell, D.S.; Ungar, D.R.; Prakash, D.; Dole, M.G.; Castle, V.P.; Hernandez, R.J.; Koeppe, R.A.; Hutchinson, R.J. Neoplasms in a pediatric population: 2-[F-18]-fluoro-2-deoxy-D-glucose PET studies. Radiology 1995, 194, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Connolly, L.P.; Fahey, F.H.; Shulkin, B.L. PET and PET/CT in pediatric oncology. Semin Nucl Med 2007, 37, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Vince, D.J. Medical radiation to children with congenital heart disease. Can Med Assoc J 1964, 91, 1345–1349. [Google Scholar] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010, 76, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Yu, X.; Prosnitz, R.G.; Zhou, S.M.; Hardenbergh, P.H.; Blazing, M.; Hollis, D.; Lind, P.; Tisch, A.; Wong, T.Z.; Borges-Neto, S. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005, 63, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Boerma, M.; Hauer-Jensen, M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract 2010, 2011. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.H.; Marmagkiolis, K.; Balanescu, D.V.; Hakeem, A.; Donisan, T.; Finch, W.; Virmani, R.; Herrman, J.; Cilingiroglu, M.; Grines, C.L.; et al. Radiation-Induced Vascular Disease-A State-of-the-Art Review. Front Cardiovasc Med 2021, 8, 652761. [Google Scholar] [CrossRef] [PubMed]
- Tapio, S. Pathology and biology of radiation-induced cardiac disease. J Radiat Res 2016, 57, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, O.; Subramanian, V.; Sievert, W.; Merl-Pham, J.; Oleksenko, K.; Rosemann, M.; Multhoff, G.; Atkinson, M.J.; Tapio, S. Activation of PPARα by Fenofibrate Attenuates the Effect of Local Heart High Dose Irradiation on the Mouse Cardiac Proteome. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Peters, J.M.; Gonzalez, F.J.; Schedin, S.; Dallner, G. Influence of peroxisome proliferator-activated receptor alpha on ubiquinone biosynthesis. J Mol Biol 2000, 297, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Djujic, I.S.; Jozanov-Stankov, O.; Mandic, M.; Demajo, M.; Vrvic, M.M. Selenium content and distribution in rat tissues irradiated with gamma rays. Biological trace element research 1992, 33, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Knapp, F.F., Jr.; Goodman, M.M.; Callahan, A.P.; Kirsch, G. Radioiodinated 15-(p-iodophenyl)-3,3-dimethylpentadecanoic acid: a useful new agent to evaluate myocardial fatty acid uptake. J Nucl Med 1986, 27, 521–531. [Google Scholar] [PubMed]
- Ogata, M. [Myocardial uptake of 125I-BMIPP in rats treated with adriamycin]. Kaku Igaku 1989, 26, 69–76. [Google Scholar] [PubMed]
- Wakasugi, S.; Fischman, A.J.; Babich, J.W.; Callahan, R.J.; Elmaleh, D.R.; Wilkinson, R.; Strauss, H.W. Myocardial substrate utilization and left ventricular function in adriamycin cardiomyopathy. J Nucl Med 1993, 34, 1529–1535. [Google Scholar] [PubMed]
- Niitsu, N.; Yamazaki, J.; Umeda, M. [Clinical usefulness of 123I-BMIPP (beta-methyl iodophenyl pentadecanoic (acid) myocardial SPECT in patients with hematological malignancies with adriamycin-induced cardiomyopathy]. Gan To Kagaku Ryoho 1996, 23, 1793–1797. [Google Scholar] [PubMed]
- Piwnica-Worms, D.; Chiu, M.L.; Kronauge, J.F. Detection of adriamycin-induced cardiotoxicity in cultured heart cells with technetium 99m-SESTAMIBI. Cancer Chemother Pharmacol 1993, 32, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Inubushi, M.; Tadamura, E.; Kudoh, T.; Hattori, M.; Kubo, S.; Koshiji, T.; Nishimura, K.; Komeda, M.; Tamaki, N.; Konishi, J. Simultaneous assessment of myocardial free fatty acid utilization and left ventricular function using 123I-BMIPP-gated SPECT. J Nucl Med 1999, 40, 1840–1847. [Google Scholar] [PubMed]
- Saito, K.; Takeda, K.; Okamoto, S.; Okamoto, R.; Makino, K.; Tameda, Y.; Nomura, Y.; Maeda, H.; Ichihara, T.; Nakano, T. Detection of doxorubicin cardiotoxicity by using iodine-123 BMIPP early dynamic SPECT: quantitative evaluation of early abnormality of fatty acid metabolism with the Rutland method. J Nucl Cardiol 2000, 7, 553–561. [Google Scholar] [CrossRef]
- Saito, K.; Takeda, K.; Imanaka-Yoshida, K.; Imai, H.; Sekine, T.; Kamikura, Y. Assessment of fatty acid metabolism in taxan-induced myocardial damage with iodine-123 BMIPP SPECT: comparative study with myocardial perfusion, left ventricular function, and histopathological findings. Ann Nucl Med 2003, 17, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Sarocchi, M.; Bauckneht, M.; Arboscello, E.; Capitanio, S.; Marini, C.; Morbelli, S.; Miglino, M.; Congiu, A.G.; Ghigliotti, G.; Balbi, M.; et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med 2018, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Pastorino, F.; Castellani, P.; Cossu, V.; Orengo, A.M.; Piccioli, P.; Emionite, L.; Capitanio, S.; Yosifov, N.; Bruno, S.; et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. J Nucl Cardiol 2019, 27, 2183–2194. [Google Scholar] [CrossRef]
- Umezawa, R.; Takase, K.; Jingu, K.; Takanami, K.; Ota, H.; Kaneta, T.; Takeda, K.; Matsushita, H.; Ariga, H.; Takahashi, S.; Yamada, S. Evaluation of radiation-induced myocardial damage using iodine-123 β-methyl-iodophenyl pentadecanoic acid scintigraphy. J Radiat Res 2013, 54, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Jingu, K.; Nemoto, K.; Kaneta, T.; Takai, Y.; Ichinose, A.; Ogawa, Y.; Yamada, S. [A case of high FDG-uptake into the myocardium after radiotherapy for esophageal cancer]. Nihon Igaku Hoshasen Gakkai Zasshi 2005, 65, 266–269. [Google Scholar] [PubMed]
- Jingu, K.; Nemoto, K.; Kaneta, T.; Oikawa, M.; Ogawa, Y.; Ariga, H.; Takeda, K.; Sakayauchi, T.; Fujimoto, K.; Narazaki, K.; et al. Temporal change in brain natriuretic Peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys 2007, 69, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Song, J.; Wu, Z.; Guo, M.; Liu, J.; Li, J.; Hao, X.; Li, S. Detection of Myocardial Metabolic Abnormalities by 18F-FDG PET/CT and Corresponding Pathological Changes in Beagles with Local Heart Irradiation. Korean J Radiol 2015, 16, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hayashi, D.; Yamazaki, T.; Mizuno, T.; Kanda, Y.; Komuro, I.; Kurabayashi, M.; Yamaoki, K.; Mitani, K.; Hirai, H.; et al. Elevated B-type natriuretic peptide levels after anthracycline administration. Am Heart J 1998, 136, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.T.; Salvatici, M.; Cardinale, D.; Zorzino, L.; Passerini, R.; Lentati, P.; Leon, M.; Civelli, M.; Martinelli, G.; Cipolla, C.M. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clinical chemistry 2005, 51, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Zaucha-Prażmo, A.; Sadurska, E.; Drabko, K.; Kowalczyk, J.R. Can we find a good biochemical marker of early cardiotoxicity in children treated with haematopoietic stem cell transplantation? Contemp Oncol (Pozn) 2016, 20, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kuittinen, T.; Jantunen, E.; Vanninen, E.; Mussalo, H.; Vuolteenaho, O.; Ala-Kopsala, M.; Nousiainen, T.; Hartikainen, J. Cardiac effects within 3 months of BEAC high-dose therapy in non-Hodgkin's lymphoma patients undergoing autologous stem cell transplantation. European journal of haematology 2006, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, B.; Hegde, S.; Tannu, M.; Stacey, R.B.; Kalogeropoulos, A.; Schocken, D.D. Preventing new-onset heart failure: Intervening at stage A. Am J Prev Cardiol 2023, 16, 100609. [Google Scholar] [CrossRef] [PubMed]
- Kouloubinis, A.; Sofroniadou, S.; Panoulas, V.F.; Makaritsis, K.; Revela, I.; Karavolias, G.; Voudris, V.; Adamopoulos, S. The role of TNF-α, Fas/Fas ligand system and NT-proBNP in the early detection of asymptomatic left ventricular dysfunction in cancer patients treated with anthracyclines. Int J Cardiol Heart Vasc 2015, 6, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, L.; Hesham, D.; Abdel Hamid, M.; Youssef, G. The combined role of NT-proBNP and LV-GLS in the detection of early subtle chemotherapy-induced cardiotoxicity in breast cancer female patients. Egypt Heart J 2021, 73, 20. [Google Scholar] [CrossRef] [PubMed]
- Borde, C.; Kand, P.; Basu, S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J Radiol 2012, 4, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Montravers, F.; McNamara, D.; Landman-Parker, J.; Grahek, D.; Kerrou, K.; Younsi, N.; Wioland, M.; Leverger, G.; Talbot, J.N. [18F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging 2002, 29, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Howard, S.C.; McCarville, E.B.; Krasin, M.J.; Kogos, P.G.; Hudson, M.M. 18F-FDG-avid sites mimicking active disease in pediatric Hodgkin's. Pediatr Radiol 2005, 35, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Metser, U.; Avrahami, G.; Dvir, R.; Valdman, D.; Sira, L.B.; Sayar, D.; Burstein, Y.; Toren, A.; Yaniv, I.; Even-Sapir, E. Role of 18F-FDG PET/CT in staging and follow-up of lymphoma in pediatric and young adult patients. J Comput Assist Tomogr 2006, 30, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Jingu, K.; Kaneta, T.; Nemoto, K.; Ichinose, A.; Oikawa, M.; Takai, Y.; Ogawa, Y.; Nakata, E.; Sakayauchi, T.; Takai, K.; et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys 2006, 66, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Gorla, A.K.; Sood, A.; Prakash, G.; Parmar, M.; Mittal, B.R. Substantial Increase in Myocardial FDG Uptake on Interim PET/CT May Be an Early Sign of Adriamycin-Induced Cardiotoxicity. Clin Nucl Med 2016, 41, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M. Doxorubicin effect on myocardial metabolism: a translational 18F-FDG PET/CT approach. 2020. [Google Scholar] [CrossRef]
- Bauckneht, M.; Ferrarazzo, G.; Fiz, F.; Morbelli, S.; Sarocchi, M.; Pastorino, F.; Ghidella, A.; Pomposelli, E.; Miglino, M.; Ameri, P.; et al. Doxorubicin Effect on Myocardial Metabolism as a Prerequisite for Subsequent Development of Cardiac Toxicity: A Translational 18F-FDG PET/CT Observation. J Nucl Med 2017, 58, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Cossu, V.; Miceli, A.; Donegani, M.; Capitanio, S.; Morbelli, S.; Marini, C.; Sambuceti, G. FDG-PET Imaging of Doxorubicin-Induced Cardiotoxicity: a New Window on an Old Problem. Current Cardiovascular Imaging Reports 2019, 12. [Google Scholar] [CrossRef]
- Haider, A.; Bengs, S.; Schade, K.; Wijnen, W.J.; Portmann, A.; Etter, D.; Fröhlich, S.; Warnock, G.I.; Treyer, V.; Burger, I.A.; et al. Myocardial 18F-FDG Uptake Pattern for Cardiovascular Risk Stratification in Patients Undergoing Oncologic PET/CT. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Gherghe, M.; Lazar, A.M.; Mutuleanu, M.D.; Bordea, C.I.; Ionescu, S.; Mihaila, R.I.; Petroiu, C.; Stanciu, A.E. Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact? Cancers (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.L.C.; Dompieri, L.T.; Leitao, G.M.; Mourato, F.A.; Santos, R.G.G.; Almeida Filho, P.J.; Markman Filho, B.; Melo, M.D.T.; Brandao, S.C.S. Aumento de Captação Cardíaca de 18F-FDG Induzida por Quimioterapia em Pacientes com Linfoma: Um Marcador Precoce de Cardiotoxicidade? Arq Bras Cardiol 2022, 118, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.T.; Rezende, M.F. Medicina de Precisão: A Tomografia por Emissão de Pósitrons com 18F-FDG pode Identificar Fenótipos de Cardiotoxicidade? Arq Bras Cardiol 2022, 119, 109–110. [Google Scholar] [CrossRef]
- Cadour, F.; Thuny, F.; Sourdon, J. New Insights in Early Detection of Anticancer Drug-Related Cardiotoxicity Using Perfusion and Metabolic Imaging. Front Cardiovasc Med 2022, 9, 813883. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, M.T.; Inserra, M.C.; Passaniti, G.; Celona, A.; D'Angelo, T.; Romeo, P.; Basile, A. Role of advanced cardiovascular imaging in chemotherapy-induced cardiotoxicity. Heliyon 2023, 9, e15226. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.M.C.; Arruda, G.F.A.; Berenguer, D.R.F.; Buril, R.O.; Cardinale, D.; Brandão, S.C.S. Anthracycline cardiotoxicity: current methods of diagnosis and possible role of (18)F-FDG PET/CT as a new biomarker. Cardiooncology 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. Proof of concept of the WOMED model of benign thyroid disease: Restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin 2015, 3, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. Applying a systems approach to thyroid physiology: looking at the whole with a mitochondrial perspective instead of just TSH values or why we should know more about mitochondria to understand metabolism. BBA Clin 2017, 7, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. Translating results from the WOMED model of benign thyroid disease to a practical approach to treat fatigue in COVID-19 patients based on combined supplementation with magnesium, selenium, and coenzyme Q10: a treatment strategy against fatigue. Cardiol Vasc Res 2020, 4, 1–4. Available online: https://scivisionpub.com/abstract-display.php?id=1443.
- Moncayo, R.; Moncayo, H.; Reisenzahn, J. Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical, and genetic data identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quality control of thyroglobulin. J Transl Genet Genom 2020, 4, 356–383. [Google Scholar] [CrossRef]
- Moncayo, R.; Moncayo, H. Practical Guidelines for Diagnosing and Treating Thyroid Disease Based on the WOMED Metabolic Model of Disease Focusing on Glycolysis and Coenzyme Q10 Deficiency—A Clinical Alternative to the 2021 Retired Clinical Practice Guidelines of the Endocrine Society. Diagnostics 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Liparulo, I.; Bergamini, C.; Bortolus, M.; Calonghi, N.; Gasparre, G.; Kurelac, I.; Masin, L.; Rizzardi, N.; Rugolo, M.; Wang, W.; et al. Coenzyme Q biosynthesis inhibition induces HIF-1α stabilization and metabolic switch toward glycolysis. FEBS J 2021, 288, 1956–1974. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. From the thyroid to the heart. Global Journal of Medical Research 2023, 23, 1–36. [Google Scholar] [CrossRef]
- Davis, L.E.; Brown, C.E. Peripartum heart failure in a patient treated previously with doxorubicin. Obstet Gynecol 1988, 71, 506–508. [Google Scholar]
- Kyvernitakis, A.; Kyvernitakis, I.; Yang, A.; Albert, U.-S.; Schmidt, S.; Arabin, B. Can peripartum cardiomyopathy be caused by chemotherapy and radiation of breast cancer? Case Reports in Perinatal Medicine 2013, 2, 29–32. [Google Scholar] [CrossRef]
- Colović, N.; Seferović, P.; Plećić, M.; Vidović, A.; Suvajdzić, N.; Tomin, D. Peripartum cardiomyopathy in a patient treated for acute myeloid leukemia. Srp Arh Celok Lek 2016, 144, 77–80. [Google Scholar] [CrossRef]
- Chait-Rubinek, L.; Mariani, J.A.; Goroncy, N.; Herschtal, A.; Wheeler, G.C.; Dwyer, M.K.; Seymour, J.F.; Campbell, B.A. A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, J.A.; Francis, S.A.; Sawyer, D.B. Anthracycline and Peripartum Cardiomyopathies. Circulation research 2019, 124, 1633–1646. [Google Scholar] [CrossRef]
- Ritchie, C. Clinical Contributions to the Pathology, Diagnosis, and Treatment of Certain Chronic Diseases of the Heart. Edinb Med Surg J 1849, 72, 325–339. [Google Scholar] [PubMed]
- Porak, C. De l’influence reciproque de la grossesse et del maladies du Coeur [thesis]. Medical Faculty of Paris, France 1880. [Google Scholar]
- Gouley, B.A.; Mcmillan, T.M.; Bellet, S. Idiopathic myocardial degeneration associated with pregnancy and especially the puerperium. Am J Med Sci 1937, 19, 185–199. [Google Scholar] [CrossRef]
- Hull, E.; Hafkesbring, E. Toxic postpartal heart disease. New Orleans Med Surg J 1937, 89, 550–557. [Google Scholar]
- Hull, E.; Hidden, E. Postpartal heart failure. Southern Medical Journal 1938, 31, 265–270. [Google Scholar] [CrossRef]
- Demakis, J.G.; Rahimtoola, S.H. Peripartum cardiomyopathy. Circulation 1971, 44, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.M.; Trevitt, L.; Parry, E.H. Perpartum cardiac failure. An explanation for the observed geographic distribution in Nigeria. Bull World Health Organ 1974, 51, 203–208. [Google Scholar] [PubMed]
- Ezem, B.U.; Otubu, J.A. A complication of a traditional puerperal practice in Nigeria. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 1980, 18, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Jha, A.K. Peripartum cardiomyopathy. Heart Fail Rev 2021, 26, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Cénac, A.; Simonoff, M.; Moretto, P.; Djibo, A. A low plasma selenium is a risk factor for peripartum cardiomyopathy. A comparative study in Sahelian Africa. Int J Cardiol 1992, 36, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Cénac, A.; Djibo, A.; Djangnikpo, L. [Peripartum dilated cardiomyopathy. A model of multifactor disease?]. Rev Med Interne 1993, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Cénac, A.; Simonoff, M.; Djibo, A. Nutritional status and plasma trace elements in peripartum cardiomyopathy. A comparative study in Niger. J Cardiovasc Risk 1996, 3, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Cénac, A.; Sparfel, A.; Amiel-Lebigre, F.; Cleuziou, A.; Pennec, Y.; Le Goff, P.; Mottier, D. [Effect of stressful life events on clinical development of temporal arteritis and/or polymyalgia rheumatica]. Presse Med 2002, 31, 873–879. [Google Scholar] [PubMed]
- Cénac, A.; Touré, K.; Diarra, M.B.; Sergeant, C.; Jobic, Y.; Sanogo, K.; Dembele, M.; Fayol, V.; Simonoff, M. [Plasma selenium and peripartum cardiomyopathy in Bamako, Mali]. Med Trop (Mars) 2004, 64, 151–154. [Google Scholar] [PubMed]
- Karaye, K.M.; Yahaya, I.A.; Lindmark, K.; Henein, M.Y. Serum selenium and ceruloplasmin in nigerians with peripartum cardiomyopathy. International journal of molecular sciences 2015, 16, 7644–7654. [Google Scholar] [CrossRef]
- Vadhanavikit, S.; Ganther, H.E. Decreased ubiquinone levels in tissues of rats deficient in selenium. Biochem Biophys Res Commun 1993, 190, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Vadhanavikit, S.; Ganther, H.E. Selenium deficiency and decreased coenzyme Q levels. Molecular aspects of medicine 1994, 15 Suppl, s103–107. [Google Scholar] [CrossRef]
- Thet Thet, L.; Takeda, T.; Wu, J.; Fumikura, Y.; Iida, K.; Yamaguchi, I.; Itai, Y. Diffuse and marked breast uptake of both 123I-BMIPP and 99mTc-TF by myocardial scintigraphy. Ann Nucl Med 2000, 14, 315–318. [Google Scholar] [CrossRef]
- Ohtsuki, K.; Sugihara, H.; Umamoto, I.; Nakamura, T.; Nakagawa, T.; Nakagawa, M. Clinical evaluation of hypertrophic cardiomyopathy by myocardial scintigraphy using 123I-labelled 15-(p-iodophenyl)-3-R, S-methylpentadecanoic acid (123I-BMIPP). Nuclear medicine communications 1994, 15, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Oki, T.; Yamamoto, T.; Tanaka, H.; Tabata, T.; Wakatsuki, T.; Nomura, M.; Ito, S.; Thomas, J.D. Potential application of tissue Doppler imaging to assess regional left ventricular diastolic function in patients with hypertrophic cardiomyopathy: comparison with 123I-beta-methyl iodophenyl pentadecanoic acid myocardial scintigraphy. Clin Cardiol 2004, 27, 33–39. [Google Scholar] [CrossRef]
- Bello, N.; Rendon, I.S.H.; Arany, Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol 2013, 62, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, A.S.; Bojer, A.S.; Hauge, M.G.; Johansen, M.; Damm, P.; Gustafsson, F.; Vejlstrup, N.G. Long-Term Cardiac Function After Peripartum Cardiomyopathy and Preeclampsia: A Danish Nationwide, Clinical Follow-Up Study Using Maximal Exercise Testing and Cardiac Magnetic Resonance Imaging. Journal of the American Heart Association 2018, 7, e008991. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, R.; Shah, Z.; Arany, Z.; Gupta, K. Peripartum cardiomyopathy: An epidemiologic study of early and late presentations. Pregnancy Hypertens 2018, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lindley, K.J. Heart Failure and Pregnancy: Thinking Beyond Peripartum Cardiomyopathy. J Card Fail 2021, 27, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Stout, M.J.; Rosenbloom, J.I.; Olsen, M.A.; Joynt Maddox, K.E.; Deych, E.; Davila-Roman, V.G.; Lindley, K.J. Preeclampsia Predicts Risk of Hospitalization for Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2021, 78, 2281–2290. [Google Scholar] [CrossRef]
- Rajapreyar, I.; Sinkey, R.; Pamboukian, S.V.; Tita, A. Did a shared thioredoxin-reductase gene mutation lead to maternal peripartum cardiomyopathy and fatal dilated cardiomyopathy in her son? A case report. Case Rep Womens Health 2020, 26, e00196. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.; Powis, G. Thioredoxin reductase. The Biochemical journal 2000, 346 Pt 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxidants & redox signaling 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Bomer, N.; Grote Beverborg, N.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; J, I.J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur J Heart Fail 2019. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Kurihara, T. Is I-123-beta-methyl-p-iodophenyl-methylpentadecanoic acid imaging useful to evaluate asymptomatic patients with hypertrophic cardiomyopathy? I-123 BMIPP imaging to evaluate asymptomatic hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 2003, 19, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Teran, E.; Racines-Orbe, M.; Vivero, S.; Escudero, C.; Molina, G.; Calle, A. Preeclampsia is associated with a decrease in plasma coenzyme Q10 levels. Free Radic Biol Med 2003, 35, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Teran, E.; Chedraui, P.; Racines-Orbe, M.; Vivero, S.; Villena, F.; Duchicela, F.; Nacevilla, L.; Schwager, G.; Calle, A. Coenzyme Q10 levels in women with preeclampsia living at different altitudes. Biofactors 2008, 32, 185–190. [Google Scholar] [CrossRef]
- Teran, E.; Hernández, I.; Tana, L.; Teran, S.; Galaviz-Hernandez, C.; Sosa-Macías, M.; Molina, G.; Calle, A. Mitochondria and Coenzyme Q10 in the Pathogenesis of Preeclampsia. Front Physiol 2018, 9, 1561. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Patergnani, S.; Pinton, P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta 2014, 1837, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pobezhimova, T.; Voinikov, V.; Varakina, N. Inactivation of complex I of the respiratory chain of maize mitochondria incubated in vitro by elevated temperature. Journal of Thermal Biology 1996, 21, 283–288. [Google Scholar] [CrossRef]
- Ludwig, P.; Bartels, M.; Schewe, T.; Rapoport, S. Selective inactivation of the NADH-ubiquinone segment of the respiratory chain of submitochondrial particles by endogenous free fatty acids during hyperthermia. FEBS Lett 1978, 95, 181–184. [Google Scholar] [CrossRef]
- Kommuru, T.R.; Ashraf, M.; Khan, M.A.; Reddy, I.K. Stability and bioequivalence studies of two marketed formulations of coenzyme Q10 in beagle dogs. Chem Pharm Bull (Tokyo) 1999, 47, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Kinugawa, S.; Utsumi, H.; Kang, D.; Hattori, N.; Uchida, K.; Arimura, K.; Egashira, K.; Takeshita, A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circulation research 1999, 85, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr Cardiol Rev 2018, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Seneş, M.; Erbay, A.R.; Yilmaz, F.M.; Topkaya, B.C.; Zengi, O.; Doğan, M.; Yücel, D. Coenzyme Q10 and high-sensitivity C-reactive protein in ischemic and idiopathic dilated cardiomyopathy. Clin Chem Lab Med 2008, 46, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Ranek, M.J.; Stachowski, M.J.; Kirk, J.A.; Willis, M.S. The role of heat shock proteins and co-chaperones in heart failure. Philos Trans R Soc Lond B Biol Sci 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Littmann, A.E.; Chang, S.H.; Wester, L.A.; Knipper, J.S.; Shields, R.K. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train 2012, 47, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Burk, A.; Timpmann, S.; Kreegipuu, K.; Tamm, M.; Unt, E.; Ööpik, V. Effects of heat acclimation on endurance capacity and prolactin response to exercise in the heat. European journal of applied physiology 2012, 112, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Stendig-Lindberg, G.; Moran, D.; Shapiro, Y. How significant is magnesium in thermoregulation? Journal of basic and clinical physiology and pharmacology 1998, 9, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Chien, W.C.; Chu, P.; Chung, C.H.; Lin, C.Y.; Tsai, S.H. The association between heat stroke and subsequent cardiovascular diseases. PLoS One 2019, 14, e0211386. [Google Scholar] [CrossRef] [PubMed]
- Nzvere, F.P.; Tariq, E.; Nishanth, K.; Arshid, A.; Cancarevic, I. Long-Term Cardiovascular Diseases of Heatstroke: A Delayed Pathophysiology Outcome. Cureus 2020, 12, e9595. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.W.; Pearce, S.F.; Zhong, R.; Silverstein, R.L.; Frazier, W.A.; Bouck, N.P. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 1997, 138, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Rac, M.; Kurzawski, G.; Safranow, K.; Rac, M.; Sagasz-Tysiewicz, D.; Krzystolik, A.; Poncyljusz, W.; Olszewska, M.; Dawid, G.; Chlubek, D. Association of CD36 gene polymorphisms with echo- and electrocardiographic parameters in patients with early onset coronary artery disease. Arch Med Sci 2013, 9, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Kazantzis, M.; Wang, J.; Venkatraman, S.; Goncalves, R.L.; Quinlan, C.L.; Ng, R.; Jastroch, M.; Benjamin, D.I.; Nie, B.; et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 2015, 10, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Son, N.H.; Basu, D.; Samovski, D.; Pietka, T.A.; Peche, V.S.; Willecke, F.; Fang, X.; Yu, S.Q.; Scerbo, D.; Chang, H.R.; et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest 2018, 128, 4329–4342. [Google Scholar] [CrossRef] [PubMed]
- Daquinag, A.C.; Gao, Z.; Fussell, C.; Immaraj, L.; Pasqualini, R.; Arap, W.; Akimzhanov, A.M.; Febbraio, M.; Kolonin, M.G. Fatty acid mobilization from adipose tissue is mediated by CD36 posttranslational modifications and intracellular trafficking. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc Res 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Ahlbom, H. Castration by Roentgen Rays as An Auxiliary Treatment in the Radiotherapy of Cancer Mammae at Radiumhemmet, Stockholm. Acta Radiologica 1930, 11, 614–635. [Google Scholar] [CrossRef]
- Peck, W.S.; McGreer, J.T.; Kretzschmar, N.R.; Brown, W.E. Castration of the Female by Irradiation. Radiology 1940, 34, 176–186. [Google Scholar] [CrossRef]
- Brinkley, D.; Haybittle, J.L.; Murrell, D.S. The X-ray menopause in 267 cases. J Obstet Gynaecol Br Commonw 1963, 70, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, D.; Haybittle, J.L. The late effects of artificial menopause by X-radiation. Br J Radiol 1969, 42, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.L.; Gray, R.J.; Solin, L.J.; Robert, N.J.; Martino, S.; Tripathy, D.; Ingle, J.N.; Wood, W.C. Efficacy of radiotherapy for ovarian ablation: results of a breast intergroup study. Cancer 2004, 101, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Lushbaugh, C.C.; Casarett, G.W. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer 1976, 37, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Shalet, S.M.; Crowne, E.C.; Morris-Jones, P.H.; Gattamaneni, H.R. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll Radiol) 1989, 1, 75–79. [Google Scholar] [CrossRef]
- Marcello, M.F.; Nuciforo, G.; Romeo, R.; Di Dino, G.; Russo, I.; Russo, A.; Palumbo, G.; Schilirò, G. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer 1990, 66, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.C.; Müller, J.; Schmiegelow, K.; Rechnitzer, C.; Andersen, A.N. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. The Journal of clinical endocrinology and metabolism 2003, 88, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Chemaitilly, W.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Yasui, Y.; Robison, L.L.; Sklar, C.A. Acute ovarian failure in the childhood cancer survivor study. The Journal of clinical endocrinology and metabolism 2006, 91, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Sklar, C.A.; Boice, J.D., Jr.; Mulvihill, J.J.; Whitton, J.A.; Stovall, M.; Yasui, Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009, 27, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, E.; Shalet, S.M.; Blackledge, G.; Todd, I.; Crowther, D.; Beardwell, C.G. The effect of combination chemotherapy on ovarian function in women treated for Hodgkin's disease. Cancer 1983, 52, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 2000, 169, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Whitton, J.A.; Ginsberg, J.P.; Green, D.M.; Leisenring, W.M.; Stovall, M.; Robison, L.L.; Armstrong, G.T.; Sklar, C.A. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; Tissing, W.J.; Shankar, S.; Sieswerda, E.; et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015, 16, e123–136. [Google Scholar] [CrossRef]
- van Dorp, W.; Mulder, R.L.; Kremer, L.C.; Hudson, M.M.; van den Heuvel-Eibrink, M.M.; van den Berg, M.H.; Levine, J.M.; van Dulmen-den Broeder, E.; di Iorgi, N.; Albanese, A.; et al. Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report From the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. J Clin Oncol 2016, 34, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Sherief, L.M.; El-sheikh, A.; Saleh, S.H.; Shahbah, D.A.; Kamal, N.M.; Sherbiny, H.S.; Ahmad, H. NT-proBNP as early marker of subclinical late cardiotoxicity after doxorubicin therapy and mediastinal irradiation in childhood cancer survivors. Dis Markers 2015, 2015, 513219. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Sato, S.; Tanaka, Y.; Nakamura, A.; Fujisawa, A.; Yamamoto, Y.; Kashiba, M. Method for detecting CoQ10 incorporation in the mitochondrial respiratory chain supercomplex. J Clin Biochem Nutr 2023, 72, 207–214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




