1. Introduction
Chronic heart failure (CHF) is among major complications of acute myocardial infarction (MI) and a typical outcome of the cardiovascular disease continuum, affecting disability and mortality worldwide. As reported by the Heart Failure Society of America, the incidence of post-MI CHF has not changed over the years, despite significant advances in treating this disease in the acute phase [
1]. According to the Cardiovascular Disease in Norway (CVDNOR) Project data, 18.7% of patients experienced CHF during hospitalization for acute MI, 13% of patients – within 30 days of the index event, and 32.6% of patients – within a year of hospital discharge [
2].
LVEF is the central measure of left ventricular contractile function, which determines the prognosis of patients in the post-MI period [
3]. The frequency of systolic dysfunction ranges from 13-36% in the acute and subsequent MI stages, depending on its assessment criteria [
4,
5]. Wherein, being a significant predictor of mortality, reduced LVEF (<40%) is closely associated with an increase in the number of hospitalizations and low quality of life, as compared to preserved LVEF [
6].
Currently, transthoracic echocardiography is used both for initial assessment of ischemic injury and LV systolic and diastolic dysfunction [
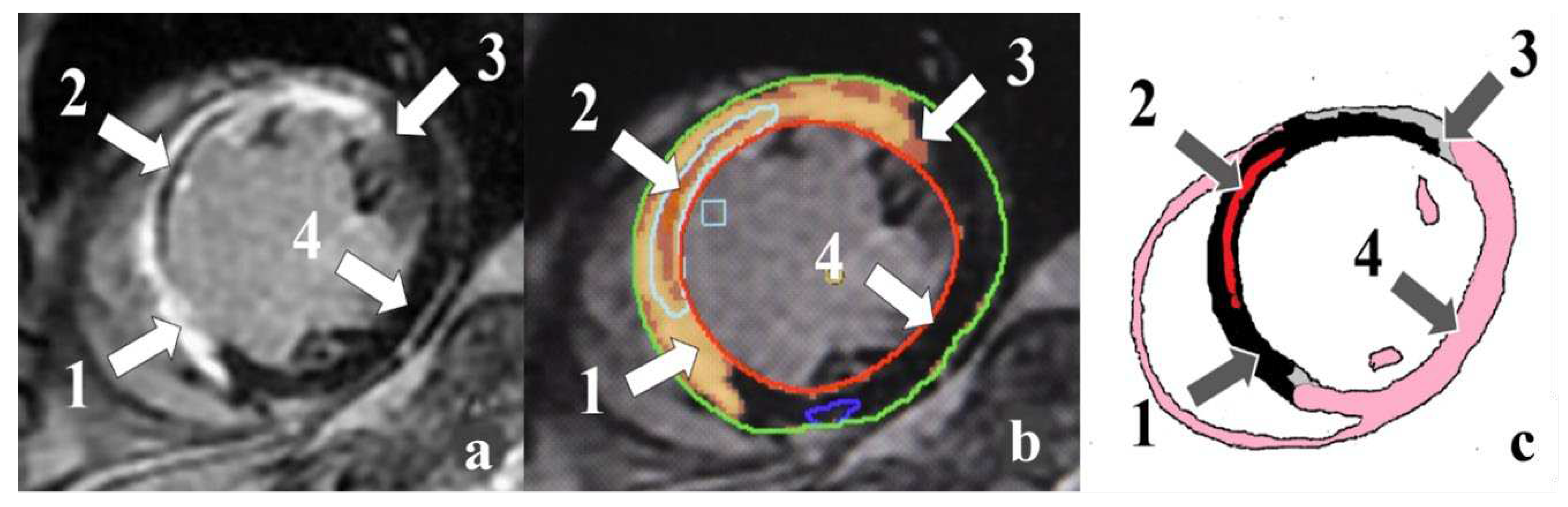
7]. An alternative to echocardiography is contrast-enhanced cardiac magnetic resonance imaging (MRI). This method allows estimating LV volumes and functions with high accuracy. Cardiac MRI provides a potential method to characterize scar and peri-infarct zones (PIZ), interstitial edema, microvascular obstruction (MVO), and intramyocardial hemorrhage (IMH) (
Figure 1) [
8].
The purpose of the study was to identify factors characterizing a decrease in LV global systolic function in patients with ST-segment elevation myocardial infarction (STEMI) after revascularization using cardiac MRI-based ischemic injury pattern and laboratory parameters sensu left ventricular global systolic function.
2. Material and Methods
A total of 109 STEMI patients were examined in the Emergency Cardiology Department of the Regional Clinical Hospital n. a. N.N. Burdenko (Penza, Russia) in 2020-2022 (protocol code CONTRAST-2, NCT04347434 at clinicaltrials.gov). The study protocol and the case report form were approved by the Local Ethics Committee of Penza State University. Written informed consent was obtained from all patients.
The study included patients having met the following criteria: aged 30-70 years; acute STEMI confirmed by an electrocardiogram, a diagnostically significant increase in troponin I, and the presence infarct-related artery determined by coronary angiography.
The exclusion criteria were as follows: repeated or recurrent MI; hemodynamically significant stenosis (>30%) of the left main coronary artery; pronouced concomitant diseases; NYHA classes III-IV of CHF.
All patients underwent coronary angiography. Primary percutaneous coronary intervention (PCI) on the infarct-related artery (IRA) was performed in 68 patients, and 41 patients experienced a pharmaco-invasive strategy.
The STEMI patients received treatment in compliance with current clinical practice guidelines.
The patients underwent contrast-enhanced cardiac MRI with a 1.5 Tesla GE SIGNA Voyager (GE HealthCare, USA) on the 7th-10th days from the onset of the disease. Being a paramagnetic contrast agent, Clariscan™ (gadoteric acid) (GE Healthcare, Norway), was used to enhance visualization in MRI procedures. A non-contrast scan protocol involved anatomical slices; LV long-axis cine images in 2- and 4-chamber projections; LV short-axis cine images; and T1-, T2-, and T2*-mapping. In accordance with post-contrast MRI studies, two-dimensional (2D) myocardial delayed enhancement (MDE) cardiac imaging was obtained along the short axis at the 7th minute and along the long axis in 2- and 4-chamber projections at the 10th-12th minute. Post-contrast T1 mapping was conducted in the two-dimensional modified Look-Locker imaging (2D MOLLI) sequence at the 15th minute. Scanning was done using MDE of fast-imaging employing steady-state acquisition (MDE FIESTA) sequences along the short axis at the 20th minute. Image processing was performed using cvi42
® software platform (Circle Cardiovascular Imaging Inc., Canada). The following LV indicators were determined: end-diastolic volume index (EDVI); end-systolic volume index (ESVI); LVEF; left ventricular mass index (LVMI); local contractility index (LCI). The ischemic injury pattern was analyzed by post-ischemic MDE, identifying scar zone and PIZ heterogeneity, MVO, and IMH. A quantitative assessment of necrosis and PIZ masses and sizes relative to LV myocardial mass as well as MVO and IMH masses and sizes relative to the scar tissue mass was carried out. The lesion depth was studied using the global contrast index (GCI) [
9].
The severity of coronary artery lesion was determined by quantitative coronary angiography in accordance with the SYNTAX score scale.
N-Terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity C-reactive protein (hsCRP) were assessed in the blood on the 7th-9th days. High-sensitivity troponin I (hsTI) was recorded three times, the maximum values of the indicator being presented in the paper.
Statistical data analysis was carried out using STATISTICA 13.0 software package (StatSoft, USA). Quantitative data were presented as the mean and standard deviation (M±SD) with normal distribution or as the median and interquartile range (Me (Q 25%; Q 75%)) with nonparametric distribution. The significance of differences between parametric data was assessed using the Student’s t-test, and the significance of differences between nonparametric data was evaluated by the Mann–Whitney U test. Qualitative data were compared using the Chi-squared test (χ2). The logistic regression analysis was used to identify variables predicting a decrease in LVEF. Statistical significance was assumed at p<0.05.
3. Results
According to cardiac MRI analysis, the patients were divided into the following groups with regard to LVEF values: group 1 – patients with LVEF ≥50% (n=74); group 2 – patients with mildly reduced LVEF 40-49% (n=27); group 3 – patients with low LVEF <40% (n=8). There was neither difference between the compared groups in certain anthropometric and anamnestic data (
Table 1). The patients in group 3 were older than those in group 1. Female trial participants were only observed in patient group with LVEF ≥50%.
According to coronary angiography data, hemodynamically significant stenosis was detected in 50% of cases within one coronary artery; 32% – within two coronary arteries; 18% – within three or more coronary arteries (group 1); 30%, 37%, and 33% (group 2); 25% (р1-2;1,2-3>0.05), 25% (р1-2;1,2-3>0.05), and 50% of cases (р1-3=0.032), respectively (group 3). When analyzing the SYNTAX score scale, the lowest number of points was observed in patients with LVEF ≥50% versus other groups. A lesion of anterior descending artery (ADA) as the IRA was detected significantly more often in the group with low LVEF as compared to the group with preserved LVEF. In turn, atherothrombosis of the right coronary artery (RCA) was observed in 37.8% of patients only in group 1. Wherein, there were no cases of RCA atherothrombosis in group 3.
A pharmaco-invasive strategy was used as a reperfusion therapy in patients with LVEF <40%. However, pain-to-needle time was 2.5 times longer in this patient group versus group 1. Pain-to-balloon time in patients with LVEF 40-49% exceeded that in group 1 with LVEF ≥50%.
According to cardiac MRI analysis, the compared groups differed in the majority of the analyzed structural and functional LV characteristics (
Table 2). With comparable EDVI, the patients with LVEF ≥50% had the lowest ESVI values; the intermediate values were recorded in the group with LVEF 40-49%, and the highest values were observed in the patients with LVEF <40%. Similar differences were found for LCI. LVMI predominated in patients with low LVEF as compared to the group with preserved LVEF.
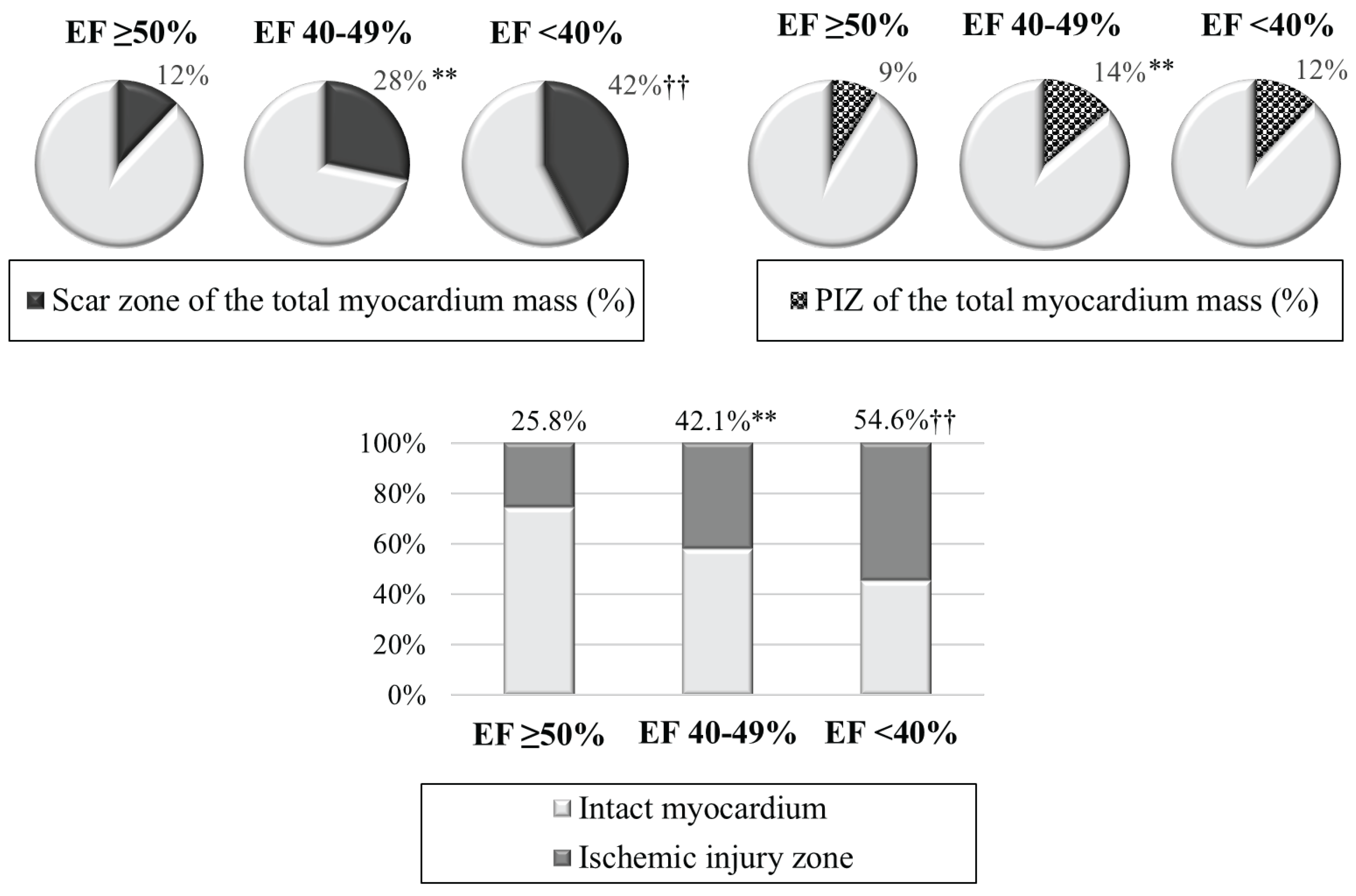
When analyzing ischemic injury pattern, the worst infarct zone characteristics were revealed (
Table 2,
Figure 2). The values of the scar tissue mass were minimal in group 1; intermediate values – in group 2; and maximum values – in group 3. The size of the scar tissue of the total myocardial mass (%) prevailed in patients with LVEF 40-49% and LVEF <40% versus patient group with LVEF ≥50%. As for PIZ characteristics, the differences were only found between patients with preserved and mildly reduced LVEF. The infarct zone, including the scar tissue and PIZ, occupied an average of 25.8% of the total myocardial mass in the group with LVEF ≥50%; whereas it was 1.6 times larger with LVEF 40-49%, and 2.1 times larger in the group with LVEF <40%.
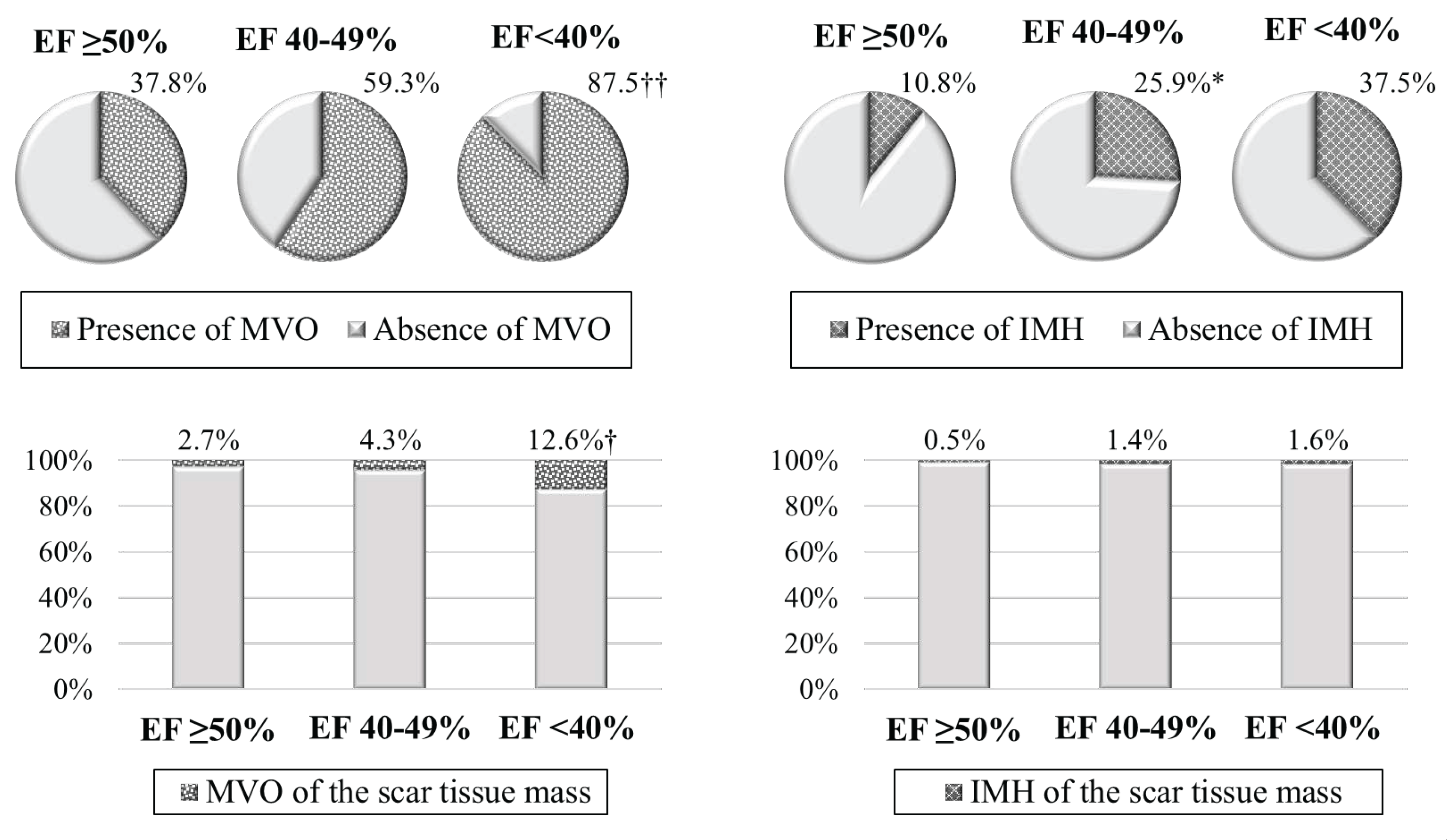
In contrast to the group with preserved LVEF, the majority of patients with low LVEF experienced MVO (
Figure 3). However, when analyzing MVO mass, its values were minimal in group 1; intermediate values – in group 2; and maximum values – in group 3. The frequency of IMH detection prevailed in patients with mildly reduced LVEF versus patient group with LVEF ≥50%.
Notable results were obtained when analyzing the GCI. The lowest values were found in group 1; intermediate values – in group 2; and the highest values – in group 3.
In the early period of MI, hsTI amounted to 23,719.6 (5,497.0; 49,239.2) pg/mL in patients with LVEF ≥50%; 21,217.4 (8,663.0; 84,972.0) pg/mL – in patients with LVEF 40-49%; 133,112.5±98,672.5 pg/mL (р1,2-3<0.05) – in patients with LVEF <40%. Similar differences were also noted for BNP. Its indicator was equal to 154.0 (61.2; 373.9) ng/mL in group 1; 274.4 (196.4; 703.7) ng/mL – in group 2; 669.3 (276.1; 700.0) ng/ml (р1-3=0.035) – in group 3. The groups did not differ in hsCRP levels.
Risk factors for a decrease in LVEF <50% in patients with MI were identified using univariate linear regression analysis (
Table 3). The largest regression coefficient
β (>0.500) was observed for the scar tissue mass, for the total mass of ischemic injury and the size thereof of the total myocardium mass (%), for GCI, and LCI. A less pronounced relationship was noted for PIZ, MVO, and IMH characteristics. Gender, the number of points on the SYNTAX score scale, type of IRA, and the time interval from the onset of anginal attack to revascularization were also associated with unfavorable values of LV global systolic function. The laboratory parameters for NT-proBNP and hsTI levels evidenced an autonomous position thereof in reducing LVEF <50%.
4. Discussion
Cardiac MRI is considered the “gold” standard for assessing such LV characteristics as volumes, LVEF, and myocardial mass. It is recommended for the diagnosis of CHF in patients with suboptimal visualization based on transthoracic echocardiography. High MRI resolution provides for highly accurate and reproducible quantification of the above parameters [
7]. In the present study, an increase in ESVI and LCI in patients with LVEF <40% was common manifestation of a decrease in LV systolic function, and myocardial hypertrophy did not increase its contractility.
Cardiac MRI is the most advantageous technique among the methods for visualizing post-MI changes due to the unique characteristics of myocardial tissue, high resolution, and the ability to quantify myocardial injury. In patients with MI, cardiac MRI can be used to determine the size of the infarction and the intact myocardium, MVO, and IMH, i.e., the main significant prognostic injury markers [
8,
9,
10].
The sizes of infarct zones visualized by MRI were calculated in grams or as a percentage of the LV mass. Local contrast enhancement can be used to detect infarcts as small as 1 g, subendocardial infarction being an example [
11]. In the present study, the scar tissue mass in the patient groups with LVEF 40-49% and LVEF <40% was 2.6 times and 4 times, respectively, larger than that in the patients with preserved LVEF.
Post-ischemic myocardial injury is morphologically heterogeneous. In addition to the infarct core, its structure includes PIZ, an area next to the intact myocardium, consisting of necrotic, ischemic, and intact cardiomyocytes [
12]. In this study, the patients with mildly reduced LVEF were distinguished by higher values of PIZ mass and size of the total LV myocardium mass versus group 1. According to Jensch P.J. et al., PIZ volume >14 mL was considered as a predictor of major adverse cardiovascular events such as death, recurrent MI, and congestive heart failure within one year after MI [
13]. According to Jones R.E. et al., in patients with stable coronary artery disease (CAD), PIZ and infarct core masses were independent predictors of sudden cardiac death [
14].
In MI patients with LVEF >50% who underwent primary PCI, the presence and severity of MVO were associated with the development of fatal and non-fatal cardiovascular events during the 5-year monitoring [
15]. Notably, MVO volume ≥2.6% of LV mass improved long-term risk stratification of adverse outcome after STEMI compared with such standard MRI indicators as LV volumes and LVEF [
16]. The combination of MVO and IMH is a predictor of decreased LVEF in patients with primary STEMI. In addition, the correlation analysis results revealed a relationship between the area of IMH based on MRI, and LV systolic dysfunction based on echocardiography [
17].
According to the results of this study, the frequency of MVO and its size relative to the scar tissue mass (%) prevailed in group 3 versus group 1. In addition, a progressive increase in MVO mass was revealed with a decrease in systolic LVEF. When comparing IMH, lesser variations were obtained. In particular, IMH was identified 2.4 times more frequently in patients with LVEF 40-49% versus patient group with LVEF ≥50%. There were no differences recorded in the quantitative characteristics of this parameter.
The GCI serves as an indicator of the injury depth in MRI [
8]. An increase in the frequency of MVO and higher values of myocardial necrosis markers were noted in patient group with a high GCI level [
9]. In this study, the severity of systolic dysfunction was associated with progressive deterioration of GCI. The level of LVEF<40% was characterized by the most adverse parameter values.
Key diagnostic biomarkers used in routine clinical practice for patients with various CAD forms include cardiospecific isoforms of troponins and natriuretic peptides. Troponin I is recommended to be determined both for the diagnosis of myocardial injury in acute coronary syndrome and for assessing the risk of adverse cardiovascular events in the general population [
18]. A number of studies have revealed a positive correlation between the mass of scar tissue, PIZ, and MVO according to MRI and the level of troponin I in patients with MI [
9,
19]. In patients without cardiovascular disease at the time of their inclusion in the study, the initially higher NT-proBNP was associated with a decrease in LVEF and the risk of myocardial scar formation according to MRI after 10 years of observation [
20]. We have established the predominance of both hs TI and NT-proBNP in the group of patients with LVEF <40%.
5. Conclusion
In this study, the presence of LV systolic dysfunction in patients with primary STEMI was distinguished by natural deterioration of structural and volumetric parameters, and a decrease in the efficiency of cardiac contraction according to MRI.
A predominance of most parameters of the ischemic injury pattern was noted in patients with mildly reduced and low LVEF versus patient group with LVEF ≥50%. Differences in the ischemic injury mass were recorded between the groups with LVEF 40-49% and LVEF <40% due to scar tissue, MVO mass, LCI and GCI, which indicates transmural injury.
The following risk factors for a decrease in LVEF <50% systolic function in STEMI patients after revascularization were revealed: male gender; time from the onset of the anginal attack to revascularization; coronary artery status; ESVI; LVMI; ischemic injury characteristics, including scar tissue and PIZ; presence and severity of MVO; IMH mass; global contrast and local contractility indices; NT-proBNP and hs TI levels.
Supplementary Materials
Figure S1: Cardiac MRI-based ischemic injury pattern (own observation): a – myocardial delayed enhancement; b – post-processing; c – schematic representation. Infarct zones: 1 – necrosis; 2 –microvascular obstruction; 3 – peri-infarct zone heterogeneity; 4 – intact myocardium. Figure S2: Char-acteristics of ischemic injury zone in the compared groups. Note: **р<0.01 – significant differences between group 1 and group 2; ††р<0.01 – significant differences between group 1 and group 3; LVEF – left ventricular ejection fraction; PIZ – peri-infarct zone heterogeneity. Figure S3: Characteristics of reperfusion injury in the compared groups. Note: *р<0.05 – significant differences between group 1 and group 2; †р<0.05, ††р<0.01 – significant differences between group 1 and group 3; IMH – intramyocardial hemor-rhage; LVEF – left ventricular ejection fraction; МVО – microvascular ob-struction. Table S1: Comparative characteristics of groups. Table S2: Car-diac MRI indicators in the compared groups. Table S3: Risk factors for a decrease in LVEF <50% in patients with acute MI based on univariate lin-ear regression analysis data
Author Contributions
Salyamova L.I. Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing. Oleynikov V.E. Conceptualization, methodology, validation, resources, data curation, writing—original draft preparation, writing—review and editing, supervision, project administration, funding acquisition. Donetskaya N.A. Formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, Vdovkin A.V. Formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, Chernova A.A. Software, formal analysis, data curation, writing—original draft preparation. Avdeeva I.V. Validation, methodology, resources, data curation, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding source
The research was supported by the grant from the Russian Science Foundation, project No. 23-
25-00381.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Penza State University. Protocol code CONTRAST-2 (NCT04347434 at clinicaltrials.gov, date of approval 27Sep2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Declaration of Conflicting Interests
The authors declare no conflicts of interest.
References
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; Ibrahim, N.E.; Jones, L.M.; Khan, S.S.; Khazanie, P.; Koelling, T.; Krumholz, H.M.; Khush, K.K.; Lee, C.; Morris, A.A.; Page, R.L. 2nd.; Pandey, A., Piano, M.R., Stehlik, J., Stevenson, L.W., Teerlink, J.R., Vaduganathan, M., Eds.; Ziaeian, B. Writing Committee Members. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412-1451. [Google Scholar] [CrossRef]
- Sulo, G.; Igland, J.; Vollset, S.E.; Nygård, O.; Ebbing, M.; Sulo, E.; Egeland, G.M.; Tell, G.S. Heart Failure Complicating Acute Myocardial Infarction; Burden and Timing of Occurrence: A Nation-wide Analysis Including 86 771 Patients From the Cardiovascular Disease in Norway (CVDNOR) Project. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Krikunov, P.V.; Vasyuk, Yu.A.; Krikunova, O.V. Predictive value of echocardiography in post myocardial infarction setting. Part 1. Russ. J. Cardiol. 2017, 12, 120–128. [Google Scholar] [CrossRef]
- Hamilton, E.; Desta, L.; Lundberg, A.; Alfredsson, J.; Christersson, C.; Erlinge, D.; Kellerth, T.; Lindmark, K.; Omerovic, E.; Reitan, C.; Jernberg, T. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail. 2023, 10, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Shalaby, G. Severe Left Ventricular Dysfunction Earlier after Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention: Predictors and In-Hospital Outcome- A Middle Eastern Tertiary Center Experience. J. Saudi Heart Assoc. 2023, 34, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Im, M.S.; Kim, H.L.; Kim, S.H.; Lim, W.H.; Seo, J.B.; Chung, W.Y.; Zo, J.H.; Kim, M.A.; Park, K.W.; Koo, B.K.; Kim, H.S.; Chae, I.H.; Cho, D.J. ; Ahn, Y; Jeong, M.H. Other Korea Acute Myocardial Infarction Registry (KAMIR) and Korea Working Group on Myocardial Infarction (KorMI) Investigators. Different prognostic factors according to left ventricular systolic function in patients with acute myocardial infarction. Int. J. Cardiol. [CrossRef]
- Pozo, E.; Sanz, J. Imaging techniques in the evaluation of post-infarction function and scar. Rev. Esp. Cardiol (Engl Ed). [CrossRef]
- Souto, A.L.M.; Souto, R.M.; Teixeira, I.C.R.; Nacif, M.S. Myocardial Viability on Cardiac Magnetic Resonance. Arq. Bras. Cardiol. 2017, 108, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Mochula, O.V.; Sulejmanova, A.S.; Sukhareva, A.E.; Ryabov, V.V.; Zavadovsky, K.V. Relationship between the degree of myocardial damage according to contrast-enhanced cardiac magnetic resonance imaging and laboratory data in patients with acute myocardial infarction. Russ. J. Cardiol. 2022, 27, 5226. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation. 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Locca, D.; Bucciarelli-Ducci, C.; Ferrante, G.; La Manna, A.; Keenan, N.G.; Grasso, A.; Barlis, P.; Del Furia, F.; Prasad, S.K.; Kaski, J.C.; Pennell, D.J.; Di Mario, C. New universal definition of myocardial infarction applicable after complex percutaneous coronary interventions? JACC Cardiovasc. Interv. 2010, 3, 950–8. [Google Scholar] [CrossRef] [PubMed]
- Robbers, L.F.H.J.; Delewi, R.; Nijveldt, R.; Hirsch, A.; Beek, A.M.; Kemme, M.J.B.; van Beurden, Y.; van der Laan, A.M.; van der Vleuten, P.A.; Tio, R.A.; Zijlstra, F.; Piek, J.J.; van Rossum, A.C. Myocardial infarct heterogeneity assessment by late gadolinium enhancement cardiovascular magnetic resonance imaging shows predictive value for ventricular arrhythmia development after acute myocardial infarction. Eur. Heart J. Cardiovasc. Imaging. 2013, 14, 1150–1158. [Google Scholar] [CrossRef]
- Jensch, P.J.; Stiermaier, T.; Reinstadler, S.J.; Feistritzer, H.J.; Desch, S.; Fuernau, G.; de Waha-Thiele, S.; Thiele, H.; Eitel, I. Prognostic relevance of peri-infarct zone measured by cardiovascular magnetic resonance in patients with ST-segment elevation myocardial infarction. Int. J. Cardiol. 2022, 347, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Zaidi, H.A.; Hammersley, D.J.; Hatipoglu, S.; Owen, R.; Balaban, G.; de Marvao, A.; Simard, F.; Lota, A.S.; Mahon, C.; Almogheer, B.; Mach, L.; Musella, F.; Chen, X.; Gregson, J.; Lazzari, L.; Ravendren, A.; Leyva, F.; Zhao, S.; Vazir, A.; Lamata, P.; Halliday, B.P.; Pennell, D.J.; Bishop, M.J.; Prasad, S.K. Comprehensive Phenotypic Characterization of Late Gadolinium Enhancement Predicts Sudden Cardiac Death in Coronary Artery Disease. JACC Cardiovasc. Imaging. 2023, 16, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Galea, N.; Dacquino, G.M.; Ammendola, R.M.; Coco, S.; Agati, L.; De Luca, L.; Carbone, I.; Fedele, F.; Catalano, C.; Francone, M. Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur. Radiol. 2019, 29, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Pontone, G.; Schwitter, J.; Francone, M.; Iglesias, J.F.; Barison, A.; Zalewski, J.; de Luca, L.; Degrauwe, S.; Claus, P.; Guglielmo, M.; Nessler, J.; Carbone, I.; Ferro, G.; Durak, M.; Magistrelli, P.; Lo Presti, A.; Aquaro, G.D.; Eeckhout, E.; Roguelov, C.; Andreini, D.; Vogt, P.; Guaricci, A.I.; Mushtaq, S.; Lorenzoni, V.; Muller, O.; Desmet, W.; Agati, L.; Janssens, S.; Bogaert, J.; Masci, P.G. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance After ST-Segment Elevation Myocardial Infarction: A Study of the Collaborative Registry on CMR in STEMI. JACC Cardiovasc. Imaging. 2018, 11, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, Ya.V.; Vyshlov, E.V.; Mochula, O.V.; Ussov, V.Yu.; Ryabov, V.V. Effect of intramyocardial haemorrhage on structural and functional echocardiographic parameters of myocardium after ST-segment elevation myocardial infarction with. Russ. J. Cardiol. 2020, 25, 4032. [Google Scholar] [CrossRef]
- Kontsevaya, A.V.; Myrzamatova, A.О.; Drapkina, O.M. Biomarkers in predicting cardiovascular risk: new prospects of troponin I. Cardiovascular Therapy and Prevention. 2020, 19, 2584. [Google Scholar] [CrossRef]
- Salatzki, J.; Giannitsis, E.; Hegenbarth, A.; Mueller-Hennessen, M.; André, F.; Katus, H.A.; Frey, N.; Biener, M. Correlation of serial high-sensitivity cardiac Troponin T values to infarct mass determined by cardiac magnetic resonance imaging: a validation study. Eur. Heart J. Acute Cardiovasc. Care. 2022, 11, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Rahsepar, A.A.; Bluemke, D.A.; Habibi, M.; Liu, K.; Kawel-Boehm, N.; Ambale-Venkatesh, B.; Fernandes, V.R.S.; Rosen, B.D.; Lima, J.A.C.; Carr, J.C. Association of Pro-B-Type Natriuretic Peptide With Cardiac Magnetic Resonance-Measured Global and Regional Cardiac Function and Structure Over 10 Years: The MESA Study. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).