2. Materials and Methods
Cell culture
The human SUM159PT breast cancer cell line was purchased from Asterand Biosceince (BioIVT, Hicksville, USA), MDA MB 231 and HCC70 cells were purchased from ATCC (Manassas, USA), and cultured in the recommended media at 37°C with 5% CO2.
Irradiation
Cells were irradiated as monolayers at room temperature and at a density of approximately 5500 cells/cm2. The irradiation was performed in the radiotherapy department of Centre Oscar Lambret de Lille using a low-energy electron accelerator (DARPAC 2000 X-Ray) at a dose rate of 0.95 Gy/min. Analysis of CSCs was performed 5 days after irradiation.
Aldefluor assay
Breast cancer stem cells were identified based on their high aldehyde dehydrogenase (ALDH) activity, as described before by Ginestier et al. [
16], using the Aldefluor
® kit (StemCell Technologies, Vancouver, Canada). Only 30% of the most negative cells were collected as ALDH
- cells. Flow cytometry data were acquired on CyAn
TMADP cytometer (Beckman Coulter, Brea, USA) with Summit software. All analyses were performed with FlowJo (v10.4.1, BD, Franklin Lakes, USA).
RNA extraction, reverse transcription, and Real-Time RT-PCR
Total RNA from the cell lines was isolated using the RNeasy kit (Qiagen, Venlo, The Netherlands). cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System for RT (Invitrogen, Carlsbad, USA). Quantitative PCR was carried out using the CFX96 Real-Time System (Bio-Rad, Hercules, USA) with Quantification SyBR Green master mix (Qiagen, Venlo, The Netherlands). All primers were synthesized by Eurogentech (Sox2 5’-3’: AACCCCAAGATGCACAACTC; Sox2 3’-5’: CGGGGCCGGTATTTATAATC; Oct4 5’-3’: GAAGGATGTGGTCCGAGTGT; Oct4 3’-5’: GTGAAGTGAGGGCTCCCATA; Nanog 5’-3’: TTCAGTCTGGACACTGGCTG; Nanog 3’-5’: CTCGCTGATTAGGCTCCAAC; GAPDH 5’-3’: AGCCACATCGCTCAGACAC; GAPDH 3’-5’: GCCCAATACGACCAAATCC). The Ct for each gene was determined after normalization to GAPDH (ΔCt). We then obtained ΔΔCt by subtracting the ΔCt of the sample from the ΔCt of the control condition, and 2-ΔΔCt was used to calculate the ratio of expression differences.
Conditioned medium preparation and treatments
Conditioned medium (CM) was withdrawn from the cell culture 5 days after irradiation and centrifuged at 300 g for 10 min to eliminate dead cells and debris. CM treatments consisted of replacing 50% of the culture medium with CM freshly obtained from irradiated cells.
Cytokine & chemokine arrays
Protein arrays were performed with CM following the manufacturers’ instructions (Cytokine array panel A ARY005, Chemokine array ARY017, Biotechne, Minneapolis, USA). The signal was detected using a LAS-4000 camera (Fujifilm, Tokyo, Japan). Control samples were performed with fresh medium incubated without cells for 6 days at 37°C and 5% CO2 and were used to normalize and compare each sample.
Enzyme-linked immunosorbent array (ELISA)
ELISA assays for CXCL1 (Duoset CXCL1, DY275, Biotechne), CCL5 (Duoset CCL5, DY278, Biotechne) and CCL19 (DuoSet CCL19, DY361, Biotechne) were performed in non-diluted CM following the fabricant instructions. The plates were read with Multiskan FC (Thermo Scientific, Waltham, USA) at 450 nm and 540 nm for blank correction. The average of each duplicate was calculated after blank correction. Each optical density was plotted on the standard curve to determine the chemokine concentrations.
Treatment of cells with human recombinant cytokines
Cells were treated with recombinant cytokines from Bio-Techne. The concentrations used were equal to 10 times the ED50 indicated by the manufacture datasheet: CXCL12 (2 ng/ml), CCL19, CCL20 (5 ng/ml), CXCL1, CCL4, CCL5, MIF (10 ng/ml), CCL3 (30 ng/ml) and CXCL9 (1 μg/ml). All cytokines were reconstituted in PBS containing 0.1% BSA. For dose effect treatments, the chosen concentrations were 1, 10, or 100 times the ED50.
Sphere-forming capacity (SFC) and sphere generations
Cells were trypsinized and cultured in sphere medium consisting of phenol red-free DMEM-F12, 0.4% BSA (Sigma, St. Louis, USA), 10 ml B27 additive (Invitrogen) per 500 ml medium, 5 μg/ml insulin (Sigma), 4 μg/ml heparin, and 20 ng/ml epidermal growth factor (EGF) and fibroblast growth factor (FGF) (Biotechne). Cells were plated in 96-well low adhesion plates, ranging from 1024 cells to 1 cell. The number of spheres per well was assessed 7 days later. Cells were also cultured as spheres in the same medium, at 10,000 cells/ml in low adhesion flasks for 10 days, to perform sphere generations. Spheres were then dissociated with Accutase (Invitrogen) and re-cultured for secondary SFC tests.
Antibody staining for flow cytometry
For the chemokine receptors studies, receptors were stained with coupled antibodies from Miltenyi (Bergisch Gladbach, Germany) targeting CCR1 (130100371, PE-Vio770), CCR3 (130097068, VioBlue), CCR4 (13010395, Biotin and anti-Biotin, 130097022, VioGreen), CCR5 (130106224, APC), CCR6 (130100375, PE), CCR7 (130099153, Biotin), CXCR2 (130100908, APC-Vio770), and CXCR3 (130101377, APC or 130107463, PE-Vio615). Antibodies at a dilution of 1/50 were incubated for 10 min on ice in the dark in a staining buffer (PBS, 0.5% BSA, 2 mM EDTA), centrifuged, and washed with a staining buffer. If necessary, cells were first stained using the Aldefluor assay, and 50 μM Verapamil was added to the staining buffer to limit the loss of ALDH staining. REA controls (REAfinity Recombinant Antibody, Miltenyi) were performed at the same concentrations as those of corresponding specific antibodies.
For ALDH1, NANOG, SOX2, and OCT4 analysis on tumor cells, tumors were dissociated using enzymatic digestion (Tumor dissociation kit, Miltenyi 130-095-929) and mechanical dissociation (gentle MACS Dissociator). Fresh samples were then stained for ALDH activity (as previously described). Part of the samples was fixed with 4% paraformaldehyde solution and stored at 4°C before SOX2 and OCT2 immunostaining. Cells were incubated in PBS 0.1% Triton X100 for 15 min and rinsed in PBS. Cells (1x106) were then incubated with Biolegend antibodies targeting NANOG (2.5μg/ml, BLE653706, AF488), SOX2 (10 μg/mL, BLE656112, Pacific Blue), or OCT4 (BLE653706, AF488), or corresponding isotypes, for 30 min at RT. Cells were rinsed and analyzed by flow cytometry.
All samples were acquired on CyanTMADP (Beckman Coulter) and data were analyzed using FlowJo 10.
Cytokine treatment and inhibition
Cells were treated with recombinant cytokines from Bio-Techne. The concentrations used were equal to 10 times the indicated ED50: CXCL12 (2 ng/ml), CCL19, CCL20 (5 ng/ml), CXCL1, CCL4, CCL5, MIF (10 ng/ml), CCL3 (30 ng/ml) and CXCL9 (1 μg/ml). All cytokines were reconstituted in PBS containing 0.1% BSA. For dose effect treatments, the chosen concentrations were 1, 10, or 100 times the ED50. Cytokine inhibitions were performed using neutralizing antibodies from Bio-Techne. Different concentrations were tested, and the most effective one was chosen anti-CXCL1 (MAB275, 2 μg/ml), and anti-CCL5 (MAB278, 0.1 μg/ml). Corresponding isotype controls were used at the same concentrations as that of corresponding specific antibodies. Antibody treatments were carried out using ALDH- cells before radiotherapy. Cytokine depletion in the CM was performed by incubating CM with neutralizing antibodies at the concentrations cited above for 1h. Then, 50% of the cell medium was replaced with this mixture of CM and antibodies.
Receptor inhibition
To assess the chemokine receptor involvement in phenotypic plasticity, ALDH- cells were treated one time, 1 h before radiotherapy, with pharmacological inhibitors of, CXCR2 (SB225002), CCR1 (BX471), and CCR5 (Maraviroc) purchased from Biotechne. Different concentrations were tested, and the least toxic one was chosen (100 nM). Inhibitors were reconstituted in DMSO, and the control treatment consisted of DMSO alone.
In vivo study
Animal experiments were approved by the French Animal Experiment Ethics Committee (authorization #5935076 and project #01989.02). Female SCID mice (8 weeks) were purchased from Pasteur Institute, Lille, and maintained under pathogen-free conditions. SUM159PT cells were suspended in PBS (106 cells/ 100 μL) and subcutaneously injected in both flanks of each mouse. Tumor volumes were monitored every week by measuring the length (l) and width (w), and calculating the volume with the following formula: (π*l*w2)/6.
Treatments
Once the tumor volumes reached approximately 200 mm3, the mice were treated with neutralizing antibodies (Biotechne) and/or radiotherapy (Oncovet, Villeneuve d’Ascq, France). Mice were divided into different groups: “isotype controls” (IgG2B, 80 μg/mouse, MAB004; IgG1, 32 μg/mouse, MAB002), “antiCXCL1” (antiCXCL1, 80 μg/mouse, MAB275; IgG1), “antiCCL5” (antiCCL5, 32 μg/mouse, MAB278; IgG2B), and “antiCXCL1 + antiCCL5” (antiCXCL1; antiCCL5). Concentrations were determined according to the literature. Each group was subdivided into 2 subgroups: one was not irradiated (“0 Gy”) and the second subgroup received 5*3 Gy radiotherapy (“5*3 Gy”). The treatment protocol consisted of 6 intraperitoneal antibody injections over 2 weeks (3 injections per week, every 2 days) and 5 irradiations (5*3 Gy) daily during the first week. Mice were euthanized when the tumor volume reached an ethical limit or when the welfare score was too low.
Irradiation procedure
A Precise (Elekta, Stockholm, Sweden) linear accelerator was used for irradiation. A 6 MV photons (X-ray) beam was selected to permit the treatment of a large field, at a standard treatment distance (100 cm), through the cover of the container (thin plastic). The beam was delivered at a rate of 200 cGy/min.
The mice were placed by 6 in a sterile isolation container under 3% Isoflurane / O2 (0.9 L/min). Each container was placed on 5 cm PMMA plates to allow for radiation back-scatter. A 20 cm x 3 cm field was designed to have all mice from one group aligned under anesthesia, under the beam. Once under anesthesia, the mice were positioned in line along the long axis of the container with their hind limbs under the field light centered on the masses.
The point of prescription was located 0.5 cm in depth from the skin. A 1 cm tissue equivalent material (sterile water-soaked gauze) was placed over and around the hind limbs between each mouse to allow for dose build-up and lateral radiation scatter (and avoid the skin-sparing effect of megavoltage irradiation). Dose calculations were made by hand (2 cm in depth at a standard 100 cm SAD (Source Axis Distance).
Gene expression analysis from public databases and clinicopathological correlations
Analysis of gene expression profiles of clinical samples collected from 38 public data sets (Supplementary Table 1-2) required pre-analytic processing. The first step was to normalize each data set separately: we used quantile normalization for the available processed data from non-Affymetrix-based sets (Agilent, SweGene, and Illumina), and Robust Multichip Average (RMA) with the non-parametric quantile algorithm for the raw data from the Affymetrix-based data sets. Normalization was done in R using Bioconductor and associated packages. The probes were then mapped based on their EntrezGeneID. When multiple probes were mapped to the same GeneID, we retained the one with the highest variance in a particular dataset. We log2-transformed the available TCGA RNAseq data that were already normalized. We applied different multigene classifiers in each data set separately, including several cancer stem cells (CSC) signatures: Prat’s claudin-low, Creighton’s CD44+/CD24-, Prat’s subpopulation transition signature, Lim’s signature, and Charafe’s ALDH1 [
17,
18,
19,
20,
21]. Estrogen receptor (ER), progesterone receptor (PR), and ERBB2 expressions (negative/positive) were defined at the transcriptional level using gene expression data of ESR1, PGR, and ERBB2 respectively, as previously described [
22]. Expression levels of CXCL1, CCL5, and their receptors were extracted from each of the 38 normalized data sets. Before analysis, gene expression data were standardized within each data set using the PAM50 luminal A population as a reference [
23]. This allowed us to exclude biases due to laboratory-specific variations and population heterogeneity and to make data comparable across all sets. Metastasis-free survival (MFS) was calculated from the date of diagnosis until the date of distant relapse. Follow-up was measured from the date of diagnosis to the date of last news for event-free patients. Survivals were calculated using the Kaplan-Meier method and curves were compared with the log-rank test. All statistical tests were two-sided at the 5% level of significance. Statistical analysis was done using the survival package (version 2.30) in the R software (version 2.15.2;
http://www.cran.r-project.org/). We followed the reporting REcommendations for tumor MARKer prognostic studies (REMARK criteria) [
24].
Statistical analysis
All results are expressed as the means of at least 3 independent biological replicates. Data were analyzed using Prism (GraphPad) software. A difference with a p-value of 0.05 or less was considered statistically significant with the two-sided Student’s t-test and two-way ANOVA. Error bars are presented as SEM. The log-rank (Mantel-Cox) test was performed for survival analysis. A difference with a p-value of 0.05 or less was considered statistically significant. All Figures of the articles have been composed using Adobe Illustrator.
4. Discussion
In breast cancer, therapies resistance and recurrence drastically affect the long-term survival of patients. While tremendous efforts have been made to target resistant populations, including the CSC population, recent studies have disrupted the hierarchical dogma by demonstrating that non-CSCs can reacquire a CSC phenotype. Those studies have shown that ionizing radiation significantly induces expression of the embryonic transcription factors OCT3/4, SOX2 and NANOG, and enriches for CSC [
7,
30,
31]. Interestingly, considering the absolute number of CSCs post-treatment, these increases are not easily explained by selective killing of non-CSCs and their proliferation activation. Indeed, we have previously demonstrated that radiation, which induces the expression of these factors, reprograms non-CSCs into CSCs with the acquisition of the
in vivo functional CSC fate [
7]. While phenotypic plasticity has also been demonstrated in other conditions such as hypoxia or chemotherapies [
5,
6], only molecular mechanisms involved in pluripotency-associated signaling pathways have been identified. Although these pathways are necessary for CSC maintenance, they might not be involved in the process of regenerating them. Therefore, the underlying molecular pathways remain to be elucidated. This plasticity is thought to be responsible for enriching much of the CSC pool and increasing tumor resistance. In the current study, we used
in vitro and
in vivo models combined with clinical data to investigate the molecular mechanisms of reprogramming. Our findings demonstrate that irradiated non-CSCs secrete soluble factors that can activate NOTCH pathway and induce reprogramming of non-CSCs into CSCs.
We next aimed to investigate what factors are secreted by irradiated non-CSCs. Interestingly, cytokines, well-known secreted, chemotactic proteins have been associated with CSCs chemoresistance and enrichment in breast cancer [
11,
52]. We demonstrated that non-CSCs express basal levels of chemokine receptors, and that radiation can increase their expression, but most importantly, ionizing radiation also induces the release of chemokines, such as CXCL1 and CCL5, confirming the pro-inflammatory effect of radiation [
32]. As observed, CXCL1 expression seems to occur earlier that CCL5 expression, which might indicate the sequential role of each other. Also, we identified several other radiation-induced cytokines. Levels of induction can differ from cell types but could drive to the same phenotype switch due to cytokine signaling redundancy [
33]. While CXCL1 has been associated with a poor patient outcome [
34] and resistance through the recruitment of CD11b+Gr1+ myeloid cells into the tumor [
35], we demonstrated herein, for the first time, the direct role of CXCL1 in generating CSCs from non-CSCs. Interestingly, some molecules, such as curcumin, which is known to target CSC [
36], can sensitize breast cancer therapies by reducing CXCL1 expression [
37].
Like CXCL1, CCL5 has been shown to be associated with high-grade breast cancer [
38]. Moreover, Norton et al. recently demonstrated that breast cancer stem cells and CCR5+ cells affect the overall growth and morphology of breast tumors [
39]. Interestingly, similarly to our group, Norton et al. only observed a slight reduction in tumor growth under CCR5 inhibition conditions. Similar results, i.e., decreases in tumor growth and CSC frequency, have been observed using the CXCR1 inhibitor reparixin conjugated to docetaxel [
40]. However, these studies only focused on global enrichment instead of reprogramming; Herein, we observed that CXCL1 and CCL5 affected a non-CSC population and not an unsorted population. In a xenograft tumor mouse model derived from SUM159PT cells, the cells are usually fast growing and relatively resistant to anti-cancer treatments, as expected for a triple-negative breast cancer model. In this study, we demonstrated, for the first time, that targeting CXCL1 and CCL5 prevents radiation-induced reprogramming of non-CSCs into CSCs and CSC enrichment
in vivo, leading to an increase of the radiosensitivity of the tumor and mice survival.
Assuming that chemokines are involved in the reprogramming process, we wondered if we could identify a “reprogrammable” population, i.e., a non-CSC subpopulation that responds to chemokines and is preferentially able to re-acquire a CSC phenotype. According to this hypothesis, this specific population could be the one expressing the receptors for the identified chemokines. Chemokines and their receptors are connected in a complex network: chemokines can be ligands of various receptors and receptors can link several chemokines [
41]. Therefore, we studied several receptors as a whole instead of individually. While isolated CCR+/CXCR+ cells were not able to undergo reprogramming at a higher rate, we observed that sorted CCR-/CXCR- cells re-exposed CCR and CXCR at the membrane within 24h, resulting in a new population of non-CSCs with reprogramming potential. Surprisingly, individual receptor inhibition was not able to induce a significant decrease in reprogramming, while CCR/CXCR combined inhibitions do. This might be due to the CXCR/CCR signaling redundancy [
42]. Indeed, chemokines and their receptors may induce several common pathways that could be involved in the process, including JAK/STAT3 or NF-kB. As Notch was the first pathway demonstrated to be involved in reprogramming [
7], we studied the link between Notch signaling and CXCL1/CCL5 signaling. Using different inhibitors, we demonstrated that CXCL1/CCL5 signaling activation occurs before Notch one, as Notch inhibition fails to prevent CXCL1/CCL5 secretion and CXCL1/CCL5 treatment induces the expression of Notch and activation of the Notch pathway. Hsu et al. recently demonstrated that IL6 could induce the Notch pathway through the activation of the 𝛾-Secretase to regulate stemness [
43]. Also, CCL2 has been shown to induce NOTCH1 expression and the CSC features in breast cancer cells with crosstalk between stromal cells and cancer cells [
28].
By analyzing a database of 9236 breast cancer patients, we identified that expression of CXCL1, CCL5, and their receptors as well as combined C(X)CL/R gene pairs were highly correlated with CSC-associated profiles, indicating an implication of those C(X)C axes in CSC phenotype. It is worth noting that Creighton’s CD44high/CD24
-/low classification matches with the cytokine/receptor expression profile, the lack of correlation with the ALDH1
+ profile could be explained by the technique of detection (global mRNA) which can’t distinguish between expression in cancer cells and stromal cells. Indeed, Bednarz-Knoll et al. recently demonstrated that stroma expression of ALDH1 indicates reduced tumor progression [
44]. Their implication could be performed through direct enrichment or as we investigated through a permissive environment allowing reprogramming of non-CSC into CSC. As CXCL1 [
35,
36,
45,
46,
47] and CCL5 [
48,
49,
50] have been shown to be associated with therapy resistance and poor prognosis, we confirmed these observations, adding that CXCL1 and CCL5 are especially valuable for prognosis in patients receiving radiation treatment. Moreover, we evaluated the influence of the C(X)C paired gene, CXCL1/CXCR2, and CCL5/CCR1 or CCR5 axes on patient overall survival.
Interestingly, overexpression of the C(X)C gene pairs was only associated with poor prognosis in the case of radiotherapy treatment. These results are in step with enrichment by reprogramming of non-CSC into CSC during radiation treatment. While C(X)C axes could be involved in cancer stromal cell recruitments [
51,
52], we highlighted the potential role of reprogramming in the process of tumor progression. Thus, while the activation of CCL5/CCR1/CCR5 axes in tumor cells could promote tumor growth through the recruitment of immunosuppressive myeloid cells [
53] or angiogenesis [
54], C(X)C axes could also directly generate, by reprogramming resistant CSC from non-CSC within specific contexts and distant environments, and so affect patient prognosis (reduced OS). This observation completes recent data demonstrating that the inflammatory cytokine CXCL8 can stimulate dormant disseminated tumor cells in the liver to induce metastasis [
55].
Figure 1.
CSC induction after ionizing radiation and CM treatments. (A) Experimental procedure for testing the conditioned medium effect on reprogramming: cells were stained with the Aldefluor© assay, and ALDH-/low non-CSCs were sorted and seeded as monolayers. The cells were irradiated with 0 or 8 Gy. Five days after radiation, conditioned medium (CM) was withdrawn from irradiated cultures and applied to treat freshly sorted non-CSCs. (B) Reprogramming was analyzed 5 days after CM treatment or IR by performing an Aldefluor© assay in SUM159PT and MDA MB 231 cells. (C) CM-treated non-CSCs from SUM159PT cells were seeded in low-adhesion 96-well plates 5 days post-radiation. The number of primary spheres was counted 10 days later. In parallel, CM-treated non-CSCs were seeded in low-adhesion flasks 5 days after treatment and allowed to grow for 10 days. After 10 days, the spheres were dissociated and reseeded for secondary sphere-forming capacity tests. The sphere-forming capacity (SFC) of the cells was evaluated up to 4 generations (4G). (D) SFC of CM-treated non-CSCs SUM159PT after 10 days of culture. (E) Representative images of SUM159PT primary spheres at 10 days. Photos were obtained with a Nikon Eclipse Ti microscope, 10x lens (scale = 100 µm). (F) Expression of OCT4, SOX2, and NANOG by qRT-PCR in irradiated or CM-treated non-CSCs 5 days post-IR in SUM159PT. Gene expression was normalized to GAPDH expression. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
Figure 1.
CSC induction after ionizing radiation and CM treatments. (A) Experimental procedure for testing the conditioned medium effect on reprogramming: cells were stained with the Aldefluor© assay, and ALDH-/low non-CSCs were sorted and seeded as monolayers. The cells were irradiated with 0 or 8 Gy. Five days after radiation, conditioned medium (CM) was withdrawn from irradiated cultures and applied to treat freshly sorted non-CSCs. (B) Reprogramming was analyzed 5 days after CM treatment or IR by performing an Aldefluor© assay in SUM159PT and MDA MB 231 cells. (C) CM-treated non-CSCs from SUM159PT cells were seeded in low-adhesion 96-well plates 5 days post-radiation. The number of primary spheres was counted 10 days later. In parallel, CM-treated non-CSCs were seeded in low-adhesion flasks 5 days after treatment and allowed to grow for 10 days. After 10 days, the spheres were dissociated and reseeded for secondary sphere-forming capacity tests. The sphere-forming capacity (SFC) of the cells was evaluated up to 4 generations (4G). (D) SFC of CM-treated non-CSCs SUM159PT after 10 days of culture. (E) Representative images of SUM159PT primary spheres at 10 days. Photos were obtained with a Nikon Eclipse Ti microscope, 10x lens (scale = 100 µm). (F) Expression of OCT4, SOX2, and NANOG by qRT-PCR in irradiated or CM-treated non-CSCs 5 days post-IR in SUM159PT. Gene expression was normalized to GAPDH expression. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
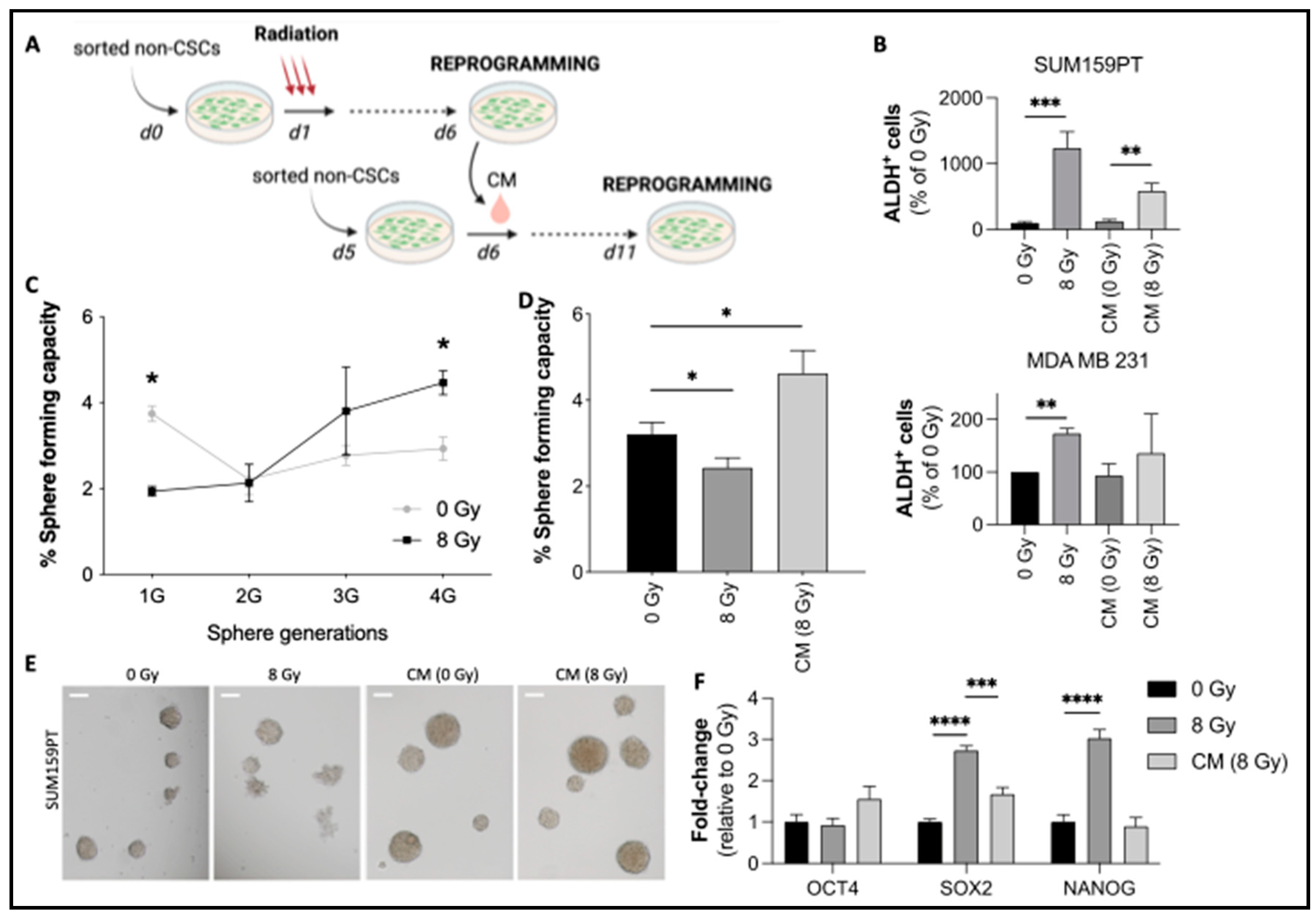
Figure 2.
Radiation-induced expression of chemokines and their effects on reprogramming. (A) CM from irradiated ALDH-/low non-CSCs SUM159PT was collected and analyzed by chemokine array. The control condition consists of a fresh medium incubated for 6 days without cells at 37°C and 5% CO2. Black arrows indicate CXCL1 (1), CCL5 (2), and CXCL8 (3). (B) Relative expression of chemokines post-IR. The expression was normalized to the internal positive controls. (C, D) Relative quantification of CXCL1 (C) and CCL5 (D) by ELISA for 5 days post-IR. (E, F) Freshly sorted non-CSCs were seeded and treated 24 hours later with CXCL1 or CCL5 (concentrations are indicated in Materials and Methods). The control condition consisted of PBS 0.1% BSA. (E) Aldefluor flow cytometry analysis at 5 days post-treatment. (F) Sphere-forming capacity of treated cells. (G) Freshly sorted non-CSCs were treated 24 hours after sorting with radiation and/or neutralizing antibodies against CXCL1 and CCL5 (or isotype controls). ALDH+ cells were analyzed by flow cytometry 5 days after treatment. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
Figure 2.
Radiation-induced expression of chemokines and their effects on reprogramming. (A) CM from irradiated ALDH-/low non-CSCs SUM159PT was collected and analyzed by chemokine array. The control condition consists of a fresh medium incubated for 6 days without cells at 37°C and 5% CO2. Black arrows indicate CXCL1 (1), CCL5 (2), and CXCL8 (3). (B) Relative expression of chemokines post-IR. The expression was normalized to the internal positive controls. (C, D) Relative quantification of CXCL1 (C) and CCL5 (D) by ELISA for 5 days post-IR. (E, F) Freshly sorted non-CSCs were seeded and treated 24 hours later with CXCL1 or CCL5 (concentrations are indicated in Materials and Methods). The control condition consisted of PBS 0.1% BSA. (E) Aldefluor flow cytometry analysis at 5 days post-treatment. (F) Sphere-forming capacity of treated cells. (G) Freshly sorted non-CSCs were treated 24 hours after sorting with radiation and/or neutralizing antibodies against CXCL1 and CCL5 (or isotype controls). ALDH+ cells were analyzed by flow cytometry 5 days after treatment. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
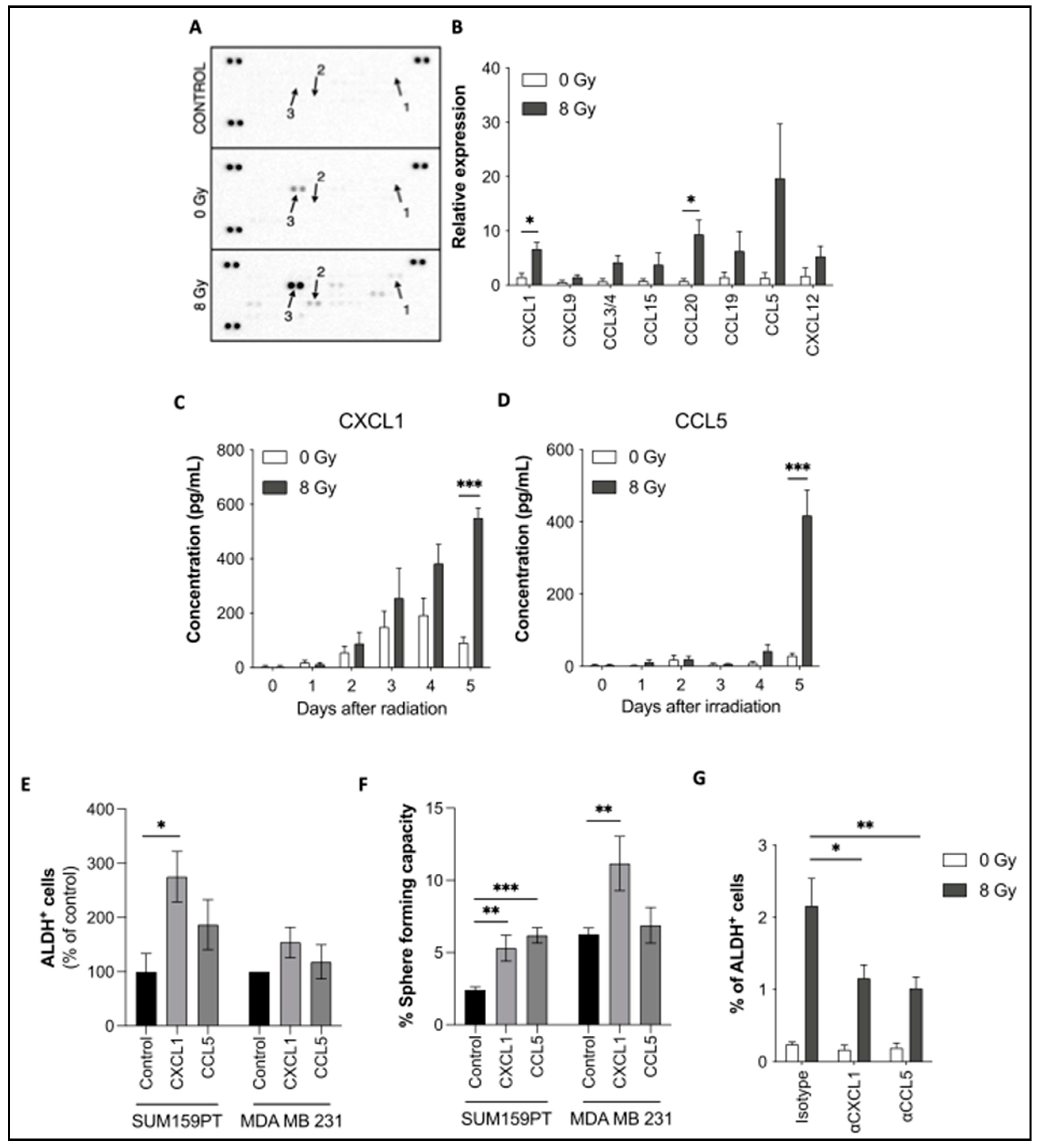
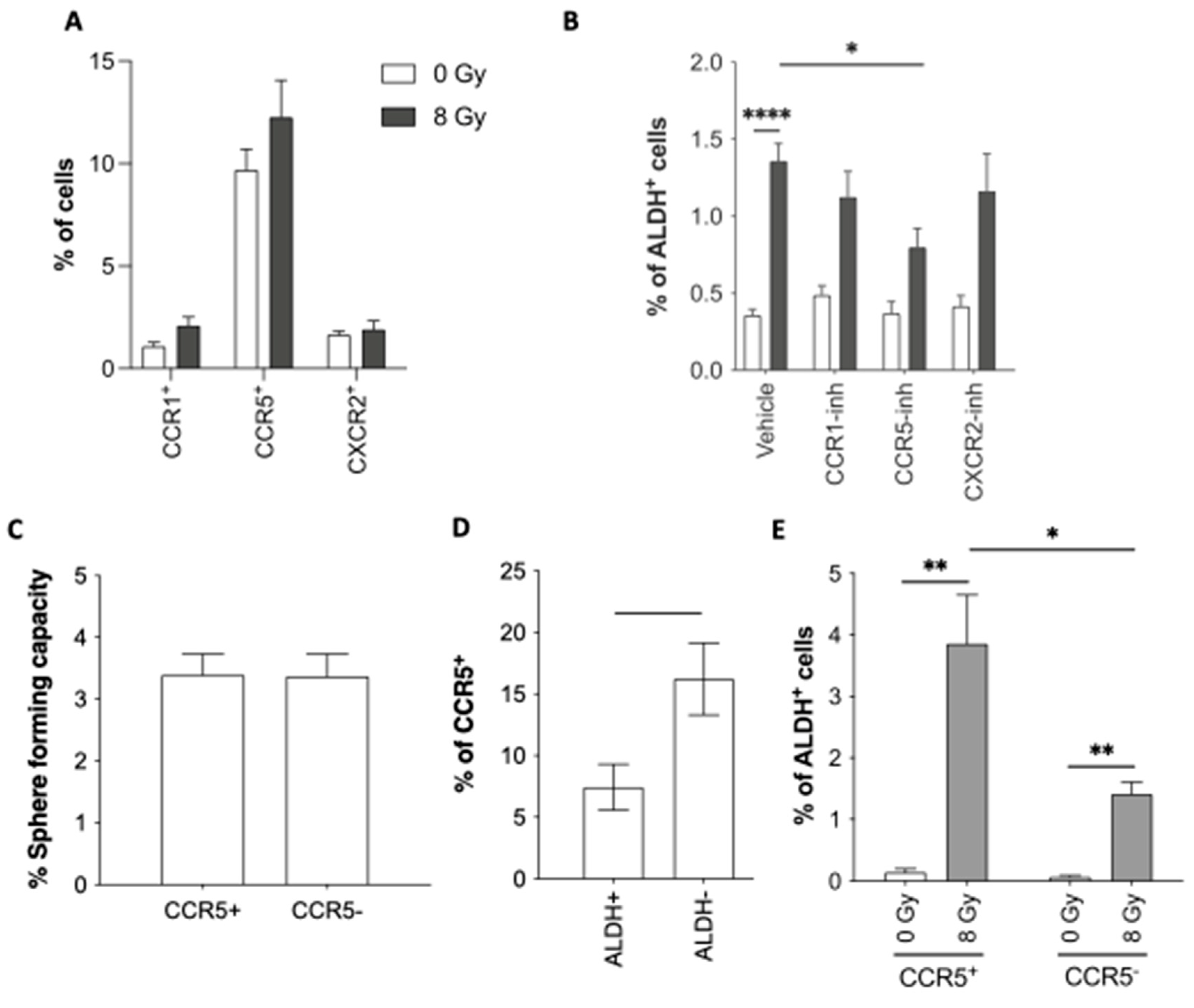
Figure 3.
Chemokine receptor expression and their role in reprogramming. (A) Immunostaining of CCR1, CCR5, and CXCR2 was performed in SUM159PT and analyzed by flow cytometry, with or without radiation. (B) Sorted ALDH- non-CSCs were treated with BX471 (CCR1-inhibitor), Maraviroc (CCR5-inhibitor), or SB225002 (CXCR2-inhibitor), followed by radiation. Reprogramming (ALDH+ cells) was analyzed by flow cytometry. (C) CCR5+ and CCR5- SUM159PT cells were sorted and plated in low-adhesion plates to measure their sphere-forming capacity. (D) Co-staining of ALDH and CCR5 was performed and the percentage of CCR5+ cells was determined in ALDH+ and ALDH- SUM159PT cells. (E) ALDH-, CCR5+, and CCR5- SUM159PT cells were sorted and treated with radiation 24 hours later. The reprogramming capacity was assessed 5 days later by flow cytometry (ALDH+ cells). All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
Figure 3.
Chemokine receptor expression and their role in reprogramming. (A) Immunostaining of CCR1, CCR5, and CXCR2 was performed in SUM159PT and analyzed by flow cytometry, with or without radiation. (B) Sorted ALDH- non-CSCs were treated with BX471 (CCR1-inhibitor), Maraviroc (CCR5-inhibitor), or SB225002 (CXCR2-inhibitor), followed by radiation. Reprogramming (ALDH+ cells) was analyzed by flow cytometry. (C) CCR5+ and CCR5- SUM159PT cells were sorted and plated in low-adhesion plates to measure their sphere-forming capacity. (D) Co-staining of ALDH and CCR5 was performed and the percentage of CCR5+ cells was determined in ALDH+ and ALDH- SUM159PT cells. (E) ALDH-, CCR5+, and CCR5- SUM159PT cells were sorted and treated with radiation 24 hours later. The reprogramming capacity was assessed 5 days later by flow cytometry (ALDH+ cells). All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t test, n ≥ 3.
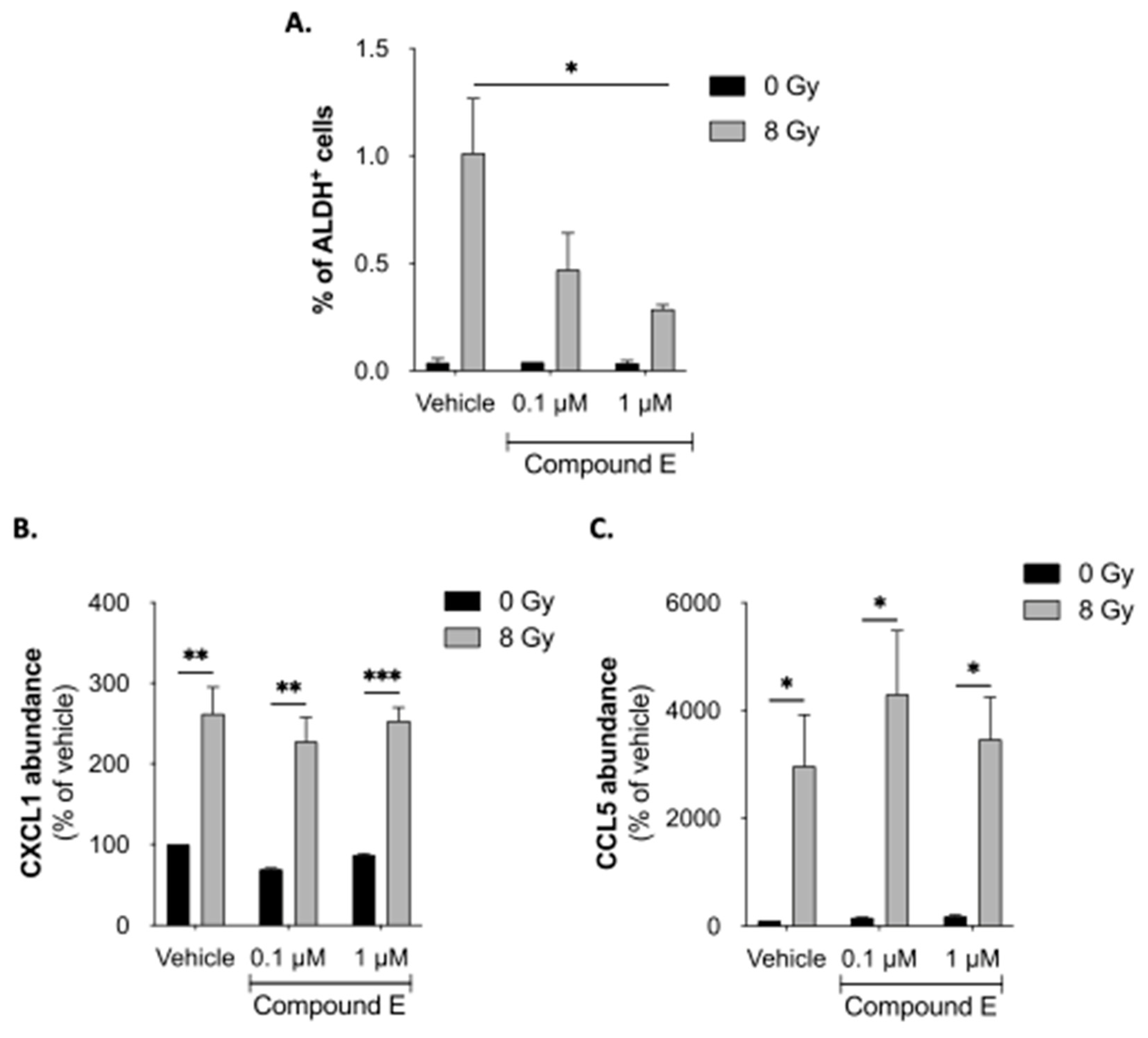
Figure 4.
Notch pathway is involved in reprogramming. (A) Sorted ALDH- non-CSCs were treated with Compound E (Notch-inhibitor), followed by radiation. Reprogramming (ALDH+ cells) was analyzed by flow cytometry. (B, C) Relative quantification of CXCL1 (B) and CCL5 (C) by ELISA for 5 days post-treatment. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t-test, n ≥ 3.
Figure 4.
Notch pathway is involved in reprogramming. (A) Sorted ALDH- non-CSCs were treated with Compound E (Notch-inhibitor), followed by radiation. Reprogramming (ALDH+ cells) was analyzed by flow cytometry. (B, C) Relative quantification of CXCL1 (B) and CCL5 (C) by ELISA for 5 days post-treatment. All data are represented by means ± SEM. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001, t-test, n ≥ 3.
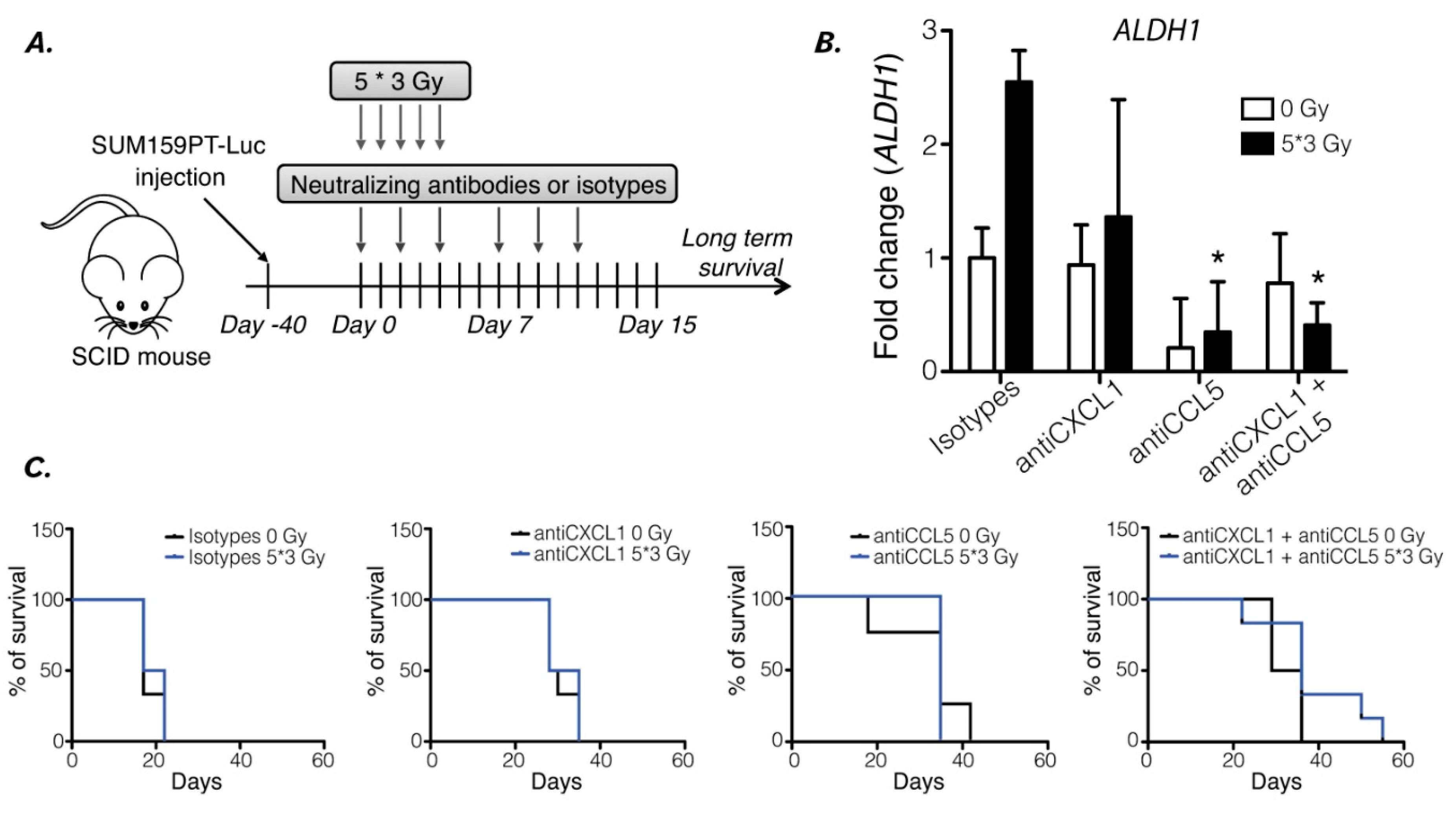
Figure 5.
Reprogramming inhibition increases xenografted mouse survival. (A) In vivo experiment protocol: mice were injected with 106 SUM159PT-Luc cells in both flanks. When tumors were detectable, the mice were either irradiated or not and then treated with either or both antiCXCL1 and antiCCL5 neutralizing antibodies or isotypes controls. (B) RNA extraction from xenografted tumors was performed, and ALDH1 expression was evaluated by qPCR. Human ß2-microglobulin expression was used as a control. t-test, n=6 mice per group, *, p<0.05. (C) Kaplan-Meier survival curves of xenografted SCID mice irradiated or not, treated with isotype controls, antiCXCL1, antiCCL5 or both. Survival medians are 17 days, 29 days, 35 days, and 32.5 days at 0 Gy (Log-rank (Mantel-Cox), p=0.0031), and 19.5 days, 31.5 days, 35 days, and 36 days at 5*3 Gy (Log-rank (Mantel-Cox), p=0.0028) in “isotypes", "antiCXCL1", "antiCCL5" and “antiCXCL1+antiCCL5" groups, respectively, n=6 mice per group.
Figure 5.
Reprogramming inhibition increases xenografted mouse survival. (A) In vivo experiment protocol: mice were injected with 106 SUM159PT-Luc cells in both flanks. When tumors were detectable, the mice were either irradiated or not and then treated with either or both antiCXCL1 and antiCCL5 neutralizing antibodies or isotypes controls. (B) RNA extraction from xenografted tumors was performed, and ALDH1 expression was evaluated by qPCR. Human ß2-microglobulin expression was used as a control. t-test, n=6 mice per group, *, p<0.05. (C) Kaplan-Meier survival curves of xenografted SCID mice irradiated or not, treated with isotype controls, antiCXCL1, antiCCL5 or both. Survival medians are 17 days, 29 days, 35 days, and 32.5 days at 0 Gy (Log-rank (Mantel-Cox), p=0.0031), and 19.5 days, 31.5 days, 35 days, and 36 days at 5*3 Gy (Log-rank (Mantel-Cox), p=0.0028) in “isotypes", "antiCXCL1", "antiCCL5" and “antiCXCL1+antiCCL5" groups, respectively, n=6 mice per group.
Figure 6.
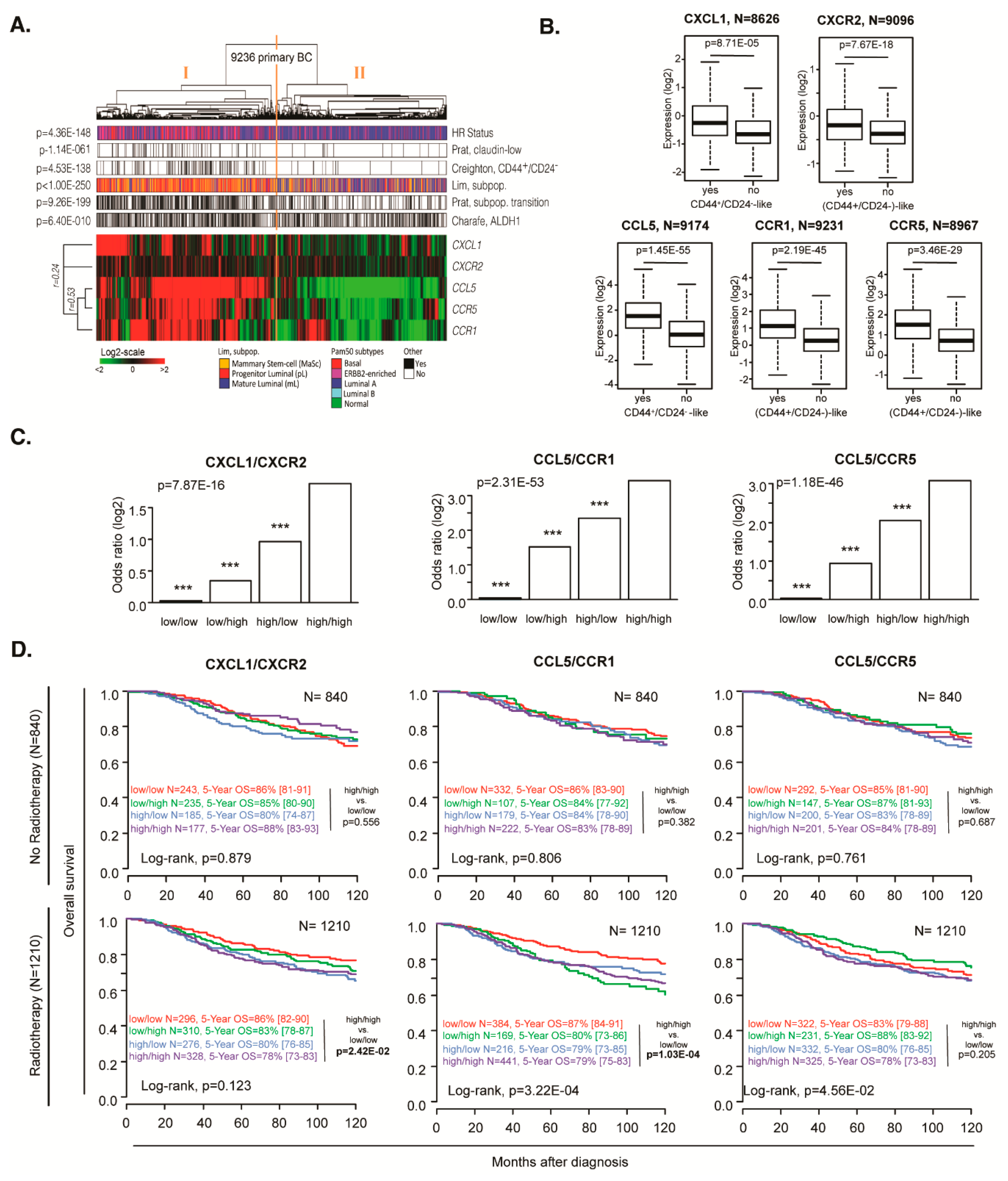
Breast cancer patient analyses to determine the correlation of C(X)CR and C(X)CL expression. (A) Hierarchical clustering of 9236 samples and 5 genes with the Cluster program (53) using complete linkage and uncentered Pearson correlation as parameters. Results were displayed using TreeView program. Below the dendrogram are indicated from top to bottom as colored bars the molecular subtypes (blue: HR+/HER2-, magenta: HER2+, red: TN), Prat’s claudin-low (black: claudin-low, white: non claudin-low), Creighton’s CD44high/CD24-/low (black: CD44high/CD24-/low-like, white: non CD44high/CD24-/low-like), Prat’s subpopulation transition signature (black: mammary stem cell to progenitor Luminal, white: progenitor Luminal to mature Luminal), Lim’s signature (orange: mammary stem cell, red: progenitor Luminal, blue: mature Luminal) and Charafe’s ALDH1 (black: ALDH1-like positive, white: ALDH1-like negative). (B) Box plot of expression level of each ligand and receptor according to the Creighton’s CD44high/CD24-/low CSC profile. (C) Bar plot of Creighton’s CD44high/CD24-/low CSC signature correlation with each ligand/receptor couple, where each bar represents the logistic regression coefficient of each expression modality. (D) Overall survival with or without radiotherapy, in CXCL1/CXCR2, CCL5/CCR1 or CCL5/CCR5-expressing tumors.
Figure 6.
Breast cancer patient analyses to determine the correlation of C(X)CR and C(X)CL expression. (A) Hierarchical clustering of 9236 samples and 5 genes with the Cluster program (53) using complete linkage and uncentered Pearson correlation as parameters. Results were displayed using TreeView program. Below the dendrogram are indicated from top to bottom as colored bars the molecular subtypes (blue: HR+/HER2-, magenta: HER2+, red: TN), Prat’s claudin-low (black: claudin-low, white: non claudin-low), Creighton’s CD44high/CD24-/low (black: CD44high/CD24-/low-like, white: non CD44high/CD24-/low-like), Prat’s subpopulation transition signature (black: mammary stem cell to progenitor Luminal, white: progenitor Luminal to mature Luminal), Lim’s signature (orange: mammary stem cell, red: progenitor Luminal, blue: mature Luminal) and Charafe’s ALDH1 (black: ALDH1-like positive, white: ALDH1-like negative). (B) Box plot of expression level of each ligand and receptor according to the Creighton’s CD44high/CD24-/low CSC profile. (C) Bar plot of Creighton’s CD44high/CD24-/low CSC signature correlation with each ligand/receptor couple, where each bar represents the logistic regression coefficient of each expression modality. (D) Overall survival with or without radiotherapy, in CXCL1/CXCR2, CCL5/CCR1 or CCL5/CCR5-expressing tumors.