Submitted:
05 February 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
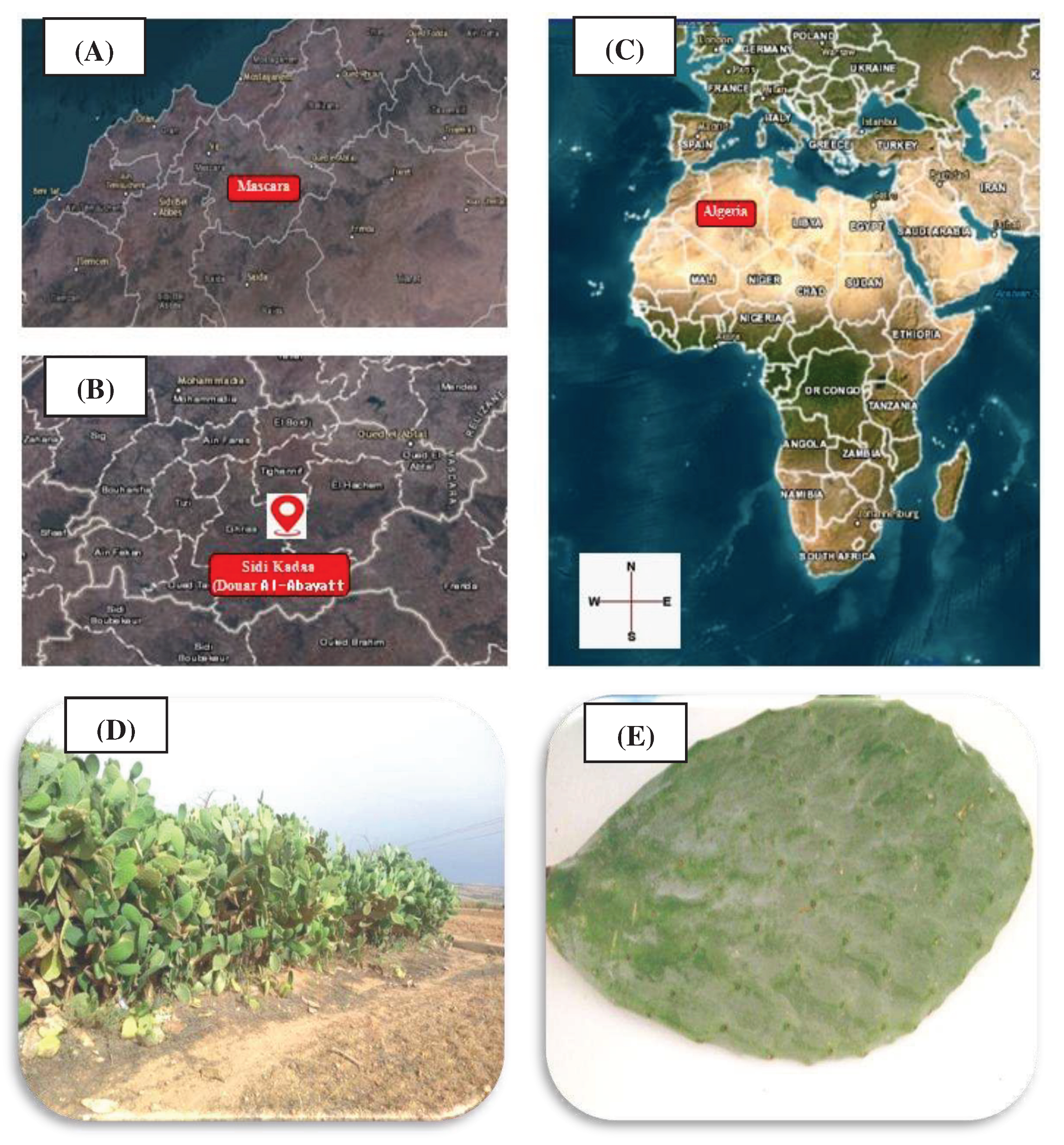
2.1. Sampling of Plant Material
2.1.1. Opuntia Ficus Indica
2.1.2. Tomato Fruits
2.2. Isolation and Identification of Fungal Agents
2.2.1. Isolation
2.2.2. Purification
2.2.3. Identification
2.2.3.1. Macroscopic Identification
2.2.3.2. Microscopic Identification
2.3. Screening of Bioactive Molecules
2.3.1. Preparation of the Methanolic Extract
2.3.2. Determination of Total Polyphenols
2.3.3. Determination of Total Flavonoids
2.3.4. Determination of Condensed Tannins
2.3.5. Determination of Carotenoids
2.3.6. Determination of Antioxidant Activity Using DPPH Test

2.4. Antifungal Activity
2.4.1. Preparation of Ethanolic Extract
2.4.2. Preparation of PDA Medium with Different Concentrations of Cladode Hydro-Ethanolic Extract
2.4.3. Antifungal Activity
2.4.3.1. Cladode hydro-Ethanolic Effect on Mycelial Growth

2.4.3.2. Cladodehydro-ethanoliceffect on Sporulation

3. Results
3.1. Characterisation of Tomato Fungal Diseases
3.2. Identification of Fungal Agents
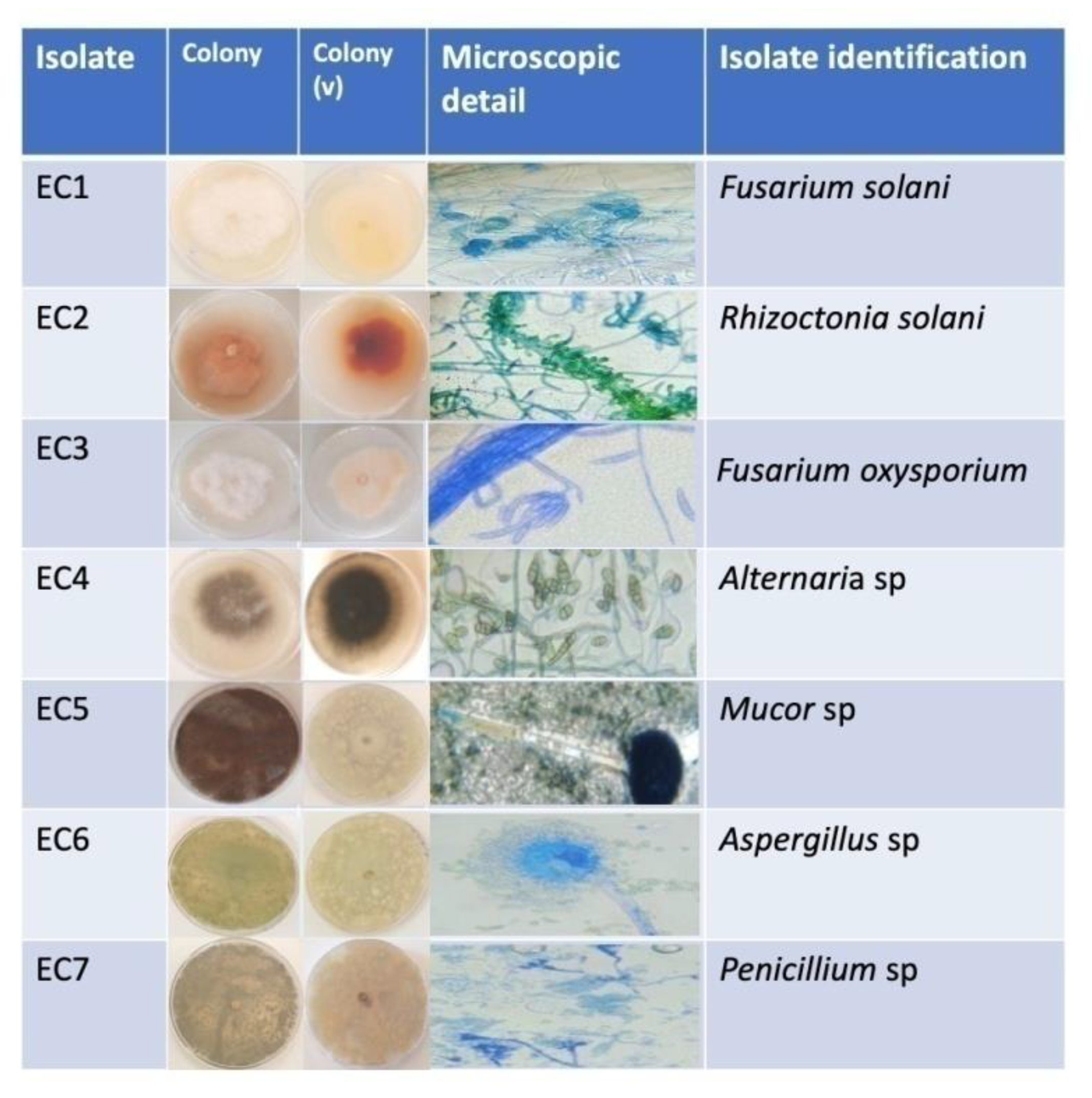
3.2.1. Macroscopic Identification
3.2.2. Microscopic Identification
3.3. Screening of Bioactive Molecules
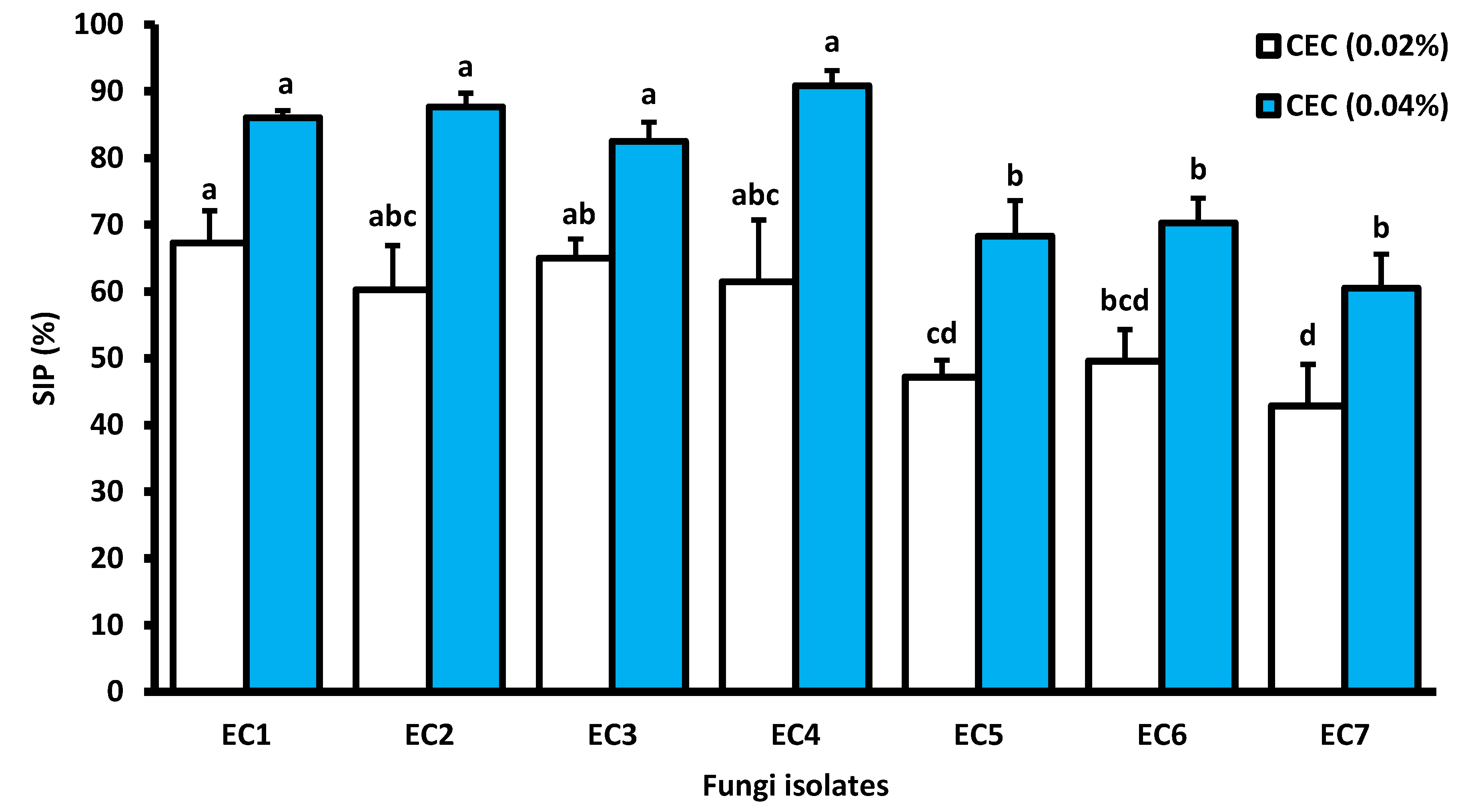
3.4. Antifungal Activity
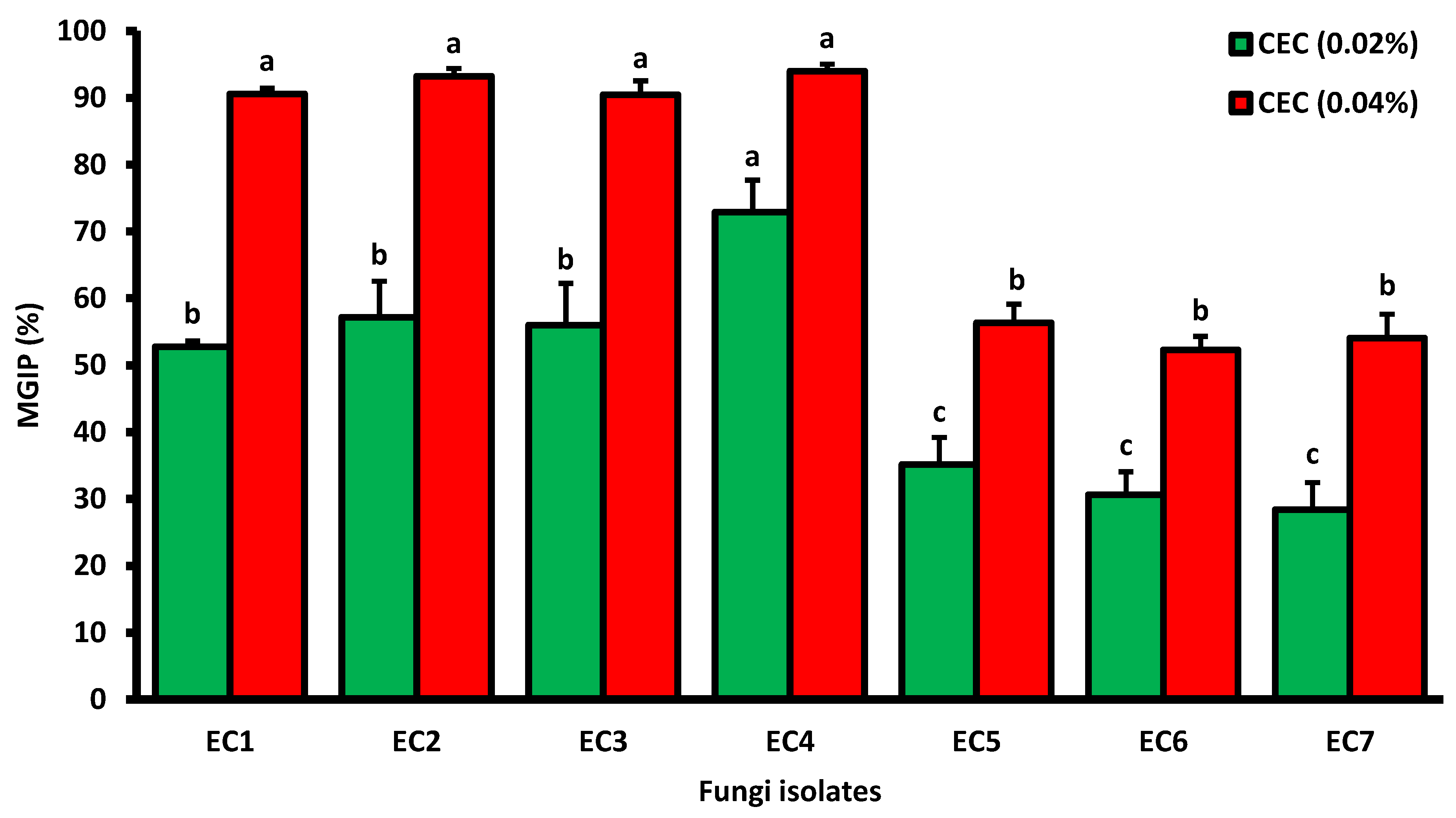
3.4.1. Cladode Hydro-Ethanoliceffecton Mycelial Growth
3.4.2. Cladode Hydro-Ethanolic Effect on Sporulation
4. Discussion
5. Conclusion
References
- Bekkar, K. Etude de l’effet des facteurs abiotiques et nutritionnels sur la production d’oospores chez Phytophthora infestans (Mont.) De Bary. Thèse de doctorat, Ecole Nationale Supérieure Agronomique d’El-Harrach, Alger, Algérie, 2014.
- Blancard, D.;Marchoux, G.; Laterrot, H.; Candresse, T. Les Maladies De La Tomate: Identifier, connaître, maîtriser. Editions Quae, Versailles, FR, 2009; p. 691.
- Branthôme, F.X. Worldwide (total fresh) tomato production in 2021. Available online: https://www.tomatonews.com/en/worldwide-total-fresh-tomato-production-in-2021_2_1911.html (accessed on 1 November 2023).
- Messiaen, C.M.; Blancard, D.; Rouxel, F.; Lafon, R. Les maladies des plantes maraichères. Ed. Institutnati. Rech. Agro., Paris, FR, 1993; p. 568.
- Naika, S.;Jeud, J.V.L.; Jeffau, M.; Hilmi, M.; Vandam, B. Culture de la tomate: production, transformation et commercialisation. (5eme edn). Digigrafi, Wageningen, NL, 2005; p.105.
- Zhang, G.; Wang, L.; Pan, J. Probing the binding of the flavonoid diosmetin to human serum albumin by multispectroscopic techniques. J. Agric. Food Chem. 2012, 60, 2721-2729. [CrossRef]
- Doyle, M.P.; Beuchat, L.R.; Montville, T.J. Food microbiology: Fundamentals and frontiers. ASM press, Washington DC, USA, 1998 ; pp. 598-611.
- Meyer, A.;Deiana, J.; Bernard, A. Cours de microbiologie générale: avec problèmes et exercices corrigés. Wolters Kluwer, Paris, FRA, 2004.
- Yang, Z.; Yuan, L.; Duan, Y. The investigation and prevention of tomato root knot nematode in Yunnan Yuanmou. Plant Prot Technol. 2011, 44–45;
- Merghid, M.;Debbache, M.; Foughali, I. Impacts des pesticides utilisés dans la plasticulture sur la santé humaine En Algérie. Etude de cas la wilaya de Constantine. Mémoire de Master, Université des Frères Mentouri Constantine, Constantine, Algérie, 2017.
- Alleche, N. Activité antifongique de quelques extraits d’une plante endémique sur des moisissures du blé stocké. Mémoire de Master, Université des Frères Mentouri Constantine, Constantine, Algérie, 2017.
- Abouthiam, A. Problématique de I’utilisation des insecticides chimiques dans la lutte anti-acridienne au Sahel. In La lutte anti-acridienne. Ed. AUPELF-UREF, Montreal, CA, 1991. pp.193-206.
- Bruneton, J. Pharmacognosie, phytochimie, plantes Médicinales. Éditions Tec & Doc, 3e édition, Lavoisier, Paris, FRA, 1999.
- Msaddak, L. Propriétés techno-fonctionnelles et substances bioactives de deux ingré-Dients alimentaires : cladodes du figuier de barbarie et feuilles de vigne. Thèse de doctorat, Université de Gabès, Tunis, Tunisie, 2018.
- Belmadadi, I.M.A. Etude du potentiel antioxydant d’Aloe vera et de la figue de Barbarie. Mémoire de Master, Université Mohamed El Bachir El Ibrahimi, Bordj Bou Arreridj, Algérie, 2018.
- Benattia, F.K. Analyse et Application des Extraits de pépains de Figues de Barbarie.Thèse de doctorat, Université Abou BekrBelkaid, Tlemcen, Algérie, 2018.
- Salem, H.B.; Nefzaoui, A.; Salem, L.B. Supplementing spineless cactus (Opuntia ficus-indica f. inermis) based diets with urea-treated straw or old man saltbush (Atriplex nummularia). Effects on intake, digestion and sheep growth. J. Agric. Sci. 2002, 138, 85-92. [CrossRef]
- Arba, M. Le cactus Opuntia, une espèce fruitière et fourragère pour une agriculture durable au Maroc. In Actes du Symposium International AGDUMED-durabilité des systèmes de culture en zone méditerranéenne et gestion des ressources en eau et en sol. Cana Print, Rabat, MAR, 2009; pp. 14-16.
- Yahia, E.M.; Sáenz, C. Cactus pear (Opuntia species). In book: Postharvest biology and technology of tropical and subtropical fruits. Acai to citrus Publisher: Woodhead Publishing, ENG, 2011; pp. 290-329.
- Felker, P.; Rodriguez, S.; del, C.; Casoliba, R.M.; Filippini, R.; Medina, D.; Zapata, R. Comparison of Opuntia ficus indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J. Arid Environ. 2005, 60, 405–422. [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128-136. [CrossRef]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and morphology of cladodes and spines of Opuntia ficus-indica. Cellulose extraction and characterisation. Carbohydr. Polym. 2003, 51, 77-83. [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S., Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442-451. [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037-2049. [CrossRef]
- Goudjil, S.;Naceri, K.; Noura, S. Caractérisation physicochimique et effet antibactérien de la cladode d’Opuntia ficus indicainermis(L) Mill. de la région de contribuTtioianret, en vue d’explorer son potentiel thérapeutique. Thèse de doctorat, Université Ibn khaldoun, Tiaret, Algérie, 2018.
- Contreras-Padilla, M.; Perez-Torrero, E.; Hernández-Urbiola, M.I.; Hernández-Quevedo, G.; del Real, A.; Rivera-Muñoz, E.M.; Rodríguez-García, M.E. Evaluation of oxalates and calcium in nopal pads (Opuntia ficus-indica var. redonda) at different maturity stages. J.Food Compos. Anal. 2011, 24, 38-43. [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [CrossRef]
- Cassagne, H. Milieux de culture et leurs applications. Edition de la Tourelle, Paris, FRA, 1966;379 p.
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd Ed. Springer Dordrecht, Heidelberg, London, New York, 2009, 524 p.
- Bessadat, N. Isolement, identification et caractérisation des Alternariasp. Résponsable de la détérioration des plantes maraichères par des systèmes enzymatiques et moléculaires. Thèse de doctorat, Université d’Oran es-senia, Oran, Algérie, 2014.
- Botton, B.; Bretton, A.; Fever, M.; Gautier, S.; Guy, P.; Larpent, J.P.; Reymond, P.; Sanglier, J.; Vayssier, Y.; Veau, P. Moisissures utiles et nuisibles. Importance industrielle, (edn) Masson, collection biotechnologie, Paris, FRA, 1990; pp. 309-512.
- Ghoul, M.; Minet, J.; Bernard, T.; Dupray, E.; Cornier, M. Marine macroalgae as a source for osmoprotection for Escherichia coli. Microb. Ecol.1995, 30, 171-81. [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152-178. [CrossRef]
- Dehpeur, A.A.; Ebrahimzadeh, A.A.; Fazel, N.S.; Nabavi, S.M. Antioxidant activity of the methanol extract of Ferulaassafoetida and its essential oil composition. Grasas Aceites. 2009, 60, 405–412. [CrossRef]
- Joslyn, M. Tannins and related phenolics. In Methods in food analysis, 701-725. J. Cell. Biochem. 1970, 22, 188-919. [CrossRef]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.M.; Toth-Markus, M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res. Int. 2005, 38, 1023-1029. [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects–A comprehensive review. Food Res. Int. 2018, 112, 328-344. [CrossRef]
- Sanchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Int. J. Foods Sci. Tech. 2002, 8, 121-137. [CrossRef]
- Serhan, A.R. Additional interaction of mint leaf extracts with fungi that have an antagonistic property on certain fungi associated with legume seeds. Arab. J. Plant Prot. 2006, 24, 118-124 (In Arabic).
- Kismoune, S. L’effet de l’extrait aqueux de cyprès sur la croissance de champignon Phythophthorainfestans. Mémoire de Master, Université Mouhamed SeddikBenyahia, Jijel, 2021.
- Leroux, P.;Credet, A. Document sur l’étude de l’activité des fongicides. INRA, Versailles, FRA, 1978, 12 p.
- Chabasse, D.;Bouchara, J.; De Gentile, L.; Brun, S.; Cimon, B.; Penn, P. Les moisissures d’intérêt médical. Cahier N°25 de formation de biologiemédicale. 2002; 157 p. [CrossRef]
- Guiraud, J.P.Microbiologie alimentaire. Dunod, Paris, FRA, 1998; pp.7-330.
- Jernejc, K.; Cimerman, A. Morphological characteristics, extracellular and inracellular protein and enzyme patterns of five Aspergillus species. Food Technol. Biotechnol. 2001, 39, 333-340. [CrossRef]
- Blancard, D. Biologie, épidémiologie. Available online: http://ephytia.inra.fr/fr/C/5217/Tomate-Biologie-epidemiologie (accessed on 1 November April 2023).
- Ventura-Aguilar, R.I.;Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065-5073. [CrossRef]
- Hadj Sadok,T.;Aid, F.;Bellal, M.; Abdul Hussain, M.S. Composition chimique des jeunes cladodes d’Opuntia ficus indicaet possibilité de valorisation alimentaire. Thèse de doctorat, Ecole NationaleSupérieurAgronomique, Alger, Algérie, 2010.
- Boutakiout, A. Etude physico-chimique, biochimique et stabilité d’un nouveau Produit: jus de cladode du figuier de Barbarie marocain (Opuntia ficus-indica et Opuntia megacantha). Thèse de doctorat, Université d’Angers, Angers, France, 2015.
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: effect on dough characteristics and cake making. Ind Crops Prod. 2009, 30, 40-47. [CrossRef]
- Aganga, A.A.; Mosase, K.W. Tannins content, nutritive value and dry matter digestibility of Lonchocarpuscapassa, Ziziphus mucronata, Sclerocaryabirrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol. 2003, 91, 107-113. [CrossRef]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065-5073. [CrossRef]
- Bruneton, J. Pharmacognosie. 5 Édition. Phytochimie– Plantes médicinales. Tec and Doc, Lavoisier, Paris, FRA, 2015; 1504 p.
- Šaponjac, V.T.; Canadanovi’c-Brunet, J.; Cetkovi’c, G.; Djilas, S. Detection of Bioactive Compounds in Plants’and Food Products. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food, 1st ed.; Nedovi’c, V., Raspor, P., Levi’c, J., TumbasŠaponjac, V., Barbosa-Cánovas, G.V., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 81–109.
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; De Leo’n-Rodrı’guez, A.; Fomsgaard, I.S.; de la Rosa, A.P.B. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compos. Anal.2010,23, 525-532. [CrossRef]
- Boukhalfa, S.; Hamdi, S. Évaluation phytochimique et étude des activités biologiques des extraits bruts des plantes médicinales locales: Opuntia ficus indica et Thymus lanceolatus. Mémoire de Master, Université des Frères Mentouri Constantine, Constantine, Algérie 2016.
- Maataoui, B.S.;Hmyene, A.; Hilali, S. Activites anti-radicalaires d’extraits de jus de fruits du figuier de barbarie (Opuntia ficus indica). Leban. Sci. J. 2006, 7, 3-8.
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184-196.
- Middleton,Jr.E.;Kandaswami, C.;Theoharides, T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673-751.
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002,13, 184-196.
- Youla, A.;Latrous, I.E. Contribution à l’étude phytochimique des flavonoïdes chez l’espèce (Melissa officinalis L.) et évaluation de leur pouvoir antibactérien. Mémoire de Master, Université des Frères Mentouri Constantine, Constantine, Algérie, 2017.
- Skadhauge, B.; Gruber, M.Y.; Thomsen, K.K.; Von Wettstein, D. Leucocyanidin reductase activity and accumulation of proanthocyanidins in developing legume tissues. Am. J. Bot. 1997, 84, 494-503. [CrossRef]
- Tirilly, Y.; Bourgeois, C. M. Technologie des légumes. Editions Tec & Doc, Lavoisier, FRA, 1999 ; 558 p.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S-2085S. [CrossRef]
- Collingborn, F.M.; Gowen, S.R.; Mueller-Harvey, I. Investigations into the biochemical basis for nematode resistance in roots of three musa cultivars in response to radopholussimilis infection. J. Agric. Food Chem. 2000,48, 5297-5301. [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for Sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol.2008, 147, 116-139. [CrossRef]
- Hassanpour, S.; Maheri-Sis, N.; Eshratkhah, B.; Mehmandar, F.B. Plants and secondary metabolites (tannins): A review. Int. J. For.Soil Eros. 2011, 1, 47–53.
- Dias, M.G.; Camões, M.F.G Oliveira, L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009, 113, 808-815. [CrossRef]
- López-Lázaro, M.; Martín-Cordero, C.; Ayuso, M.J. Two new flavonol glycosides as DNA topoisomerase I poisons. Z Naturforsch C. 2000, 55, 898-902. [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003, 1000, 657-691. [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. Trends Anal. Chem. 2014, 56, 49-73. [CrossRef]
- Jaramillo-Flores, M.E.; González-Cruz, L.; Cornejo-Mazon, M.; Dorantes-Alvarez, L.;Gutierrez-Lopez, G.F.; Hernandez-Sanchez, H. Effect of thermal treatment on the antioxidant activity and content of carotenoids and phenolic compounds of cactus pear cladodes (Opuntia ficus-indica). Food Sci Technol Int.2003, 9, 271-278. [CrossRef]
- Ootaki, T.; Yamazaki, Y.; Noshita, T.; Takahashi, S. Excess carotenoids disturb prospective cell-to-cell recognition system in mating responses of phycomycesblakesleeanus. Mycoscience. 1996, 37, 427-435. [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459-516. [CrossRef]
- Msaddak, L. Propriétés techno-fonctionnelles et substances bioactives de deux ingré-dients alimentaires : cladodes du figuier de barbarie et feuilles de vigne. Thèse de doctorat, Université de Gabès, Tunis, Tunisie, 2018.
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306-312. [CrossRef]
- Benhamama, L. Contribution à l’étude phytochimique et évaluation de l’activitéAntioxydante de la plante médicinale Crataegus monogyna. Mémoire de Master, Université des Frères Mentouri Constantine, Constantine, Algérie, 2015.
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244-282. [CrossRef]
- Cristani, M.; D’arrigo, M.; Giuseppina, M.; Castelli, F.; Sarpietro, M.; Micieli, M.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem. 2007, 55, 6301-6305. [CrossRef]
- Guignard, J.L. Abrégé de biochimie végétale, Ed. Masson, Paris, FRA;160 p.
- Alghamdi, A.; Alshehri, W.; Sajer, B.; Ashkan, M.; Ashy, R.; Gashgari, R.; Hakmi, H. Biological Activities and GC-MS Analysis of Aloe vera and Opuntia ficus-indica Extracts. J. Chem. 2023, 2023, 1-15. https://doi.org/10.1155/2023/6504505. [CrossRef]
- Hajar, N.; Nawal, A.; Amjad, D. A study to determine total phenolic content of Opuntia ficus-indica extracts and their activity against some pathogenic fungi. World J Pharm Pharm. Sci. 2018, 8, 98-109.
- Ali, N.; Nasser, H.; Deeb, A. Evaluating The inhibitory Efficacy Of Cactus plant Extracts (Opuntia ficus-indica) Against The Isolation Of Aspergillus niger Fungus. Tishreen University Journal-Basic Sciences Series. 2018, 40, 227-241.
- Paiva, P.M.; Santana, G.M.; Souza, I.F.; Albuquerque, L.P.; Agra-Neto, A.C.; Albuquerque, A.C.; Luz, L.A.; Napoleão, T.H.; Coelho, L.C.B.B. Effect of lectins from Opuntia ficus-indica cladodes and Moringa oleifera seeds on survival of Nasutitermes corniger. Int. Biodeterior. Biodegradation. 2011, 65, 982-989. [CrossRef]
- de Santana Souza, C.; Procópio, T.F., do Rego Belmonte, B.; Paiva, P.M.G.; de Albuquerque, L.A.; Pontual, E.V.; Napoleão, T.H. Effects of Opuntia ficus-indica lectin on feeding, survival, and gut enzymes of maize weevil, Sitophilus zeamais. Appl Biol Chem. 2018, 61, 337–343. [CrossRef]
- Ferrreira, E.C.B.; Nova, I.C.V.; de Almeida, W.A.; dos Santos Albuquerque, F.M.; dos Santos Cruz, G.; da Costa, H.N.; Procópio, T.F.; da Silva, W.A.V.; Ferreira, M.R.A.; Paiva, P.M.G.; Soares, L.A.L.; Teixeira, A.A.C.; Teixeira, V.W.; Napoleão, T.H.; Barros, R.; Pontual, E.V. Opuntia ficus-indica cladode extract is an embryotoxic, larvicidal, and oviposition-deterrent agent for the diamondback moth, Plutella xylostella. Crop Prot. 2021, 139, 105351. [CrossRef]
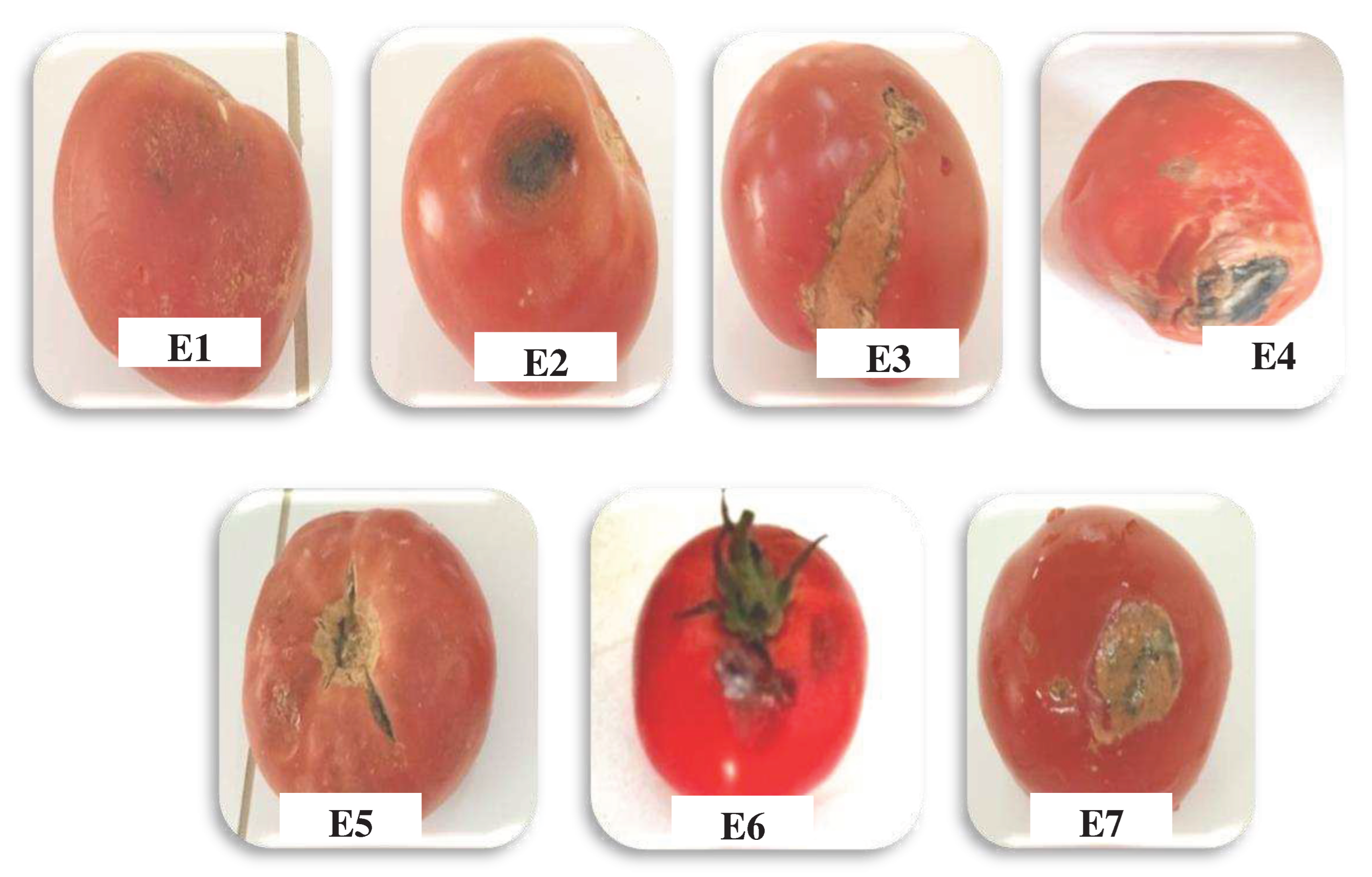

| Samples | Symptom | Fungiisolated | Disease |
|---|---|---|---|
| E1 | White spots | Fusarium solani | Fusariosis |
| E2 | Black spots | Rhizoctonia solani | Brown rot |
| E3 | Brown spots | Fusarium oxysporium | Fusariosis |
| E4 | Black spots | Alternaria sp | Alternariosis |
| E5 | Soft, dampfabric | Mucor sp | Mucormycosis |
| E6 | Grey stains | Aspergillus sp | Aspergillosis |
| E7 | Green stains | Penicillium sp | Penicillosis |
| Isolate code | Identification (Genus/species) |
|---|---|
| EC1 | Fusarium solani |
| EC2 | Rhizoctonia solani |
| EC3 | Fusarium oxysporium |
| EC4 | Alternaria sp |
| EC5 | Mucor sp |
| EC6 | Aspergillus sp |
| EC7 | Penicillium sp |
| Component | Content | |
|---|---|---|
| Total polyphenols | 86.63±0 .008 mg GAE/100g FW | |
| Flavonoids | 13.40±0.01 mg QE/100g FW | |
| Condensed tannins | 08.90±0.11mg TAE/100g FW | |
| Carotenoids | 0.94±0.02 mg β-CE /100g FW | |
| Antioxidant activity(DPPH test) | IC 50 (%) | |
| Cladode extract | Ascorbic acid | |
| 0.64±0.005 mg/ml | 0.39±0.003 mg/ml | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





