Submitted:
05 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Runners Characteristics
3.2. Effect of Beetroot Intake on the Runners’ Oxidative Status
| With beetroot (n=6) | Without beetroot (n=26) | |
|---|---|---|
| MDA baseline (µM) | 1.1 ± 0.5 | 0.6 ± 0.1 |
| MDA finish line (µM) | 1.0 ± 0.2 | 1.3 ± 0.1 † |
| MDA 24h post-race (µM) | 0.8 ± 0.1 | 0.8 ± 0.1 |
| MDA 48h post-race (µM) | 1.2 ± 0.1 | 1.4 ± 0.1† |
| CG baseline (nmol/mL) | 1.26 ± 0.09 | 1.4 ± 0.1 |
| CG finish line (nmol/mL) | 2.7 ± 1.1 | 2.1 ± 0.2 † |
| CG 24h post-race (nmol/mL) | 1.7 ± 0.3 | 1.9 ± 0.12 |
| CG 48h post-race (nmol/mL) | 1.6 ± 0.4 | 2.2 ± 0.3 † |
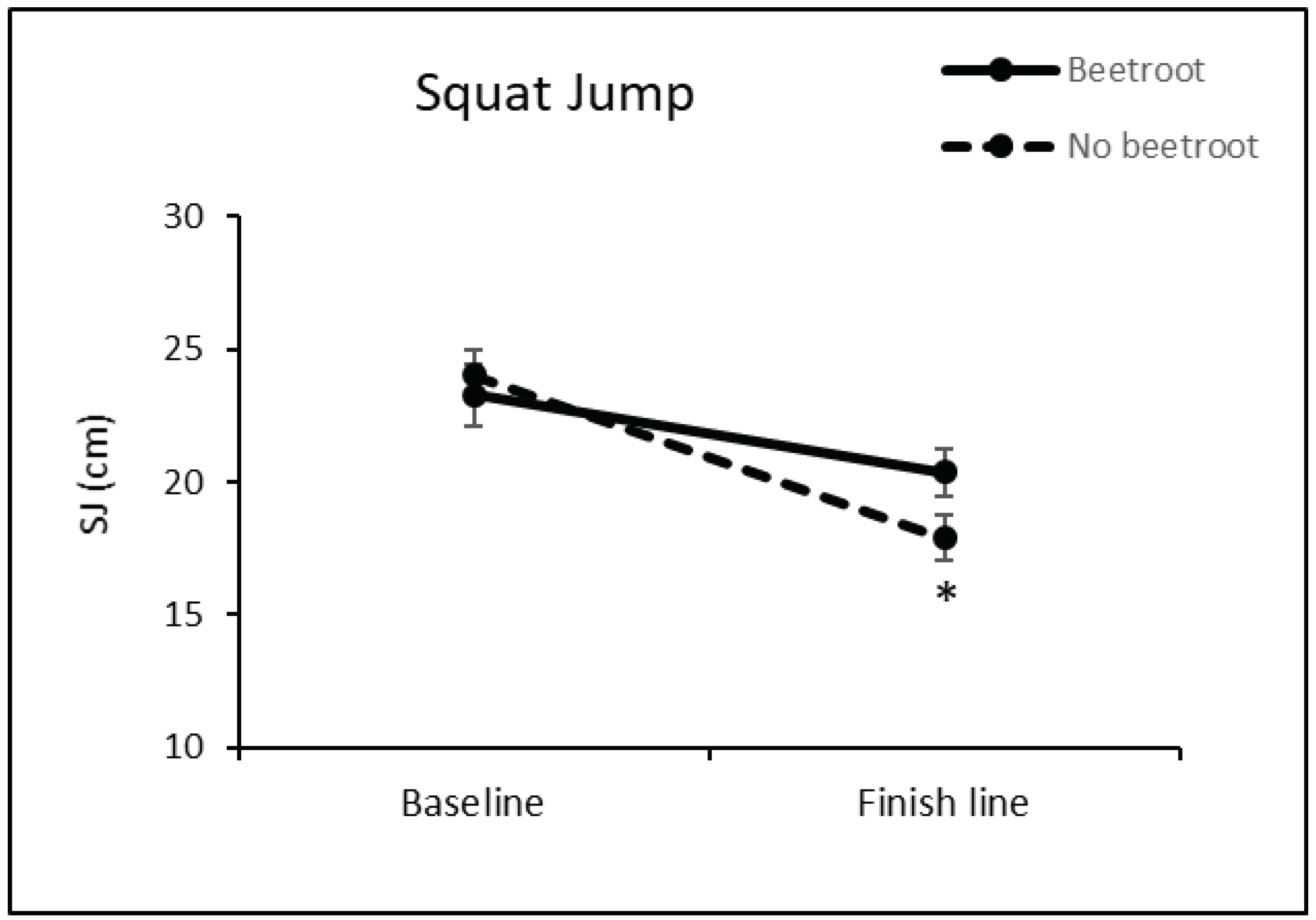
3.3. Effect of Beetroot Intake on Runners’ Muscle Damage and Inflammatory Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devrim-Lanpir, A.; Bilgic, P.; Kocahan, T.; Deliceoğlu, G.; Rosemann, T.; Knechtle, B. Total Dietary Antioxidant Intake Including Polyphenol Content: Is It Capable to Fight against Increased Oxidants within the Body of Ultra-Endurance Athletes? Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Viljoen, C.; du Toit, E.; van Niekerk, T.; Mashaba, S.; Ndaba, Z.; Verster, M.; Bellingan, A.; Ramagole, D.; Jansen van Rensburg, A.; Botha, T.; Janse van Rensburg, D.C. Training for shorter ultra-trail races results in a higher injury rate, a more diverse injury profile, and more severe injuries: 2022 Mac ultraraces. Phys Ther Sport 2023, 65, 7–13. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: past, present and future. J Physiol 2016, 594(18), 5081–92. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Collado, E.; Hernando, C.; Hernando, B.; Hernando, C. Inflammation, muscle damage and postrace physical activity following a mountain ultramarathon. J Sports Med Phys Fitness 2021, 61(12), 1668–1674. [Google Scholar] [CrossRef]
- León-López, J.; Calderón-Soto, C.; Pérez-Sánchez, M.; Feriche, B.; Iglesias, X.; Chaverri, D.; Rodríguez, F.A. Oxidative stress in elite athletes training at moderate altitude and at sea level. Eur J Sport Sci 2018, 18(6), 832–841. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; Montorsi, M.; Vezzoli, A. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS One 2015, 10(11), e0141780. [Google Scholar] [CrossRef]
- Spanidis, Y.; Stagos, D.; Orfanou, M.; Goutzourelas, N.; Bar-Or, D.; Spandidos, D.; Kouretas, D. Variations in Oxidative Stress Levels in 3 Days Follow-up in Ultramarathon Mountain Race Athletes. J Strength Cond Res 2017, 31(3), 582–594. [Google Scholar] [CrossRef]
- Guerrero, C.; Collado-Boira, E.; Martinez-Navarro, I.; Hernando, B.; Hernando, C.; Balino, P.; Muriach, M. Impact of Plasma Oxidative Stress Markers on Post-race Recovery in Ultramarathon Runners: A Sex and Age Perspective Overview. Antioxidants (Basel) 2021, 10(3), 355. [Google Scholar] [CrossRef] [PubMed]
- Elkington, L.J.; Gleeson, M.; Pyne, D.B.; Callister, R.; Wood, L.G. Inflammation and Immune Function: Can Antioxidants Help the Endurance Athlete? In Antioxidants in Sport Nutrition; Lamprecht M; CRC Press/Taylor & Francis: Boca Raton (FL), 2015; Chapter 11. [Google Scholar]
- Slattery, K.M.; Dascombe, B.; Wallace, L.K.; Bentley, D.J.; Coutts, A.J. Effect of N-acetylcysteine on cycling performance after intensified training. Med Sci Sports 2014, 46, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic Biol Med 2016, 98, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Redox interventions to increase exercise performance. J Physiol 2016, 594(18), 5125–33. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int J Environ Res Public Health 2020, 17(22), 8452. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary Supplementation for Attenuating Exercise-Induced Muscle Damage and Delayed-Onset Muscle Soreness in Humans. Nutrients 2021, 14(1), 70. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J Int Soc Sports Nutr 2018, 15, 2. [Google Scholar] [CrossRef]
- Behrens, C.E. Jr.; Ahmed, K.; Ricart, K.; Linder, B.; Fernández, J.; Bertrand, B.; Patel, R.P.; Fisher, G. Acute beetroot juice supplementation improves exercise tolerance and cycling efficiency in adults with obesity. Physiol Rep 2020, 8(19), e14574. [Google Scholar] [CrossRef] [PubMed]
- Samozino, P.; Morin, JB.; Hintzy, F.; Belli, A. A simple method for measuring force, velocity and power output during squat jump. J Biomech 2008, 41(14), 2940–5. [Google Scholar] [CrossRef] [PubMed]
- Panizo González, N.; Reque Santivañez, J.E.; Hernando Fuster, B.; Collado Boira, E.J.; Martinez-Navarro, I.; Chiva Bartoll, Ó.; Hernando Domingo, C. Quick Recovery of Renal Alterations and Inflammatory Activation after a Marathon. Kidney Dis (Basel) 2019, 5(4), 259–265. [Google Scholar] [CrossRef]
- León-López, J.; Calderón-Soto, C.; Pérez-Sánchez, M.; Feriche, B.; Iglesias, X.; Chaverri, D.; Rodríguez, F.A. Recovery of Inflammation, Cardiac, and Muscle Damage Biomarkers After Running a Marathon. J Strength Cond Res 2021, 35(3), 626–632. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Sánchez-Gómez, J.M.; Collado-Boira, E.J.; Hernando, B.; Panizo, N.; Hernando, C. Cardiac Damage Biomarkers and Heart Rate Variability Following a 118-Km Mountain Race: Relationship with Performance and Recovery. J Sports Sci Med 2019, 18(4), 615–622. [Google Scholar]
- Lawrence, R.A.; Parkhill, L.K.; Burk, R.F. Hepatic cytosolic non selenium-dependent glutathione peroxidase activity: Its nature and the effect of selenium deficiency. J. Nutr 1978, 108, 981–987. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,50-dithiobis(2- nitrobenzoic acid). Anal. Biochem 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Guiraud, P.; Meo, J.; Favier, A. High-performance liquid chromatographic separation of malondialdehydethiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J. Chromatogr. B Biomed. Sci. Appl 1992, 577, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect 1998, 106, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.L. Determination of carbonyl content in oxidatively modified proteins. Methods Enzym 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Tiana, L.; Caib, Q.; Wei, H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med 1998, 24, 1477–1484. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; Meeusen, R.; van Loon, L.; Shirreffs, S.M.; Spriet, L.L.; Stuart, M.; Vernec, A.; Currell, K.; Ali, V.M.; Budgett, R.G.M.; Ljungqvist, A.; Mountjoy, M.; Pitsiladis, Y.; Soligard, T.; Erdener, U.; Engebretsen, L. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int J Sport Nutr Exerc Metab 2018, 28(2), 104–125. [Google Scholar] [CrossRef]
- Clifford, T.; Allerton, D.M.; Brown, M.A.; Harper, L.; Horsburgh, S.; Keane, K.M.; Stevenson, E.J.; Howatson, G. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metab 2017, 42(3), 263–270. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics following Simulated Match Play in Soccer Players. J Am Coll Nutr 2021, 40(1), 61–69. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; Peña Amaro, J.; Berral-Aguilar, A.J.; Berral-de la Rosa, F.J. Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review. Sports Health 2022, 14(4), 556–565. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery after Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15(17), 3763. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, R.; Sinaga, F.A.; Sinaga, R.N. Effect of Beetroot Juice (Beta vulgaris L) During Training on Malondialdehyde Level in Athletes. Int. J. Sci. Res 2018, 8, 4. [Google Scholar]
- Kozłowska, L.; Mizera, O.; Gromadzińska, J.; Janasik, B.; Mikołajewska, K.; Mróz, A.; Wąsowicz, W. Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers. Antioxidants (Basel) 2020, 9(7), 571. [Google Scholar] [CrossRef]
- Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8(8), 506. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J.K. Bioactive potential of beetroot (Beta vulgaris). Food Res Int 2022, 158, 111556. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Castro, J.M.; Campos-Perez, J.; Ranchal-Sanchez, A.; Durán-López, N.; Domínguez, R. Acute Effects of Beetroot Juice Supplements on Lower-Body Strength in Female Athletes: Double-Blind Crossover Randomized Trial. Sports Health 2022, 14(6), 812–821. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Price, K.M.; Wideen, L.E.; Lincoln, I.G.; Karl, S.T.; Seals, J.P.; Paniagua, K.K.; Hagen, D.W.; Tchaprazian, I.; Bailey, S.J.; Pennell, A. Dietary nitrate ingested with and without pomegranate supplementation does not improve resistance exercise performance. Front Nutr 2023, 10, 1217192. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of Beetroot Juice Supplementation on Muscle Soreness and Performance Recovery after Exercise-Induced Muscle Damage in Female Volleyball Players. Nutrients 2023, 15(17), 3763. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11(6), 1289. [Google Scholar] [CrossRef]
| Total (n=32) | Women (n=13) | Men (n=19) | |
|---|---|---|---|
| Average time (h) | 21,38 ± 0,61 | 21,39 ± 0,88 | 21,36 ± 0,89 |
| Average age (years) | 40,9 ± 1,0 | 42,2 ± 1,7 | 40,1 ± 1,2 |
| Years runninng | 8,0 ± 0,5 | 8,0 ± 0,6 | 8,1 ± 0,9 |
| Training hours/week | 9,6 ± 4,6 | 10 ± 0,9 | 9 ± 1,3 |
| Pre-race weight (kg) | 66,1 ± 1,9 | 55,7 ± 1,4 | 73,2 ± 1,5 * |
| BMI | 22,9 ± 0,4 | 21,7 ± 0,6 | 23,8 ± 0,4 * |
| Pre-race % Muscular Mass | 84,1 ± 1,1 | 79,1 ± 1,3 | 87,6 ± 0,8 * |
| Beetroot consumption (nº of runners) | 6 | 3 | 3 |
| With beetroot (n=6) | Without beetroot (n=26) | |
|---|---|---|
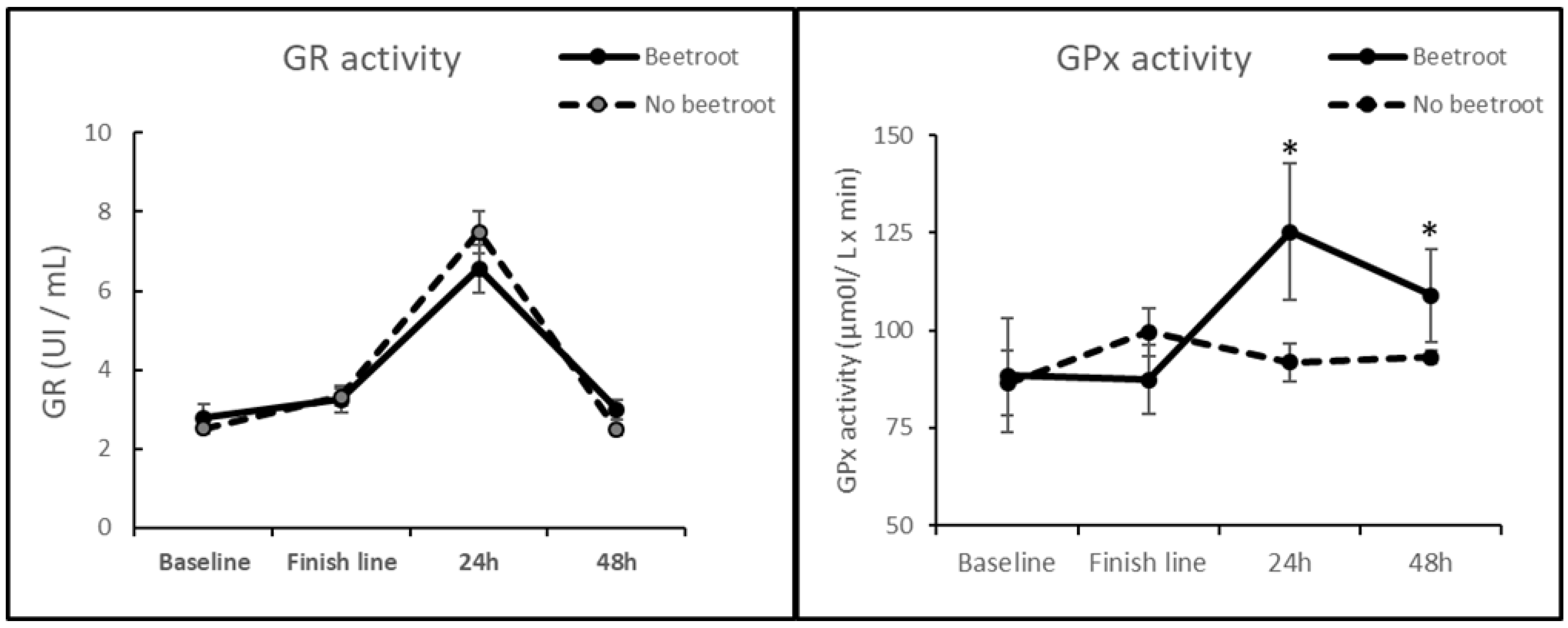
| GPx baseline (µmol/L x min) | 88.4 ± 14.6 | 86.5 ± 8.3 |
| GPx finish line (µmol/L x min) | 87.4 ± 8.8 | 99.5 ± 6.2 |
| GPx 24h post-race (µmol/L x min) | 125,3 ± 17,4 † | 91,7 ± 4,9 * |
| GPx 48h post-race (µmol/L x min) | 109,0 ± 11,8 | 93,1 ± 1,5 * |
| GR baseline (UI/mL) | 2.7 ± 0.3 | 2.5 ± 0.1 |
| GR finish line (UI/mL) | 3.2 ± 0.3 | 3.3 ± 0.2 |
| GR 24h post-race (UI/mL) | 6.5 ± 0.6 ** | 7.4 ± 0.5 ** |
| GR 48h post race (UI/mL) | 2.9 ± 0.2 | 2.4 ± 0.1 |
| With beetroot (n=6) | Without beetroot (n=26) | |
|---|---|---|
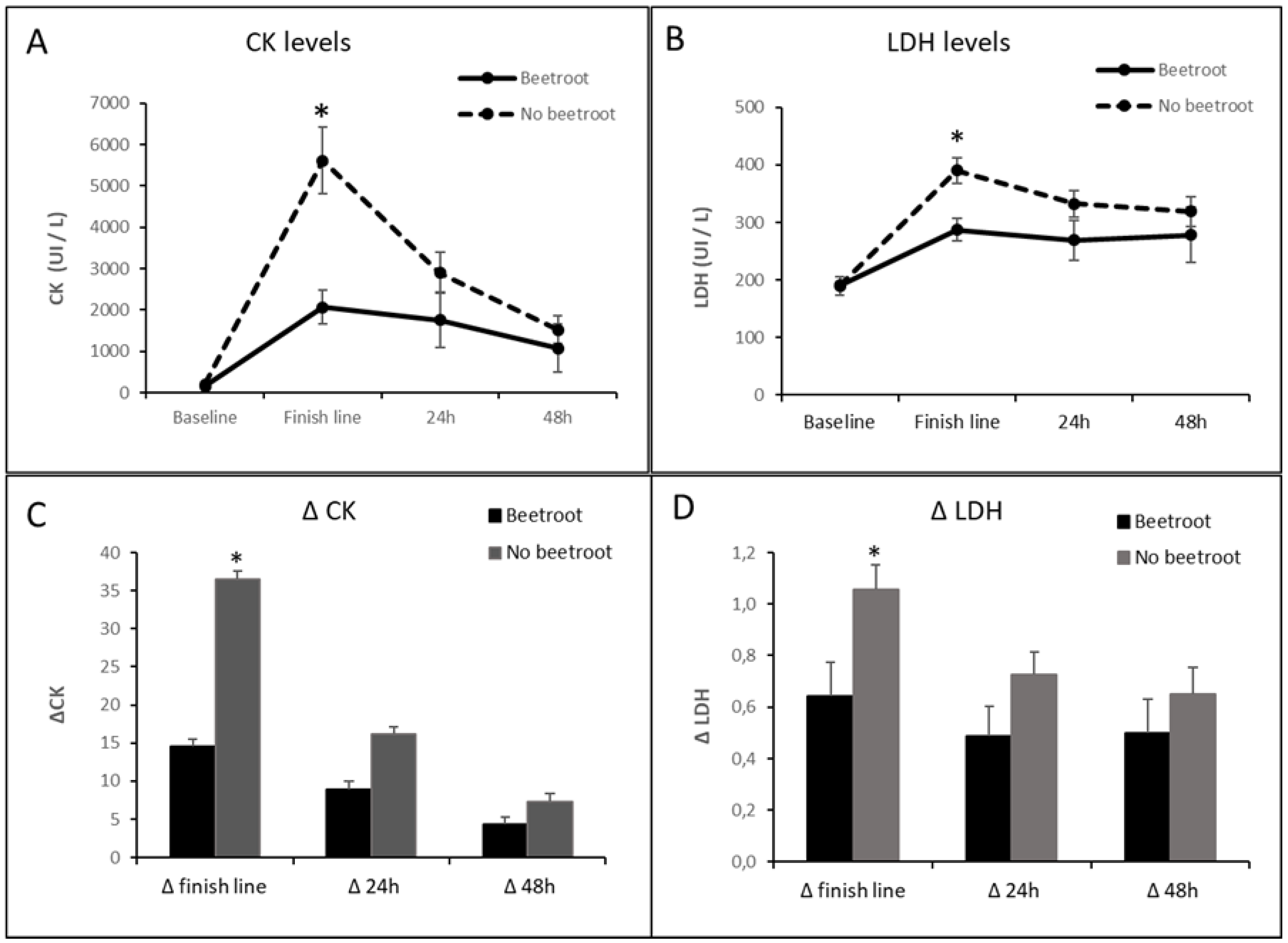
| CK baseline value (UI/L | 159.5 ± 33.5 | 213.9 ± 47.4 |
| CK finish line value (UI/L) | 2071.7 ± 406.7 | 5616.01 ± 816.1 *† |
| CK 24h post-race value (UI/L) | 1758.5 ± 663.7 | 2901.6 ± 503.4† |
| CK 48h post race value (UI/L) | 1076.2 ± 588.5 | 1521.6 ± 338.3† |
| LDH baseline value (UI/L) | 185.2 ± 15.2 | 190.4 ± 5.9 |
| LDH finish line value (UI/L) | 299.4 ± 20.7† | 390.6 ± 22.4 *† |
| LDH 24h post-race value (UI/L) | 277.0 ± 32.4 | 332.5 ± 23.2† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





