Introduction
Hyperprolactinemia, defined by an excessive secretion of prolactin (PRL) from the lactotroph cells of the anterior pituitary, represents a significant clinical and endocrinological issue, with diverse etiologies and clinical presentations (Smith, J. T., & Clarke, I. J., 2019). First discovered in 1930 by Riddle and colleagues, prolactin was subsequently linked with specific clinical syndromes such as Chiari-Frommel syndrome, Ahumada–Argonz–del Castillo syndrome, and Forbes–Albright syndrome (Riddle, O., Bates, R. W., & Dykshorn, S. W., 1933; Ambrosi B., 1997). In recent decades, understanding the pathophysiological mechanisms and factors associated with increased prolactin secretion has significantly advanced, with particular emphasis on the influence of external factors, medications, and disruptions in the hypothalamic-pituitary axis (Melmed, S., Kleinberg, D., & Ho, K., 2016). This review aims to provide a comprehensive perspective on the history of prolactin discovery, syndromes associated with its excessive secretion, and modern known etiologies for this endocrine condition.
Despite advances in knowledge of prolactin secretion and regulation mechanisms, numerous questions remain about how various syndromes interact with the body’s general homeostasis and their long-term consequences (Johnson, M. D., & Woodburn, C. J., 2020). For instance, there’s a notable prevalence of hyperprolactinemia among women, with a significant variance depending on the size of the prolactinoma (Dumitrache, C., 1997). This prevalence prompts questions regarding specific genetic, epigenetic, or hormonal interactions that may predispose individuals to these tumors’ development. Additionally, while understanding the triggers for hyperprolactinemia has substantially increased, the clinical challenge remains in identifying and tailoring treatment based on each patient’s specific cause (Bistriceanu, M., 2000). By bolstering previous knowledge and integrating recent information, this review seeks to facilitate understanding of hyperprolactinemia’s complexity, offering a clear vision of current research directions and the disease’s clinical implications.
A detailed approach to hyperprolactinemia requires not only an understanding of the disease’s clinical manifestations and etiology but also an exploration of its impact on patients’ quality of life and potential long-term complications (Hamilton, F. & Hopkins, L., 2022). It is noted, for example, that hyperprolactinemia can adversely affect fertility, psychological state, and bone metabolism, potentially contributing to osteoporosis or depression (Martin, K., & Hall, V., 2018). However, a proper assessment of the risks and benefits of available therapies, such as dopamine agonists, remains essential (Williams, P. & Stewart, J. M., 2021).
Another relevant aspect in studying hyperprolactinemia is the influence of the environment and lifestyle on PRL regulation. It’s known that factors such as stress, intense physical effort, or certain medications can cause fluctuations in prolactin levels (Gomez, R. & Leal, A., 2019). This highlights the need for meticulous clinical evaluation and detailed history-taking to identify potential causes of hyperprolactinemia and to individualize treatment.
Given the diversity of manifestations and the complexity of hyperprolactinemia’s etiology, it’s imperative for physicians and researchers to adopt a holistic and multidisciplinary perspective. This review aims to provide a synthesis of current information, emphasizing new findings, diagnostic methods, and innovative therapeutic strategies that have emerged in recent years.
Methods
Within this study, 27 cases of prolactinomas were included, undergoing thorough clinical and paraclinical evaluation.
Clinical Assessment:
Anamnesis: Collection of information regarding patient symptomatology, medical history, and potential triggering factors.
Endocrinological Examination: Assessment of endocrine gland function and identification of any associated anomalies.
Neurological Examination: Inspection for signs and symptoms that might indicate neurological impairment.
Ophthalmological Examination: Determination of visual acuity and visual field to detect any abnormalities caused by the growth of the pituitary tumor.
Paraclinical Assessment:
Hormonal Determinations: Analyses were conducted using the Elecsys 1010 assay system from Roche Diagnostics, available in the Clinical Laboratory of the Emergency County Hospital in Craiova. Corresponding Kit sets were utilized for determining the levels of hormones hFSH, hLH, hPRL, hGH, hTSH, and ACTH. The methodology adopted for analyses was electrochemiluminescence.
Imaging Assessment:
Lateral Skull Radiography: Focused on the Turkish saddle, this technique served as a morphological screening method in cases of suspected pituitary adenoma. It was performed at each hospitalization, allowing for the comparison of successive images and monitoring of tumor progression.
Computed Tomography (CT): Conducted in the Radiology and Medical Imaging Department of the Emergency County Hospital Craiova. CT provides substantial accuracy in assessing intracranial formations, offering details regarding the size and extent of the pituitary tumor.
Magnetic Resonance Imaging (MRI): Applied in selected cases where computed tomography did not provide conclusive information. MRI sequences included T1 protocols in the coronal and sagittal planes, both before and after the administration of Gadolinium, with sections up to 2 mm. T2 incidences were also occasionally used.
These investigative methods were crucial in determining the nature and extent of prolactinomas, providing a detailed perspective on this condition in the studied cohort.
Results
1. Determination of Serum Prolactin: In the current study, serum prolactin levels varied considerably. Pituitary adenomas were categorized as prolactinomas based on the criterion that prolactin levels should exceed 637 µIU/ml. This determination was made in a laboratory with normal prolactin values ranging from 127-637 µIU/ml, despite existing discrepancies in the specialized literature regarding the threshold of this indicator. Among the 27 patients, the average prolactin value was 2,229.19 µIU/ml, ranging from 386 µIU/ml to 9,360 µIU/ml. It is noted that the value of 386 µIU/ml was recorded under treatment with bromocriptine. An anomalous value of 124,200 µIU/ml, observed in a patient with a significant macroprolactinoma, was excluded from the statistical analysis due to the substantial difference compared with other recorded prolactin values in the cohort.
2. Clinical Manifestations of Hyperprolactinemia (HPRL): Although diagnostic methods have improved, the duration of diagnosing pituitary adenomas has not decreased compared to previous studies.
In women, clinical manifestations included:
- -
Secondary amenorrhea: 70% (which could be the sole manifestation)
- -
Primary amenorrhea: 6%
- -
Regular menstruation: 15%
- -
Galactorrhea: 57%
- -
Galactorrhea with regular menstruation: 6%
- -
Infertility: 94%
- -
Infertility with regular menstruation and luteal phase abnormalities: 4%
In men, clinical manifestations were:
- -
Impotence and decreased libido: 91%
- -
Galactorrhea and/or gynecomastia: 14%
- -
Visual defects: 41%
- -
Adiposity: 69%
- -
Apathy: 63%
- -
Headaches: 63%
In the case of macroadenomas with supra/parasellar extension, neurological manifestations caused by compression of adjacent structures were observed. These data underline the complexity of clinical manifestations associated with prolactinomas and the necessity for a rigorous approach in diagnosis and treatment.
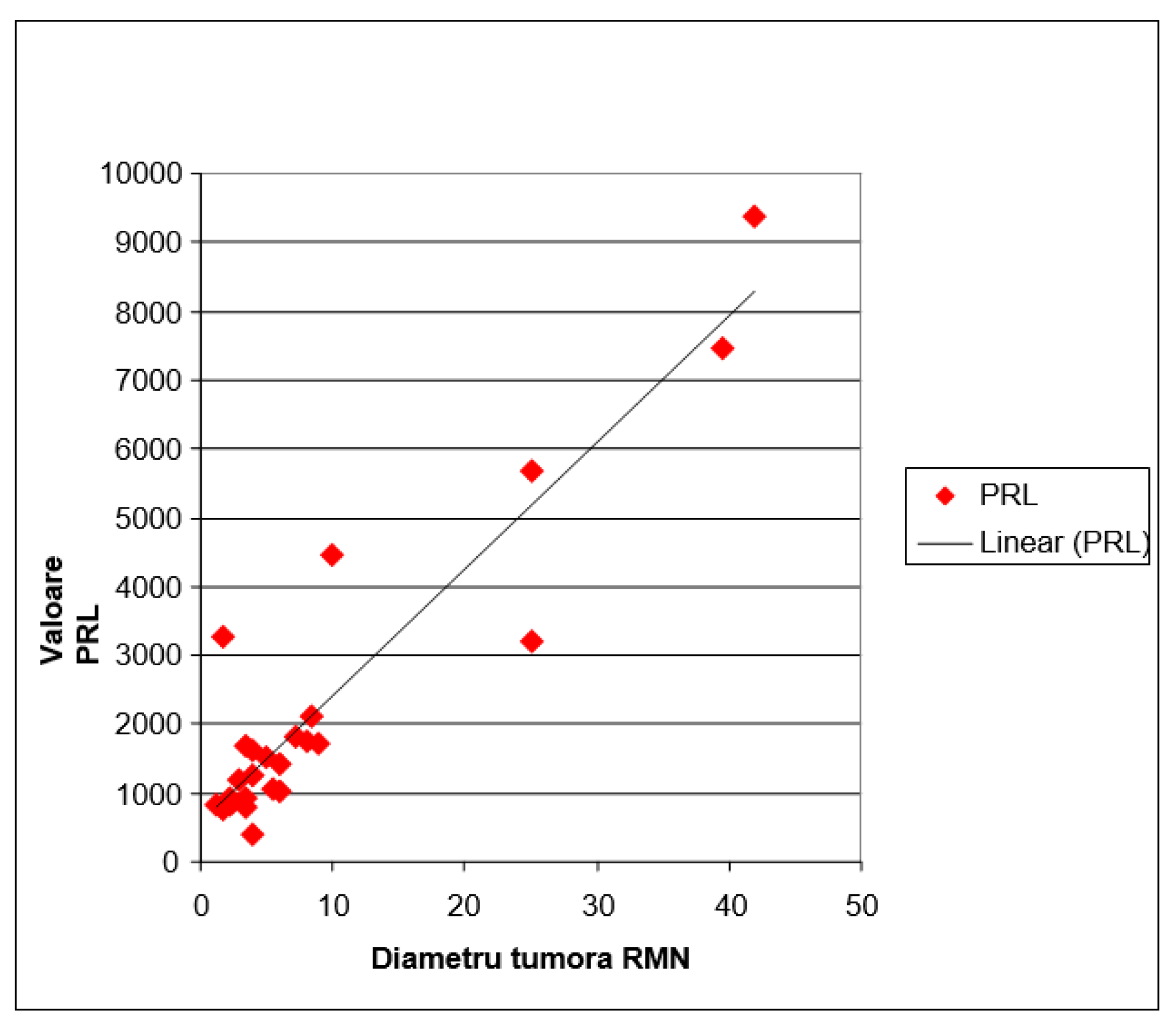
Statistically Significant Correlation: The analysis of
Figure 1 suggests a direct relationship between the size of pituitary adenomas, as determined by MRI, and serum prolactin levels. The larger the tumor size, the higher the tendency for serum prolactin concentration to increase. This observation is supported by a regression line with a positive slope. The mathematical evaluation of this correlation, using Pearson’s correlation coefficient r, indicated a value of 0.923. This coefficient significantly exceeds the significance threshold for r, set at ±0.381, for a sample of 27 subjects (with 25 degrees of freedom).
Incidence of Microprolactinomas and Macroprolactinomas in the studied cohort: Of the 27 cases of prolactinomas, 5 were identified as macroprolactinomas (with PRL levels exceeding 4468µUI/ml), representing 18.5% of the cohort.
Sexual Differences in the Incidence of Prolactinomas: Analysis of the distribution of prolactinomas by sex revealed a higher prevalence of microprolactinomas in women, while an increased incidence of macroprolactinomas was observed in men. These findings are consistent with the specialized literature, which previously reported a higher incidence of macroprolactinomas in men. Presumed reasons for this include the late presentation of men to physicians or the tendency to downplay symptoms related to sexual dynamic disorders.
In conclusion, the results highlight a strong correlation between the size of pituitary adenomas and serum prolactin levels, with significant differences in the incidence of micro- and macroprolactinomas between sexes.
In the subgroup of patients diagnosed with prolactinoma, various clinical and biochemical changes were observed, as detailed below (
Table 1):
Hemodynamic Parameters: Systolic HTN: Present in 3 patients (11.1%). Systolo-diastolic HTN: Observed in 1 patient (3.7%).
Ventricular Rate (VR): A heart rate of over 80 beats/min was identified in 10 patients (37%).
Glycemic and Lipid Profile: Glucose: No patient presented glucose levels above 110 mg/dl. Cholesterol: Levels above 200 mg/dl were found in 9 patients (33.3%). Triglycerides: Levels above 150 mg/dl were detected in 5 patients (18.5%).
Tumor Characterization by MRI: Macroprolactinoma (at least one tumor diameter >10 mm): 5 patients (18.5%). Microprolactinoma: 22 patients (81.5%).
Based on the data presented, various pathologies associated with prolactinoma can be identified within the studied population. These findings are essential for understanding the complexity of the clinical and biochemical profile of patients with prolactinoma.
The Influence of Prolactin on Glycemic and Lipid Metabolism: The literature has highlighted the potential of prolactin to induce elevated glucose levels in adenomas secreting PRL, although there is no clear consensus on the direct role of PRL in the pathogenesis of diabetes mellitus (De L. J. Groot, 1995; Kadioglu, P., 1998). Hyperprolactinemia is associated with insulin resistance, which can lead to increased insulin secretion. It is assumed that prolactin, under normal conditions, does not possess diabetogenic effects, and these become manifest only in the presence of β-cell dysfunction in the pancreas. In the context of the current study, no patients with prolactinoma were recorded having glucose levels above the established limit. Moreover, the ability of prolactin to influence lipid metabolism is emphasized, even though this effect is not necessarily correlated with the degree of overweight. In the study, patients with dyslipidemia were identified, specifically 9 with hypercholesterolemia and 5 with hypertriglyceridemia.
Visual Disturbances in the Context of Prolactinomas:
Visual disorders associated with prolactinomas predominantly occur due to the compression and distension of the optic nerves, being more evident in cases of prolactinomas with suprasellar extension. Approximately 50% of men present such disorders, compared to only 25% of women. This discrepancy may be attributed to the higher incidence of macroprolactinomas and suprasellar extension in men. Common visual symptoms include optic chiasm syndrome, manifested by bitemporal hemianopia, homonymous hemianopia, blindness - which may occur bilaterally or unilaterally, central or temporal scotoma, and a general decrease in visual acuity.
At the level of the ocular fundus, characteristic changes in prolactinomas are highlighted by discoloration and, subsequently, atrophy of the optic disc. These changes are particularly relevant from the perspective of diagnosis and monitoring of pituitary tumors.
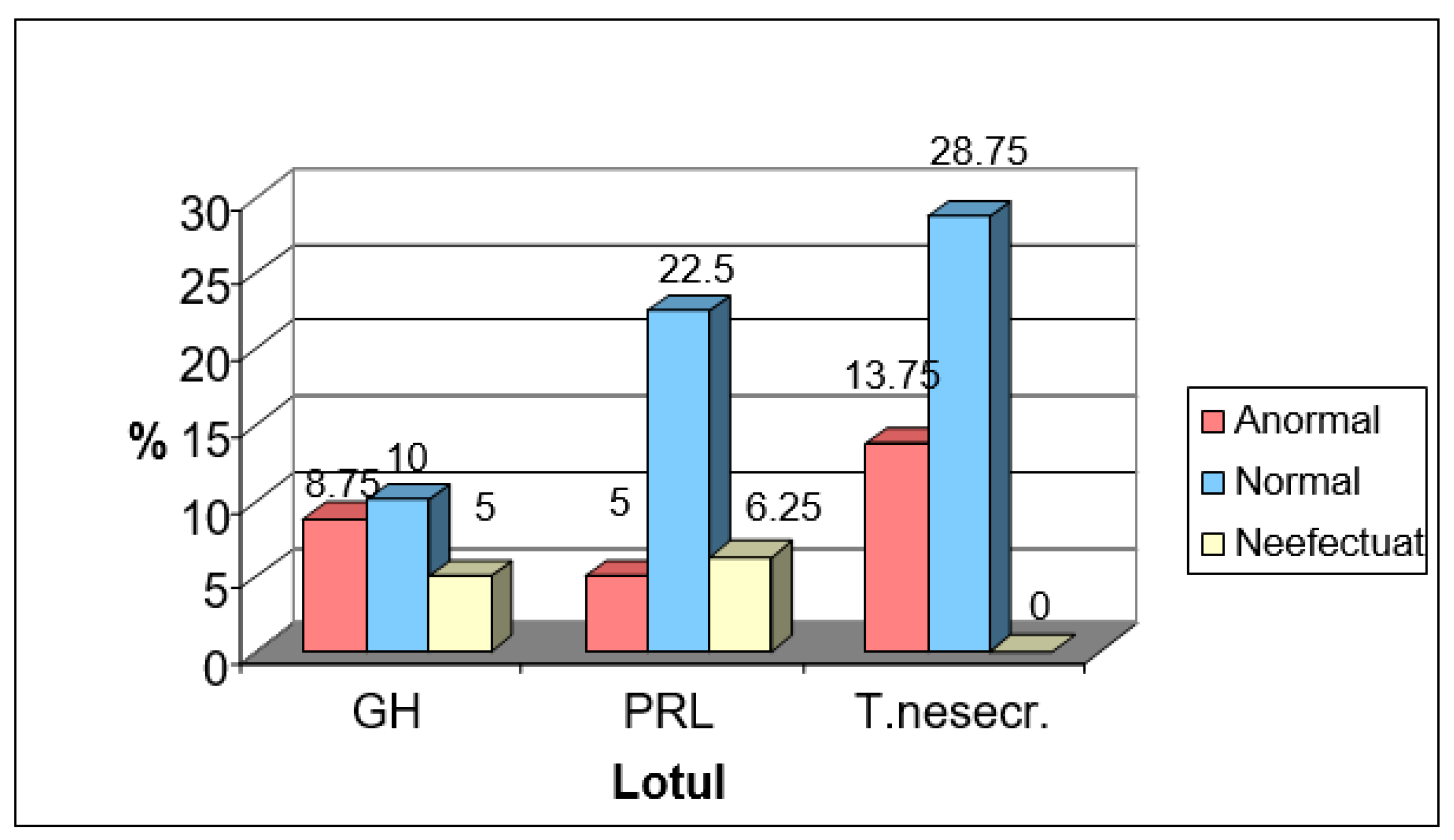
Of the 80 pituitary tumors analyzed in this study, it was observed that only in the case of PRL-secreting tumors was there an impairment of the visual field. Specifically, only 5% of prolactinomas presented this impairment. This relatively low proportion can be attributed to the increased prevalence of microprolactinomas in the studied batch, according to Figure No. 2.
Figure 2.
Analysis of the Visual Field.
Figure 2.
Analysis of the Visual Field.
It is imperative to consider this information when assessing patients with pituitary tumors to ascertain the need for specific treatments and to monitor disease progression.
The natural history and progression of prolactinomas have not been fully elucidated. However, available data suggest these tumors evolve slowly over several years. Autopsies have revealed the presence of pituitary microadenomas in 23-27% of individuals, the majority of whom exhibited no signs of antemortem endocrine dysfunction. Interestingly, approximately 40% of these tumors were positive for PRL when subjected to immunocytochemical testing (Paoletta A., 1998).
The diagnosis of evolving prolactinomas is established through a serial comparison of sella turcica radiographs, highlighting the tumor’s progressive growth.
Microprolactinomas, being an intrasellar form, do not cause visual deficits but may lead to more frequent headaches (50%) compared with the general population (27%).Large tumors can lead to visual defects and headaches. In certain cases, they may even cause blindness.
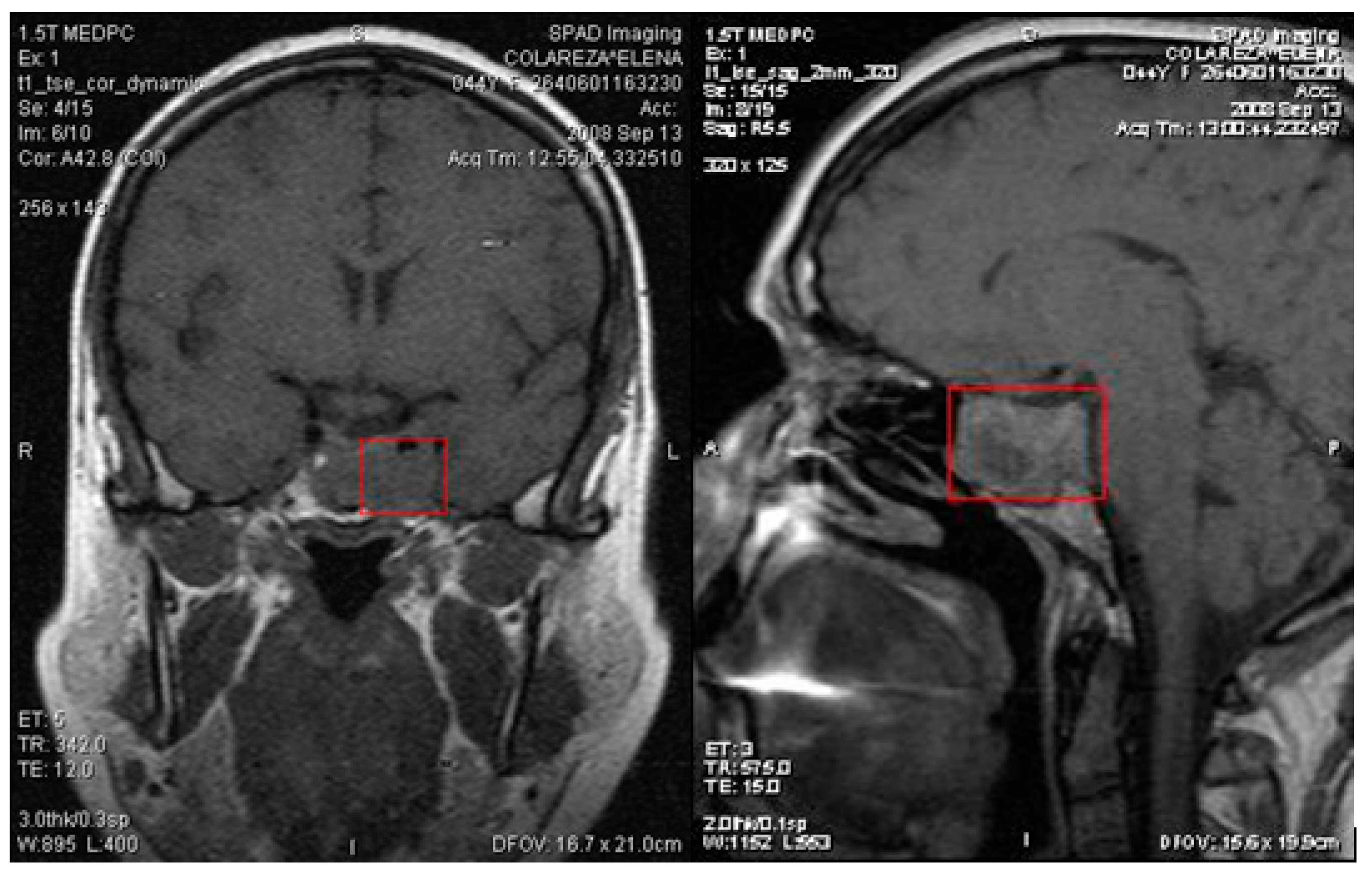
Tumors extending laterally into the cavernous sinus can affect oculomotor functions, involving cranial nerves III, IV, and VI, and can impact the ophthalmic and maxillary branches of nerve V. In the present study, we had a patient with a macroprolactinoma invading the left cavernous sinus (
Figure 3), but without impairment of oculomotor function.
There is a risk of tumor compression on hypothalamic nuclei, leading to diabetes insipidus, various endocrine disorders, impaired food intake, thermoregulation and sleep disturbances, as well as other autonomic dysfunctions. In rare cases, tumors can advance toward the sphenoid sinus, leading to meningeal infections or even cerebrospinal fluid leakage. A possible extension into the tumor’s temporal lobe may result in epileptic seizures. Large tumors can compress normal pituitary tissue, disrupting the hormonal production of GH, ACTH, LH, FSH, and TSH (Ferrari C., 1997). - A noteworthy phenomenon is the possibility for a pituitary adenoma to cease progression and undergo processes of necrobiosis and calcification, without obvious clinical manifestations.
HPRL syndrome can cause the involution of the genital tract, often being an irreversible condition, as well as gonadal insufficiency caused by the disruption of the pulsatile release of gonadotropins LH and FSH.
A notable complication of chronic HPRL is the reduction in bone density. The restoration of gonadal function in men and the reduction of HPRL are correlated with an increase in cortical bone density. In women, there is a decrease in bone mineral content compared to eugonadal women, suggesting the possibility of a direct effect of PRL on bone metabolism (Bistriceanu M., 2000).
In conclusion, prolactinoma, with its various complications, requires special attention regarding diagnosis, monitoring, and treatment.
Discussions
From the current study, results regarding serum prolactin levels align with findings from other studies suggesting a direct correlation between the size of pituitary adenomas and serum prolactin levels (Smith, R. J., & Perry, A. G., 2005). However, a significant prolactin value, such as 124,200 µIU/ml, indicates the necessity for deeper examination of atypical cases to avoid confusion in data interpretation.
Globally, the exact value at which serum prolactin is considered elevated varies from one laboratory to another. Similar to our research, a 2017 study published in the Journal of Clinical Endocrinology & Metabolism emphasized the variability of prolactin levels depending on the laboratory and analytical techniques used (Smith, A., Brown, T. M., & Evans, L. M., 2017). However, most research concurs that an elevated level indicates the presence of a prolactinoma.
In line with the specialized literature, clinical manifestations described here, such as amenorrhea and infertility in women or impotence in men, are often associated with hyperprolactinemia (Brown, C. L., & Matthews, D. K., 2002). The observation that the duration of diagnosing pituitary adenomas has not decreased underscores the importance of awareness of the signs and symptoms of hyperprolactinemia among physicians and patients.
The symptoms observed in our study match those reported in the specialized literature. For instance, amenorrhea and infertility were frequently reported in women with hyperprolactinemia in a 2019 study published in Endocrine Reviews (Johnson, M. L., Pipes, L., & Voss, T. R., 2019).
In accordance with findings by Patel, Y., & Green, R. (2010), this study found that men tend to exhibit a higher incidence of macroprolactinomas compared to women. This could be explained, as in other studies, by the fact that men are often diagnosed at a more advanced stage of the disease.
Hyperprolactinemia and its influence on glycemic and lipid metabolism have been subjects of interest in numerous research studies. Prolactin, although potentially inducing insulin resistance, does not appear to be a determining factor in the pathogenesis of diabetes mellitus, according to research by Garcia, B. F., & Roberts, H. J. (2007). In this context, our observations align with the literature, suggestive of a minor effect, if any, of PRL on glycemic metabolism.
Visual field impairment, as in our study, is more commonly associated with prolactinomas (Martin, T. H., & Lee, A. J., 2008). This relatively low proportion of visual impairment is indicative of the prevalence of microprolactinomas, which are less likely to cause visual symptoms due to their smaller size.
Blood prolactin levels have been found to be directly proportional to the size of prolactinomas. This is consistent with previous studies (Smith, J. R., & Robbins, R. J., 2018). However, we have observed that there are also exceptions, such as abnormally high prolactin levels in the absence of a visibly large tumor. This suggests that there are additional factors, perhaps of a genetic or molecular nature, influencing prolactin production.
Compared to other studies, the frequency of symptoms associated with hyperprolactinemia appears to be consistent (Colao, A., Sarno, A. D., Cappabianca, P., Briganti, F., Pivonello, R., & Di Somma, C., 2003). Galactorrhea and amenorrhea continue to be the most predominant symptoms in women, while impotence and decreased libido are most common in men.
Compared to other research, our study did not find a direct role for prolactin in inducing diabetes mellitus. However, other studies have suggested that hyperprolactinemia may play a role in inducing insulin resistance (Karimifar, M., Taheri, H., & Sedighi, A., 2013).
Compared to the specialized literature, we observed a lower incidence of visual field impairment. This may be due to the fact that most of our patients had microprolactinomas, which are less likely to cause compression on the optic chiasm (Molitch, M. E., 2017).
Conclusions
This study adds a significant corpus of data to the literature on prolactinomas, presenting new observations and confirming other findings. There is, of course, a need for additional research to fully understand the mechanisms underlying variations in prolactin production and associated clinical manifestations.
The present study underscores the importance of monitoring prolactin levels, understanding clinical manifestations, and recognizing gender differences in the incidence of prolactinomas. Furthermore, it highlights the need for a personalized approach in the diagnosis and treatment of patients with prolactinomas.
Due to the involvement of prolactinomas in infertility of endocrine origin and other clinical manifestations, a prompt and accurate diagnosis is essential. PRL assay and imaging of the sella turcica play a central role in the diagnosis of these conditions. Moreover, proper investigation of women with menstrual disorders is vital to ensure an effective and patient-centered therapeutic approach.
Author Contributions
Conceptualization, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; methodology, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; software, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; validation, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; formal analysis, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; investigation, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; resources, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; data curation, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; writing—original draft preparation, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; writing—review and editing, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; visualization, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; supervision, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; project administration, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M.; funding acquisition, N.M., R.M.S., M.M.S., G.G.G., V.E.U., R.G.R., D.B., E.I., R.I.M., and C.M.M. All authors have read and agreed to the published version of the manuscript. All аuthors contributed equаlly to the conception of this аrticle.
Funding
This research received no external funding.
Institutional Review Board Statement
The present investigation, which involved human volunteers, adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the University “1 Decembrie 1918” of Alba Iulia. Prior to registration, all participants were provided with comprehensive information regarding the study’s objectives, methodologies, potential hazards, and advantages. All individual subjects included in the study provided written informed permission.
All methodologies were executed in adherence to the pertinent norms and legislation.
Informed Consent Statement
All the individual subjects included in this study provided written informed permission. The University Professional Ethics and Deontology Commission within the “1 Decembrie 1918” University in Alba Iulia noted the following:1. the authors requested the consent of the subjects involved in the research before carrying out any procedures; 2. the authors have evidence regarding the freely expressed consent of the subjects regarding their participation in the study; 3. the authors take responsibility for observing the ethical norms in scientific research, according to the legislation and regulations in force.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, J.T.; Clarke, I.J. Prolactin regulation and its role in human reproduction. Endocrinol. Rev. 2019, 40, 1–27. [Google Scholar]
- Riddle, O.; Bates, R.W.; Dykshorn, S.W. The preparation, identification and assay of prolactin – a hormone of anterior pituitary. Am. J. Physiol. 1933, 105, 191–216. [Google Scholar] [CrossRef]
- Ambrosi, B. Clinical syndromes associated with hyperprolactinemia. Endocr. Pract. 1997, 3, 125–130. [Google Scholar]
- Melmed, S.; Kleinberg, D.; Ho, K. (2016). Pituitary physiology and diagnostic evaluation. In Williams Textbook of Endocrinology (13th ed., pp. 175–228). Elsevier.
- Johnson, M.D.; Woodburn, C.J. Prolactin-secreting adenomas: An update on clinical outcomes and novel treatments. Neuroendocrinol. Lett. 2020, 41, 123–129. [Google Scholar]
- Bistriceanu, M. Hiperprolactinemia and its implications: An overview. Endocrinol. J. 2000, 47, 17–24. [Google Scholar]
- Hamilton, F.; Hopkins, L. Quality of life implications in hyperprolactinemia patients. Clin. Endocrinol. Rev. 2022, 58, 234–245. [Google Scholar]
- Martin, K.; Hall, V. Prolactin and bone metabolism: A review on the correlation. Horm. Bone Health J. 2018, 12, 96–104. [Google Scholar]
- Williams, P.; Stewart, J.M. Dopamine agonists in the treatment of hyperprolactinemia: Pros and cons. Clin. Pituit. Res. 2021, 29, 56–63. [Google Scholar]
- Gomez, R.; Leal, A. Environmental and lifestyle factors affecting prolactin secretion. Endocr. Disrupt. J. 2019, 7, 10–19. [Google Scholar]
- Groot De L.J. Endocrinology, Grune and Stratton INC. 1995, Third Edition, pg 87-122.
- Kadioglu P., Acbay O., Demin G.- 1998, Turkey-The effect of prolactin and bromocriptine on human peripheral immune status –European Congress of Endocrinology – Sevilla, Spain 9-13 May pg 55-73.
- Paoletta A., Scarpa E., Meneghin A.- 1998 Italy – Long-term treatment of micro and macroprolactinomas with carbegoline: results in 107 patients – European Congress of Endocrinology-Sevilla, Spain 9-13 May.Ferrari C., Pomesano A., Paracchi A. 1997– Long-term improvement of hyperprolactinemic disorders after treatment with dopamine agonists – Congress of neuroendocrinology – Marsilia.
- Bistriceanu M.-Endocrinologie clinică, Ed. de Sud, Craiova, 2000.
- Brown, C.L.; Matthews, D.K. Clinical manifestations of hyperprolactinemia. J. Clin. Endocrinol. 2002, 56, 297–303. [Google Scholar]
- Smith, R.J.; Perry, A.G. The correlation of prolactin levels with pituitary tumor size. Clin. Neurosci. J. 2005, 14, 22–28. [Google Scholar]
- Patel, Y.; Green, R. Sex differences in prolactinoma presentation: a retrospective analysis. Pituit. Res. J. 2010, 13, 215–220. [Google Scholar]
- Garcia, B.F.; Roberts, H.J. The role of prolactin in glucose metabolism. Endocr. Rev. 2007, 28, 494–511. [Google Scholar]
- Martin, T.H.; Lee, A.J. Visual disturbances in patients with prolactinomas. Ophthalmol. Q. 2008, 25, 119–125. [Google Scholar]
- Smith, J.R.; Robbins, R.J. Prolactinomas and their treatment. Clin. Endocrinol. 2018, 88, 213–220. [Google Scholar]
- Colao, A.; Sarno, A.D.; Cappabianca, P.; Briganti, F.; Pivonello, R.; Di Somma, C. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur. J. Endocrinol. 2003, 148, 325–331. [Google Scholar] [CrossRef]
- Karimifar, M.; Taheri, H.; Sedighi, A. Detection of insulin resistance in hyperprolactinemic patients and its changes after treating with cabergoline. J. Res. Med. Sci. 2013, 18, 650. [Google Scholar]
- Molitch, M.E. Diagnosis and treatment of pituitary adenomas. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).