1. Introduction
Sorghum is a genus of plants belonging to the family of
Poaceae, widely used as forage crop as well as human food and for biofuel production. Globally, the most cultivated species is
Sorghum bicolor (L.) Moench, also known as broomcorn or the great millet, that is particularly spread in the Americas and Africa, which in 2017-2021 accounted together for more than 80% of total production. In 2021, Europe produced 1.9% of global Sorghum, with France being the first country with 386,040 t [
1]. In Italy, the second European producer, Sorghum yielding reached 242,855 t in 2023, being the fourth cereal after wheat, corn and rice [
2]. Some wild species are also exploited for animal feeding, like the widespread
Sorghum halepense (L.) Pers., commonly referred to as Johnson grass, originating from the Mediterranean and Western Asia regions and now reported as an invasive weed across all the continents [
3].
When used as feed, Sorghum management must be particularly careful because of a cyanogenic glycoside called dhurrin ((S)-4-Hydroxymandelonitrile β-D-glycoside) [
4], which is synthesised as a secondary metabolite in its tissues. This molecule contains a cyanide group (CN¯) that can be released upon hydrolysis and is extremely toxic to every eukaryotic cells. CN¯ inhibits cellular respiration by binding to the Fe
+++ of cytochrome oxidase resulting in cell inability to utilize molecular oxygen and ultimately to synthetise ATP [
5]. Rumen microbiota is able to rapidly hydrolyse dhurrin, further accelerating cyanide leak, making therefore ruminants much more sensitive to CN¯ than monogastric species [
6,
7]. Such a rapid and massive CN¯ release can cause severe, often lethal, poisonings in ruminants, particularly upon the ingestion of large amounts of high dhurrin-containing fodder [
6].
In Sorghum plants dhurrin is produced especially during early growth phases [
5,
8]. Thanks to this glycoside,
Sorghum species are quite resistant to herbivores, including insects [
9]. Mature plants generally contain lower amount of dhurrin and are therefore considered safe for animal feeding; however, dhurrin content is reported to increase under the following conditions [
10]:
Prolonged drought, frost, wilting, chewing and any other condition causing plant cell injury;
Massive herbicide treatments;
Extensive use of nitrogen-based fertilizers.
When used as feed, Sorghum can be directly grazed by animals, or harvested for green forage, silage and hay production. Generally, ensiling process leads to a dispersal of CN¯ from plant tissues. Still, in some cases high CN¯ concentrations could remain in plants that have undergone rapid desiccation and subsequent conservation in large bales [
9]. Because of CN¯ poisoning potential, Sorghum harvesting and its use requires a cautious management in order to minimise intoxication risks, with special attention when used for feeding ruminants. Young leaves and new shoots, including the sprouts, are the most dangerous parts as they can concentrate large amounts of dhurrin [
6].
Although Sorghum toxicity is long known, no poisoning cases in bovines have been reported in Europe in the last decades [
11,
12], with the exception of two cases in Spain quoted in a review on plant poisoning [
13]. A search on grey literature also revealed no results in Europe, but several cases in both the Americas and Australia [
14]. Likewise, data on Sorghum poisonings were found -both in scientific databases and online search engines- in extra-European countries, especially in semi-arid regions from South America [
15,
16,
17] and India [
18,
19,
20], where Sorghum cultivation for fodder purposes is common.
In August 2022, 66 bovines died in Piedmont (a region in North-western Italy) after being exposed to
S. bicolor x
S. sudanense -i.e.,
S. bicolor ssp. sudanense (P.) Stapf- cultivar called Suzy [
21] or to forage containing
S. halepense. The aim of this study is to provide a detailed overview of this outbreak, with special emphasis on the diagnosis and the therapeutic management of the toxicosis. Results of dhurrin concentration monitoring from August to November 2022 in both cultivated and wild
Sorghum samples from the affected farms and elsewhere are also presented. A preliminary short report of the outbreak has been published in Italian in 2023 [
22].
1.1. Poisoning cases (August 2022)
Five outbreaks of Sorghum poisoning occurred in August 2022 in Piedmont.
Figure 1 shows the epidemiological data concerning the poisoning cases.
1.1.1. Case A - 6th of August: Sommariva del Bosco (Cuneo)
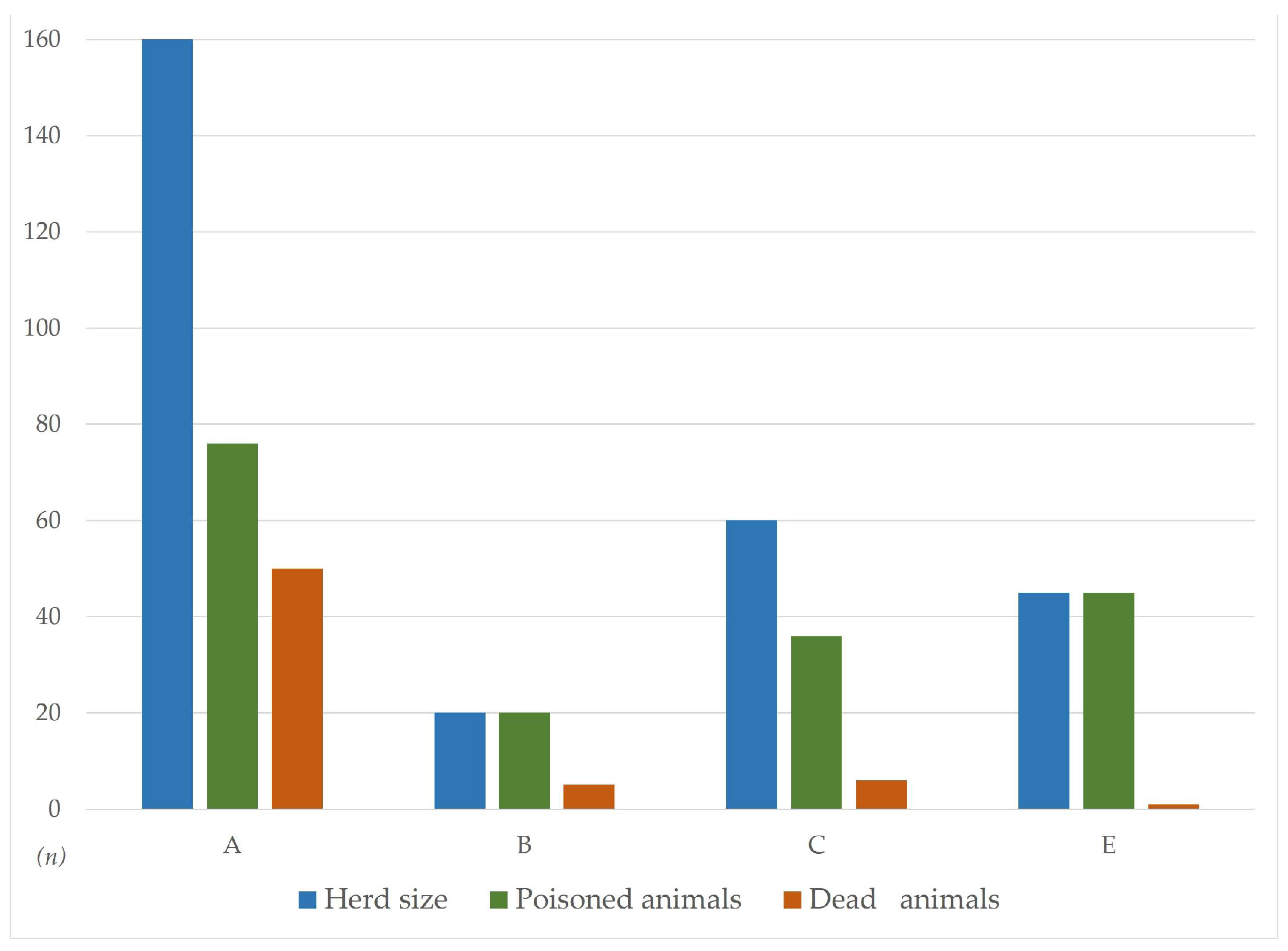
A herd of 160 cows, mainly of Piedmontese breed, was allowed free access to a field entirely cultivated with
S. bicolor x
S. sudanense cultivar Suzy. As animals were hungry due to overnight fasting, they rapidly ingested Sorghum plants, and namely sprouts with a height range of 30-45 cm. Around half of the animals were poisoned; forty-six of them rapidly died 20-30 min after the ingestion (
Figure 2a), while in 4 further individuals death ensued in the following hours. Most of the dead individuals were pregnant. The survived ones were immediately moved away. Based on the clinical picture, the sudden deaths and the gross lesions (see below), a cyanogenic glycoside poisoning was promptly suspected.
1.1.2. Case B - 11th of August: Moretta (Cuneo)
A group of 20 adult mixed breeds cows and bulls (mainly Friesian x Piedmontese or other meat breeds) housed in tie stalls were fed green chop (fresh forage) mainly composed by
S. halepense. This is a common farming practice in Piedmont especially during the warm season, when green and high-quality forage is scarce. All the affected animals were lactating cows aged more than 3 years; five of them suddenly died after being offered the contaminated feed (
Figure 2b). The forage was promptly removed from the troughs after the onset of the clinical signs of poisoning.
1.1.3. Case C - 11th of August: Bra (Cuneo)
Sixty adult cows of Piedmontese breed housed in a free stall barn were fed green chop, mainly consisting of Johnson grass. Poisoning signs were noticed during the subsequent night in 36 individuals: 4 of these suddenly died after grass ingestion, while in 2 further individuals death ensued few days later. As in case B, the forage was removed after the first symptoms and no further mortality was recorded.
1.1.4. Case D - 12th of August: Asti
This case occurred in a cow-calf operation farm consisting of about 60 Piedmontese breed heads (cows and calves) housed in a free stall barn. Animals are daytime allowed to graze on pastures in the proximity of the farm for most of the year. Four cows died after the ingestion of S. halepense, which was found to contaminate the pasture. This episode was lately and poorly reported to the veterinarians, so that it was not possible to collect reliable epidemiologic information.
1.1.5. Case E - 25th of August: Cossato (Biella)
Farm characteristics are similar to that from case D, i.e. a cow-calf operation farm with about 45 heads mainly of the Piedmontese breed (but also meat crossbreds). For most of the year, animals are free to graze on pastures surrounding the farm. All cows showed the typical signs of cyanide poisoning, mainly respiratory distress and tendency to recumbency; overall, symptoms were less severe than in case A, B and C, resulting in the loss of just one cow. Also in this case, the cause of poisoning was pasture contamination with S. halepense.
2. Materials and Methods
2.1. Necropsies and histological analysis
Due to unfavourable conditions (high external temperatures and the limited availability of veterinarians) necropsies were performed on few animals (N=6 in total) directly at farms. Heart, lung, brain, liver, kidney, spleen, reticulum, rumen, omasum, abomasum, and intestine samples were collected, fixed in 10% buffered formalin (4% formaldehyde), dehydrated and embedded in paraffin wax blocks. Each sample was then sectioned at 4-5 µm-thickness, mounted on glass slides and stained with haematoxylin and eosin to reveal histopathological alterations. Slides were examined by two independent veterinary pathologists.
2.2. Sorghum sample collection
To confirm the suspect of cyanogenic glycoside poisoning, samples of Sorghum cattle were exposed to were collected at each farm involved in the outbreak (
Figure 3) and submitted for dhurrin determination (see below). It was also decided to collect and analyse additional specimens of both wild and cultivated Sorghum in order to measure dhurrin content in plants from different areas of the Piedmont region. In particular, the selection process was based on three main factors:
In addition, in certain instances samples were collected from different plant portions and at diverse growth stages (
Figure 3).
As regards cultivated Sorghum, the
S. bicolor x
S. sudanense cultivar Suzy was involved in case A. Two cultivars,
S. bicolor ssp. drummondii Piper and
S. bicolor x
S. sudanense Sudal [
24], were then sampled in a farm in Montechiaro d’Asti (Asti), which was experiencing similar drought conditions as the farm of case A; Sorghum had not yet been harvested due to the severe outbreak occurred in Sommariva del Bosco.
The common Johnson grass, which is frequently used as fodder by Piedmontese farmers, was the cause of poisoning cases B, C, D and E. Further sites for S. halepense sampling were Verrua Savoia (Torino province), Montiglio Monferrrato (Asti province), Cuneo, Faule, Sampeyre and Sanfrè (Cuneo province).
One pooled sample composed of a minimum of 500 g of fresh plant materials was collected randomly from different areas inside pasture fields or directly taken from the green forage offered to animals in the stalls. In one case (A) also the rumen content was collected from a dead cow. The whole sampling activities were accomplished from August to November 2022.
2.3. Dhurrin determination
Samples were analysed using an in-house liquid chromatography-tandem mass spectrometry (LC-MS/MS) method at the National Reference Laboratory for Plant Toxins, Food Chemical Department of Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna (IZSLER), located in Bologna. Samples were grounded into flour and 1 ± 0.1 g of each one was extracted with 6 mL of aqueous methanol (80%). The sample was shaken vigorously for 30 seconds and placed in an ultrasonic water bath for 15 minutes. The mixture was centrifugated for 5 min at 4000 x g and the supernatant was collected in another tube. This extraction was repeated twice, the supernatant was combined and made up to a volume of 20 mL with water. Thereafter, 1 mL of solution was evaporated to dryness under a stream of nitrogen at 40°C. The residue was dissolved in 0.5 mL of 10% methanol aqueous solution, diluted and analysed by using LC-MS/MS.
The LC-MS/MS analysis was performed on a XEVO Tq-XS Acquity ultra-performance liquid chromatograph (UPLC) I Class Plus Waters (Milford, MA, USA). The chromatographic separation was achieved on an Acquity UPLC C8 BEH 100 mm × 2.1 mm, 1.7 µm column (Water Corporation, Milford, MA, USA). Data acquisition and processing were carried out by MassLynx software v. 4.2. SCN1012. The mobile phase A consisted of 0.1% formic acid in water/acetonitrile (95:5, v/v), and the mobile phase B consisted in 0.1% acid formic in acetonitrile. The following gradient was used: 0-0.5 min, isocratic 2% B; 0.5-4 min linear gradient 2-50% B, it returned to initial conditions in 0.5 min and holding for 1 min. The total run time was 6 min. The flow rate was 0.4 mL/min. The injection volume was set at 5µL. The ESI source operated in positive ionization mode with the following instrumental parameters: capillary voltage of 0.5 kV, cone voltage of 40 V, source temperature of 120°C, and a desolvation temperature of 600°C. The conditions of ionization and PA fragmentation were identified by continuous infusion of the tuning solutions and gradual adjustment of the parameters. According to SANTE/12089/2016 [
25], dhurrin was identified by the retention time, ion fragments and ion ratio. The retention time was within ± 0.2 min of the reference peaks. The peaks showed similar shapes and overlapped with each other. The ion ratio was within ± 30% of the average of the calibration standards from the same sequence. The peaks were within the linear range of the detector with an S/N ≥ 3 [
26]. The LC-MS/MS method’s selectivity was evaluated by acquiring the data in MRM mode and monitoring one precursor ion and two daughter ions for each molecule [
25].
Multi-level calibration curve-concentration levels from lowest to highest (0.2-0.5-1-2.5-5-10-15 µg/mL) was prepared in 10% methanol aqueous solution. A correlation coefficient (R
2) ≥ 0.99 and a normal distribution of residuals lower than 20% were achieved into every analytical batches. The calibration curve, a representative chromatogram of dhurrin reference material (2.5 µg/mL) and a chromatogram of a Sorghum sample are shown in
Figure S1, S2 and S3.
The limit of quantification (LOQ) of dhurrin in feed was 50 mg/kg, corresponding to 4.3 mg/kg hydrogen cyanide (HCN), i.e. cyanide. It has been evaluated under conditions of accuracy and precision, verifying signal-to-noise ratio to be at least equal to 10. The recovery % (70-120) of the quality control spiked at LOQ was in line with the guidance document on performance criteria of the European Union Reference Laboratory for Mycotoxin and Plant Toxins [
26]. According to EFSA Journal [
27], 1 g of dhurrin releases 86.7 mg of HCN potential, representing the total amount of HCN released under conditions of complete hydrolysis of the present dhurrin. For the sake of simplicity, in this paper the HCN potential is referred to as HCN/cyanide concentration.
2.4. Clinical picture
Poisoned bovines showed multiple symptoms, with variable distribution among individuals. Many cows were found in sternal or lateral recumbency, mainly on the right side. Respiratory distress was observed in most of the poisoned animals consisting in tachypnoea, dyspnoea, panting and gasping. Several cows also displayed astonishment, convulsions, and muscle twitching with vocalizations (mooing). Sialorrhea was an additional common symptom among intoxicated bovines. Moreover, light to moderate tympanism was detected in a few individuals. Hyperthermia, nystagmus, mydriasis and wheezes were occasionally observed.
2.5. Therapeutical protocols
Table 1 depicts the treatment performed in each case and the relative success rate.
2.5.1. Case A
Although, as mentioned above, a cyanogenic glycoside poisoning was suspected, it was difficult to find the proper remedies also because this outbreak happened during the weekend. Thirty animals were treated intravenously with a mix of rehydrating solutions (Ringer’s lactate, physiological and glucose solutions), coupled with 60 mL of the multivitamin Dobetin B1® (cyanocobalamin 1 mg/mL, thiamine hydrochloride 100 mg/mL). Considered the hot external temperature -over 38 °C-, the cows were also cooled down by spraying with water taken from the mobile drinking troughs. Twenty-six of the treated animals survived.
2.5.2. Case B
Owing to the similarity with the clinical picture described for the Sommariva del Bosco poisoning (case A) and based on the first analytical results revealing the massive presence of dhurrin in sorghum samples from that case, the antidotal therapy was immediately started. However, due to the limited availability of sodium thiosulphate (Na
2S
2O
3), it was decided to treat only the most severely affected individuals (
n=5), lying in sternal/lateral recumbency with panting and vocalizations. Antidote solution was prepared by dissolving 5 g Na
2S
2O
3 in 4 L of Ringer’s lactate, which was slowly administered i.v. (
Figure 4).
Further 15 g of Na2S2O3 were dissolved in 10 L of cold water and then given orally through drench guns. After 10-15 min from antidote administration, breathing started to improve and vocalizations almost ceased; cows were again able to stand in about one hour.
2.5.3. Case C
As mentioned above, poisoning symptoms were noticed during the night and this implied difficulties in getting Na2S2O3 in sufficient amounts to treat all the affected animals (N=30). It was therefore decided to administer first methylene blue i.v. (10 g dissolved in 4 L of Ringer’s lactate), which however was only partially effective in reducing the severity of the clinical signs. As soon as Na2S2O3 was fully available (late in the morning), it was readily administered i.v. (5 g dissolved in 4 L rehydrating solution) to all previously treated cows. This led to a rapid improvement of the clinical picture as described for case B. Twenty-eight cows survived, while 2 died few days later.
2.5.4. Case D
No treatment was performed.
2.5.5. Case E
Due to the alert system set up on purpose to tackle the cyanogenic glycoside outbreaks, the antidote Na2S2O3 was made readily available to veterinarians. Accordingly, all poisoned animals were treated with the antidote as soon as 1 hr after the onset of clinical signs and a rapid recovery ensued within 2 hr from therapeutic intervention. Treatment schedule was the one detailed for case B.
4. Discussion
The rapid onset of clinical signs in cows shortly after the ingestion of Sorghum, followed sometimes by sudden death, had immediately suggested cyanide poisoning. Respiratory distress, stupor, sternal or lateral recumbency, convulsions, muscle tremors, and sialorrhea are typically reported in cyanide poisoning in cattle [
6,
29]. In addition, the recorded intense sweet odour of “bitter almonds”, the bright cherry red colour of venous blood, lung congestion and emphysema as well as the presence of froth in trachea are consistently recorded in cyanogenic glycoside poisoned bovines [
30]. The detected abomasitis, that featured oedematous-haemorrhagic and neutrophilic granulocyte infiltrations, has been also associated with cyanide poisoning [
6]. Finally, myocardial haemorrhages further pointed to cyanide poisoning [
31].
The gold-standard therapy for cyanide toxicosis [
6,
32] consists of supplying a chemical agent able to induce the formation of methaemoglobin (MetHb), i.e. oxidized (Fe
+++) haemoglobin, which is unable to bind O
2 and making it available to tissues. However, cyanide shows a higher affinity toward the Fe
+++ central haem iron of MetHb than the Fe
+++ of cytochrome oxidase. This causes the release of cyanide from the enzyme, the formation of cyanMetHb and the reactivation of cell respiration. MetHb formation in large animals may be primarily accomplished by administering sodium nitrite i.v. (10 to 20 mg/kg bw); this treatment should be repeated with great care, because of the danger of producing nitrite toxicosis, with further impairment of cellular respiration and severe hypotension [
30]. Methylene blue at high dosages (1 to 3 g/~250 kg bw) has been recommended as an alternative to nitrites [
31]. This treatment must be coupled with the sulphur donor Na
2S
2O
3, which, in the presence of rhodanese, reacts with HCN yielding thiocyanate (SCN¯); this metabolite lacks any detrimental effects on cellular respiration and is rapidly excreted via the kidneys. In the hereby reported cases, coupling methylene blue and Na
2S
2O
3 administration did not seemingly result in a visible improvement of the therapeutic efficacy; a significant and rapid relief of the clinical signs was indeed obtained only after Na
2S
2O
3 treatment, which was successfully used alone in cases B and E with 100% efficacy. It has been actually reported that in cattle there is no benefit in administering i.v. a MetHb-inducing agent over Na
2S
2O
3 alone [
32]. In addition, the prompt oral dosing with Na
2S
2O
3 may help in detoxifying HCN released in the rumen even before the onset of clinical signs [
33]. The overall good success of the antidotal treatment further confirmed the diagnosis of cyanide poisoning. It should be noted that treated cows from case A had a relatively high survival rate (87%) even though they did not receive specific antidotes, but only a palliative fluid therapy with a multivitamin complex. The prompt removal from the contaminated pasture, i.e. after the first sudden deaths, was likely the cause of the high recovery rate.
According to the European Directive 2002/32/EC [
34], a threshold of 50 mg/kg cyanide has been established for animal feed and raw materials. Under field conditions, concentrations over 200 mg/kg are considered sufficient to induce overt toxicosis [
6,
28,
31].
It is generally assumed that crop plants are less resistant to parasites and herbivores with respect to their wild counterparts due to the artificial genetic selection aiming at reducing the content of specific defence compounds (e.g., cyanogenic glycosides) that may prove harmful for humans and livestock [
35]. However, this assumption cannot be generalized for Sorghum. Unexpectedly, broomcorn cultivars such as Suzy (a
S. bicolor x
S. sudanense variety, Sommariva del Bosco, case A) and the mixture of Piper and Sudal (Montechiaro d’Asti), revealed very high HCN concentrations in August 2022. Both cultivars are specifically marketed for animal feeding purposes; however, guidelines for use reported on seeds’ envelopes do recommend not to feed animals when plants are below 70/80 cm (70 cm for the mixture Piper and Sudal, and 80 cm for Suzy) and yet without any information on the potential related danger [
36]. In case A, the farmer decided to allow his herd to graze on the field despite the sorghum plants were below the recommended height. As many other farmers during that summer, his farm was experiencing a shortage of forage due to its high cost and the scarce availability of green pastures. The increase in forage prices were a direct consequence of a lower offer on the market that, in turn, was caused by a diffuse drought particularly affecting the North-western of Italy. A parallel survey was conducted on cultivated hybrids (
S. bicolor ssp. Drummondii Piper and
S. bicolor x
S. sudanense Sudal) from different fields surrounding a farm in the Asti province (Montechiaro d’Asti) near to poisoning case D; HCN concentrations > 200 mg/kg were detected in 50% of specimens collected in August 2022, with peaks of 847-868 mg/kg. Overall, our findings confirm that bovines should not be fed on young plants even of cultivated hybrids, including regrowth after cutting, because of the high risk of cyanide poisoning.
In the outbreak of cyanogenic glycoside poisoning in cows described herein,
S. halepense was implied in 4 out 5 cases. Johnson grass is considered among the most invasive and dangerous weeds in Europe and extra European countries; beside the potential accumulation of toxic amounts of cyanogenic glycosides, several potentially adverse effects have been reported, including displacement of natural flora, competition with other crops, synthesis of allelochemicals interfering with crop growth, and hosting of plant pathogens (for a review, see Peerzada et al. 2017 [
37], and the numerous literature references therein). Despite that, the free growth of Johnson grass is rarely counteracted, and even, as reported in four cases (B to E), farmers traditionally employ Johnson grass as a fodder plant (hay or pasture) during periods of droughts. Similar to other Sorghum species, several factors, including soil chemical composition, plant age, use of nitrogen fertilizers, weather conditions as well as damage to plant tissues, are reported to affect dhurrin content and hence the potential HCN release in Johnson grass [
34]. There is scant information on dhurrin and HCN content of
S. halepense, particularly from European countries. In a study performed in India, calculated HCN concentrations (colorimetric method) of uncultivated Johnson grass from farm bunding averaged around 900 mg/kg at 30 days post weeding but fall to 120 mg/kg at 25% flowering stage [
38]. Therefore, similar to cultivated Sorghum species, cattle should not be fed with Johnson grass at the early stage of crop. In the outbreaks reported here, 3 poisoning cases concerned with
S. halepense revealed HCN concentrations in the range 419-690 mg/kg (cases B, C, D). The cause of the relatively low amount of HCN (below 50 mg/kg) detected in plant specimens from case E is probably attributable to uncorrected sampling procedures. For comparison, samples of
S. halepense were collected in a more scattered way during August and September 2022 in fields from farms located in different areas of Piedmont, even near to poisoning cases (Sanfrè, Faule, Montiglio Monferrato); of note, only in one case HCN amounts > 200 mg/kg were detected in plant specimens, likely pointing to the occurrence of different pedo-climatic conditions not resulting in remarkable accumulation of dhurrin as it was reported for the areas of the outbreak.
As a matter of fact, in summer 2022 unfavourable weather conditions were registered all across Europe, and Northern Italy, particularly certain areas of Piedmont, resulted one of the driest regions [
39]. According to the Piedmont Regional Agency for Environmental Protection (ARPA), summer 2022 was as one of the hottest and driest of the last 30 years in Piedmont [
23]. Indeed, during that summer unprecedented temperatures were registered, occasionally reaching values at an all-time high (
Figure S4). Also, the number of tropical nights (T > 20°C) and days (T > 30°C) were higher than in previous years (
Table S1). Moreover, rainfalls were irregular, both in terms of quantity and regional distribution, with a decrease of 50-60% with respect to previous years especially in areas where cyanide poisoning outbreaks occurred (
Figures S5 and S6). Finally, hydric balance was in deficit since the previous winter (
Figure S7), also due to limited snow reserves. These conditions were reasonably responsible for the excessive accumulation of dhurrin observed in most
Sorghum specimens collected in the outbreak and surrounding areas.
Figure 1.
Epidemiological data (herd size, morbidity and mortality rate) of the five reported poisoning cases: A-Sommariva del Bosco; B-Moretta; C-Bra; E-Cossato. Case D-Asti is not shown due to the lack of reliable information.
Figure 1.
Epidemiological data (herd size, morbidity and mortality rate) of the five reported poisoning cases: A-Sommariva del Bosco; B-Moretta; C-Bra; E-Cossato. Case D-Asti is not shown due to the lack of reliable information.
Figure 2.
Case A-Sommariva del Bosco (a) and B-Moretta (b): poisoned/dead animals in lateral recumbency, mostly on the right side.
Figure 2.
Case A-Sommariva del Bosco (a) and B-Moretta (b): poisoned/dead animals in lateral recumbency, mostly on the right side.
Figure 3.
Map of the Piedmont region showing the location of the poisoning cases (blue triangles: A: Sommariva del Bosco, B-Moretta, C-Bra, D-Asti, E-Cossato), and the other farms selected for cultivated Sorghum sampling (red circle: M-Montechiaro d’Asti) or wild Sorghum sampling (red circles: 1-Cuneo, 2-Faule, 3-Montiglio Monferrato, 4-Sampeyre, 5-Sanfrè, 6-Verrua Savoia). All the samplings (N=57) were performed from August to November 2022. Orange lines indicate province borders.
Figure 3.
Map of the Piedmont region showing the location of the poisoning cases (blue triangles: A: Sommariva del Bosco, B-Moretta, C-Bra, D-Asti, E-Cossato), and the other farms selected for cultivated Sorghum sampling (red circle: M-Montechiaro d’Asti) or wild Sorghum sampling (red circles: 1-Cuneo, 2-Faule, 3-Montiglio Monferrato, 4-Sampeyre, 5-Sanfrè, 6-Verrua Savoia). All the samplings (N=57) were performed from August to November 2022. Orange lines indicate province borders.
Figure 4.
Moretta (case B), a poisoned cow receiving the antidote (sodium thiosulfate) i.v. Note the cherry red blood on the neck of the animal.
Figure 4.
Moretta (case B), a poisoned cow receiving the antidote (sodium thiosulfate) i.v. Note the cherry red blood on the neck of the animal.
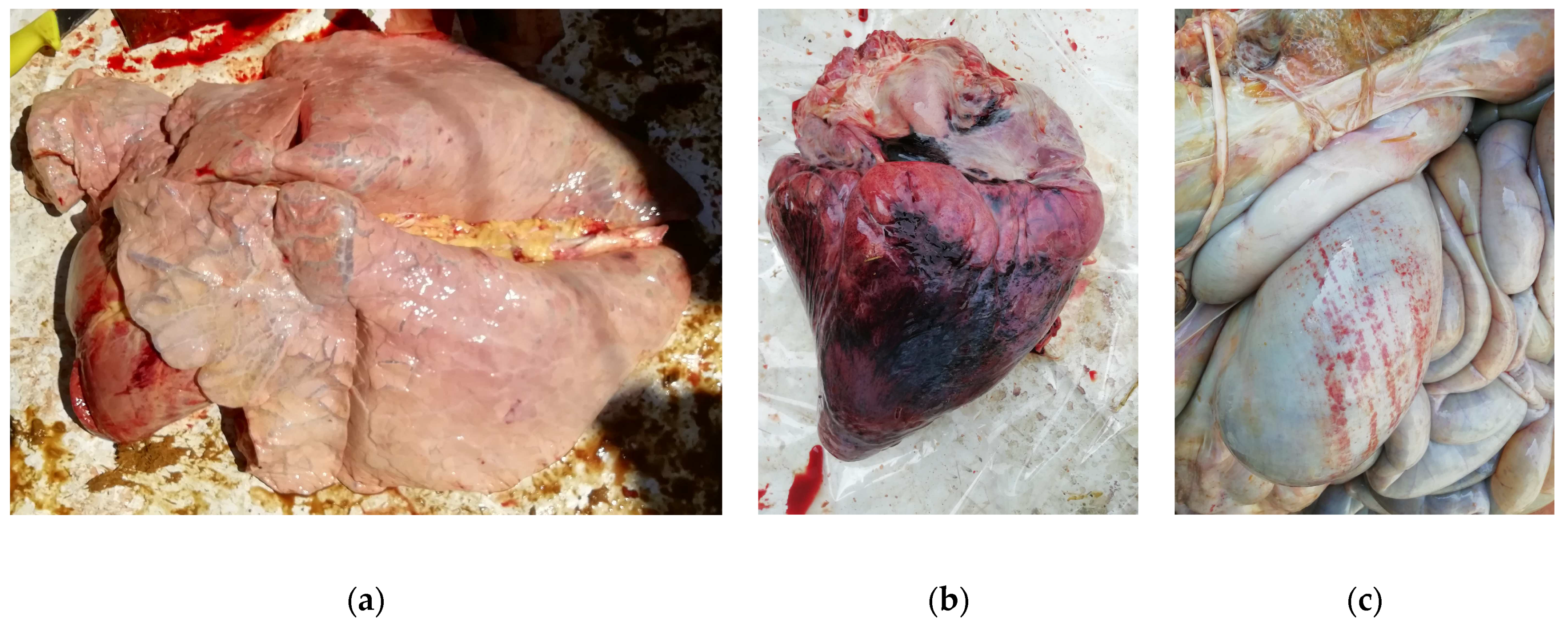
Figure 5.
Necropsy findings in Moretta case (B), revealing lung emphysema (a), myocardial haemorrhages (b) and abomasitis (c).
Figure 5.
Necropsy findings in Moretta case (B), revealing lung emphysema (a), myocardial haemorrhages (b) and abomasitis (c).
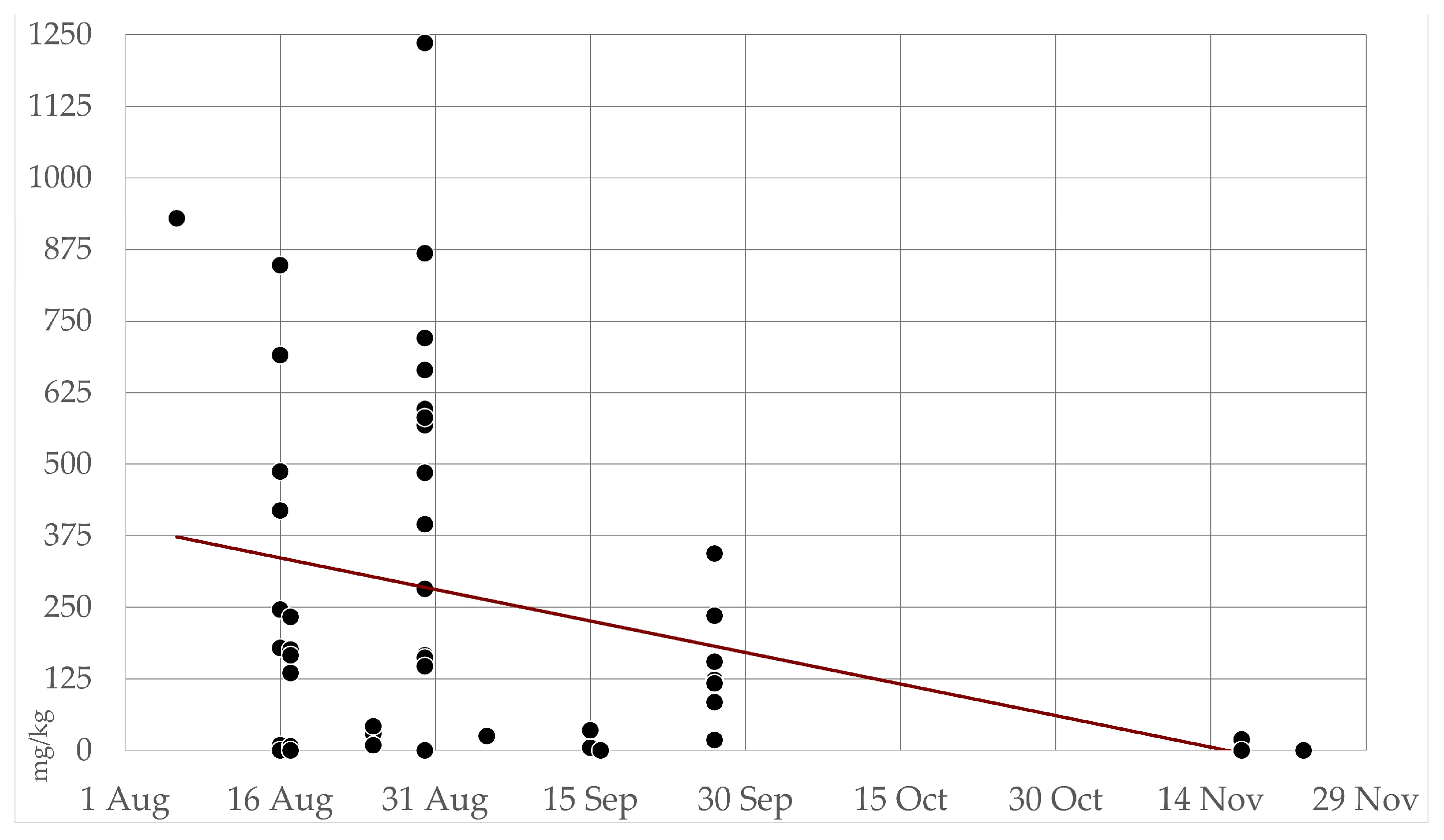
Figure 6.
Seasonal trend of hydrogen cyanide (HCN) concentration in all cultivated and wild collected Sorghum samples. Figure shows aggregate data (N=57) of the whole sampling in Piedmont, including all areas from August to November 2022.
Figure 6.
Seasonal trend of hydrogen cyanide (HCN) concentration in all cultivated and wild collected Sorghum samples. Figure shows aggregate data (N=57) of the whole sampling in Piedmont, including all areas from August to November 2022.
Table 1.
Treatments given, n. of survived animals and therapeutical success rate in the described outbreak of Sorghum toxicosis. Case D is not included because cows were not subjected to any treatment.
Table 1.
Treatments given, n. of survived animals and therapeutical success rate in the described outbreak of Sorghum toxicosis. Case D is not included because cows were not subjected to any treatment.
| Case |
Type of treatment |
Treated animals |
Survived animals |
Success rate |
| A |
Rehydrating solutions + multivitamin complex |
30 |
26 |
87% |
| B |
Sodium thiosulfate |
5 |
5 |
100% |
| C |
Methylene blue and (later) sodium thiosulfate |
30 |
28 |
93% |
| E |
Sodium thiosulfate |
40 |
40 |
100% |
Table 2.
Dhurrin and hydrogen cyanide (HCN) concentrations in Sorghum samples implied in the five outbreaks of cyanogenetic glycoside poisoning occurring in Piedmont in August 2022. When plant part is not specified, analysis has been performed on the whole plant.
Table 2.
Dhurrin and hydrogen cyanide (HCN) concentrations in Sorghum samples implied in the five outbreaks of cyanogenetic glycoside poisoning occurring in Piedmont in August 2022. When plant part is not specified, analysis has been performed on the whole plant.
| Case |
Date |
Sorghum species |
Site of collection |
Dhurrin (mg/kg) |
HCN (mg/kg) |
| A |
6-Aug |
Suzy1 (sprout, height 30-45 cm) |
Pasture |
10.717 |
929 |
| B |
16-Aug |
S. halepense |
Trough |
5.627 |
487 |
| C |
16-Aug |
S. halepense |
Trough |
7.961 |
690 |
| D |
16-Aug |
S. halepense |
Pasture |
4.834 |
419 |
| |
|
S. halepense |
Pasture border |
104 |
9 |
| E |
25-Aug |
S. halepense (young plants) |
Pasture |
335 |
29 |
| |
|
S. halepense (leaves mix) |
Pasture |
488 |
42 |
| |
|
S. halepense (inflorescence) |
Pasture |
105 |
9 |
Table 3.
Time course of dhurrin and hydrogen cyanide (HCN) concentration in the Sorghum bicolor x Sorghum sudanense variety called Suzy from Sommariva del Bosco (case A). When plant part is not specified, analysis has been performed on the whole plant.
Table 3.
Time course of dhurrin and hydrogen cyanide (HCN) concentration in the Sorghum bicolor x Sorghum sudanense variety called Suzy from Sommariva del Bosco (case A). When plant part is not specified, analysis has been performed on the whole plant.
| Date |
Dhurrin (mg/kg) |
HCN (mg/kg) |
Notes |
| 6-Aug |
10.717 |
929 |
Sample relative to the outbreak (case A) |
| 14-Aug |
6.869 |
596 |
Average plants height 50 cm |
| 16-Aug |
14.246 |
1,235 |
Open-air dried |
| 16-Aug |
5.590 |
485 |
Fresh, leaves > 1 m |
| 16-Aug |
8.300 |
720 |
Fresh, leaves < 50 cm |
| 17-Aug |
< LOQ |
0 |
Bundled; cut of 14th of July |
| 21-Aug |
6.550 |
568 |
Average plants height 60 cm |
| 27-Aug |
7.661 |
664 |
Average plants height 68 cm |
| 5-Sept |
1.420 |
123 |
|
| 12-Sept |
1.798 |
155 |
|
| 23-Sept |
958 |
83 |
|
| 27-Sept |
974 |
84 |
Field “Paolorio” |
| 27-Sept |
1.354 |
117 |
Field “Luppiano” |
| 27-Sept |
2.707 |
235 |
Field “Valè” |
| 6-Oct1
|
< LOQ |
0 |
Fresh, chopped |
| 23-Nov |
< LOQ |
0 |
Mature silo (45 days), from mixed fields |
Table 4.
Time course of dhurrin and hydrogen cyanide (HCN) concentration in Sorghum samples from a farm in the Asti province (Montechiaro d’Asti), located near case D. All the samples belong to mixed individuals grown from a seed mixture of two varieties: the S. bicolor ssp. drummondii Piper, and the S. bicolor x S. sudanense Sudal. When plant part is not specified, analysis has been performed on the whole plant.
Table 4.
Time course of dhurrin and hydrogen cyanide (HCN) concentration in Sorghum samples from a farm in the Asti province (Montechiaro d’Asti), located near case D. All the samples belong to mixed individuals grown from a seed mixture of two varieties: the S. bicolor ssp. drummondii Piper, and the S. bicolor x S. sudanense Sudal. When plant part is not specified, analysis has been performed on the whole plant.
| Date |
Dhurrin (mg/kg) |
HCN
(mg/kg) |
Notes |
| 16-Aug |
9.770 |
847 |
Sowed at the beginning of June, never cut |
| 16-Aug |
2.840 |
246 |
Grown back plants |
| 16-Aug |
2.065 |
179 |
Sowed at the beginning of June, grazed in July |
| 30-Aug |
1.792 |
155 |
Field 1; leaves > 150 cm |
| 30-Aug |
1.919 |
166 |
Field 1; leaves ~ 50 cm |
| 30-Aug |
< LOQ |
0 |
Field 1; culm |
| 30-Aug |
3.251 |
282 |
Field 1; inflorescence |
| 30-Aug |
1.865 |
162 |
Field 1; grown back plants, without roots |
| 30-Aug |
10.010 |
868 |
Field 2; leaves > 150 cm |
| 30-Aug |
6.701 |
581 |
Field 2; leaves ~ 50 cm |
| 30-Aug |
1.697 |
147 |
Field 2; culm |
| 30-Aug |
4.553 |
395 |
Field 2; inflorescence |
| 26-Sept |
3.967 |
344 |
Culm and leaves |
| 26-Sept |
205 |
18 |
Inflorescence |
| 17-Nov |
229 |
19 |
Immature plants (without grains) ~ 50 cm; from ensiled bale |
| 17-Nov |
< LOQ |
0 |
Mature plants (with grains) > 150 cm; from ensiled bale |
Table 5.
Dhurrin and hydrogen cyanide (HCN) concentrations in Sorghum halepense collected from different farms and fields in Piedmont during 2022. The analysis has been performed on the whole plants.
Table 5.
Dhurrin and hydrogen cyanide (HCN) concentrations in Sorghum halepense collected from different farms and fields in Piedmont during 2022. The analysis has been performed on the whole plants.
| Date |
Location |
Province |
Dhurrin (mg/kg) |
HCN (mg/kg) |
Notes |
| 16-Aug |
Asti |
Asti |
< LOQ |
0 |
Mixed grasses |
| 17-Aug |
Faule |
Cuneo |
85 |
7 |
Cut for haymaking |
| 17-Aug |
Verrua Savoia |
Torino |
1.558 |
135 |
Field used for haymaking |
| 17-Aug |
Montiglio M.to |
Asti |
2.036 |
176 |
Field “Sant’Anna” |
| 17-Aug |
Montiglio M.to |
Asti |
2.693 |
233 |
Field “Acquedotto” |
| 17-Aug |
Montiglio M.to |
Asti |
1.917 |
166 |
Field “Vallone” |
| 5-Sept |
Bra |
Cuneo |
289 |
25 |
Field of case C; forage for silo |
| 15-Sept |
Cuneo |
Cuneo |
57 |
5 |
Plants > 50 cm |
| 15-Sept |
Cuneo |
Cuneo |
401 |
35 |
Plants < 50 cm |
| 16-Sept |
Sanfrè |
Cuneo |
< LOQ |
0 |
Mature plants (with inflorescence) |
| 23-Nov |
Sampeyre |
Cuneo |
< LOQ |
0 |
Frosted plants |