1. Introduction
In most northern regions of China, the outdoor temperature of sewage during the low-temperature season typically ranges from 6 to 15 degrees Celsius, and sometimes even drops below 5 degrees Celsius [
1]. Most aerobic denitrifying bacteria struggle to grow under low-temperature conditions, resulting in difficulty removing nitrogen [
2,
3]. Moreover, low temperatures also significantly inhibit the growth rate of bacteria and the metabolic activity of their enzymes, severely impeding the denitrification function of beneficial bacteria [
4,
5,
6]. The cold-tolerant bacteria applied in actual sewage were shown to have significant economic and environmental benefits due to their unique cold resistance mechanisms [
7]. Most domestic and international studies focus on the research of HN-AD bacteria for low-concentration nitrogen sewage [
8,
9,
10]. However, previous studies have revealed that nitrogen removal was strongly inhibited at 10 °C or less[
6]. It is essential to isolate and screen HN-AD bacteria capable of effectively treating high ammonia-nitrogen sewage below 10 °C as a crucial way to solve nitrogen contamination in cold regions.
The traditional biological nitrogen removal process is distributed into aerobic and anaerobic stages. Firstly, ammonia-oxidizing bacteria convert organic nitrogen to ammonium nitrogen under aerobic conditions, subsequently nitrifying bacteria convert ammonium nitrogen to nitrate nitrogen. Finally, denitrifying bacteria reduce nitrate nitrogen to nitrogen under anaerobic conditions. The culture conditions of denitrifying and nitrifying bacteria are pretty different, complicating the whole nitrogen removal process [
11]. In addition, denitrifying bacteria are sensitive to oxygen, and nitrifying bacteria are susceptible to high ammonium, which limits their application in high-concentration ammonia sewage treatment. Compared with the traditional biological method of nitrogen removal, heterotrophic nitrifying aerobic denitrifying bacteria have many advantages: the reaction occurs in the same container, nitrification and denitrification reactions co-occur, reducing the occupied area and the applied cost. Subsequent studies have found that HN-AD microorganisms reproduce rapidly and can tolerate extreme environments such as alkaline, high temperature, low temperature, and high salinity[
12]. Therefore, HN-AD bacteria are the current research hotspot in sewage nitrogen removal. However, most of the sewage nitrogen removal bacteria are not effective in actual sewage treatment, and more HN-AD microorganisms need to be screened for efficient nitrogen removal in actual sewage.
Currently, several cold-tolerant heterotrophic nitrification-aerobic denitrification bacterial genera have been reported.
Acinetobacter calcoaceticus TY1 was a non-mobile rod-shaped bacterium that removed 94.47% of the initial ammonia nitrogen concentration of 106 mg/L at 8°C [
13].
Rhizobium sp. WS7 removed 98.73% of the initial ammonia nitrogen concentration of 48.19 mg/L at 15 °C [
14]. However, most of the studied HN-AD strains currently have poor denitrification effects in actual sewage treatment. The level of cold tolerance and tolerance to high ammonia nitrogen concentrations is insufficient for practical application in sewage treatment. Therefore, it is necessary to isolate cold-tolerant HN-AD strains with stronger denitrification capabilities and study the actual application conditions of these strains for sewage denitrification under low-temperature conditions, laying the foundation for denitrification treatment of low-temperature chemical sewage. This study isolated and screened a bacterium from industrial sewage in Inner Mongolia, which could remove 200mg/L of ammonia nitrogen at 8°C. The denitrification capability of strain HS2 under single-factor variables was studied, and its application in actual sewage treatment was explored, aiming to provide applicable bacterial resources for sewage denitrification treatment under low-temperature conditions.
2. Materials and Methods
2.1. Bacterial Isolation
The samples for screening cold-tolerant HN-AD bacteria were collected from a chemical sewage treatment plant in Inner Mongolia. The sewage sample was added to a sterilized enrichment medium at a seeding rate of 1% for enrichment culture. This was followed by incubation at 8°C and 150 r/min on a constant temperature shaker for 5 days. Subsequently, 1 mL of the original enriched culture was added to a new bottle of enrichment medium and incubated under the same environmental conditions on a shaker for 3 days. The composition of the enrichment medium (g/L) was as follows[
15]: ammonium sulfate (4.25 g), potassium nitrate (0.72 g), brown sugar (19.02 g), trisodium citrate (8.17 g), potassium dihydrogen phosphate (3.00 g), dipotassium phosphate (8.00 g), magnesium sulfate heptahydrate (0.20 g), trace elements solution (2.00 mL). The composition of the trace elements solution (g/L) was as follows [
16]: ethylenediaminetetraacetic acid disodium salt (10.00 g), zinc sulfate heptahydrate (3.90 g), calcium chloride (7.00 g), manganese(ii) chloride tetrahydrate: (5.10 g), ferrous sulfate (5.00 g), ammonium molybdate tetrahydrate (1.10 g), copper(ii) sulfate pentahydrate (1.60 g), cobalt chloride (1.60 g).
1 mL of the second-stage enriched bacterial suspension was subjected to serial dilution using the spread plate method, diluted to 10-7, and then spread onto isolation media. The isolation media was prepared by adding 20.00 g/L agar to the enriched medium. After incubation at 8°C for 24-48 h, purified single colonies were inoculated into Bromothymol Blue (BTB) agar medium, inverted, and cultivated at 4°C. The color of the plates was observed. Composition of Bromothymol Blue (BTB) agar medium (per liter, pH = 7.00) [
15]: potassium nitrate (1.00 g), L-Asparagine (1.00 g), trisodium citrate (8.50 g), potassium dihydrogen phosphate (1.00 g), iron(III) chloride (0.05 g), calcium chloride (0.20 g), magnesium sulfate heptahydrate (1.00 g), bromothymol Blue (0.005 g).
Strains that turned from green to blue were inoculated into liquid nitrification and denitrification media. The cultures were maintained at 8°C and 150 r/min for 5 days. Samples were collected every 24 h to measure the concentrations of TN(total nitrogen) and NH4
+-N in each medium. The strain with the best nitrification and denitrification abilities was selected. Composition of nitrification medium (per liter, pH = 7.00)[
16]: ammonium sulfate (0.944 g), sodium succinate (10.13 g), potassium dihydrogen phosphate (3.00 g), potassium hydrogen phosphate (8.00 g), magnesium sulfate heptahydrate (0.20 g), trace elements (2.00 mL). Composition of denitrification medium (g/L) [
10]: potassium nitrate (2.16 g), sodium succinate (10.13 g), potassium dihydrogen phosphate (3.00 g), potassium hydrogen phosphate (8.00 g), Magnesium sulfate heptahydrate (0.20 g), Trace elements (2.00 mL).
2.2. Molecular Identification
The bacterial suspension was spread onto agar plates of the isolation medium and incubated at 4°C until colonies formed. The colony morphology was observed using a TESCAN 5136 SB scanning electron microscope (Beijing, China). Gram staining of bacterial cells was performed using a Leica DM500 optical microscope (Beijing, China). For amplification of the 16S rRNA gene sequence, a fresh culture of strain HS2 was used as a template. General primers 27F and 1492R from Sangon Biotech (Shanghai, China) were employed for polymerase chain reaction (PCR) amplification of the 16S rRNA gene in a 25µL reaction system. The PCR products were verified by Sangon Biotech (Shanghai, China). After BLAST alignment, a phylogenetic tree reflecting the evolutionary relationship of strain HS2 was constructed using MEGA 7.0. Subsequently, the obtained 16S rRNA gene sequence was submitted to NCBI to obtain a GENE BANK accession number.
2.3. Nitrification Performance under Different Temperature
To compare the denitrification performance of strain HS2 at low and common temperatures, the incubation temperatures were set at 8 °C, 16 °C, 24 °C and 32 °C. Fresh bacterial liquid was inoculated into nitrification medium at a ratio of 10% (V/V), followed by constant-temperature incubation at 120 r/min. Samples were collected every 24 h in a clean centrifuge. They were then centrifuged at 12 000 r/min for 2 min under 4 °C, and the supernatant was taken for the measurement of TN, NH4+-N, NO3--N, and NO2--N concentrations.
2.4. Nitrification Performance under Different Culture Conditions
The factors significantly affecting the growth and nitrogen removal of strain HS2 were assessed as single-factor variables. These included carbon source, carbon-to-nitrogen ratio, initial pH, and shaking speed. For the optimization of nitrogen removal conditions of strain HS2 at 8°C, a 48-hour activated bacterial culture was used for inoculation. Inoculate with a 10% (v/v) seeding volume into the optimized nitrification culture medium. Carbon sources included sodium succinate, glucose, sodium acetate, and sucrose. C/N was 5, 10, 15, and 20 respectively. Shaking speeds were set at 100, 130, 150 and 180 r/min. The initial pH of the medium was adjusted to 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0 using 1 mol/L HCl and NaOH. Samples were collected every 24 h in a clean centrifuge. They were then centrifuged at 12 000 r/min for 2 min under 4 °C, and the supernatant was taken for the measurement of TN, NH4+-N, NO3--N, and NO2--N concentrations.
2.5. Nitrogen Balance Analysis
To analyze the nitrogen balance during the HN-AD process of strain HS2, inoculating with a 10% (v/v) seeding volume into the optimized denitrification culture medium. The cultivation was carried out in a constant temperature oscillating incubator set at 150 r/min and 8°C. Sodium succinate was used as the carbon source, and the cultures were grown for 120 h. The total nitrogen at the beginning of the reaction was subtracted from the total nitrogen remaining after the reaction ended to calculate the total nitrogen removal rate of strain HS2 through denitrification. During the utilization of ammonia nitrogen by strain HS2, some nitrogen entered the cells through assimilation. The samples were crushed using an ultrasonic cell disruptor (JY92-ШD, SCIENTZ, China) to release the nitrogen inside the cells. The samples were then filtered through a 0.22 μm cellulose acetate membrane to obtain the filtrate used for measuring the remaining TDN, NO3--N, NH4+-N, and NO2--N. The calculation methods for various nitrogen concentrations are as follows:
Organic nitrogen = Total Dissolved Nitrogen - Nitrate Nitrogen - Nitrite Nitrogen - Ammonia Nitrogen
Nitrogen within cells = Total Nitrogen - Total Dissolved Nitrogen
Nitrogen loss = (Initial Total Nitrogen - Remaining Nitrate Nitrogen - Remaining Nitrite Nitrogen - Remaining Ammonia Nitrogen - Remaining Organic Nitrogen - Remaining Nitrogen within Cells) / Initial Total Nitrogen × 100%.
2.6. The Nitrogen Removal Pathway of Strain HS2
The newly cultivated strain HS2 was used to extract genomic DNA using the TIANGEN kit method (Beijing, China). The DNA was then assessed for quality and concentration using a nanodrop one ultramicro UV-visible spectrophotometer (nano-300, China). The DNA samples were distributed into two parts, one for amplifying critical genes related to nitrification and denitrification, while another for whole-genome sequencing.
Table 1.
Primers for critical enzyme genes in the denitrification process.
Table 1.
Primers for critical enzyme genes in the denitrification process.
| Gene |
Primer sequence (5’ to 3’) |
| 16S |
27F: AGAGTTTGATCMTGGCTCAG |
| 1492R: 5′-TACGGYTACCTTGTTACGACTT |
| napA |
Nap1: TCTGGACCATGGGCTTCAACCA |
| Nap2: ACGACGACCGGCCAGCGCAG |
| nirK |
Nir-F: CTACTTCTCCCATCATAC |
| Nir-R: CACAGGTTGTTGTTCACT |
| norB |
Nor-F: CGNGARTTYCTSGARCARCC |
| Nor-R: CRTADGCVCCRWAGAAVGC |
| nosZ |
Nos-F: CGYTGTTCMTCGACAGCCAG |
| Nos-R: CGSACCTTSTTGCCSTYGCG |
1 200 mL of nitrification medium was prepared in a 2 L conical flask. A 10% (v/v) inoculum of the activated strain HS2 culture was introduced into the nitrification medium. Pure oxygen was introduced into the flask and allowed to flow for approximately 5 min. The rubber tubing on one side of the gas inlet was clamped, and on the other side, a gas sampling bag was attached. The flask was then placed in a shaking incubator at 8°C and 150 r/min for cultivation. The valve on the gas sampling bag was closed after denitrification process. The gas composition was qualitatively analyzed using Gas Chromatography-Mass Spectrometry (GC-MS).
2.7. The Strain HS2 Applied in Sewage
The strain HS2 was cultured in a nitrification medium, and then inoculated into actual sewage at a 10% (v/v) inoculum during the logarithmic growth phase. The experiment was designed with four treatment groups: control group, carbon source addition (C/N ratio of 15), inoculation of strain HS2, and simultaneous addition of strain HS2 and carbon source. These groups were cultured under constant conditions of 8°C and 150 r/min for aerobic cultivation. Each experimental group was replicated three times. Samples were collected every 24 h to measure the concentrations of total nitrogen and ammonia nitrogen.
To further investigate the microbial community changes induced by the addition of strain HS2 in sewage. The experimental group was the SS+HS2 group, which was collected after denitrification and sequenced for environmental microbial diversity analysis. The control group was the SS group. Those groups were replicated three times. The extraction and sequencing of DNA sequences were conducted by Majorbio (Shanghai, China) using the Illumina platform.
2.8. Analytical Analysis
The concentrations of TN, NH
4+-N, NO
3--N, and NO
2--N were determined using standard methods[
17]. The pH was measured using a pH meter. After appropriate dilution, the optical density (OD600) of the samples was measured at a wavelength of 600 nm. Data analysis and plotting were performed using Origin Pro 2021 software (Origin Lab Corp).
3. Results
3.1. Isolation and Identification of Cold-Tolerant Strain
Six bacteria strains were isolated from industrial sewage at 8°C. Based on the color changes on BTB agar medium and the determination of nitrification ability, five strains were preliminarily selected as HN-AD bacteria. Among them, the strain HS2 exhibited better abilities in heterotrophic nitrification and aerobic denitrification. Therefore, strain HS2 was chosen for further research.
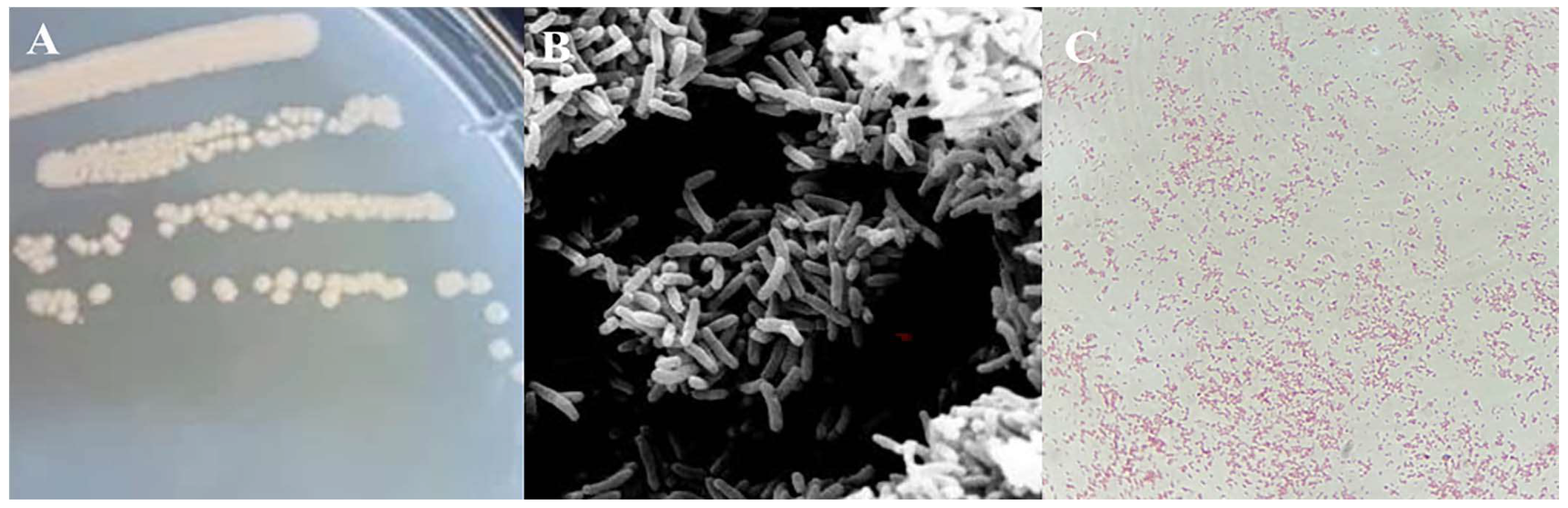
As shown in
Figure 1, the strain HS2 exhibited colonies with a pale yellow color, smooth and moist surface, and slight elevation, with a diameter of approximately 2-3 mm on isolation medium when cultured at 4°C. Gram staining indicated negative. Observation under a scanning electron microscope revealed that the bacterial cells were rod-shaped, with dimensions of approximately (2–4) μm×(0.6–1.4) μm.
The 16S rRNA sequence of strain HS2 was aligned with the GenBank database, which indicated a high similarity with Achromobacter spiritinus. A neighbor-joining phylogenetic tree was constructed by MEGA 7.0 software, demonstrating the closest phylogenetic relationship between strain HS2 and Achromobacter spiritinus, confirming the classification of strain HS2 within the Achromobacter genus (
Figure 2). The 16S rRNA sequence of strain HS2 was submitted to NCBI, resulting in the assignment of Genbank accession number ON514055.
3.2. Nitrification Performance of Strain HS2 under Different Temperature
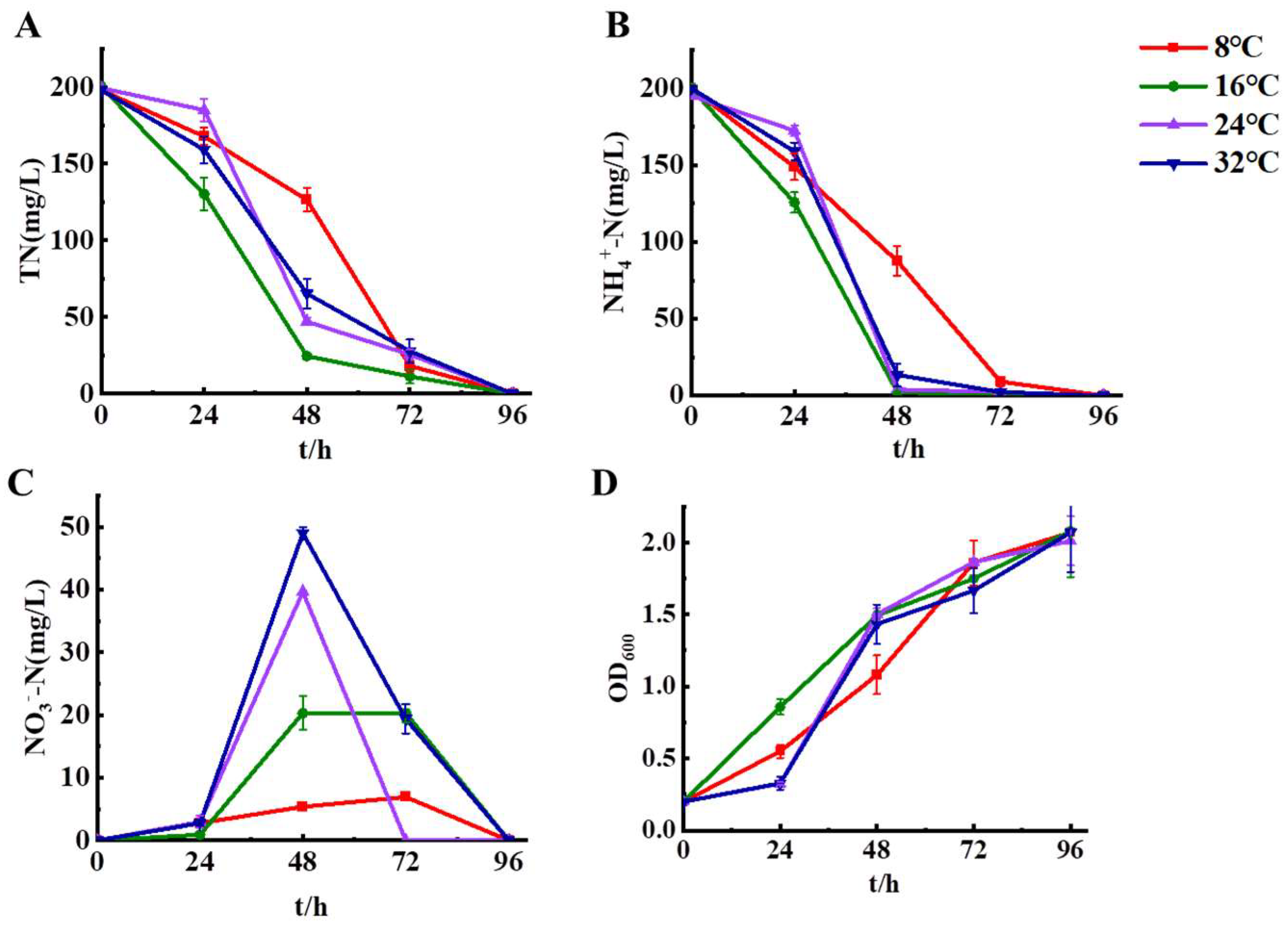
Setting the incubation temperature as 8 °C, 16 °C, 24 °C, 32 °C for examining the growth of strain HS2 in the low temperature environment, and determining the denitrification ability of the strain in 0-96 h. The results in
Figure 3 showed that strain HS2 had better nitrogen removal and growth ability at 16 °C, and strain HS2 could remove 98.84% of ammonia nitrogen and 87.73% of total nitrogen at 48 h. Strain HS2 at 8 °C still had high ability to degrade ammonia nitrogen, and could degrade 95.46% of ammonia nitrogen and remove 90.80% of total nitrogen at 72 h, during which less nitrate nitrogen was detected at 6.94 mg/L. Strain HS2 at 24 °C and 32 °C could remove more than 90% of ammonia nitrogen and more than 85% of total nitrogen at 48 h. During this period, 49 mg/L of nitrate nitrogen was produced, which was subsequently removed. No nitrite nitrogen production was detected by strain HS2 at different temperature conditions. Strain HS2 has an excellent nitrogen removal ability, which can remove over 90% of 200 mg/L ammonia nitrogen at 72 h and 100% of ammonia nitrogen at 96 h in the range of 8-32°C, and it could provide nitrogen removal strains for wastewater treatment plants under low-temperature conditions.
3.3. Nitrification Performance under Different Culture Conditions
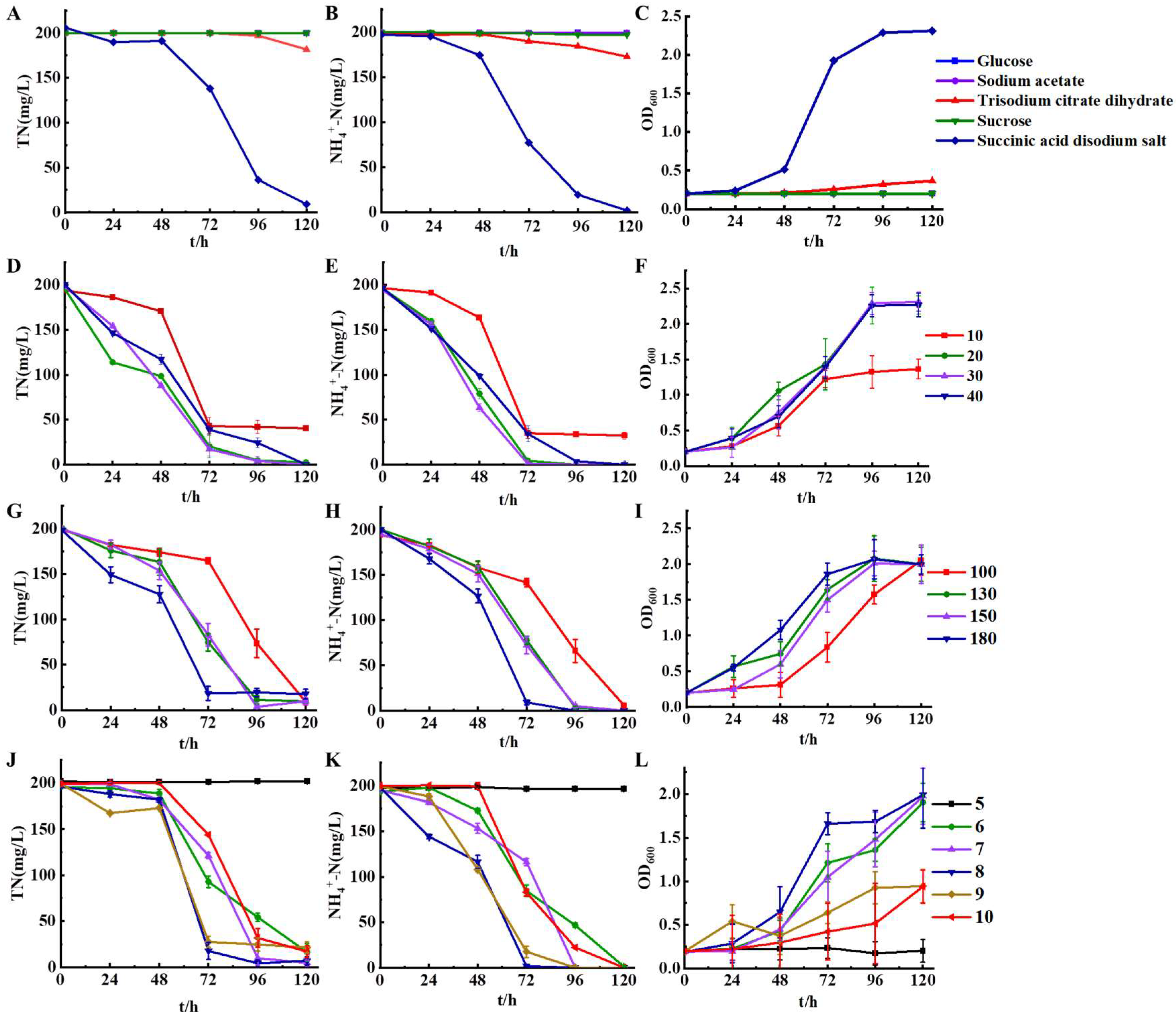
It was found that sodium succinate serves as the optimal carbon source for strain HS2 in
Figure 4 (A-C). When sodium succinate was used as the carbon source, the maximum removal rates of total nitrogen and ammonium nitrogen within 120 h were 95.54% and 99.11%, respectively. There was a slight accumulation of nitrate nitrogen during this period, which was subsequently removed. No accumulation of nitrite nitrogen was detected. Citrate sodium was the second-best carbon source, with removal rates of 9.14% for total nitrogen and 12.89% for ammonium nitrogen at 120 h. When glucose, sodium acetate, and sucrose were used as carbon sources, total nitrogen and ammonium nitrogen removal were negligible within a short period of time. This indicated the critical importance of selecting suitable carbon sources for strain HS2 to achieve efficient denitrification at low temperatures. Previous studies on
Halomonas piezotolerans HN2[
18] and
Pseudomonas stutzeri sdu10[
19] have also identified sodium succinate as the optimal carbon source. This may be attributed to the fact that succinate can enter the tricarboxylic acid (TCA) cycle directly without undergoing modification during aerobic metabolism [
20]. However, the reason why strain HS2 can only utilize sodium succinate in a short time at low temperatures warrants further investigation.
From
Figure 4 (D-F), it was evident that the optimal C/N ratio for strain HS2 was 20-30, with over 95% removal rates of total nitrogen and ammonium nitrogen within 72 h, and all experimental groups showed an increasing trend in total nitrogen and ammonium nitrogen removal rates between 24-72 h. The accumulation of nitrate nitrogen was detected with ammonium nitrogen removed, followed by the nitrate nitrogen being completely removed (
Supplementary Material S1). When C/N was 10, the trend line of the ammonium nitrogen removal rate became relatively stable after 72 h. This may be attributed to an insufficient carbon source in the culture medium, which hindered bacterial growth and subsequently affected nitrogen removal by the strain [
21]. Similarly, it can be observed that strain HS2 ceased growth after 72 h (
Figure 4F). Therefore, strain HS2 showed a better nitrogen removal effect with C/N increasing to 20-30. This may be attributed to the higher C/N ratio in the culture medium, which allows bacteria to obtain more organic matter. It generates more energy through organic matter metabolism, thereby enhancing the nitrogen removal rate of bacteria [
22,
23]. However, the rate of nitrogen removal decreased when the C/N was 40. This may be due to more organic produces more energy molecularly, ATP, as well as NADH, the limitation of electron transport system activity and enzyme activities caused by high levels of NADH [
15].
The shaking speed of the shaker determines the dissolved oxygen level in the flask. The optimal speed for strain HS2 is 180 r/min. From
Figure 4 (G-I), it can be observed that when the speed was 180 r/min, strain HS2 achieved maximum removal rates of 90.80% for total nitrogen and 95.46% for ammonium nitrogen. The trends in total nitrogen and ammonium nitrogen removal rates at different speeds are very similar. As the speed increases, strain HS2 exhibits higher removal rates for both total nitrogen and ammonium nitrogen. This may be attributed to the higher dissolved oxygen content and better growth and metabolism of the strain, leading to enhanced nitrogen removal effects [
24]. With higher speeds, there is an increased level of dissolved oxygen in the culture medium, resulting in better nitrogen removal efficiency for strain HS2. It was inferred that the presence of dissolved oxygen benefits the growth of the strain. The final coinciding with the completion of ammonia removal was no nitrate accumulation at all conditions. The results of
Fusarium solani RADF-77 [
25] and
Halomonas salifodinae [
26] are similar to the findings of this experiment, indicating that higher dissolved oxygen levels are conducive to denitrification by strains. This suggests that increasing dissolved oxygen levels appropriately in practical low-temperature sewage treatment could enhance the nitrification performance of denitrifying bacteria.
It could be observed that the optimal pH for strain HS2 was in the range of 8-9 in
Figure 3 (J-L). When pH was 8, removal rates of 86.17% and 91.19% were achieved for total nitrogen and ammonium nitrogen within 72 h, respectively. A few accumulations of nitrate nitrogen were observed at 48 h, and then removed. Between 48 and 72 h, the removal rate of total nitrogen at different initial pH levels showed an upward trend. The experimental results indicate that pH in the environment has a significant impact on denitrification by strain HS2. Both total nitrogen and ammonium nitrogen were hard to remove when the initial pH was at 5. Effective denitrification occurred within the pH range of 6-10, while the optimal pH for strain HS2 is between 8-9. Strain HS2 also exhibited a high removal rate of total nitrogen (>80%) when the pH reached 9-10, indicating its good alkaline resistance. The biological denitrification process is highly sensitive to pH values, and a weak alkaline environment (pH range of 7-9) is typically conducive to the growth of most denitrifying bacteria [
2]. This finding is consistent with the results of experiments conducted with
Alcaligenes aquatilis AS1 [
20] and
Marinobacter strain NNA5 [
27], with denitrification rates were higher within the pH range of 6-9.
3.4. Nitrogen Balance Analysis
Under the conditions with sodium succinate as the carbon source and ammonia nitrogen as the nitrogen source, strain HS2 could completely transform 198.96 mg/L of ammonia nitrogen. During this process, 58.36% of the ammonia nitrogen was converted into intracellular nitrogen, 16.17% was transformed into organic nitrogen existing in the reaction system, and 25.24% was converted into gaseous nitrogen, with relatively low concentrations of hydroxylamine generated during nitrogen removal. Only 0.26% of the ammonia nitrogen was converted to hydroxylamine.
During the whole nitrogen removal process, the utilization of ammonia nitrogen indicates that strain HS2 exhibits good nitrification performance under low-temperature conditions. Moreover, in the whole reaction system, most of the ammonia nitrogen was converted into intracellular nitrogen, which provides energy for the growth of the strain, suggesting that nitrogen removal by strain HS2 is primarily achieved through assimilation rather than the HN-AD process. Similarly,
Alphaproteobacteria W30 could remove environmental nitrogen, which was primarily driven by assimilation [
28]. In contrast, only 47.02% of the ammonia nitrogen was converted into intracellular nitrogen in the
Pseudomonas stutzeri UFV5, while 52.98% was transformed into gaseous nitrogen [
29]. This variation may be attributed to differences in the nitrogen conversion rates of different bacterial strains.
Table 2.
the nitrogen balance analysis for strain HS2.
Table 2.
the nitrogen balance analysis for strain HS2.
| Initial TN (mg/L) |
Final N (mg/L) |
Intracellular-N
(mg/L) |
N lose
(mg/L) |
| NH4+–N |
NH2OH |
NO3-–N |
NO2-–N |
Organic N |
| 198.96±0.86 |
0 |
0.52±0.12 |
0 |
0 |
32.18±1.71 |
116.12±7.52 |
50.22±6.01 |
3.5. The Nitrogen Removal Pathway of Strain HS2
The extracted genomic DNA of the strain Achromobacter spiritinus HS2 exhibited excellent integrity, with a single and clear band observed. The concentration of the genomic DNA of strain HS2 is 123.6 ng/μL, with OD260/280=1.97 and OD260/230=1.53. These results indicate that the genomic DNA sample of strain HS2 has been successfully extracted and meets the subsequent requirements for library construction and sequencing.
Table 3.
Genes involved in nitrogen metabolism in the genome of strain HS2.
Table 3.
Genes involved in nitrogen metabolism in the genome of strain HS2.
| Gene |
Gene ID |
Function |
| Denitrogen |
| NarI |
gene 2675 |
respiratory nitrate reductase subunit gamma |
| NarH |
gene 2677 |
nitrate reductase subunit beta |
| NarG |
gene 2678 |
nitrate reductase subunit alpha |
| Nar |
gene 5329 |
nitrate reductase |
| MoaA |
gene 2673 |
Molybdenum cofactor biosynthesis protein MoaA |
| MoaD |
gene 199 |
molybdenum cofactor biosynthesis protein |
| MoaE |
gene 615 |
molybdenum cofactor biosynthesis protein |
| Mob B |
gene 616 |
molybdenum cofactor biosynthesis protein B |
| MoeA |
gene 617 |
molybdenumtransferase |
| MoeB |
gene 3185 |
molybdopterin-synthase adenylyltransferase |
| Assimilatory nitrite reduction |
| NirD |
gene 776 |
nitrite reductase (NAD(P)H) small subunit |
| NirB |
gene 777 |
nitrite reductase large subunit |
| Nitrification process |
|
|
| AMO |
gene 3664 |
ammonia monooxygenase |
| Amino acid metabolism |
| GlnA |
gene 1683 |
glutamine synthetase |
| GlnH |
gene 4575 |
glutamine ABC transporter substrate-binding protein |
| GlnP |
gene 4576 |
glutamine ABC transporter permease |
| Gud |
gene 3273 |
NADP-specific glutamate dehydrogenase |
| GltA |
gene 4215 |
glutamate synthase subunit alpha |
| GltB |
gene 4216 |
glutamate synthase subunit beta |
In the genome of strain HS2, 23 genes involved in nitrogen metabolism were identified. These genes encompass processes such as denitrification, assimilatory nitrite reduction, nitrification, and amino acid metabolism. Further comparative analysis revealed one gene (HAO) participating in heterotrophic nitrification and four genes (NarI, NarH, NarG, and Nar) involved in aerobic denitrification pathways during the HN-AD process. Additionally, six genes (moaA, moaD, moaE, mobB, moeA, and moeB) related to the synthesis of the cofactor molybdopterin guanine dinucleotide were found in the genome of strain HS2. Molybdopterin guanine dinucleotide constitutes a part of the Nap large subunit, suggesting the presence of Nap genes in strain HS2. Furthermore, the existence of two genes (Nir) encoding assimilatory nitrite reductase indicates the capability of strain HS2 to reduce nitrite to intracellular nitrogen. Analysis of the predicted coding genes in the HS2 whole genome revealed some genes associated with amino acid synthesis, such as glutamate dehydrogenase, glutamine synthetase, and glutamate synthase. This suggested that strain HS2 could convert ammonium nitrogen to intracellular nitrogen[
30]. Specifically, the GlnA gene is involved in the conversion of ammonium nitrogen to L-Gln, while the GltB gene participates in the conversion of L-Gln to L-Glu[
21]. Both L-Gln and L-Glu serve as essential energy sources for cellular biosynthesis and metabolism[
31].
The generated gas during the HN-AD process of strain HS2 was N
2 (
Supplementary Material S2), which contradicts the result of the genome. Some of the functionally relevant genes involved in nitrification and denitrification processes were found to be missing in the genome of strain HS2. Therefore, it was necessary to design relevant primers for PCR amplification of critical enzyme genes involved in denitrification.
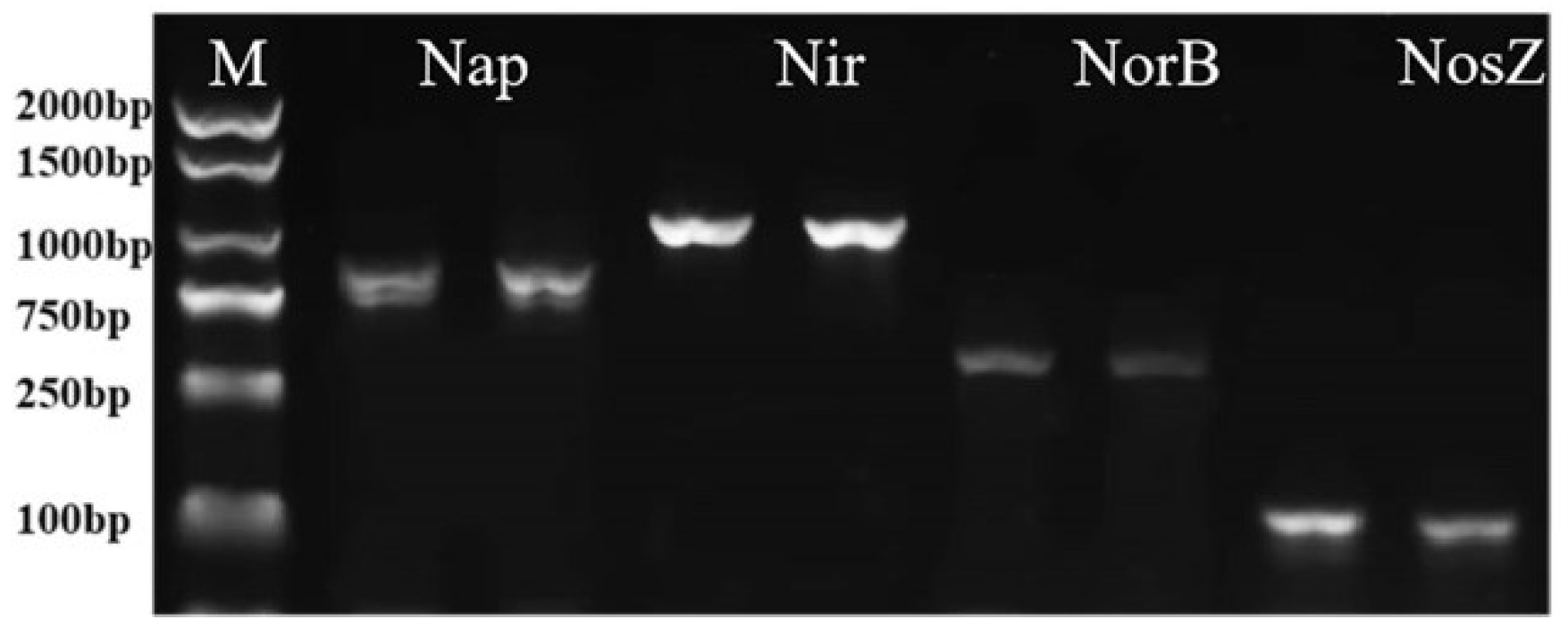
Figure 5 shows that potential denitrification genes are detected by using the PCR method. It was successful in amplifying the functional genes related to denitrification in the genome of strain HS2. These include the nitrate reductase genes Nap (with a sequence length of 844 bp), the nitrite reductase gene Nir (with a length of 1000 bp), the nitric oxide reductase gene Nor (with a length of 432 bp), and the nitrous oxide reductase gene Nos (with a length of 275 bp). Sequencing of the products was performed, and the amplified sequences of NirK, NorB, and NorZ genes were compared to the NR database. They exhibited a similarity of up to 98% with known sequences. However, the amplified product of the NirK gene did not match any similar gene sequences in the NR database. This may be due to the database does not have a similar gene as the NirK of the strain HS2 due to the detection result of N
2. It could be concluded that strain HS2 possesses complete nitrification and denitrification capabilities by combining all the above gene amplification and whole-genome analysis results.
3.6. The Application of Strain HS2 in Sewage Treatment
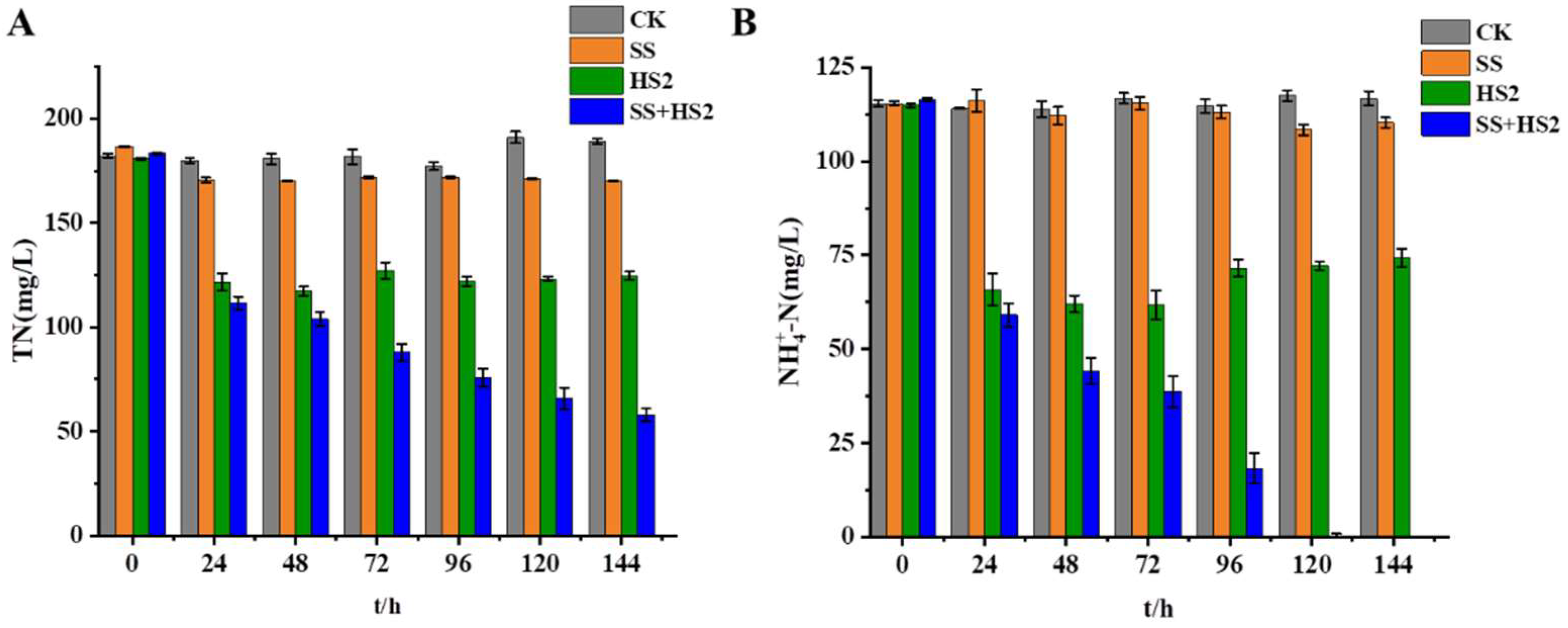
The total nitrogen and ammonium nitrogen concentrations in the sewage were 362.45 mg/L and 127.23 mg/L, respectively. As shown in
Figure 6, strain HS2 demonstrated a certain level of ammonium nitrogen removal efficacy at 8°C in industrial sewage. In the experimental group where HS2 was added to the sewage, the ammonium nitrogen removal rate was slow. At 120 h, the maximum removal rates for total nitrogen and ammonium nitrogen reached 11.34% and 43.58%, respectively. In the group where both added a carbon source and strain HS2, the maximum removal rates for total nitrogen and ammonium nitrogen were 38.78% and 100%, respectively. The final ammonium nitrogen concentration in the SS+HS2 group was 0 mg/L, indicating that the addition of strain HS2 could be utilized for denitrification in actual sewage under low-temperature conditions. Further improvement in denitrification efficiency could be achieved by introducing the optimal carbon source. While strain HS2 exhibited excellent denitrification performance in sewage treatment, achieving ideal total nitrogen removal has proven challenging. This may be attributable to the complexity of sewage composition, which may hinder microbial osmoregulation and impede intracellular enzymatic activity, thereby exerting irreversible effects on strain growth and metabolism. In the later stages, enhancing the denitrification capacity of strain HS2 can be achieved through techniques such as encapsulation of the microorganism in biopolymeric microcapsules along with organic substrates [
32,
33]. Additionally, the co-cultivation of fungi and bacteria could be employed to augment the denitrification efficiency of sewage [
34].
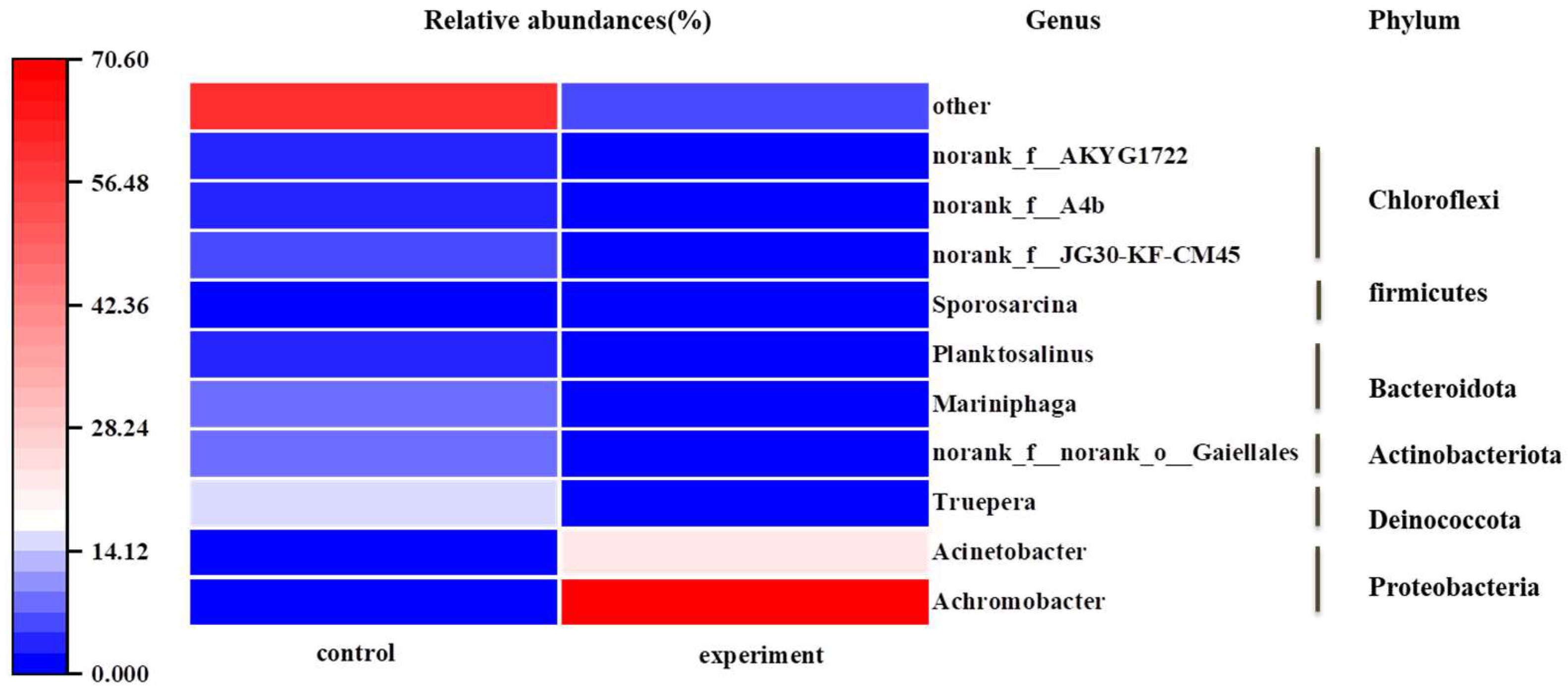
To research the microbial community structure in sewage after the ammonia nitrogen was removed by strain HS2, bacterial community diversity analysis was performed on samples from the control group and the experiment group. The control group was the control group, and the experiment group was the experiment group. As shown in
Figure 7, the dominant phylum in both samples was
Proteobacteria (29.19% in the SS group, 94.05% in the experiment group).
Proteobacteria have been reported to encompass various denitrifying bacteria [
35]. Compared to the control group, the abundance of Proteobacteria significantly increased in the experiment group, while
Chloroflexi, Bacteroidota,
Deinococcota, and
Actinobacteria were replaced by
Proteobacteria, indicating that the added strain had a significant impact on the microbial community structure during the denitrification process in sewage.
Figure 7 showed the changes in microbial community at the genus level. The relative abundance of
Achromobacter sp. was 0.18% in the control group and 70.41% in the experiment group, indicating that strain HS2 could survive in sewage for an extended period and dominate the community. Additionally, there were remarkable differences in the predominant functional genera between the two samples. In the control group,
Truepera (14.67%) played a crucial role in nitrogen removal[
36]. In the experiment group, the dominant genera were
Achromobacter (70.41%) and
Acinetobacter (22.12%). Some strains of
Acinetobacter have been reported as low-temperature denitrifying bacteria[
37], suggesting that strain HS2 not only grows rapidly in sewage but also promotes the growth of native low-temperature denitrifying bacteria.
4. Discussion
The current research on low-temperature nitrogen removal strains mainly focuses on the nitrogen removal performance of the strains, and there are serious questions about the effectiveness of the application for actual sewage. Strain HS2 can remove 100% of ammonia nitrogen in sewage at 8°C, which provides the strain for actual sewage. Besides, the removal of ammonia nitrogen by strain HS2 does not produce harmful intermediate products, such as nitrite and N
2O. Many HN-AD microorganisms have emissions of N
2O during denitrification, such as
Pseudomonas stutzeri PCN-1 [
38]and
Marinobacter hydrocarbonoclasticus NY-4 [
39]. N
2O is a major component of greenhouse gases, so it is necessary to reduce the production of N
2O during denitrification.
Marinobacter strain NNA5 had zero N
2O emissions in the same way as strain HS2 [
27]. Nitrite is harmful to water organisms, which is critical to isolate strains that have no nitrite accumulation in aquaculture sewage[
40]. The final product of ammonia nitrogen removal by
Halomonas salifodinae Y5 is nitrite nitrogen [
26], while strain HS2 would not influence the quality of the water during the removal of ammonia nitrogen.
The nitrogen metabolism pathway of HN-AD microorganisms proposed by Wehrfritz in 1993 has been widely accepted[
41]. The heterotrophic nitrification pathway is NH4
+-N→NH
2OH→NO
2--N→NO
3--N, and the aerobic denitrification pathway is NO
3--N→NO
2--N→N
2. The denitrification pathway of strain HS2 is similar to most research findings. It was determined that
Pseudomonas aeruginosa follows the denitrification pathway of NO
3--N→NO
2--N→NO→N
2O→N
2[
42].
Pseudomonas bauzanensis DN13-1 was found to have the same denitrification pathway. But there isn’t just one nitration and denitration gene during denitrification. A strain might have multiple enzyme genes coexisting [
43]. Luz et al. [
44] found
Bacillus spp. possessed both NiRS and NiRK nitrite reductase enzymes, the same as the strain HS2 possesses both Nap and NarG. Strain HS2 only detected one nitrification gene, HAO, but nitrate was accumulated during the removal of ammonium nitrogen, which may be due to strain HS2 having other genes that could convert hydroxylamine to nitrate. There were various pathways of the nitrification in HN-AD bacteria[
45], Wang et al. [
46] studied
Rhodococcus sp. strain CPZ 24 and identified its nitrification pathway as NH
4+-N→NO
2--N. Silisti et al. [
47] isolated a strain named
Paenibacillus sp. strain GLM-08 and found its nitrification pathway to be NH
4+-N→NH
2OH→NO
2--N. Tsujino et al. [
48] discovered that after NH
4+-N is converted to NH
2OH, NH
2OH and acetone produce acetoxime. This is then transformed into NO
2--N by Pyruvic oxime dioxygenase. Wu et al. [
49] investigated
Alcaligenes ammonioxydans sp. HO-1 and determined its nitrification pathway as NH
4+-N→NH
2OH→N
2. It will be worthwhile to determine the nitrification pathway of strain HS2 for further study, but one certain thing is that the strain can convert hydroxylamine to nitrate nitrogen (
Supplementary Material S1).
The whole gene sequencing and gene amplification were not deep enough to investigate the nitrogen metabolism pathway of strain HS2, and the nitrogen transformation pathway could be investigated by using isotope tracer assay to measure nitrogenous compounds, or qPCR to measure the expression of genes related to the HN-AD process[
50]. Ozkaraova et al.[
32]studied organic substrates such as peanut, walnut and almond shells and luffa sponge enhanced denitrification in wastewater treatment. The use of low-temperature biochar enhanced the nitrogen removal capacity of combinatorial bacteria in the study by Jiang et al[
9]. It is possible to increase the removal of nitrogen from HS2 in low-temperature effluents by the method described above
5. Conclusions
A strain of cold-tolerant HN-AD bacteria, named as HS2, was isolated from the industrial sewage in Inner Mongolia. Morphological characteristics and homology analysis of the 16S rRNA gene identified this strain as belonging to Achromobacter spiritinus. Strain HS2 exhibited the highest denitrification efficiency under the conditions of sodium succinate as the carbon source, a carbon-to-nitrogen ratio of 20-30, agitation at 150-180 rpm, and a pH range of 6-10. Nitrogen balance experiments demonstrated that the denitrification process of strain HS2 is primarily achieved through assimilation rather than the processes of nitrification and denitrification.
It was inferred that the HN-AD pathway of strain HS2 proceeds as follows: NH4+-N→NH2OH→NO2--N→NO→N2O→N2 by combining the results of whole-genome sequencing, gene amplification, and gas product detection. Under 8°C conditions, strain HS2 was able to remove 38.78% of total nitrogen and 100% of ammonium nitrogen from industrial sewage with initial concentrations of 362.45 mg/L and 127.23 mg/L, respectively. Dominant species analysis in the applied sewage revealed the richness of Achromobacter sp.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Nitrate accumulation of strain HS2 under various factors; Figure S2: Gas products of strain HS2 in HN-AD.
Author Contributions
Conceptualization, L.H. and Y.J.; Methodology, Y.G.; C.L., and Y.J.; Investigation, L.H., S.L. and Y.J.; Resources, L.H., H.L. and Y.J.; Data curation, Y.G. and L.H.; Writing—original draft, Y.G.; Writing—review & editing, Y.G. T.Z. Y.J. and H.L.; Visualization, Y.G.; Supervision, Y.G., T.Z., L.H., S.L., C.L., H.L. and Y.J.; Project administration, Y.J.; Funding acquisition, H.L. and Y.J. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Statement
The experiments reported in this study did not involve human participants and/or animals.
Abbreviations
HN-AD: heterotrophic nitrification–aerobic denitrification; TN: total nitrogen; PCR: polymerase chain reaction; GC-MS: Gas Chromatography-Mass Spectrometry; TCA: Tricarboxylic acid.
References
- Yang, M.; Lu, D.; Qin, B.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total. Environ. 2021, 801, 149319. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ni, J.; Chen, Q.; Borthwick, A.G. Enrichment and characterization of a bacteria consortium capable of heterotrophic nitrification and aerobic denitrification at low temperature. Bioresour. Technol. 2012, 127, 151–157. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, P.; Zhang, L.; Zhang, L.; Gao, Y.; Ai, S.; Liu, H.; Liu, X. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. TAC-1 at low temperature and high ammonia nitrogen. Bioresour. Technol. 2021, 339, 125620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, G.-M.; Wu, M.-R.; Li, S.-S.; Miao, L.-L.; Liu, Z.-P. Photobacterium sp. NNA4, an efficient hydroxylamine-transforming heterotrophic nitrifier/aerobic denitrifier. J. Biosci. Bioeng. 2019, 128, 64–71. [Google Scholar] [CrossRef]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef]
- rivastava, M., and A. K. Mishra. Nitrogen removal and metabolic profiling of a cold-adaptive and biofilm producing paddy soil bacterium Cupriavidus sp. PDN31. Archives of Agronomy and Soil Science. 2018, 1608–1621. [CrossRef]
- Xu, Y.; He, T.; Li, Z.; Ye, Q.; Chen, Y.; Xie, E.; Zhang, X. Nitrogen Removal Characteristics of Pseudomonas putida Y-9 Capable of Heterotrophic Nitrification and Aerobic Denitrification at Low Temperature. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- dos Santos, P.R.; Daniel, L.A. A review: organic matter and ammonia removal by biological activated carbon filtration for water and wastewater treatment. Int. J. Environ. Sci. Technol. 2019, 17, 591–606. [Google Scholar] [CrossRef]
- Kou, L.; Huang, T.; Zhang, H.; Wen, G.; Li, N.; Wang, C.; Lu, L. Mix-cultured aerobic denitrifying bacterial communities reduce nitrate: Novel insights in micro-polluted water treatment at lower temperature. Sci. Total. Environ. 2021, 796, 148910. [Google Scholar] [CrossRef]
- Yang, J.-R.; Wang, Y.; Chen, H.; Lyu, Y.-K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2018, 274, 56–64. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2019, 128, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, X.; Lin, Y.; Lu, X.; Lv, H.; Zhao, M.; Yu, R. Nitrogen removal by a novel heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter calcoaceticus TY1 under low temperatures. Bioresour. Technol. 2022, 353, 127148. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Luo, X.; Ma, W.; Lv, P. Biological nitrogen removal and metabolic characteristics of a novel cold-resistant heterotrophic nitrification and aerobic denitrification Rhizobium sp. WS7. Bioresour. Technol. 2022, 362, 127756. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Li, X.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification- aerobic denitrification process. Bioresour. Technol. 2021, 343, 126116. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examinations of Water and Wastewater. USA, 2017.
- Dong, L.; Ge, Z.; Qu, W.; Fan, Y.; Dai, Q.; Wang, J. Characteristics and mechanism of heterotrophic nitrification/aerobic denitrification in a novel Halomonas piezotolerans strain. J. Basic Microbiol. 2021, 62, 124–134. [Google Scholar] [CrossRef]
- Kundu, P., A. Pramanik, A. Dasgupta, S. Mukherjee, and J. Mukherjee. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by Chryseobacterium sp. R31 Isolated from Abattoir Wastewater. BioMed Research International. 2014, 1–12. [CrossRef]
- Cao, X.; Zhao, B.; Wu, Y.; Huang, J.; Wang, H.; Sun, X.; Li, S. Characterization of Alcaligenes aquatilis as a novel member of heterotrophic nitrifier-aerobic denitrifier and its performance in treating piggery wastewater. Bioresour. Technol. 2022, 354, 127176. [Google Scholar] [CrossRef]
- Padhi, S.K.; Maiti, N.K. Molecular insight into the dynamic central metabolic pathways of Achromobacter xylosoxidans CF-S36 during heterotrophic nitrogen removal processes. J. Biosci. Bioeng. 2017, 123, 46–55. [Google Scholar] [CrossRef]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: a nonconventional nitrogen removal pathway in wastewater treatment. Lett. Appl. Microbiol. 2021, 74, 159–184. [Google Scholar] [CrossRef]
- Wei, R.; Hui, C.; Zhang, Y. P.; Jiang Y., H.; Zhao, Y. H.; Du, L. N. Nitrogen removal characteristics and predicted conversion pathways of a heterotrophic nitrification-aerobic denitrification bacterium, Pseudomonas aeruginosa P-1. Environ Sci Pollut Res Int. 2021, 28, 6, 7503–7514. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W.; Huang, J.-L.; Sun, F.-L.; Chen, S. A novel Pseudomonas aeruginosa strain performs simultaneous heterotrophic nitrification-aerobic denitrification and aerobic phosphate removal. Water Res. 2022, 221, 118823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-Y.; Xu, A.-A.; Awasthi, M.K.; Kong, D.-D.; Chen, J.-S.; Wang, Y.-F.; Xu, P. Aerobic denitrification performance and nitrate removal pathway analysis of a novel fungus Fusarium solani RADF-77. Bioresour. Technol. 2019, 295, 122250. [Google Scholar] [CrossRef]
- Hu, J.; Yan, J.; Wu, L.; Bao, Y.; Yu, D.; Li, J. Simultaneous nitrification and denitrification of hypersaline wastewater by a robust bacterium Halomonas salifodinae from a repeated-batch acclimation. Bioresour. Technol. 2021, 341, 125818. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, G.-M.; Miao, L.-L.; Liu, Z.-P. Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour. Technol. 2016, 206, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Bohu, T.; Wu, S.; Bai, Z.; Zhuang, X. Nitrogen Removal Characteristics and Constraints of an Alphaproteobacteria with Potential for High Nitrogen Content Heterotrophic Nitrification-Aerobic Denitrification. Microorganisms 2022, 10, 235. [Google Scholar] [CrossRef]
- Silva, L.C.F.; Lima, H.S.; Mendes, T.A.d.O.; Sartoratto, A.; Sousa, M.P.; de Souza, R.S.; de Paula, S.O.; de Oliveira, V.M.; Silva, C.C. Physicochemical characterization of Pseudomonas stutzeri UFV5 and analysis of its transcriptome under heterotrophic nitrification/aerobic denitrification pathway induction condition. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Q.; Wu, H.; Jiang, W.; Kahaer, A.; Tang, Q.; Hu, Z.; Hong, C.; Liu, D. Integrative chemical and omics analysis of the ammonia nitrogen removal characteristics and mechanism of a novel oligotrophic heterotrophic nitrification-aerobic denitrification bacterium. Sci. Total. Environ. 2022, 852, 158519. [Google Scholar] [CrossRef]
- Jha, V.; Dafale, N.A.; Hathi, Z.; Purohit, H. Genomic and functional potential of the immobilized microbial consortium MCSt-1 for wastewater treatment. Sci. Total. Environ. 2021, 777, 146110. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liang, D.; Wang, G.; Xie, J.; Zhu, X. Enhanced denitrification of sewage via bio-microcapsules embedding heterotrophic nitrification-aerobic denitrification bacteria Acinetobacter pittii SY9 and corn cob. Bioresour. Technol. 2022, 358, 127260. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Shi, J. Micro-Polluted Surface Water Treated by Yeast-Chitosan Bio-Microcapsules. Materials 2020, 13, 3519. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhao, M.; Yuan, B.; Yao, J.; Chen, J.; Hrynshpan, D.; Savitskaya, T. A fungus–bacterium co-culture synergistically promoted nitrogen removal by enhancing enzyme activity and electron transfer. Sci. Total. Environ. 2020, 754, 142109. [Google Scholar] [CrossRef]
- Bucci, P.; Coppotelli, B.; Morelli, I.; Zaritzky, N.; Caravelli, A. Heterotrophic nitrification-aerobic denitrification performance in a granular sequencing batch reactor supported by next generation sequencing. Int. Biodeterior. Biodegradation 2021, 160. [Google Scholar] [CrossRef]
- Mupindu, P.; Zhao, Y.; Wang, X.; Hu, Y. Effect of sulfamethoxazole on nitrate removal by simultaneous heterotrophic aerobic denitrification. Water Environ. Res. 2022, 94, e10716. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; He, D.; Ma, T.; Chen, Q.; Liu, S.; Ahmad, M.; Gui, M.; Ni, J. Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour. Technol. 2014, 162, 80–88. [Google Scholar] [CrossRef]
- Li, R.; Zi, X.; Wang, X.; Zhang, X.; Gao, H.; Hu, N. Marinobacter hydrocarbonoclasticus NY-4, a novel denitrifying, moderately halophilic marine bacterium. SpringerPlus 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Neissi, A.; Rafiee, G.; Farahmand, H.; Rahimi, S.; Mijakovic, I. Cold-Resistant Heterotrophic Ammonium and Nitrite-Removing Bacteria Improve Aquaculture Conditions of Rainbow Trout (Oncorhynchus mykiss). Microb. Ecol. 2020, 80, 266–277. [Google Scholar] [CrossRef]
- Wehrfritz, J. M. R. A.; SPIRO STEPHEN , RICHARDSON DAVID J. Purification of hydroxylamine oxidase from thiosphaera pantotropha: identification of electron acceptors that couple heterotrophic nitrification to aerobic denitrification. Federation of European Biochemical Societies. 1993, 335, 2, 246-250. [CrossRef]
- Mpongwana, N.; Ntwampe, S.K.O.; Mekuto, L.; Akinpelu, E.A.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, J.; He, L.; He, X.; Pan, Z.; Wang, Y.; He, Q. High-temperature biofilm system based on heterotrophic nitrification and aerobic denitrification treating high-strength ammonia wastewater: Nitrogen removal performances and temperature-regulated metabolic pathways. Bioresour. Technol. 2022, 344, 126184. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.F.D.; Mujica, J.G.Q.; Cunayque, J.M.R.; Lucana, G.W.A.; Angulo, J.J.I.; De la Cruz, V.I.S.; Escobar, V.A.C.; Matonnier, E.M. Assessment of Heterotrophic Nitrification Capacity in Bacillus spp. and its Potential Application in the Removal of Nitrogen from Aquaculture Water. J. Pure Appl. Microbiol. 2019, 13, 1893–1908. [Google Scholar] [CrossRef]
- Shukla, S.; Rajta, A.; Setia, H.; Bhatia, R. Simultaneous nitrification–denitrification by phosphate accumulating microorganisms. World J. Microbiol. Biotechnol. 2020, 36, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Karuriya, S., and S. Choudhary. Simultaneous heterotrophic nitrification and aerobic denitrification potential of Paenibacillus sp. strain GLM-08 isolated from lignite mine waste and its role ammonia removal from mine waste water. Water Science and Technology. 2022, 3223–3235. [CrossRef]
- Zhao, Y.; Li, W.; Chen, L.; Zhou, Y.; Meng, L.; Zhang, S. Isolation and application of a thermotolerant nitrifying bacterium Gordonia paraffinivorans N52 in sewage sludge composting for reducing nitrogen loss. Bioresour. Technol. 2022, 363, 127959. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, S.; Uematsu, C.; Dohra, H.; Fujiwara, T. Pyruvic oxime dioxygenase from heterotrophic nitrifier Alcaligenes faecalis is a nonheme Fe(II)-dependent enzyme homologous to class II aldolase. Sci. Rep. 2017, 7, 39991. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. R.; Hou, T. T.; Liu, Y.; et al. Novel Alcaligenes ammonioxydans sp. nov. from wastewater treatment sludge oxidizes mmonia to N2 with a previously unknown pathway. Environmental Microbiology. 2021, 23, 11, 6965-6980. [CrossRef]
- Padhi, S.K.; Tripathy, S.; Mohanty, S.; Maiti, N.K. Aerobic and heterotrophic nitrogen removal by Enterobacter cloacae CF-S27 with efficient utilization of hydroxylamine. Bioresour. Technol. 2017, 232, 285–296. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).