Submitted:
06 February 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
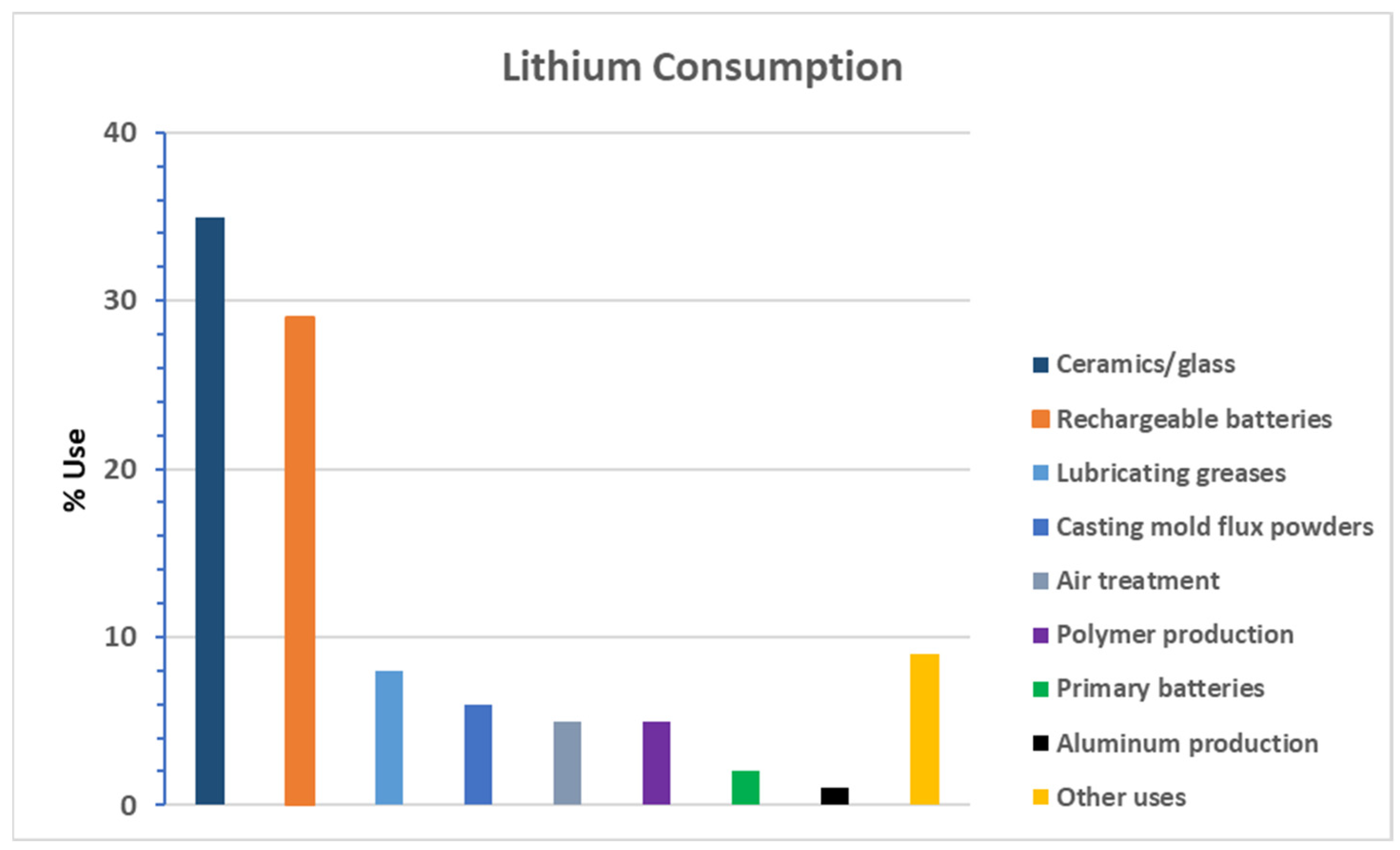
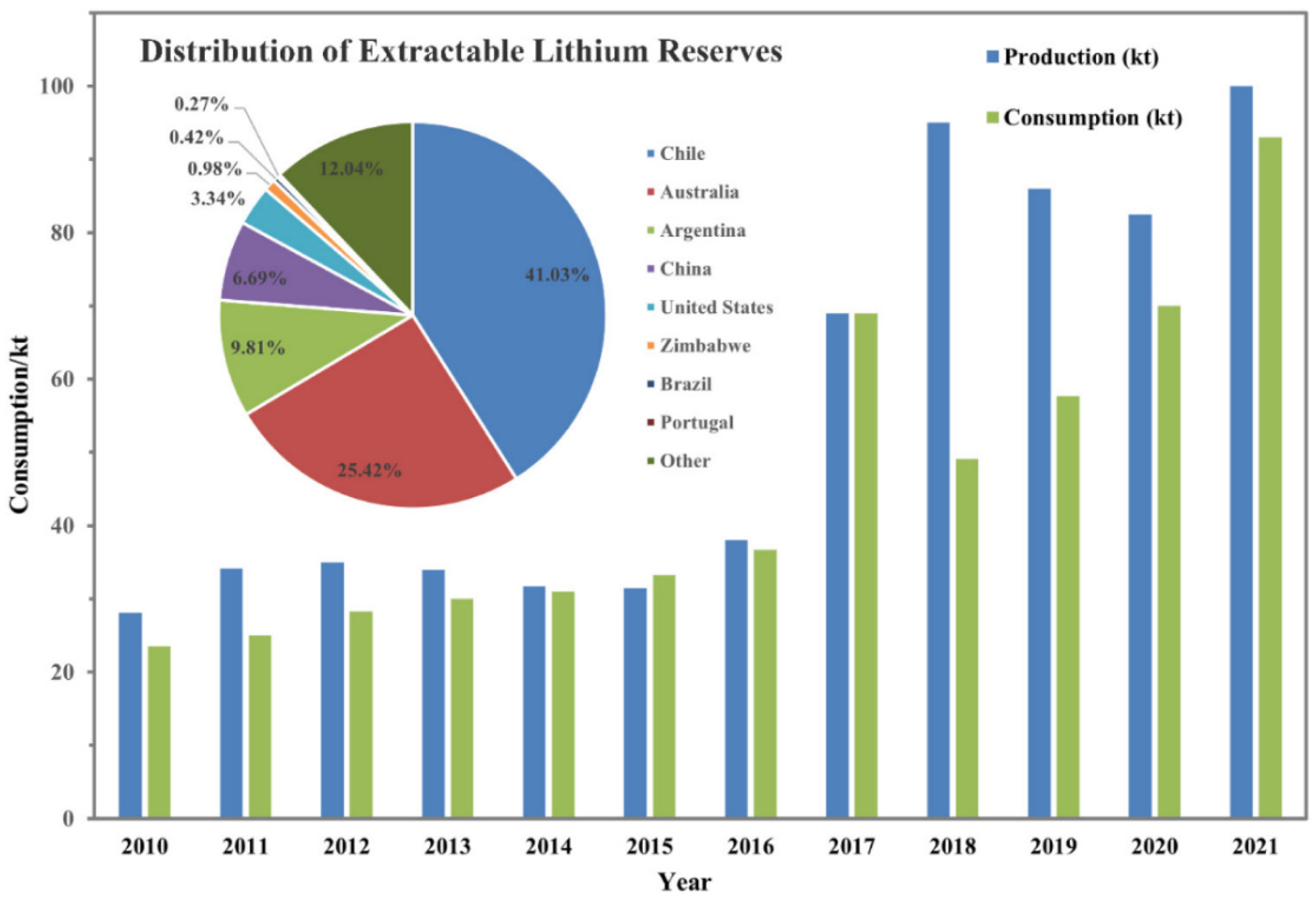
1. Introduction
2. Preparation of Lithium-Ion Sieves (LIS): An Overview
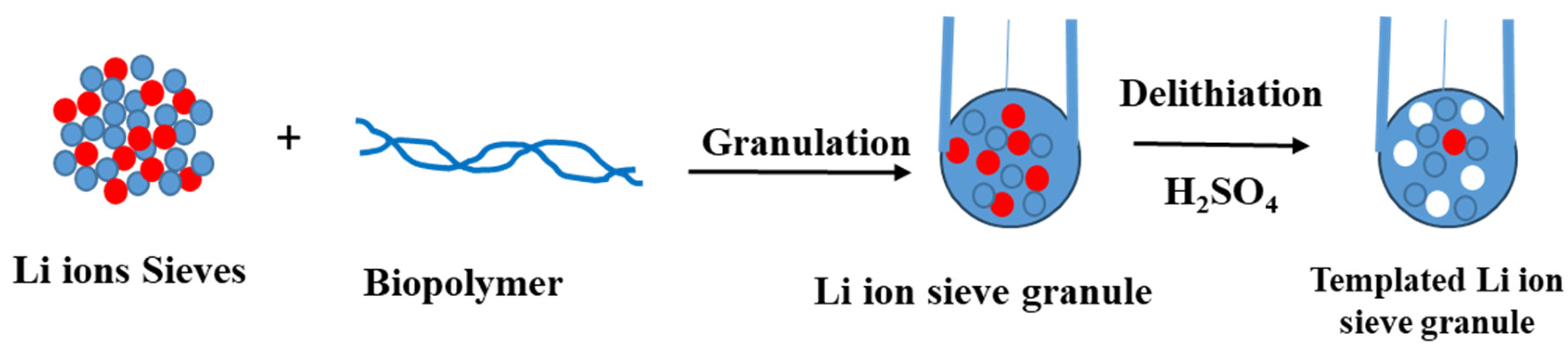
3. Granulation of LISs
3.1. Granulation of LISs Using Inorganic Materials
3.2. Granulation of LISs Using Biopolymers
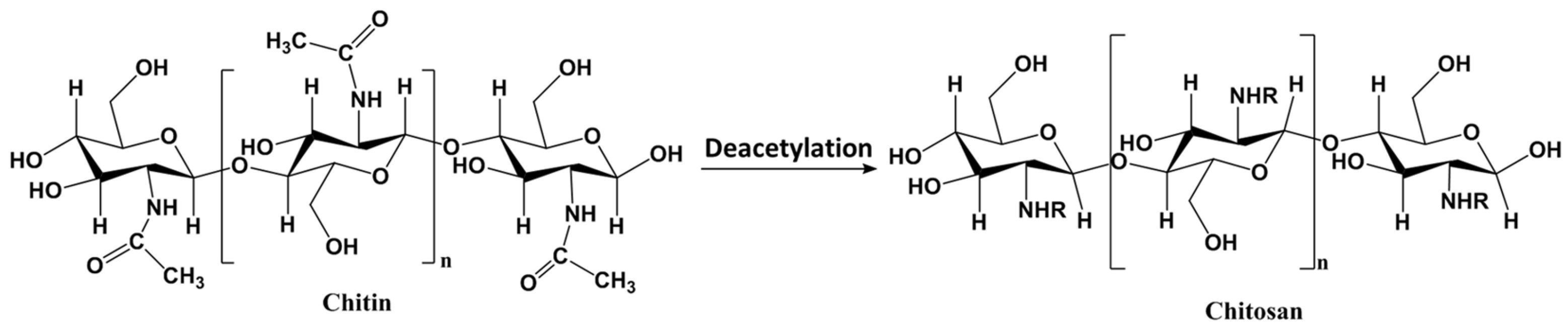
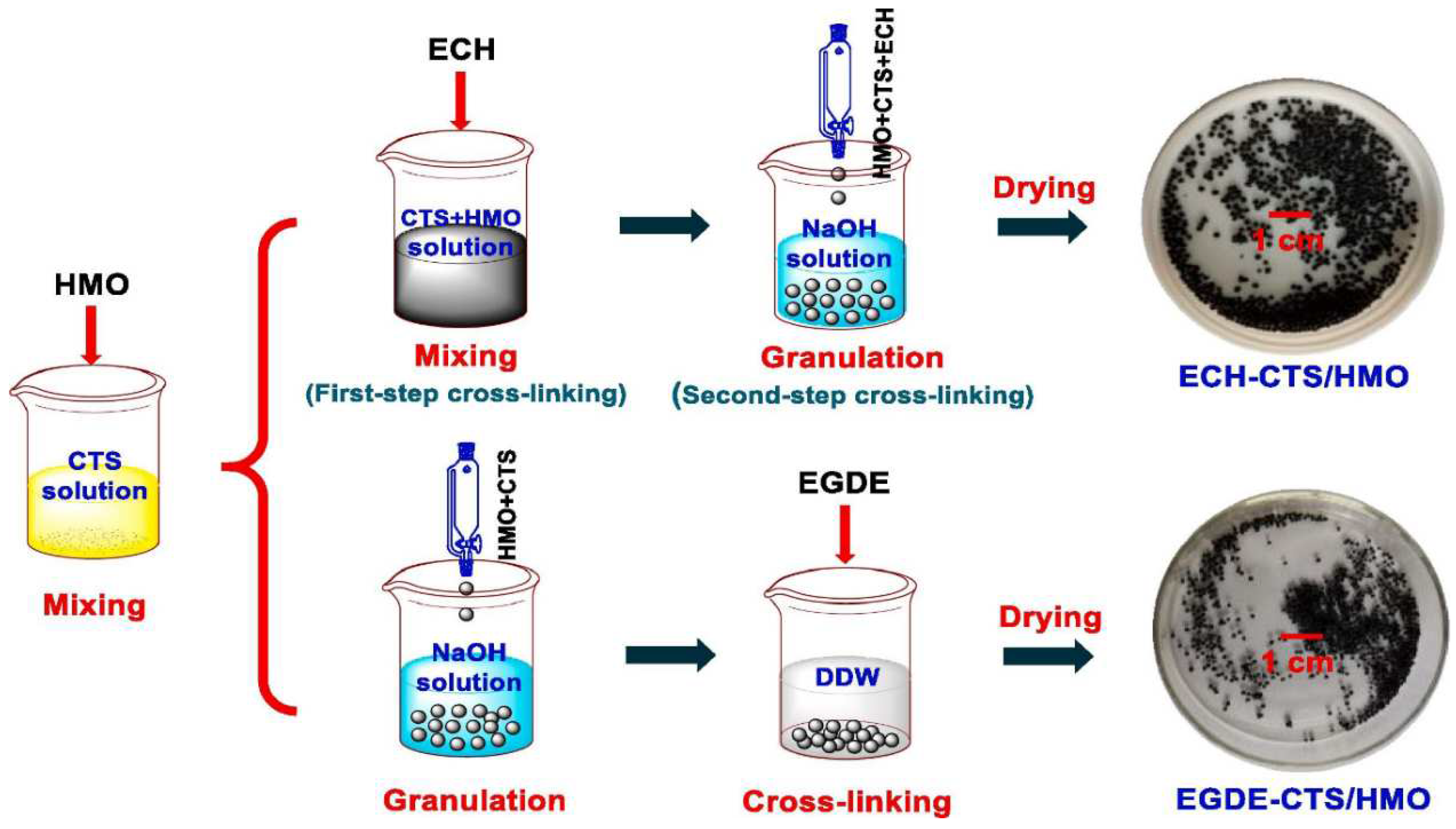
3.2.1. Metal-Based LISs Granulated with Chitosan
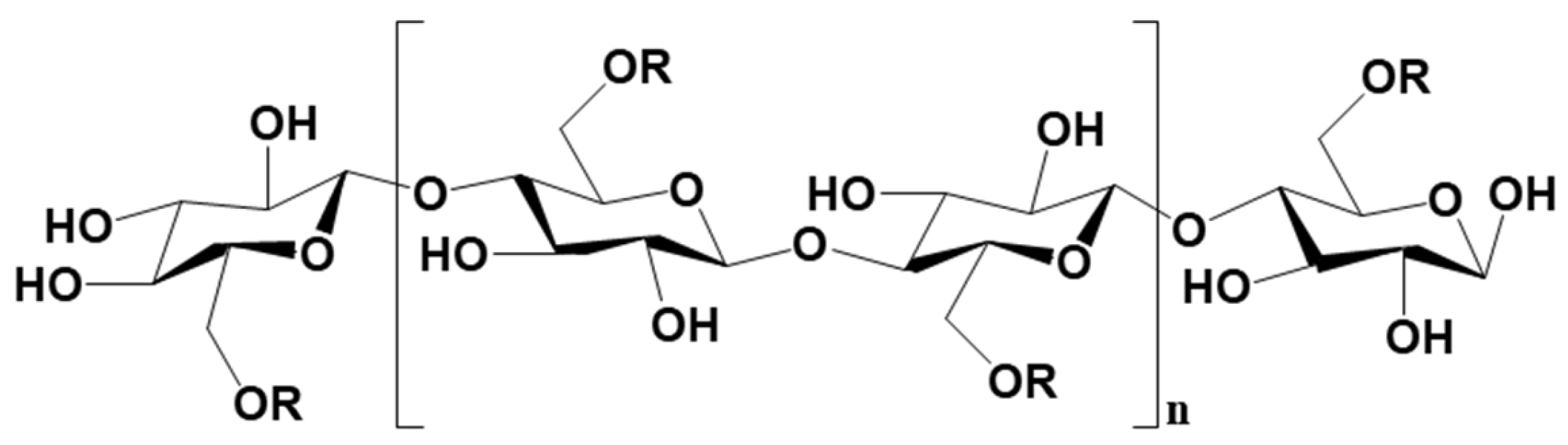
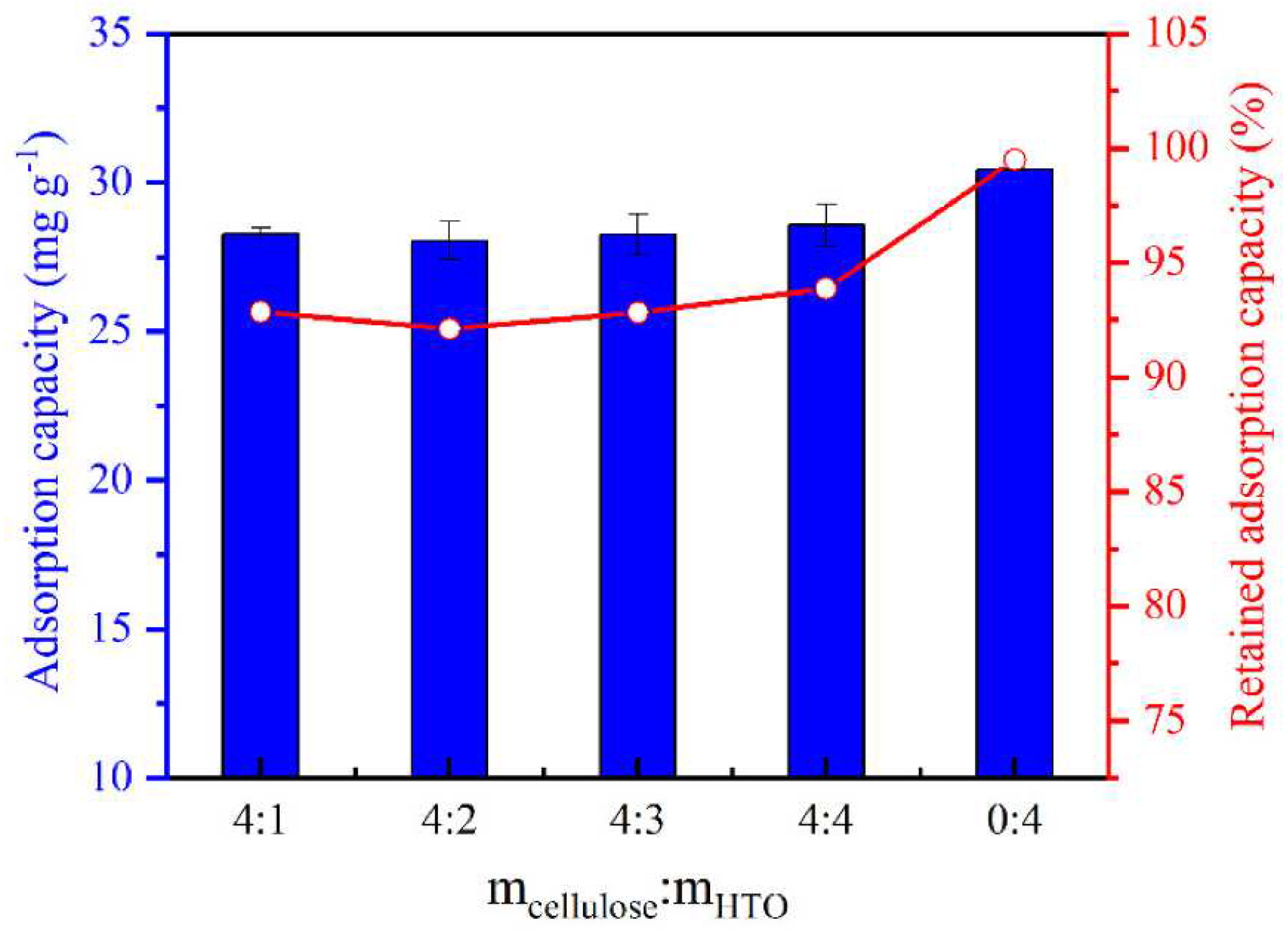
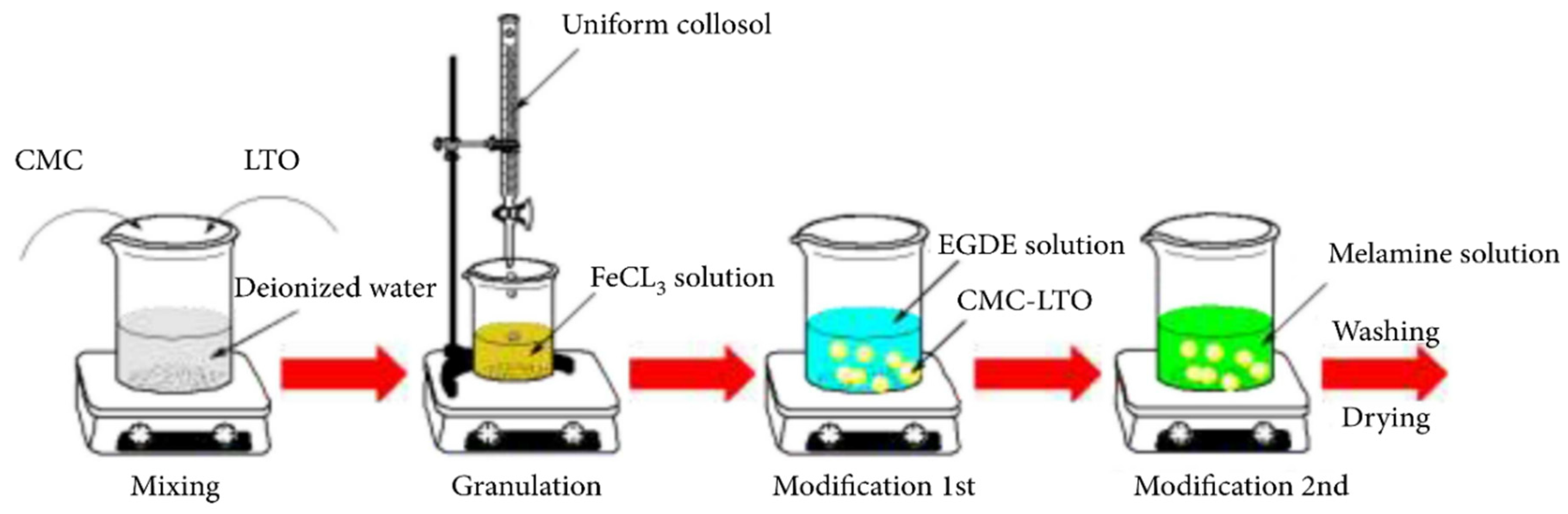
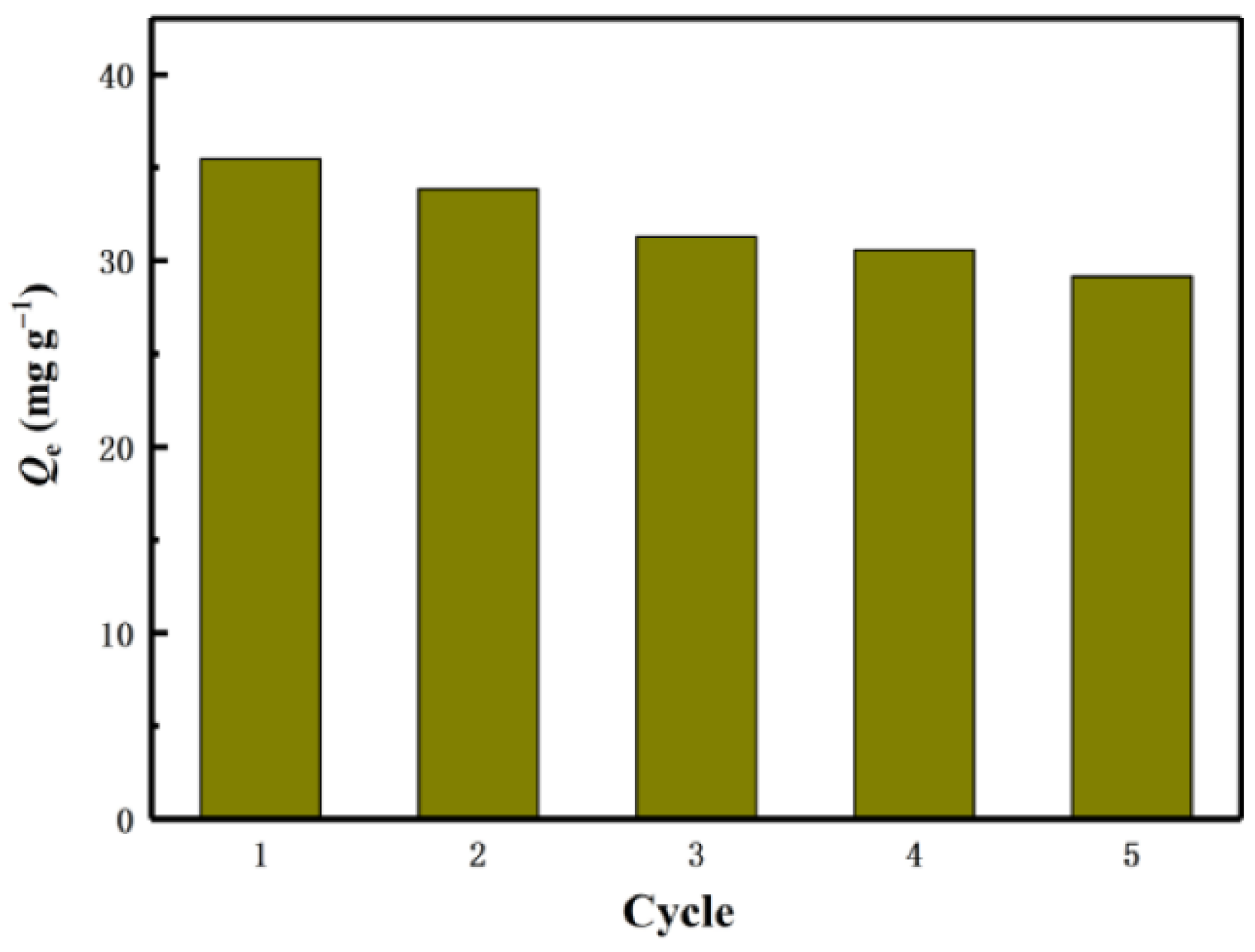
3.2.2. Metal-Based LISs Granulated with Cellulose
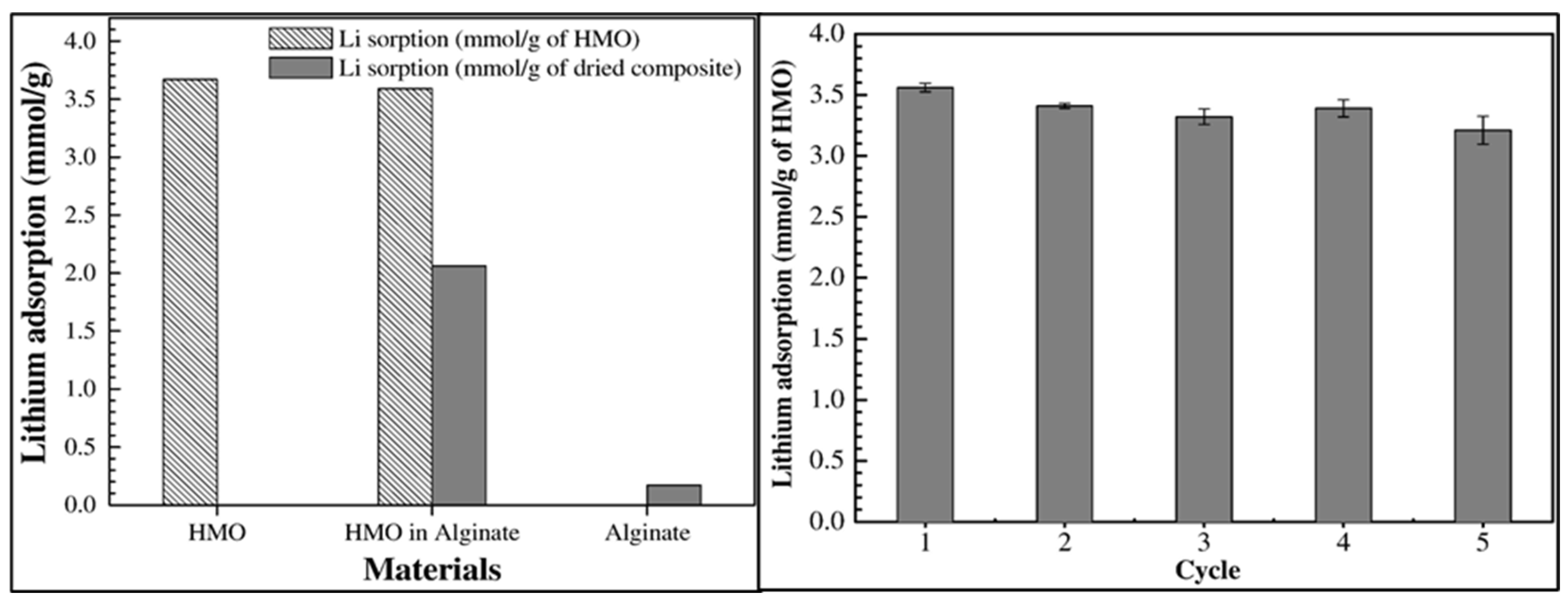
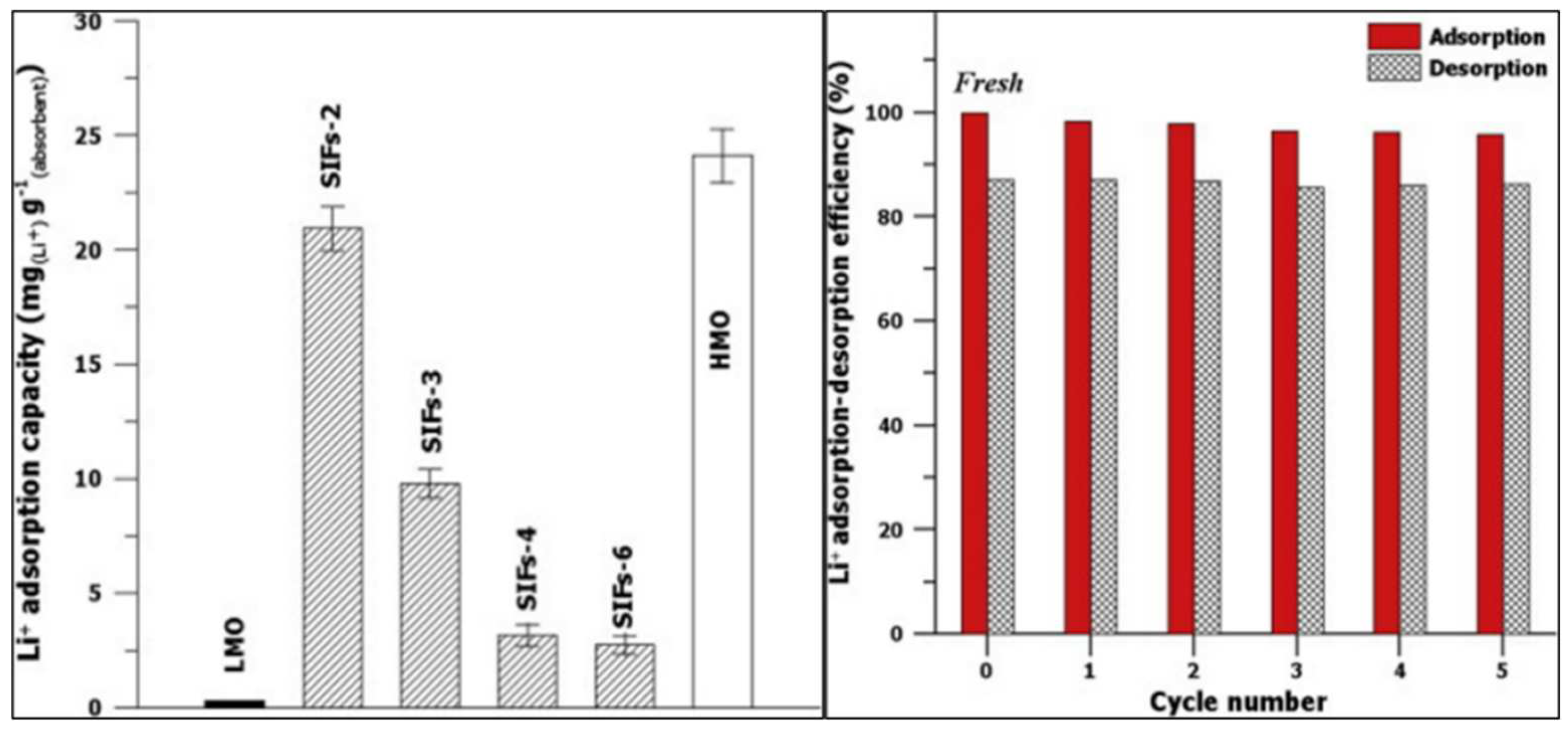
3.2.3. Metal-Based LISs Granulated with Alginate
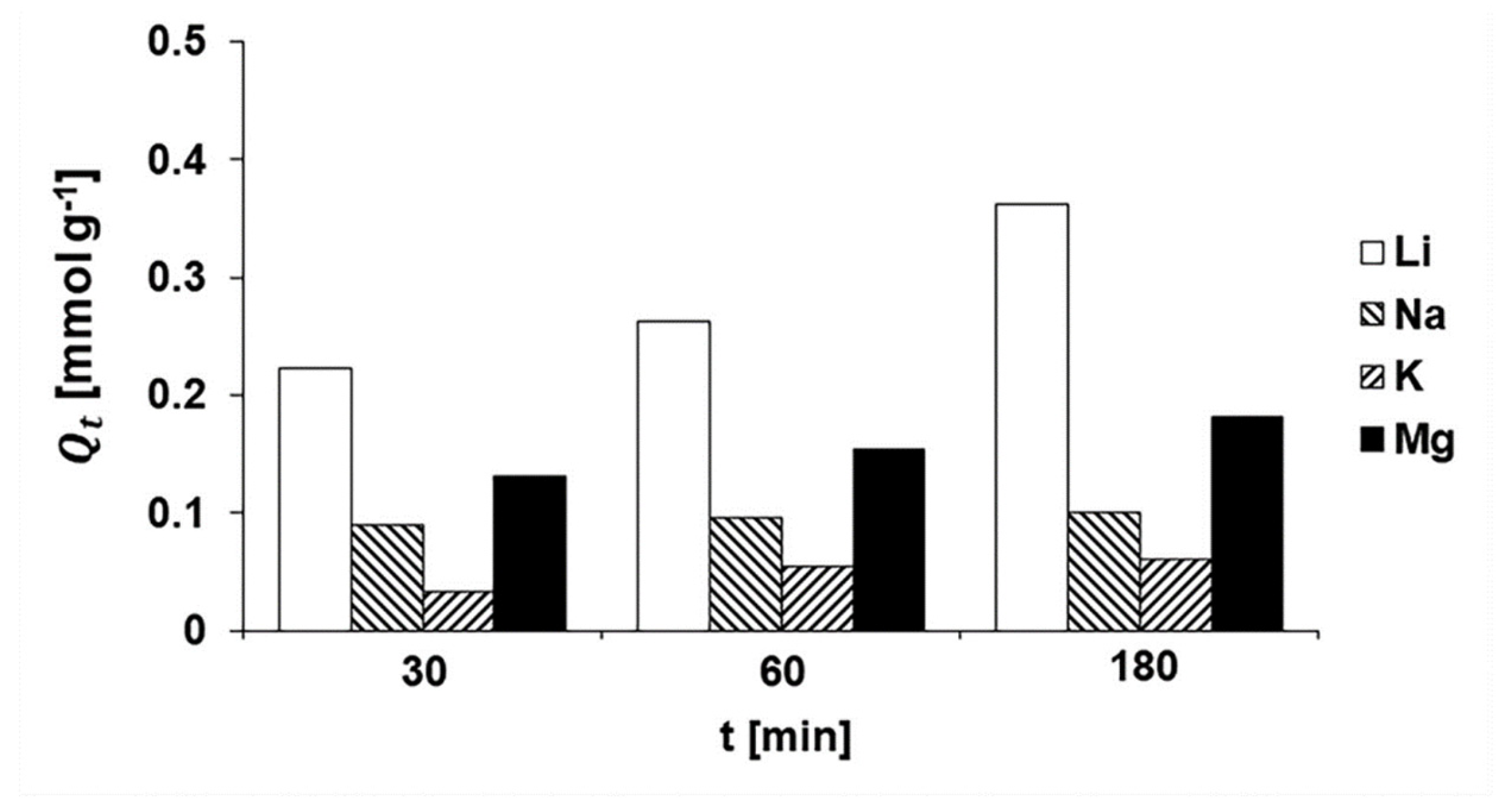
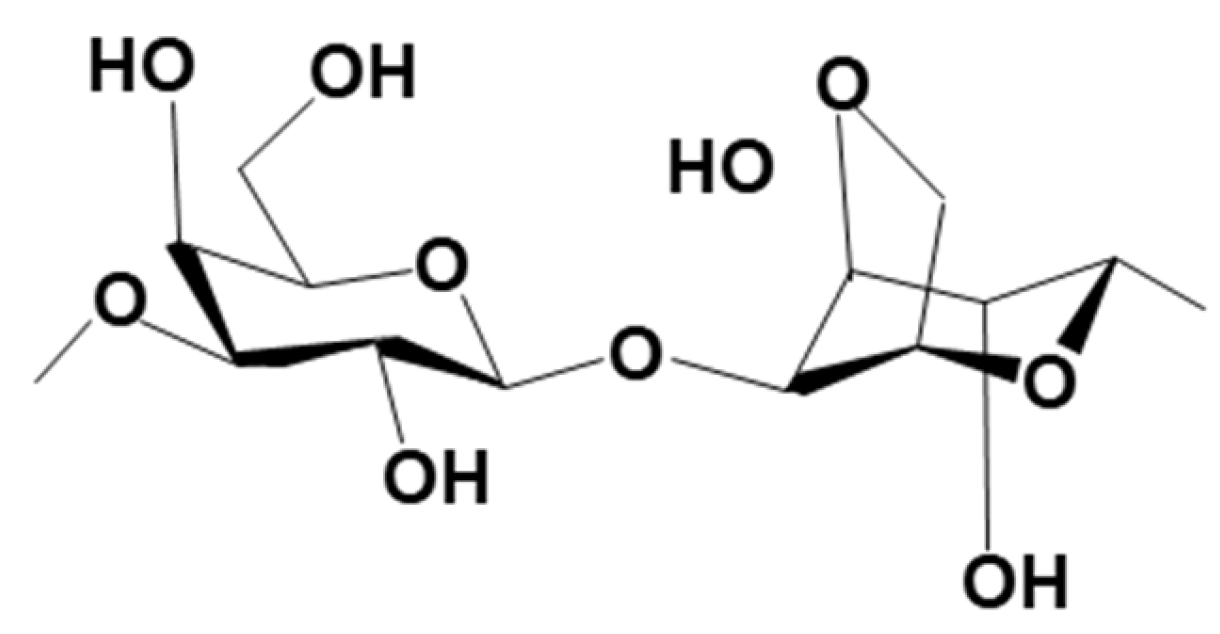
3.2.4. Metal-Based LISs Granulated with Agar
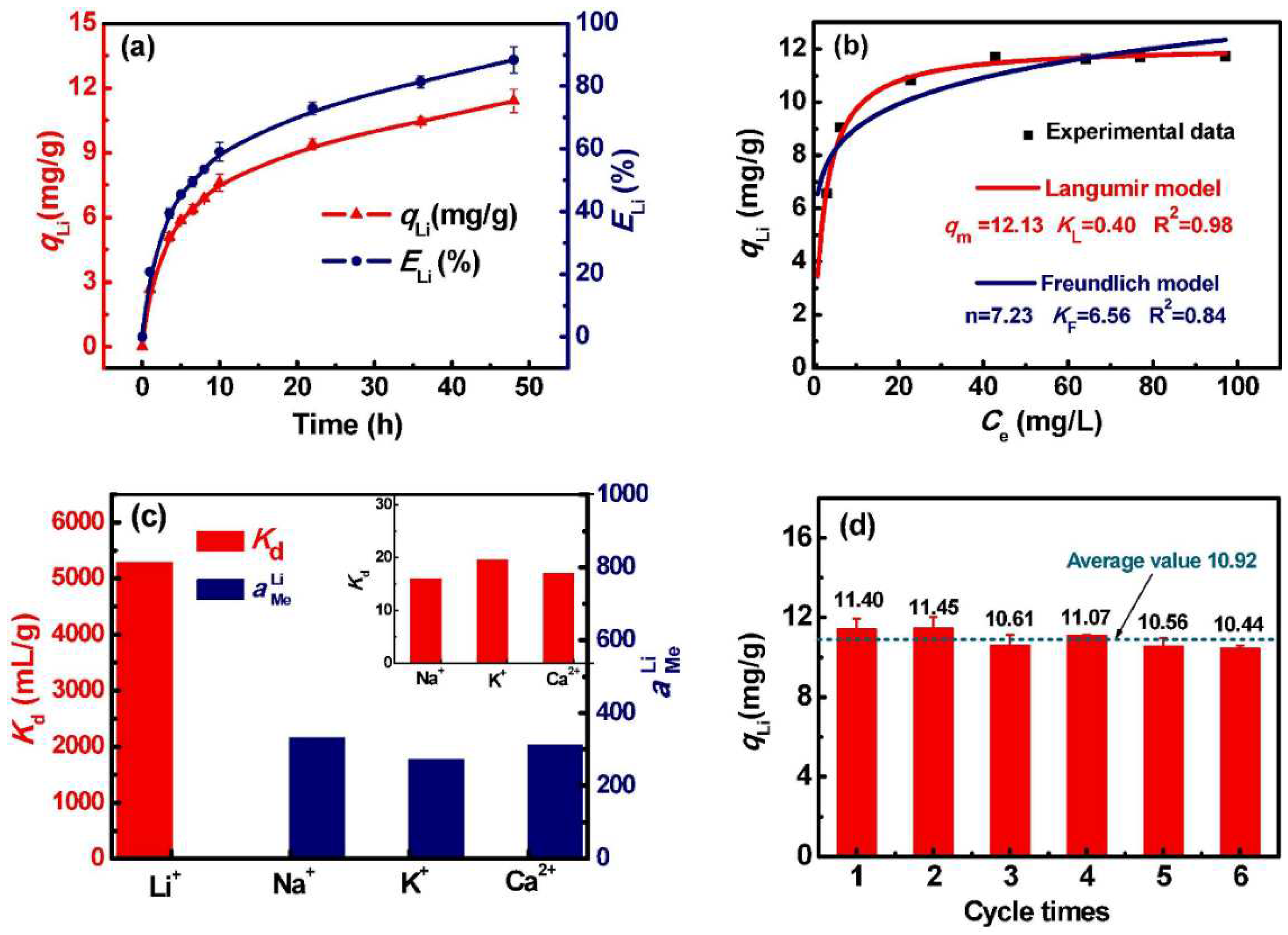
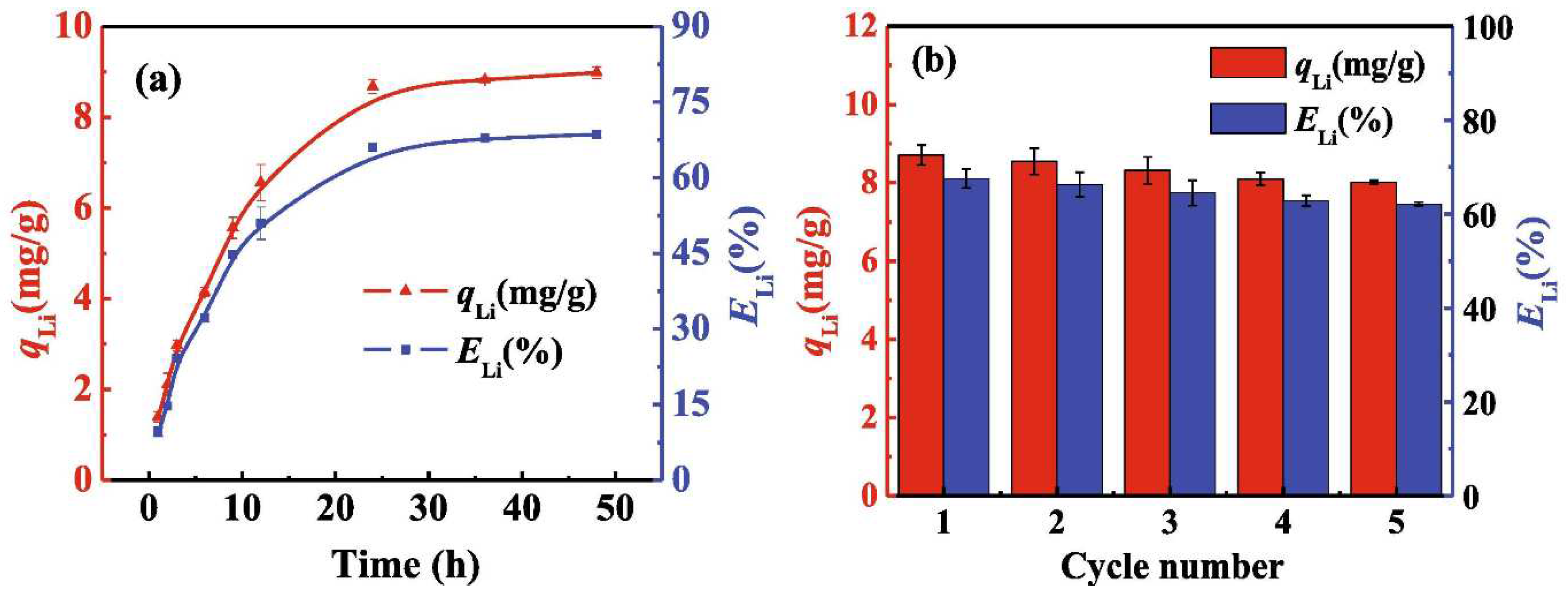
4. Comparison of the Sorption Properties of Granulated LISs
5. Conclusions
Acknowledgments/Funding
References
- Haddad, A.Z.; Hackl, L.; Akuzum, B.; Pohlman, G.; Magnan, J.-F.; Kostecki, R. How to make lithium extraction cleaner, faster and cheaper - in six steps. Nature 2023, 616, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Jaskula, B.W. Mineral Commodity Summaries. LITHIUM. US Geological Survey, Mineral Commodity Summaries. 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-lithium.pdf (accessed on 24 May 2023).
- Weng D, Duan H, Hou Y, Huo J, Chen L, Zhang F, et al. Introduction of manganese based lithium-ion Sieve-A review. Progress in Natural Science: Materials International 2020, 30, 139–152.
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 1, resource model. J Ind Ecol 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Advanced Materials 2020, 32, 1905440. [Google Scholar] [CrossRef]
- Yu H, Naidu G, Zhang C, Wang C, Razmjou A, Han DS, et al. Metal-based adsorbents for lithium recovery from aqueous resources. Desalination 2022, 539, 115951. [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. , Miner Eng 2019, 139, 105868. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.; Du, B.; Zhao, Y.; Wang, M. Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 2018, 175, 102–108. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Xu, M.; Zhang, Y.; Peng, X.; et al. Removal of calcium magnesium from lithium concentrated solution by solvent extraction method using, D2EHPA. Desalination 2020, 479, 114306. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Yang, Y.; Yu, J. Synergic and competitive adsorption of Li–Na–MgCl2 onto lithium–aluminum hydroxides. Adsorption 2020, 26, 1039–1049. [Google Scholar] [CrossRef]
- Wang., J.; Yue, X.; Wang, P.; Yu, T.; Du, X.; Hao, X.; et al. Electrochemical technologies for lithium recovery from liquid resources: A review. Renewable and Sustainable Energy Reviews 2022, 154, 111813. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Drioli, E.; Macedonio, F. Perspectives on mining from sea and other alternative strategies for minerals and water recovery – The development of novel membrane operations. J Taiwan Inst Chem Eng 2019, 94, 129–134. [Google Scholar] [CrossRef]
- Ryu, T.; Lee, D.-H.; Ryu, J.C.; Shin, J.; Chung, K.-S.; Kim, Y.H. Lithium recovery system using electrostatic field assistance. Hydrometallurgy 2015, 151, 78–83. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J Memb Sci 2019, 591, 117317. [Google Scholar] [CrossRef]
- Paredes, C.; Rodríguez de San Miguel, E. Selective lithium extraction and concentration from diluted alkaline aqueous media by a polymer inclusion membrane and application to seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep Purif Technol 2021, 256, 117807. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Z.; Qin, X.; Gao, X.; Wang, M.; Xiang, X. Recent advances in lithium extraction from salt lake brine using coupled and tandem technologies. Desalination 2023, 547, 116225. [Google Scholar] [CrossRef]
- Hu, S.; Sun, Y.; Pu, M.; Yun, R.; Xiang, X. Determination of boundary conditions for highly efficient separation of magnesium and lithium from salt lake brine by reaction-coupled separation technology. Sep Purif Technol 2019, 229, 115813. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep Purif Technol 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Hamzaoui, A.H.; M’nif, A.; Hammi, H.; Rokbani, R. Contribution to the lithium recovery from brine. Desalination 2003, 158, 221–224. [Google Scholar] [CrossRef]
- Masmoudi, A.; Zante, G.; Trébouet, D.; Barillon, R.; Boltoeva, M. Solvent extraction of lithium ions using benzoyltrifluoroacetone in new solvents. Sep Purif Technol 2021, 255, 117653. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; Ghahreman, A. Novel approaches for lithium extraction from salt-lake brines: A review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Hong, H.-J.; Ryu, T.; Park, I.-S.; Kim, M.; Shin, J.; Kim, B.-G.; et al. Highly porous and surface-expanded spinel hydrogen manganese oxide (HMO)/Al2O3 composite for effective lithium (Li) recovery from seawater. Chemical Engineering Journal 2018, 337, 455–461. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z.; Li, J. Extraction of lithium from salt lake brines by granulated adsorbents. Colloids Surf A Physicochem Eng Asp 2021, 628, 127256. [Google Scholar] [CrossRef]
- Safari, S.; Lottermoser, B.G.; Alessi, D.S. Metal oxide sorbents for the sustainable recovery of lithium from unconventional resources. Appl Mater Today 2020, 19, 100638. [Google Scholar] [CrossRef]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Shafiei, N.; Afsari, M.; Pakzad, K.; et al. Recent advances in nanomaterial development for lithium ion-sieving technologies. Desalination 2022, 529, 115624. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Song, H.S.; Limjuco, L.A.; Lawagon, C.P.; Parohinog, K.J.; et al. Design of lithium selective crown ethers: Synthesis, extraction and theoretical binding studies. Chemical Engineering Journal 2017, 326, 921–933. [Google Scholar] [CrossRef]
- Oral, I.; Tamm, S.; Herrmann, C.; Abetz, V. Lithium selectivity of crown ethers: The effect of heteroatoms and cavity size. Sep Purif Technol 2022, 294, 121142. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Q.; Li, J.; Wu, Z.; Liu, Z. Lithium adsorption properties of porous LiAl-layered double hydroxides synthesized using surfactants. Journal of Saudi Chemical Society 2022, 26, 101535. [Google Scholar] [CrossRef]
- Suresh, P.; Sreedhar, I.; Vaidhiswaran, R.; Venugopal, A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chemical Engineering Journal 2017, 328, 785–815. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Topotactic lithium (1+) insertion to. lambda.-manganese dioxide in the aqueous phase. Langmuir 1989, 5, 150–157. [Google Scholar]
- Kozawa, A.; Powers, R.A. The Manganese Dioxide Electrode in Alkaline Electrolyte; The Electron-Proton Mechanism for the Discharge Process from MnO2 to MnO1. 5. J Electrochem Soc 1966, 113, 870. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J Solid State Chem 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Yang, X.; Kanoh, H.; Tang, W.; Ooi, K. Synthesis of Li1. 33Mn1. 67O4 spinels with different morphologies and their ion adsorptivities after delithiation. J Mater Chem 2000, 10, 1903–1909. [Google Scholar]
- Chitrakar, R.; Sakane, K.; Umeno, A.; Kasaishi, S.; Takagi, N.; Ooi, K. Synthesis of orthorhombic LiMnO2 by solid-phase reaction under steam atmosphere and a study of its heat and acid-treated phases. J Solid State Chem 2002, 169, 66–74. [Google Scholar] [CrossRef]
- Takada, T.; Hayakawa, H.; Akiba, E. Preparation and crystal structure refinement of Li4Mn5O12 by the Rietveld method. J Solid State Chem 1995, 115, 420–426. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Zhou, D.; Zhang, L.; Chen, B.; Yu, L. Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties. Transactions of Nonferrous Metals Society of China 2013, 23, 253–259. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium recovery from salt lake brine by H2TiO3. Dalton Transactions, 2014; 43, 8933–8939. [Google Scholar]
- Zhang, L.; Zhou, D.; Yao, Q.; Zhou, J. Preparation of H2TiO3-lithium adsorbent by the sol–gel process and its adsorption performance. Appl Surf Sci 2016, 368:82–7.
- Wang S, Li P, Cui W, Zhang H, Wang H, Zheng S, et al. Hydrothermal synthesis of lithium-enriched β-Li 2 TiO 3 with an ion-sieve application: Excellent lithium adsorption. RSC Adv 2016, 6, 102608–16.
- Moazeni, M.; Hajipour, H.; Askari, M.; Nusheh, M. Hydrothermal synthesis and characterization of titanium dioxide nanotubes as novel lithium adsorbents. Mater Res Bull 2015, 61, 70–75. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Topotactic lithium (1+) insertion to lambda.-manganese dioxide in the aqueous phase. Langmuir 1989, 5, 150–157. [Google Scholar] [CrossRef]
- Shen, X.-M.; Clearfield, A. Phase transitions and ion exchange behavior of electrolytically prepared manganese dioxide. J Solid State Chem 1986, 64, 270–282. [Google Scholar] [CrossRef]
- Koyanaka, H.; Matsubaya, O.; Koyanaka, Y.; Hatta, N. Quantitative correlation between Li absorption and H content in manganese oxide spinel λ-MnO2. Journal of Electroanalytical Chemistry 2003, 559, 77–81. [Google Scholar] [CrossRef]
- Koyanaka, H.; Matsubaya, O.; Koyanaka, Y.; Hatta, N. Quantitative correlation between Li absorption and H content in manganese oxide spinel λ-MnO2. Journal of Electroanalytical Chemistry 2003, 559, 77–81. [Google Scholar] [CrossRef]
- Feng, Q.; Miyai, Y.; Kanoh, H.; Ooi, K. Lithium (1+) extraction/insertion with spinel-type lithium manganese oxides. Characterization of redox-type and ion-exchange-type sites. Langmuir 1992, 8, 1861–1867. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of lithium (1+) insertion in spinel-type manganese oxide. Redox and ion-exchange reactions. Langmuir 1991, 7, 1167–1171. [Google Scholar] [CrossRef]
- Shen, X.-M.; Clearfield, A. Phase transitions and ion exchange behavior of electrolytically prepared manganese dioxide. J Solid State Chem 1986, 64, 270–282. [Google Scholar] [CrossRef]
- Hong, H.-J.; Park, I.-S.; Ryu, T.; Ryu, J.; Kim, B.-G.; Chung, K.-S. Granulation of Li1.33Mn1.67O4 (LMO) through the use of cross-linked chitosan for the effective recovery of Li+ from seawater. Chemical Engineering Journal 2013, 234, 16–22. [Google Scholar] [CrossRef]
- Ryu, T.; Haldorai, Y.; Rengaraj, A.; Shin, J.; Hong, H.-J.; Lee, G.-W.; et al. Recovery of Lithium Ions from Seawater Using a Continuous Flow Adsorption Column Packed with Granulated Chitosan–Lithium Manganese Oxide. Ind Eng Chem Res 2016, 55, 7218–7225. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, J.; Liu, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of granulated H4Mn5O12/chitosan with improved stability by a novel cross-linking strategy for lithium adsorption from aqueous solutions. Chemical Engineering Journal 2021, 426, 131689. [Google Scholar] [CrossRef]
- Yüksel Özşen, A.; Kahvecioğlu, A. Adsorbent synthesis for the recovery of lithium water resources. Master of Science. Izmir Institute of Technology 2022. [Google Scholar]
- Wang, H.; Cui, J.; Li, M.; Guo, Y.; Deng, T.; Yu, X. Selective recovery of lithium from geothermal water by EGDE cross-linked spherical CTS/LMO. Chemical Engineering Journal 2020, 389, 124410. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Qin, J.; Samadiy, M.; Deng, T. Synthesis of Spherical Composite CMC-LTO-EGDE-ME for Lithium Recovery from Geothermal Water. J Chem 2022, 2022, 6884947. [Google Scholar] [CrossRef]
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium (IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour Technol 2013, 142, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K. Lithium ion rechargeable batteries: materials, technology, and new applications; John Wiley & Sons, 2012. [Google Scholar]
- Darul, J.; Nowicki, W.; Piszora, P. Unusual compressional behavior of lithium–manganese oxides: a case study of Li4Mn5O12. The Journal of Physical Chemistry C 2012, 116, 17872–17879. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Greenblatt, M.; Dismukes, G.C. Water oxidation by λ-MnO2: catalysis by the cubical Mn4O4 subcluster obtained by delithiation of spinel LiMn2O4. J Am Chem Soc 2010, 132, 11467–11469. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Tong, K.; Zhou, L.; Xiao, J.; Sun, S.; Li, P.; et al. Adsorption and Desorption Behavior of Lithium Ion in Spherical PVC–MnO2 Ion Sieve. Ind Eng Chem Res 2012, 51, 10921–10929. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, P.; Qi, P.; Gao, C. Adsorption and desorption properties of Li+ on PVC-H1.6Mn1.6O4 lithium ion-sieve membrane. Chemical Engineering Journal 2014, 235, 340–348. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Ma, P. Synthesis of H2TiO3-PVC lithium-ion sieves via an antisolvent method and its adsorption performance. Ceram Int 2022, 48, 30127–30134. [Google Scholar] [CrossRef]
- Nisola, G.M.; Limjuco, L.A.; Vivas, E.L.; Lawagon, C.P.; Park, M.J.; Shon, H.K.; et al. Macroporous flexible polyvinyl alcohol lithium adsorbent foam composite prepared via surfactant blending and cryo-desiccation. Chemical Engineering Journal 2015, 280. [Google Scholar] [CrossRef]
- Limjuco, L.A.; Nisola, G.M.; Lawagon, C.P.; Lee, S.-P.; Seo, J.G.; Kim, H.; et al. H2TiO3 composite adsorbent foam for efficient and continuous recovery of Li+ from liquid resources. Colloids Surf A Physicochem Eng Asp 2016, 504, 267–279. [Google Scholar] [CrossRef]
- Xiao, J.-L.; Sun, S.-Y.; Song, X.; Li, P.; Yu, J.-G. Lithium ion recovery from brine using granulated polyacrylamide–MnO2 ion-sieve. Chemical Engineering Journal 2015, 279, 659–666. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, J.; Guo, R. Preparation and characterization of porous HMO/PAN composite adsorbent and its adsorption–desorption properties in brine. Journal of Porous Materials 2019, 26, 705–716. [Google Scholar] [CrossRef]
- Lai, X.; Yijia, Y.; Chen, Z.; Peng, J.; Sun, H.; Zhong, H. Adsorption-Desorption Properties of Granular EP/HMO Composite and Its Application in Lithium Recovery from Brine. Ind Eng Chem Res 2020, XXXX. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Yan, X.; Chen, Y.; Lang, W. Zr-doped titanium lithium ion sieve and EP-granulated composite: Superb adsorption and recycling performance. Particuology 2022, 69, 100–110. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Miao, Y.; Yang, Y.; Li, P. Alkaline Resins Enhancing Li+/H+ Ion Exchange for Lithium Recovery from Brines Using Granular Titanium-Type Lithium Ion-Sieves. Ind Eng Chem Res 2021, 60, 16457–16468. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yang, Y.; Lin, S.; Li, P. Preparation of granular titanium-type lithium-ion sieves and recyclability assessment for lithium recovery from brines with different pH value. Sep Purif Technol 2021, 267, 118613. [Google Scholar] [CrossRef]
- Hong, H.-J.; Park, I.-S.; Ryu, J.; Ryu, T.; Kim, B.-G.; Chung, K.-S. Immobilization of hydrogen manganese oxide (HMO) on alpha-alumina bead (AAB) to effective recovery of Li+ from seawater. Chemical Engineering Journal 2015, 271, 71–78. [Google Scholar] [CrossRef]
- Qian, H.; Huang, S.; Ba, Z.; Wang, W.; Yu, F.; Liang, D.; et al. Hto/cellulose aerogel for rapid and highly selective li+ recovery from seawater. Molecules 2021, 26, 54. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Xu, T.; Zhang, Y.; Li, G.; Li, Z. Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li. Molecules 2023, 28, 3191. [Google Scholar] [CrossRef] [PubMed]
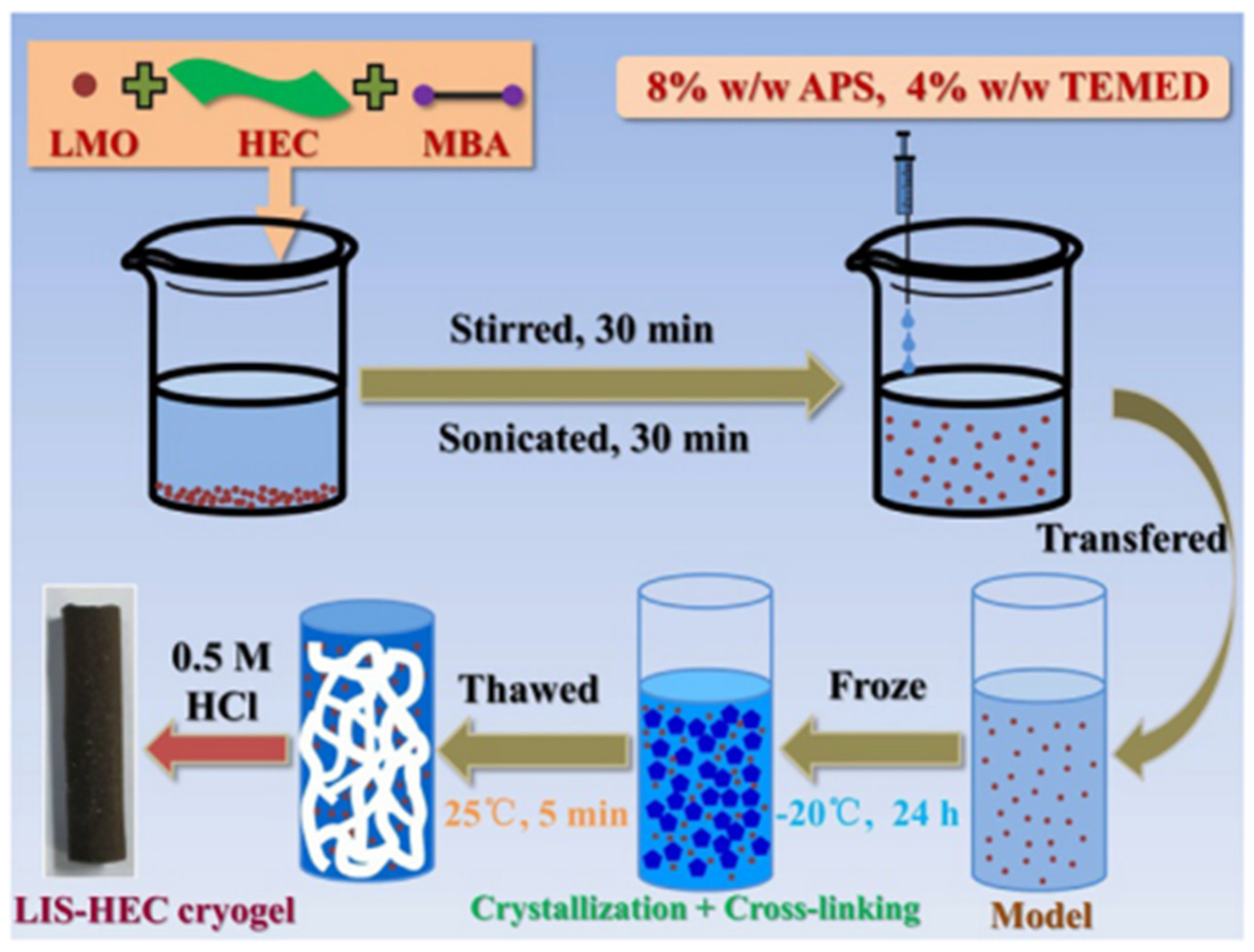
- Liu, C.; Tao, B.; Wang, Z.; Wang, D.; Guo, R.; Chen, L. Preparation and characterization of lithium ion sieves embedded in a hydroxyethyl cellulose cryogel for the continuous recovery of lithium from brine and seawater. Chem Eng Sci 2021, 229, 115984. [Google Scholar] [CrossRef]
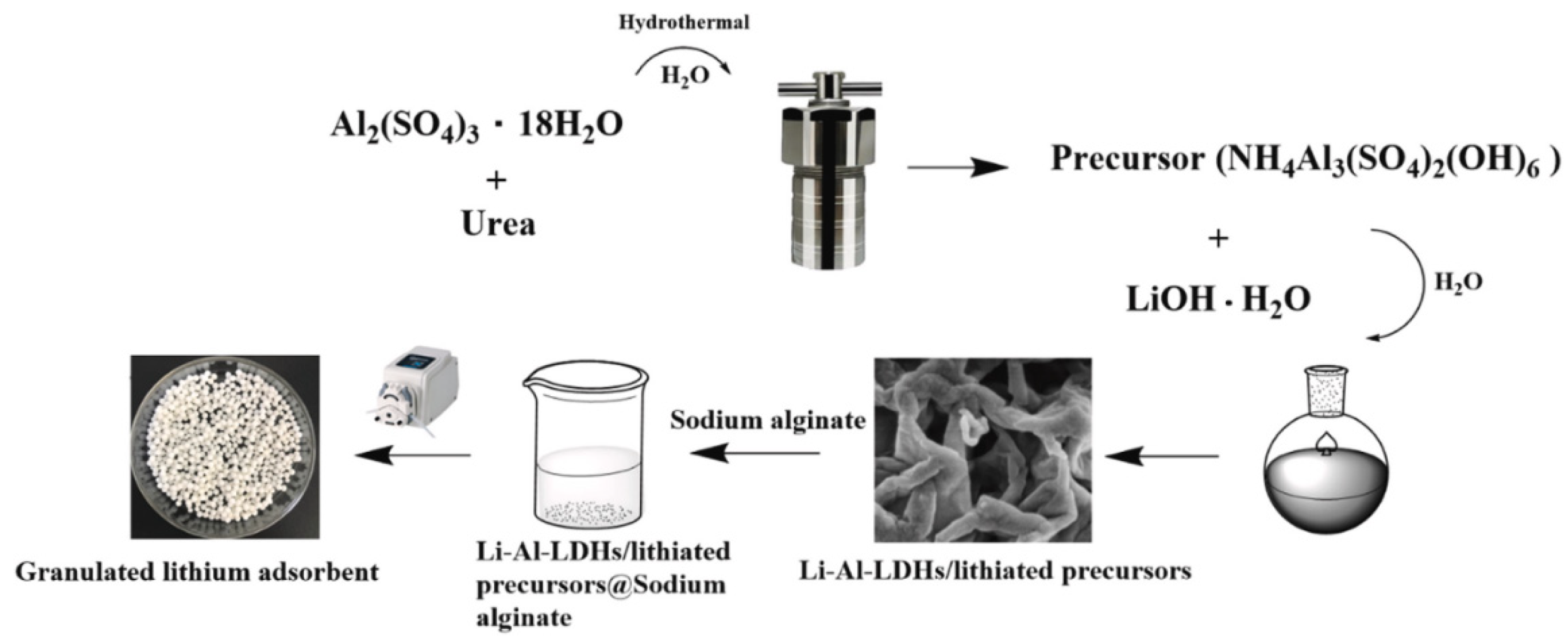
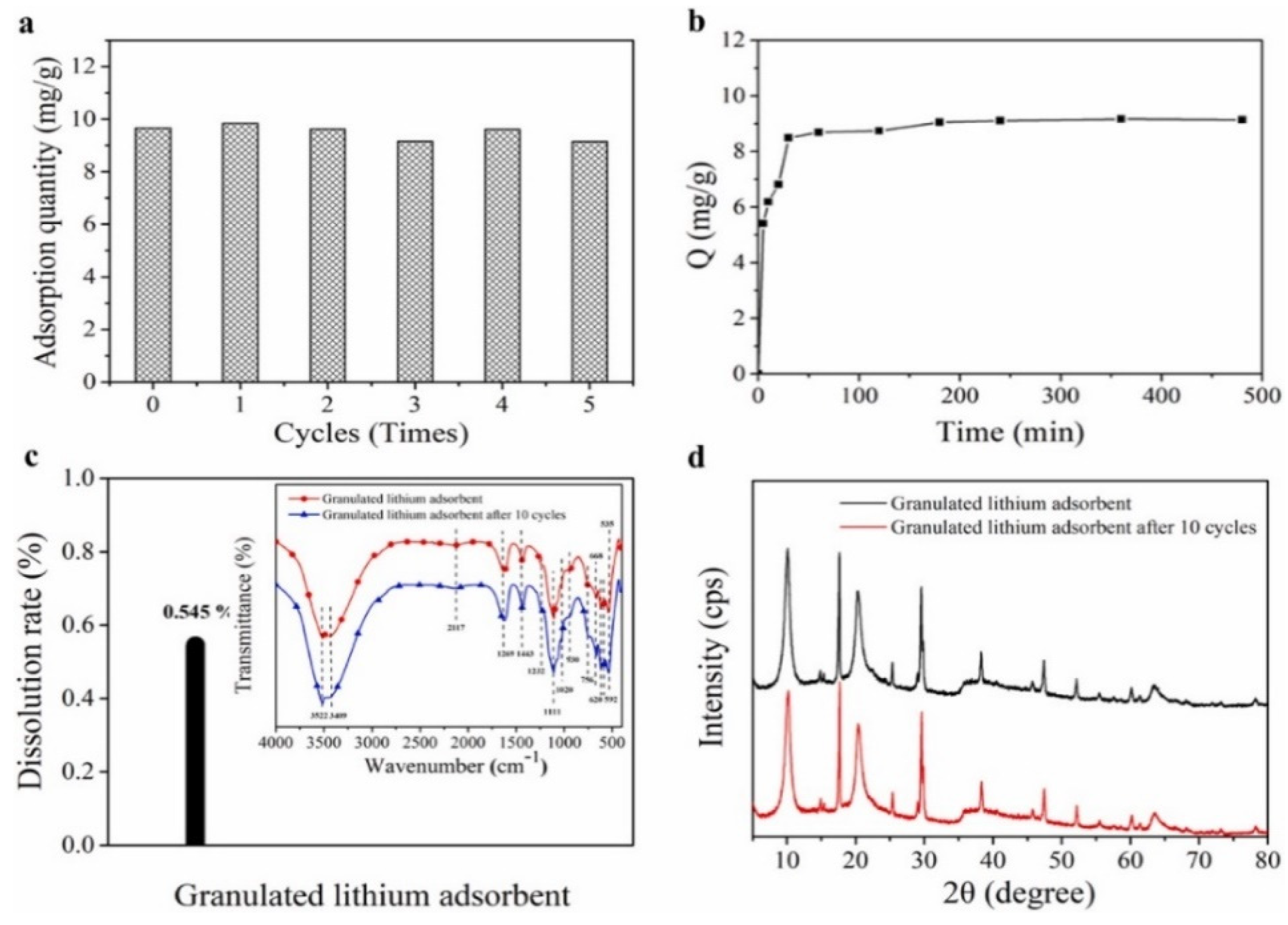
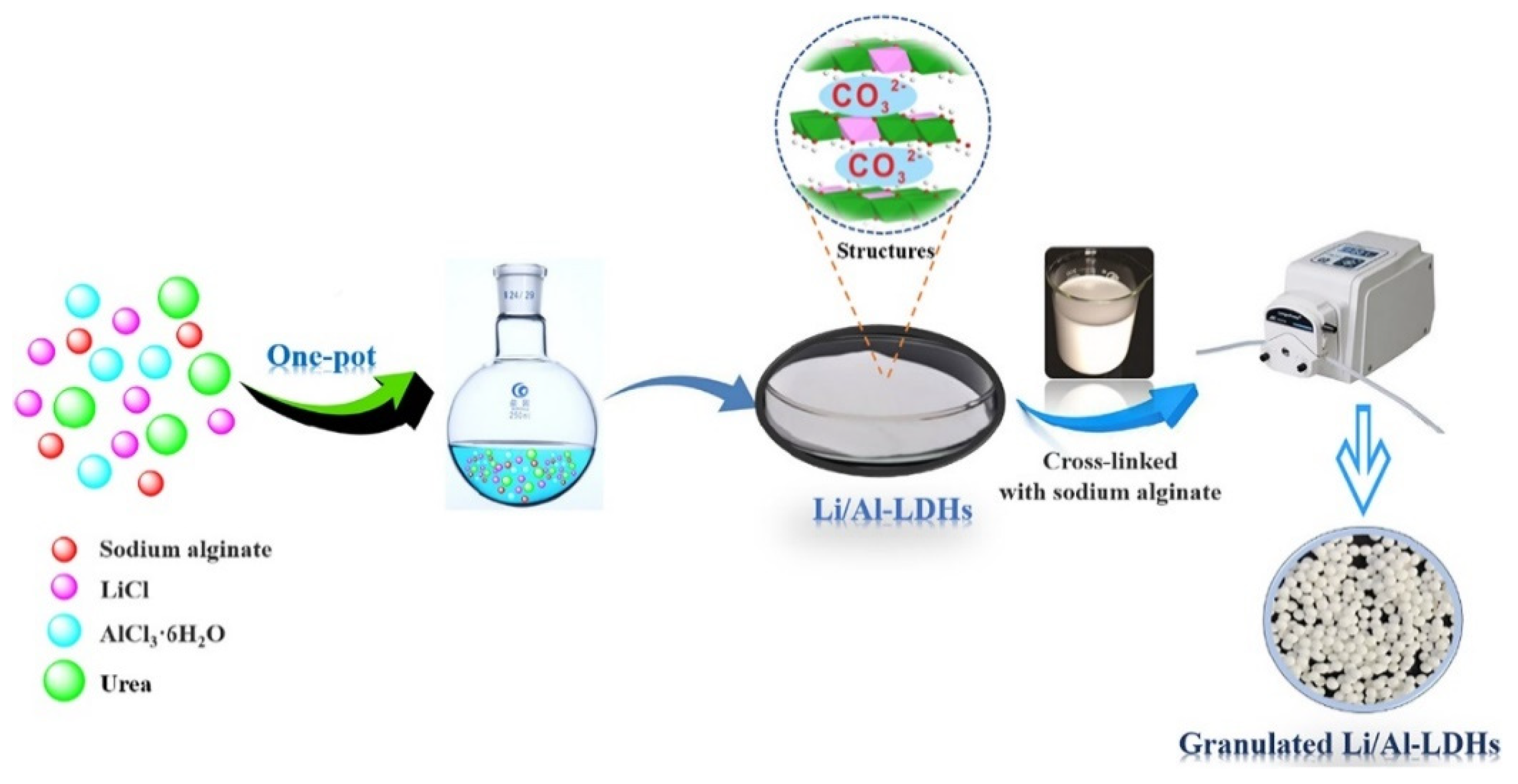
- Li, J.; Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z. Synthesis of granulated Li/Al-LDHs adsorbent and application for recovery of Li from synthetic and real salt lake brines. Hydrometallurgy 2022, 209, 105828. [Google Scholar] [CrossRef]
- Koilraj, P.; Smith, S.M.; Yu, Q.; Ulrich, S.; Sasaki, K. Encapsulation of a powdery spinel-type Li+ ion sieve derived from biogenic manganese oxide in alginate beads. Powder Technol 2016, 301, 1201–1207. [Google Scholar] [CrossRef]
- Park, S.H.; Yan, Y.-Z.; Kim, J.; Ha, C.-S.; Lee, S.J. Rapid and selective adsorption of Li+ from concentrated seawater using repulsive force of Al3+–crosslinked alginate composite incorporated with hydrogen manganese oxide. Hydrometallurgy 2022, 208, 105812. [Google Scholar] [CrossRef]
- Cheng, M.; Yao, C.; Su, Y.; Liu, J.; Xu, L.; Hou, S. Synthesis of membrane-type graphene oxide immobilized manganese dioxide adsorbent and its adsorption behavior for lithium ion. Chemosphere 2021, 279, 130487. [Google Scholar] [CrossRef]
- Han, Y.; Kim, H.; Park, J. Millimeter-sized spherical ion-sieve foams with hierarchical pore structure for recovery of lithium from seawater. Chemical Engineering Journal 2012, 210, 482–489. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wei, Z.; Hu, J.; Guo, Y.; Deng, T. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water. Chemical Engineering Journal 2021, 410, 128320. [Google Scholar] [CrossRef]
- Udoetok, I. Modified Biopolymer Sorbents for the Uptake of Naphthenic Acid Fraction Components (NAFCs) from Aqueous Solutions. Doctoral. University of Saskatchewan, 2018.
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.M.; Rozali, S. Biopolymers from Crop Plants. Reference Module in Materials Science and Materials Engineering, 2019. [CrossRef]
- Kaith, B.S.; Mittal, H.; Bhatia, J.K.; Kalia, S. Polysaccharide Graft Copolymers – Synthesis, Properties and Applications. In Biopolymers; John Wiley & Sons, Inc., 2011; pp. 35–57. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog Polym Sci 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications; Elsevier Science B.V., 2000. [Google Scholar]
- Gu, D.; Sun, W.; Han, G.; Cui, Q.; Wang, H. Lithium ion sieve synthesized via an improved solid state method and adsorption performance for West Taijinar Salt Lake brine. Chemical Engineering Journal 2018, 350. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Q.; Lin, D.; Yao, S. Effect and mechanism of sodium chloride on the formation of chitosan–cellulose sulfate–tripolyphosphate crosslinked beads. Soft Matter 2013, 9, 10354–10363. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan–Cellulose Composites and Their Anion Uptake Properties. ACS Appl Mater Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. “Pillaring Effects” in Cross-Linked Cellulose Biopolymers: A Study of Structure and Properties. Int J Polym Sci 2018, 2018, 6358254. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef]
- Yamazawa, A.; Iikura, T.; Shino, A.; Date, Y.; Kikuchi, J. Solid-, Solution-, and Gas-state NMR Monitoring of 13C-Cellulose Degradation in an Anaerobic Microbial Ecosystem. Molecules 2013, 18, 9021–9033. [Google Scholar] [CrossRef]
- Boufi, S.; Alila, S. Modified Cellulose Fibres as a Biosorbent for the Organic Pollutants 2011:483–524. [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.-Y. Kinetics of cellulose regeneration from cellulose-NaOH/thiourea/urea/H2O system. Cellulose Chemistry and Technology 2011, 45, 593. [Google Scholar]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol Biosci 2005, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, A.; Zhang, L. Effect of stirring conditions on cellulose dissolution in NaOH/urea aqueous solution at low temperature. J Appl Polym Sci 2012, 126, E470–7. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym J 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem Soc Rev 2012, 41, 1519. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J Am Chem Soc 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Moulthrop, J.S.; Swatloski, R.P.; Moyna, G.; Rogers, R.D. High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chemical Communications 2005, 1557. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angewandte Chemie International Edition 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int J Biol Macromol 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Faye, O.; Wilson, L.D. Adsorption of Phosphate Dianions by Hybrid Inorganic–Biopolymer Polyelectrolyte Complexes: Experimental and Computational Studies. ACS Appl Polym Mater 2020, 2, 899–910. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1 - Alginates: sources, structure, and properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Alginates: versatile polymers in biomedical applications and therapeutics; CRC Press, 2019. [Google Scholar]
- Yu, Q.; Morioka, E.; Sasaki, K. Characterization of lithium ion sieve derived from biogenic Mn oxide. Microporous and Mesoporous Materials 2013, 179, 122–127. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, M.; Qian, F.; Qian, Z.; Xu, N.; Wu, Z.; et al. Hydrothermal synthesis and adsorption behavior of H 4 Ti 5 O 12 nanorods along [100] as lithium ion-sieves. RSC Adv 2020, 10, 35153–35163. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications - A review. Int J Biol Macromol 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res 2019, 38, 101420. [Google Scholar] [CrossRef]
- Armisén, R, Gaiatas. 4 - Agar. In Handbook of Hydrocolloids, Second Edition; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing, 2009; pp. 82–107. [Google Scholar] [CrossRef]
- Schmidt, É.C.; Dos Santos, R.; Horta, P.A.; Maraschin, M.; Bouzon, Z.L. Effects of UVB radiation on the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): changes in cell organization, growth and photosynthetic performance. Micron 2010, 41, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B.; Akins, M. Hydrocolloids in bakery fillings. Hydrocolloids in Food Processing 2010:67–107.
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. Edible Films and Coatings for Food Applications 2009, 57–112. [Google Scholar]
- Han, Y.; Kim, S.; Kim, H.; Park, J. Preparation of sizable and uniform-sized spherical ceramic foams: drop-in-oil and agar gelation. Journal of the American Ceramic Society 2011, 94, 2742–2745. [Google Scholar] [CrossRef]





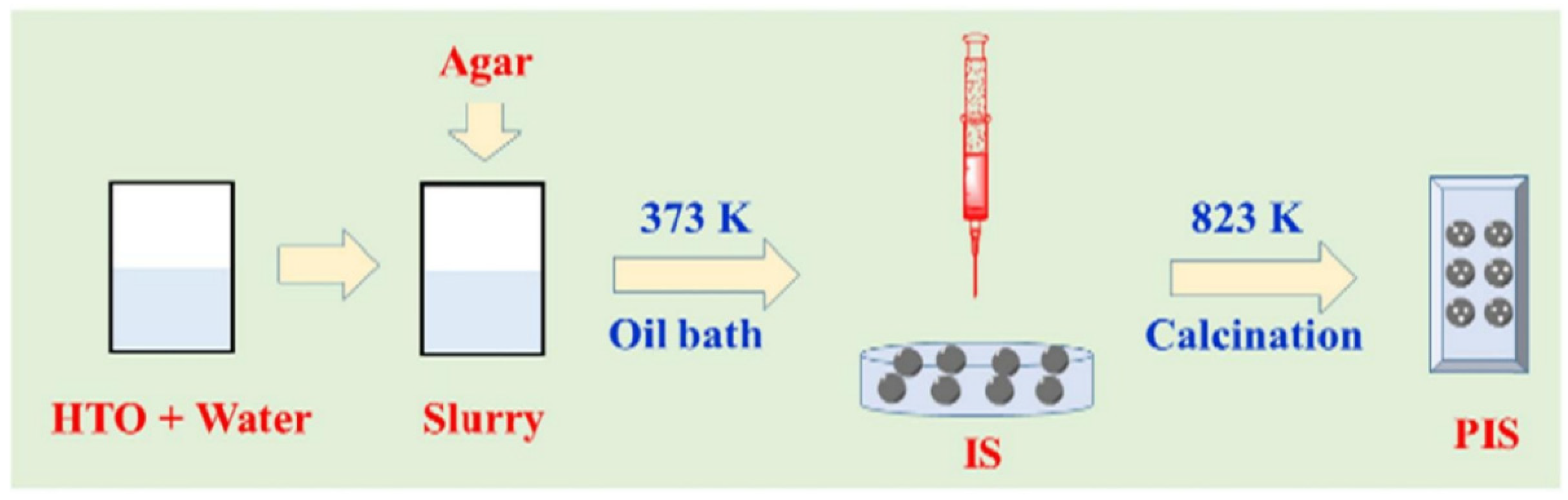
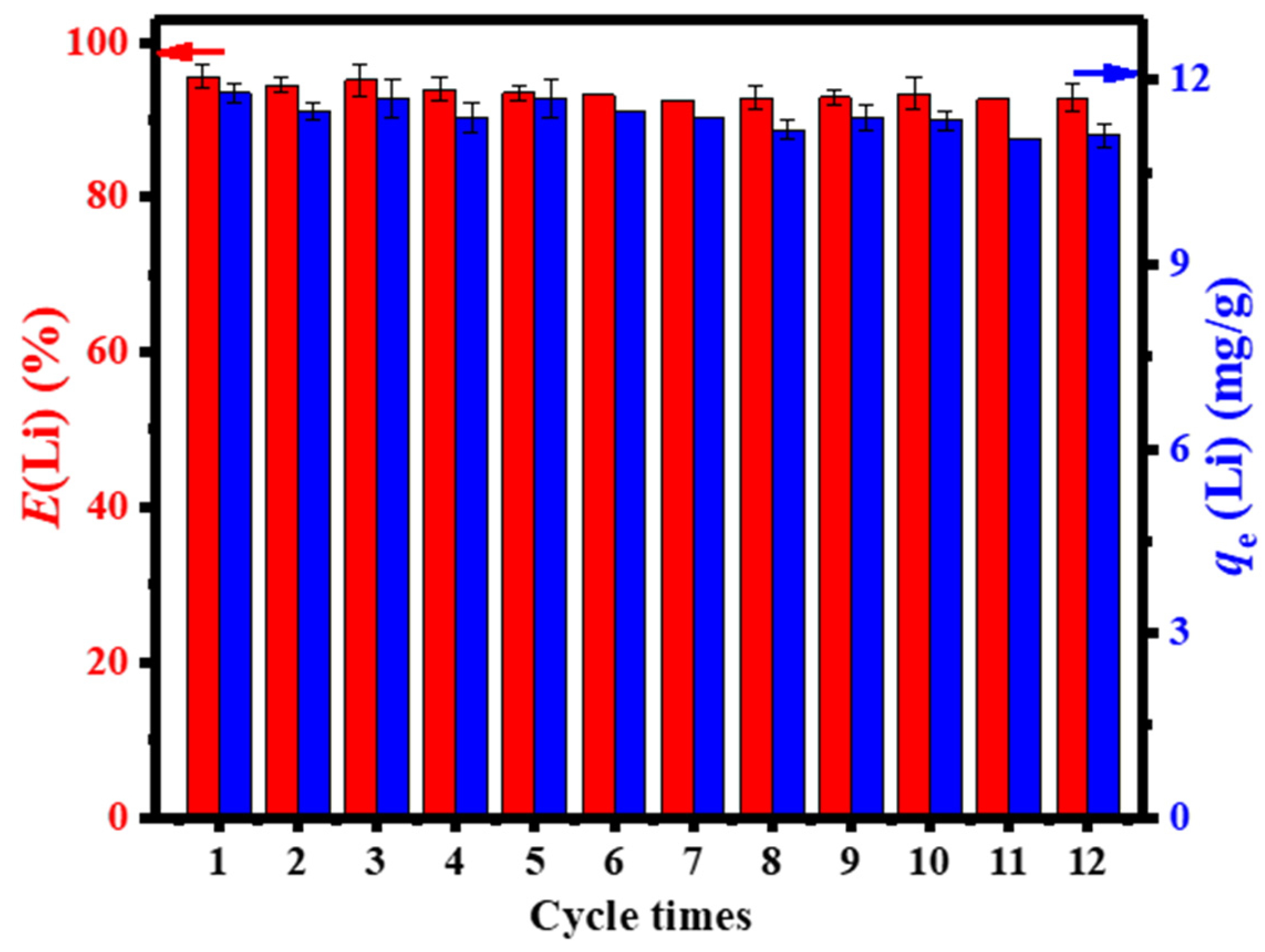
| S/N | Binder | Crosslinker | Sorbate | Uptake capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| 1 | Chitosan | Sulfuric acid | Spiked seawater | 10.0 | Hong et al. [49] |
| 2 | Chitosan | Sulfuric acid | Spiked seawater | 54.7 | Ryu et al. [50] |
| 3 | Chitosan | ECH and EDGE | Geothermal water | 11.4 | Ding et al. [51] |
| 4 | Chitosan | ECH | LiCl | 5.0 | Yüksel Özşen and Kahvecioğlu [52] |
| 5 | Chitosan | EDGE, ECH and glutaraldehyde | Geothermal water | 8.98 | Wang et al. [53] |
| 6 | Cellulose | NA | LiCl | 28.6 | Qian et al. [71] |
| 7 | Carboxymethyl cellulose | FeCl3, EDGE and ME | Geothermal water | 12.0 | Xie et al. [54] |
| 8 | Bacterial cellulose | NA | LiCl | 35.5 | Zhang et al. [72] |
| 9 | Hydroxyethyl cellulose | MBA | LiCl | 54.2 | Liu et al. [73] |
| 10 | Sodium alginate | CaCl2 | Salt Lake brine | 9.16 | Luo et al. [24] |
| 11 | Sodium alginate | CaCl2 | Salt Lake brine | 14.5 | Li et al. [74] |
| 12 | Sodium alginate | CaCl2 | LiCl | 24.9 | Koilraj et al. [75] |
| 13 | Sodium alginate | AlCl3/ ZrCl4 | Concentrated seawater | 2.52 | Park et al. [76] |
| 14 | Agar | NA | Seawater | 3.40 | Han et al. [78] |
| 15 | Agar | NA | Geothermal water | 12.3 | Chen et al. [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





