Submitted:
08 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Macroscopic and Microscopic Observation
2.2.2. Cell Maceration
2.2.3. Measuring Dimensions of Vessels and Fibers, as Well as Their Derivative Value
3. Results and Discussion
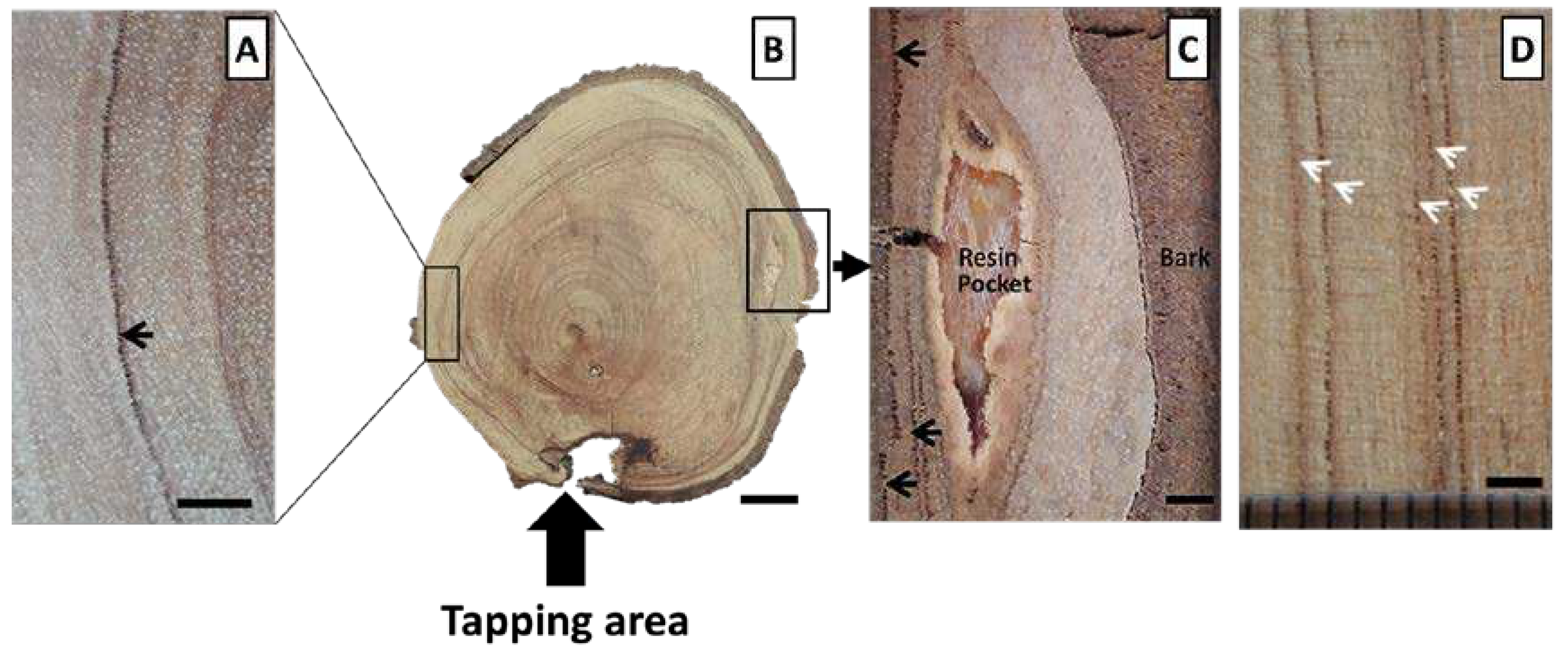
3.1. Macroscopic Characteristics in the Transverse and Longitudinal Surfaces
3.2. Microscopic Characteristics
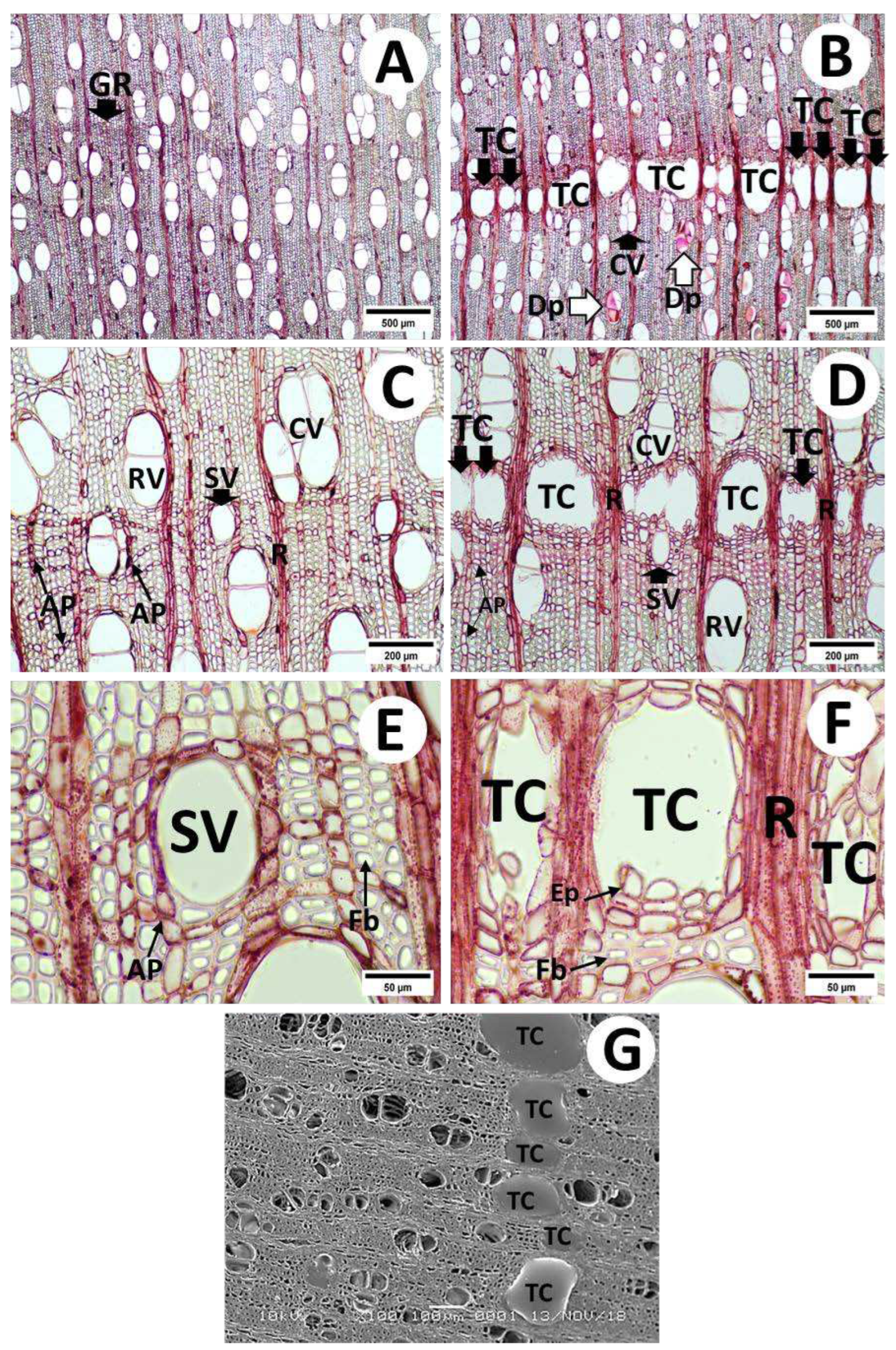
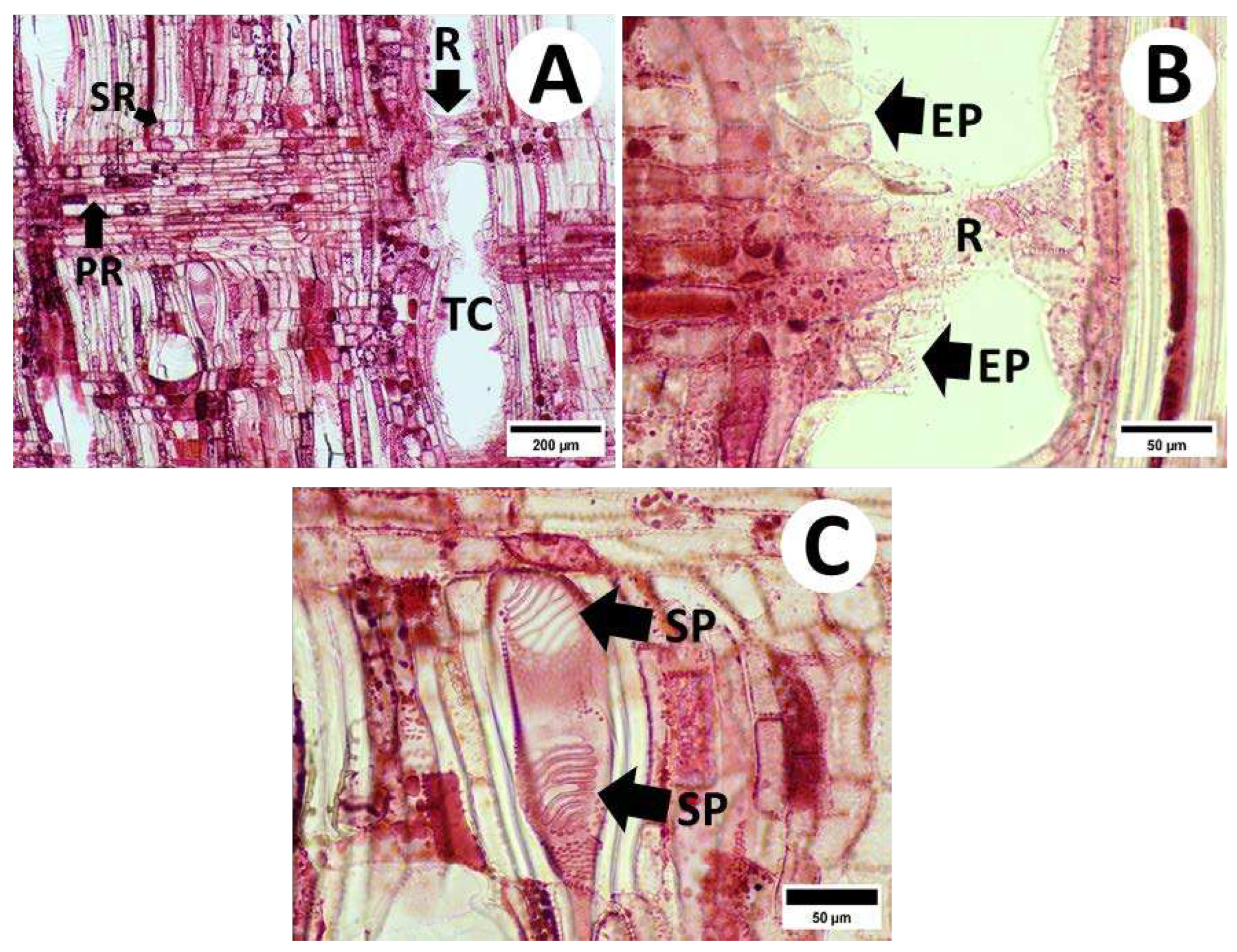
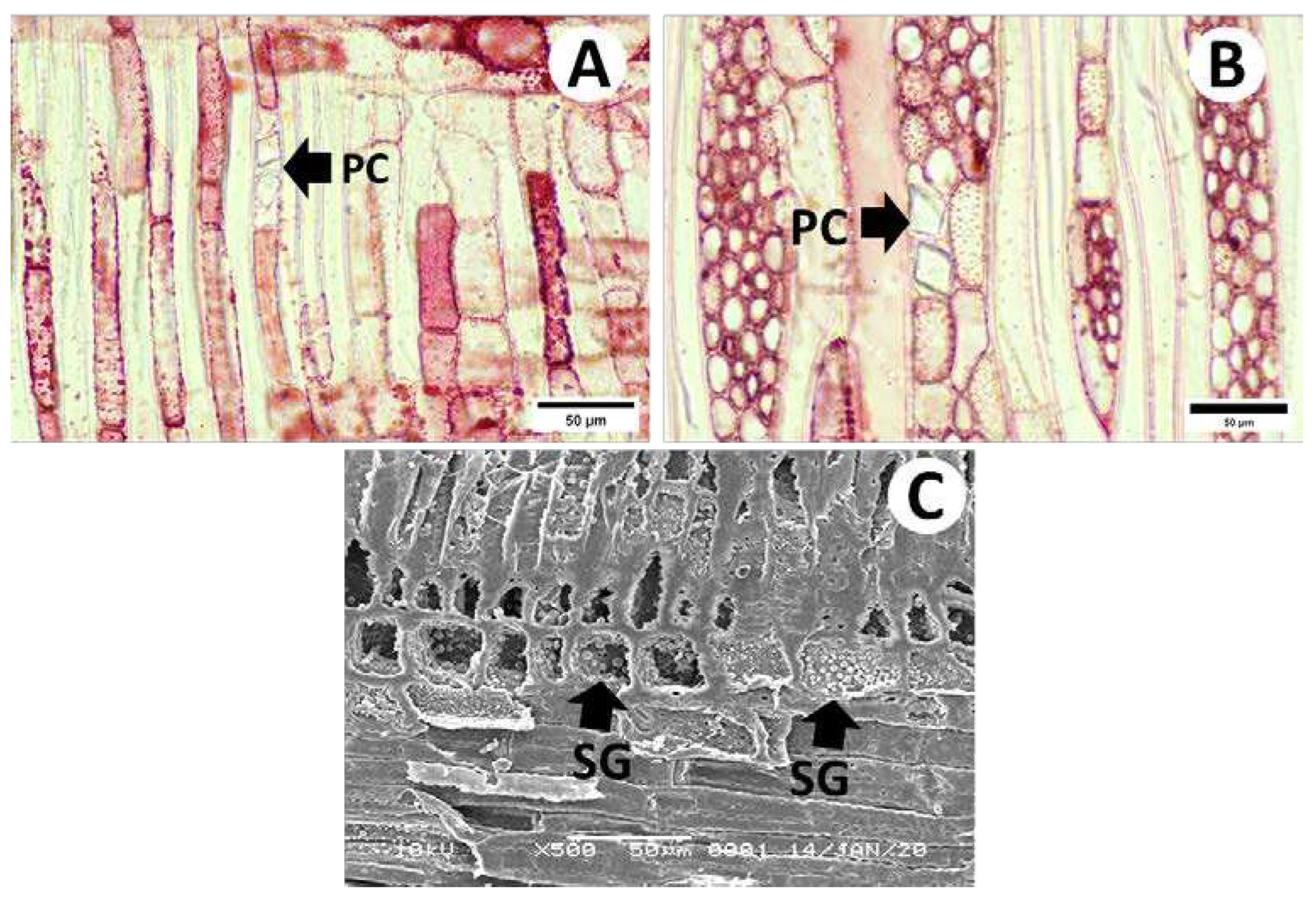
3.2.1. Cross-Sections
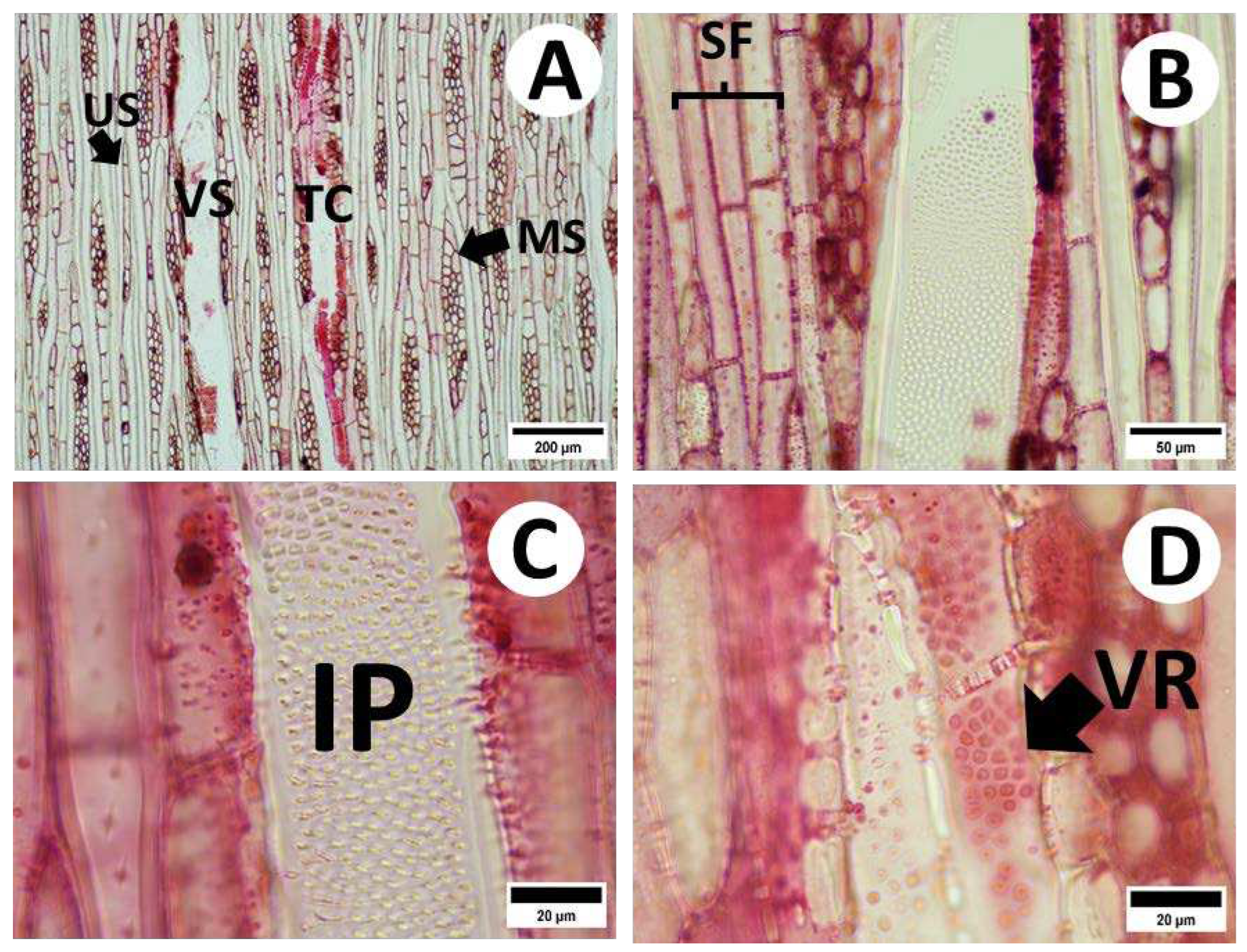
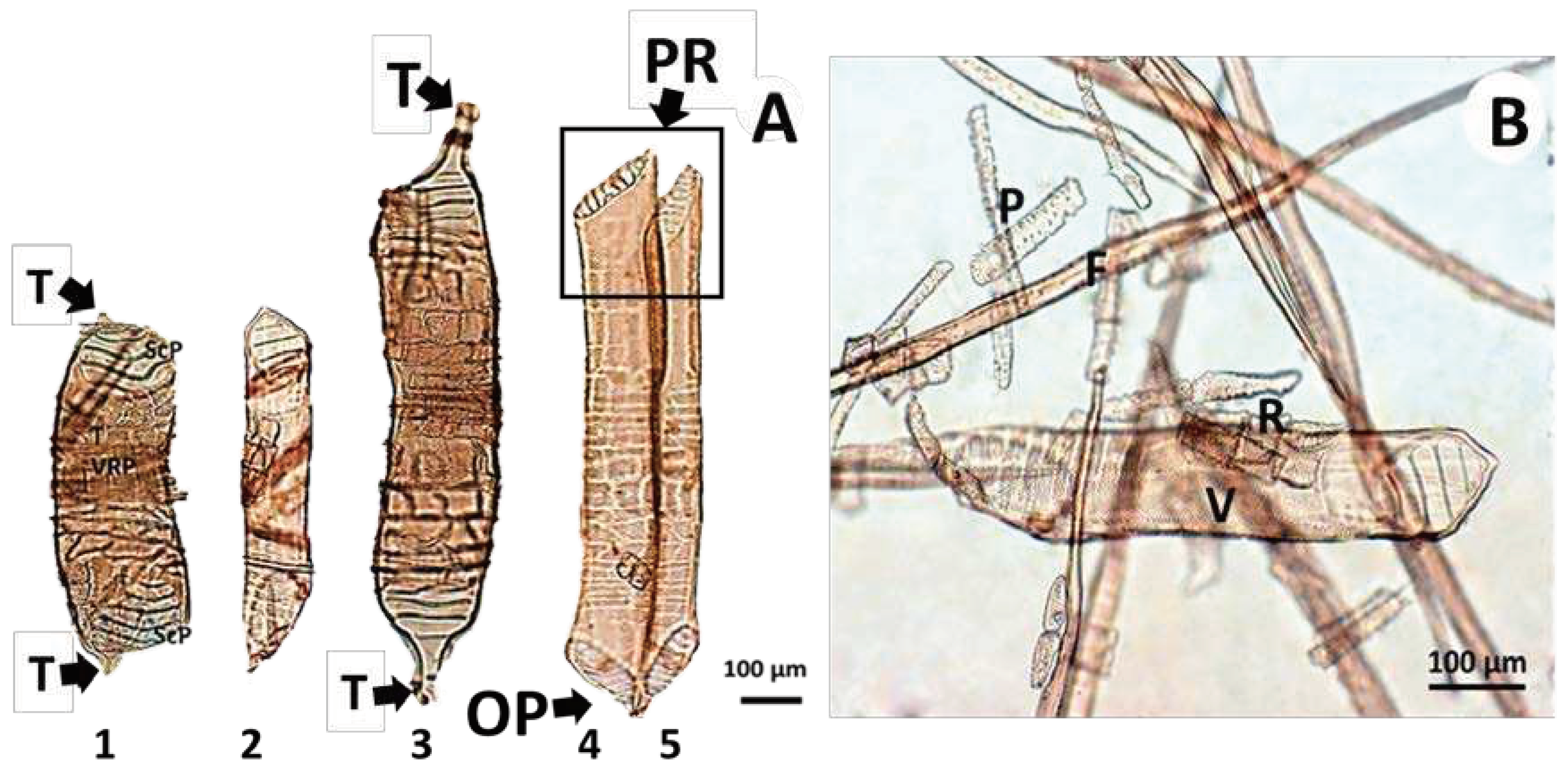
3.2.2. Radial and Tangential Sections
3.3. Vessel and Fiber Characteristics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nussinovitch, A. Plant Gum Exudates of the World: Sources, Distribution, Properties, and Applications: CRC Press, Boca Raton, 2010. [CrossRef]
- Boer, E.; Ella, A.B. Introduction. In: Boer E, Ella AB. (eds). Plant Resources of South-East Asia No 18: Plants Producing Exudates: Prosea, Bogor, Indonesia, 2001.
- Langenheim, J.H. Plant resins. American Scientist. 1990, 78, 16-24.
- Langenheim, J.H. Plant resins: chemistry, evolution, ecology, and ethnobotany: Timber Press, Portland, 2003. [CrossRef]
- Fritsch, P. W; Morton, C. M.; Chen, T.; Meldrum, C. Phylogeny and biogeography of the Styracaceae. International Journal of Plant Sciences. 2001, 162(S6), pp.S95-S116. [CrossRef]
- Hoesen, D.S.H. Styrax L. In Plant Resources of South-East Asia: Plants Producing Exudates, Boer, E., Ella, A.B., Eds; Prosea: Bogor, Indonesia. 2001, Volume 18, pp. 112–119.
- Simamora, S.W.; Muhdi; Batubara, R. Modifikasi Teknik Pemanenan Dengan Perlakuan Fisik Dalam Upaya Peningkatan Produktivitas Getah Kemenyan Toba (Styrax Sumatrana). Peronema Forestry Science Journal 2015, 4, 159-165.
- Iswanto, A.H.; Susilowati, A.; Azhar, I.; Riswan, R.; Supriyanto, S.; Tarigan, J.E.; Fatriasari, W. Physical and mechanical properties of local Styrax woods from North Tapanuli in Indonesia. J. Korean Wood Sci. Technol. 2016, 44(4), 539–550. [CrossRef]
- Iswanto, A.H.; Siregar, Y.S.; Susilowati, A.; Darwis, A.; Hartono, R.; Wirjosentono, B.; Rachmat, H.H.; Hidayat, A.; Fatriasari, W. Variations in chemical constituent of Styrax sumatrana wood growing at different cultivation sites in North Sumatra, Indonesia. BIODIVERSITAS 2019, 20 (2), 448–452. [CrossRef]
- Badan Pusat Statistik Propinsi Sumatera Utara. Indikator Pertanian Propinsi Sumatera Utara 2023: Badan Pusat Statistik Propinsi Sumatera Utara, Medan, Indonesia, 2023.
- Hovaneissian M., Archier P., Mathe C., Culioli G., Vieillescazes C. Analytical investigation of Styrax and benzoin balsams by HPLC-PAD-fluorimetry and GC-MS. Phytochemical Analysis. 2008, 19(4):301-310. [CrossRef]
- Jayusman. Recognising Incense Trees (Styrax spp.): Species with Broad Utilization Spectrums for Optimalization. IPB Press: Bogor, Indonesia, 2014.
- Dünisch O., Bass P. On the origin of intercellular canals in the secondary xylem of selected Meliaceae species. IAWA J 2006, 27:281–297. [CrossRef]
- Kuroda K, Shimaji K. Traumatic resin canal formation as a marker of xylem growth. Forest Sci 1983, 29(3):653-659. [CrossRef]
- DeRose RJ, Bekker MF, Long JN. Traumatic resin ducts as indicators of bark beetle outbreaks. Can. J. For. Res. 2017, 47:1168–1174. [CrossRef]
- Deng, X.Q.; Cheng, S.P.; Pan, N.X.; Chen, J.L. The effects of ethrel upon benzoin production and balsamic ducts of Styrax hypoglauca Perk. J. Integr. Plant Biol. 1978, 20, 26–29.
- Pasaribu, G.; Jasni, J; Damayanti, R.; Wibowo, S. Anatomical, physical, mechanical properties of Kemenyan Toba (Styrax sumatrana) and Kemenyan Bulu (Styrax paralleloneurus). J. For. Prod. Res. 2013, 31(2), 161–169.
- Franklin, G.L. Preparation of thin sections of synthetic resins and wood resin composites, and a new macerating method for wood. Nature 1945, 155(3924), 51–59. [CrossRef]
- Nurachman, R.; Siagian, M. Dimensi serat jenis kayu Indonesia. Laporan No. 75. Lembaga Penelitian: Bogor, Indonesia, 1976.
- Adi, D. S.; Wahyuni, I.; Risanto, L.; Rulliaty, S.; Hermiati, E.; Dwianto, W.; Watanabe, T. Central kalimantan’s fast growing species: Suitability for pulp and paper. Indonesian J. For. Res. 2015, 2(1), 21–29. [CrossRef]
- Dickison, W.C.; Phend, K.D. Wood anatomy of the Styracaceae: evolutionary and ecological considerations. IAWA Bulletin n.s., 1985, 6(1), 3–22.
- IAWA Committee. IAWA List of Microscopic Features for Hardwood Identification with an Appendix on Non-anatomical Information; National Herbarium of the Netherlands: Leiden, the Netherlands, 1989; pp. 219–332. [CrossRef]
- Damayanti, R.; Mandang, Y.I.; Waluyo, T.K. Anatomical properties and fiber quality of Styrax stem from North Sumatra. J. For. Prod. Res. 2007, 25(3), 273–290.
- Machado, S. R., Rodella, R. A., Angyalossy, V., Marcati, C. R. Structural variations in root and stem wood of Styrax (Styracaceae) from Brazilian forest and cerrado. IAWA Journal 2007, 28(2), 173-188. [CrossRef]
- Wistara, N.J.; Carolina, A.; Pulungan, W.S.; Emil, N.; Lee, S.H.; Kim, N.H. Effect of tree age and active alkali on kraft pulping of white jabon. J. Korean Wood Sci. Technol. 2015, 43, 566–577. [CrossRef]
- Darwis, A.; Karliati, T.; Sutrisno, Alamsyah, E.M.; Rumidatul, A.; Melani, L.; Kim, H.J.; Iswanto, A.H.; Fatriasari, W. Chemical properties, crystallinity, and fiber biometry of Jabon (Anthocephalus cadamba) wood for pulp raw material: the effect of age and position. Nord. Pulp Pap. Res. J. 2023, 1–11. [CrossRef]
| The derivative values of fiber | Equation |
|---|---|
| Runkel ratio (RR) | |
| Felting power (FP) | |
| Mulhsteph ratio (MR) | |
| Coefficient rigidity (CR) | |
| Flexibility ratio (FR) |
| Parameter | Class I | Class II | Class III | Class IV | ||||
|---|---|---|---|---|---|---|---|---|
| Value | Score | Value | Score | Value | Score | Value | Score | |
|
Fiber length (µm) |
2200 | 100 | 1600–2200 | 75 | 900–1600 | 50 | 900 | 50 |
| Runkel ratio | 0.25 | 100 | 0.2–0.5 | 75 | 0.5–0.1 | 50 | 1 | 50 |
| Felting power | 90 | 100 | 70–90 | 75 | 40–70 | 50 | 40 | 50 |
| Flexibility ratio | 0.8 | 100 | 0.6–0.8 | 75 | 0.4–0.6 | 50 | 0.6 | 50 |
|
Coefficient of rigidity |
0.1 | 100 | 0.1–0.15 | 75 | 0.15–0.2 | 50 | 0.2 | 50 |
| Mulhsteph ratio | 30% | 100 | 30–60% | 75 | 60–80% | 50 | 80% | 50 |
| Total score | 451–600 | 301–450 | 151–300 | 150 | ||||
| Min | Max | Average | SD | Pulp quality | |
|---|---|---|---|---|---|
| Dimension of fibers (μm) | |||||
| Length | 1113.25 | 1774.02 | 1390.77 | 180.27 | (50) III |
| Diameter | 24.04 | 34.72 | 29.23 | 3.07 | - |
| Lumina Diameter | 14.69 | 24.37 | 17.99 | 3.08 | - |
| Wall thickness | 4.675 | 7.51 | 5.62 | 0.78 | - |
| Biometrics | |||||
| Runkel ratio | 0.42 | 1.00 | 0.64 | 0.15 | (50) III |
| Felting power | 34.73 | 57.40 | 46.38 | 7.52 | (50) III |
| Mulhsteph ratio (%) | 50.73 | 74.98 | 62.13 | 6.53 | (50) III |
| Coefficient of rigidity | 0.15 | 0.25 | 0.19 | 0.03 | (50) III |
| Flexibility ratio | 0.50 | 0.70 | 0.61 | 0.05 | (75) II |
| Total score | (320) II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





