Submitted:
08 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Case presentation
2.2. Literature review
2.3. Statistical analysis
3. Results
Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegen, H.; Reindl, M. Recent developments in MOG-IgG associated neurological disorders. Ther Adv Neurol Disord 2020, 13, 1756286420945135. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Aktas, O.; Ayzenberg, I.; Bellmann-Strobl, J.; Berthele, A.; Giglhuber, K.; Häußler, V.; Havla, J.; Hellwig, K.; Hümmert, M.W.; et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J Neurol 2023. [Google Scholar] [CrossRef] [PubMed]
- Papp, V.; Magyari, M.; Aktas, O.; Berger, T.; Broadley, S.A.; Cabre, P.; Jacob, A.; Kira, J.I.; Leite, M.I.; Marignier, R.; et al. Worldwide Incidence and Prevalence of Neuromyelitis Optica: A Systematic Review. Neurology 2021, 96, 59–77. [Google Scholar] [CrossRef] [PubMed]
- de Mol, C.L.; Wong, Y.; van Pelt, E.D.; Wokke, B.; Siepman, T.; Neuteboom, R.F.; Hamann, D.; Hintzen, R.Q. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler 2020, 26, 806–814. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Hamilton-Shield, A.; Woodhall, M.; Messina, S.; Mariano, R.; Waters, P.; Ramdas, S.; Leite, M.I.; Palace, J. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire, UK. J Neurol Neurosurg Psychiatry 2020, 91, 1126–1128. [Google Scholar] [CrossRef]
- Wynford-Thomas, R.; Jacob, A.; Tomassini, V. Neurological update: MOG antibody disease. J Neurol 2019, 266, 1280–1286. [Google Scholar] [CrossRef]
- Ramanathan, S.; Mohammad, S.; Tantsis, E.; Nguyen, T.K.; Merheb, V.; Fung, V.S.C.; White, O.B.; Broadley, S.; Lechner-Scott, J.; Vucic, S.; et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018, 89, 127–137. [Google Scholar] [CrossRef]
- Jurynczyk, M.; Messina, S.; Woodhall, M.R.; Raza, N.; Everett, R.; Roca-Fernandez, A.; Tackley, G.; Hamid, S.; Sheard, A.; Reynolds, G.; et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017, 140, 3128–3138. [Google Scholar] [CrossRef]
- López-Chiriboga, A.S.; Majed, M.; Fryer, J.; Dubey, D.; McKeon, A.; Flanagan, E.P.; Jitprapaikulsan, J.; Kothapalli, N.; Tillema, J.M.; Chen, J.; et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol 2018, 75, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Calvo, A.; Ruiz, A.; Maillart, E.; Audoin, B.; Zephir, H.; Bourre, B.; Ciron, J.; Collongues, N.; Brassat, D.; Cotton, F.; et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology 2018, 90, e1858–e1869. [Google Scholar] [CrossRef]
- Pohl, D.; Alper, G.; Van Haren, K.; Kornberg, A.J.; Lucchinetti, C.F.; Tenembaum, S.; Belman, A.L. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology 2016, 87, S38–45. [Google Scholar] [CrossRef]
- Bennetto, L.; Scolding, N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry 2004, 75 Suppl 1, i22–28. [Google Scholar] [CrossRef]
- Baxter, R.; Lewis, E.; Goddard, K.; Fireman, B.; Bakshi, N.; DeStefano, F.; Gee, J.; Tseng, H.F.; Naleway, A.L.; Klein, N.P. Acute Demyelinating Events Following Vaccines: A Case-Centered Analysis. Clin Infect Dis 2016, 63, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, F.; Xu, Y.; Chu, X.; Zhang, J. Vaccines and the risk of acute disseminated encephalomyelitis. Vaccine 2018, 36, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J.; Mba-Jonas, A.; Dimova, R.B.; Alimchandani, M.; Zinderman, C.E.; Nair, N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. Jama 2021, 326, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. Bmj 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Lyu, R.K.; Chang, C.W.; Tseng, W.J. The First Guillain-Barr? Syndrome After SARS-CoV-2 Vaccination in Taiwan. Acta Neurol Taiwan 2022, 31(1), 46–51. [Google Scholar]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Romano, A.; Bozzao, A.; Salvetti, M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult Scler 2021, 13524585211040222. [Google Scholar] [CrossRef]
- Permezel, F.; Borojevic, B.; Lau, S.; de Boer, H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol 2021, 1–6. [Google Scholar] [CrossRef]
- Pagenkopf, C.; Südmeyer, M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol 2021, 358, 577606. [Google Scholar] [CrossRef]
- Tan, W.Y.; Yusof Khan, A.H.K.; Mohd Yaakob, M.N.; Abdul Rashid, A.M.; Loh, W.C.; Baharin, J.; Ibrahim, A.; Ismail, M.R.; Inche Mat, L.N.; Wan Sulaiman, W.A.; et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Notghi, A.A.; Atley, J.; Silva, M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin Med (Lond) 2021, 21, e535–e538. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Tsai, M.J.; Chen, Y.H.; Hsu, C.F. Acute Transverse Myelitis after COVID-19 Vaccination. Medicina (Kaunas) 2021, 57. [Google Scholar] [CrossRef]
- Vegezzi, E.; Ravaglia, S.; Buongarzone, G.; Bini, P.; Diamanti, L.; Gastaldi, M.; Prunetti, P.; Rognone, E.; Marchioni, E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: Casual or causal association? J Neuroimmunol 2021, 359, 577686. [Google Scholar] [CrossRef] [PubMed]
- Singh Malhotra, H.; Gupta, P.; Prabhu, V.; Garg, R.K.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. Qjm 2021. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Koorapati, G.; Prasad, S.; Jeelani, H.M.; Sherchan, R.; Shrestha, J.; Shayuk, M. SARS-CoV-2 Vaccination-Induced Transverse Myelitis. Cureus 2021, 13, e16624. [Google Scholar] [CrossRef]
- Badrawi, N.; Kumar, N.; Albastaki, U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: Case report & MRI findings. Radiol Case Rep 2021, 16, 3864–3867. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Ogaki, K.; Nakamura, R.; Kado, E.; Nakajima, S.; Kurita, N.; Watanabe, M.; Yamashiro, K.; Hattori, N.; Urabe, T. An 88-year-old woman with acute disseminated encephalomyelitis following messenger ribonucleic acid-based COVID-19 vaccination. eNeurologicalSci 2021, 25, 100381. [Google Scholar] [CrossRef]
- Vogrig, A.; Janes, F.; Gigli, G.L.; Curcio, F.; Negro, I.D.; D’Agostini, S.; Fabris, M.; Valente, M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg 2021, 208, 106839. [Google Scholar] [CrossRef]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alshararni, A. Acute Transverse Myelitis Associated with COVID-19 vaccine: A Case Report. International Journal of Research in Pharmaceutical Sciences 2021, Vol. 12, no. 3, 5. [Google Scholar] [CrossRef]
- McLean, P.; Trefts, L. Transverse myelitis 48 hours after the administration of an mRNA COVID 19 vaccine. Neuroimmunology Reports 2021, 1, 100019. [Google Scholar] [CrossRef]
- Kania, K.; Ambrosius, W.; Tokarz Kupczyk, E.; Kozubski, W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann Clin Transl Neurol 2021, 8, 2000–2003. [Google Scholar] [CrossRef]
- Fujikawa, P.; Shah, F.A.; Braford, M.; Patel, K.; Madey, J. Neuromyelitis Optica in a Healthy Female After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine. Cureus 2021, 13, e17961. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Tseng, H.P.; Lin, C.L.; Shiu, J.S.; Lee, M.H.; Liu, C.H. Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Shrestha, A.K.; Colantonio, M.A.; Liberio, R.N.; Sriwastava, S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol 2021, 1–12. [Google Scholar] [CrossRef]
- Fitzsimmons, W.; Nance, C.S. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. 2021.
- Ozgen Kenangil, G.; Ari, B.C.; Guler, C.; Demir, M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg 2021, 121, 1089–1091. [Google Scholar] [CrossRef]
- Erdem, N.; Demirci, S.; Özel, T.; Mamadova, K.; Karaali, K.; Çelik, H.T.; Uslu, F.I.; Özkaynak, S.S. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy Sz 2021, 74, 273–276. [Google Scholar] [CrossRef]
- Cao, L.; Ren, L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg 2021, 1–3. [Google Scholar] [CrossRef]
- Sepahvand, M.; Yazdi, N.; Rohani, M.; Emamikhah, M. Cervical longitudinally extensive myelitis after vaccination with inactivated virus-based COVID-19 vaccine. Radiol Case Rep 2022, 17, 303–305. [Google Scholar] [CrossRef]
- Chen, S.; Fan, X.R.; He, S.; Zhang, J.W.; Li, S.J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci 2021, 42, 3537–3539. [Google Scholar] [CrossRef]
- Lotan, I.; Romanow, G.; Levy, M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult Scler Relat Disord 2021, 55, 103189. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Food and Drug Administration. The report of adverse effects after COVID-19 vaccines in Taiwan. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f637814781667990040&type=2&cid=39989 (accessed on.
- Huang, C.T.; Hsu, S.Y.; Wang, C.H.; Tseng, W.J.; Yang, C.Y.; Ng, C.J.; Warkentin, T.E.; Cheng, M.H. Double high-dose immunoglobulin for ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia. Thromb Res 2021, 206, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Smith, H.; Ma, Y.; Baum, H.; Mieli-Vergani, G.; Vergani, D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol 2005, 12, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Wingerchuk, D.M.; Vukusic, S.; Linbo, L.; Pittock, S.J.; Lucchinetti, C.F.; Lennon, V.A. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 2006, 59, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, D.G.; Cañete, L.A.Q.; Dos Santos, G.A.C.; de Oliveira, R.V.; Brandão, C.O.; da Cruz, L.C.H., Jr. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: Cause or coincidence? Clin Imaging 2021, 80, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ohyama, A.; Kubota, T.; Ikeda, K.; Kaneko, K.; Takai, Y.; Warita, H.; Takahashi, T.; Misu, T.; Aoki, M. MOG Antibody-Associated Disorders Following SARS-CoV-2 Vaccination: A Case Report and Literature Review. Front Neurol 2022, 13, 845755. [Google Scholar] [CrossRef]
- Dams, L.; Kraemer, M.; Becker, J. MOG-antibody-associated longitudinal extensive myelitis after ChAdOx1 nCoV-19 vaccination. Mult Scler 2022, 28, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Mumoli, L.; Vescio, V.; Pirritano, D.; Russo, E.; Bosco, D. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol Sci 2022, 43, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.; Bansal, P.; Arora, S.; Kapila, S.; Bedi, G.S. Myelin Oligodendrocyte Glycoprotein Antibody Disease After COVID-19 Vaccination - Causal or Incidental? Cureus 2022, 14, e27024. [Google Scholar] [CrossRef]
- Garg, R.K.; Malhotra, H.S.; Kumar, N.; Pandey, S.; Patil, M.R.; Uniyal, R.; Rizvi, I. Tumefactive Demyelinating Brain Lesion Developing after Administration of Adenovector-Based COVID-19 Vaccine: A Case Report. Neurol India 2022, 70, 409–411. [Google Scholar] [CrossRef]
- Ballout, A.A.; Babaie, A.; Kolesnik, M.; Li, J.Y.; Hameed, N.; Waldman, G.; Chaudhry, F.; Saba, S.; Harel, A.; Najjar, S. A Single-Health System Case Series of New-Onset CNS Inflammatory Disorders Temporally Associated With mRNA-Based SARS-CoV-2 Vaccines. Front Neurol 2022, 13, 796882. [Google Scholar] [CrossRef] [PubMed]
- Dr. Md, R.; Tasnim, J.; Dr. Md. Biplob, H.; Dr. Beauty, S.; Dr. Subash Kanti, D.; Dr. Md, S. Post Covid19 Vaccination Acute Disseminated Encephalomyelitis: A Case Report in Bangladesh. International Journal Of Medical Science And Clinical Research Studies 2021, 1, 31–36. [CrossRef]
- Nagaratnam, S.A.; Ferdi, A.C.; Leaney, J.; Lee, R.L.K.; Hwang, Y.T.; Heard, R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol 2022, 22, 54. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Iranpour, P.; Haseli, S.; Poursadeghfard, M.; Yarmahmoodi, F. Acute disseminated encephalomyelitis (ADEM) after SARS- CoV-2 vaccination: A case report. Radiol Case Rep 2022, 17, 1789–1793. [Google Scholar] [CrossRef]
- Doi, K.; Ohara, Y.; Ouchi, T.; Sasaki, R.; Maki, F.; Mizuno, J. Cervical Transverse Myelitis Following COVID-19 Vaccination. NMC Case Rep J 2022, 9, 145–149. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Timmermans, V.M.; Dakakni, T. Acute Disseminated Encephalomyelitis After SARS-CoV-2 Vaccination. Am J Case Rep 2022, 23, e936574. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Lotlikar, R.S.; Sukumaran, S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol Sci 2022, 43, 3503–3507. [Google Scholar] [CrossRef] [PubMed]
- Al-Quliti, K.; Qureshi, A.; Quadri, M.; Abdulhameed, B.; Alanazi, A.; Alhujeily, R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.; Patel, T.H.; Meadows, I.; Ozdemir, B. Acute Disseminated Encephalomyelitis (ADEM) After Consecutive Exposures to Mycoplasma and COVID Vaccine: A Case Report. Cureus 2022, 14, e26258. [Google Scholar] [CrossRef] [PubMed]
- Motahharynia, A.; Naghavi, S.; Shaygannejad, V.; Adibi, I. Fulminant neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccination: A need for reconsideration? Mult Scler Relat Disord 2022, 66, 104035. [Google Scholar] [CrossRef]
- Anamnart, C.; Tisavipat, N.; Owattanapanich, W.; Apiwattanakul, M.; Savangned, P.; Prayoonwiwat, N.; Siritho, S.; Rattanathamsakul, N.; Jitprapaikulsan, J. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review. Mult Scler Relat Disord 2022, 58, 103414. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Saab, G.; Schneider, R. Antibody-Positive Neuromyelitis Optica Spectrum Disorder After Second COVID-19 Vaccination: a Case Report. SN Compr Clin Med 2022, 4, 130. [Google Scholar] [CrossRef]
- Caliskan, I.; Bulus, E.; Afsar, N.; Altintas, A. A Case With New-Onset Neuromyelitis Optica Spectrum Disorder Following COVID-19 mRNA BNT162b2 Vaccination. Neurologist 2022, 27, 147–150. [Google Scholar] [CrossRef]
- Netravathi, M.; Dhamija, K.; Gupta, M.; Tamborska, A.; Nalini, A.; Holla, V.V.; Nitish, L.K.; Menon, D.; Pal, P.K.; Seena, V.; et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult Scler Relat Disord 2022, 60, 103739. [Google Scholar] [CrossRef]


| No | Age/sex/PH | Vaccine/ interval (D) |
Dx | Serum | CSF | MRI | Tx | Outcome | Ref | ||||||||
| AQP4 | MOG | OCB | WBC | Lym(%) | Neut(%) | TP | Glu ratio | OCB | Brain | Spine(myelitis) | |||||||
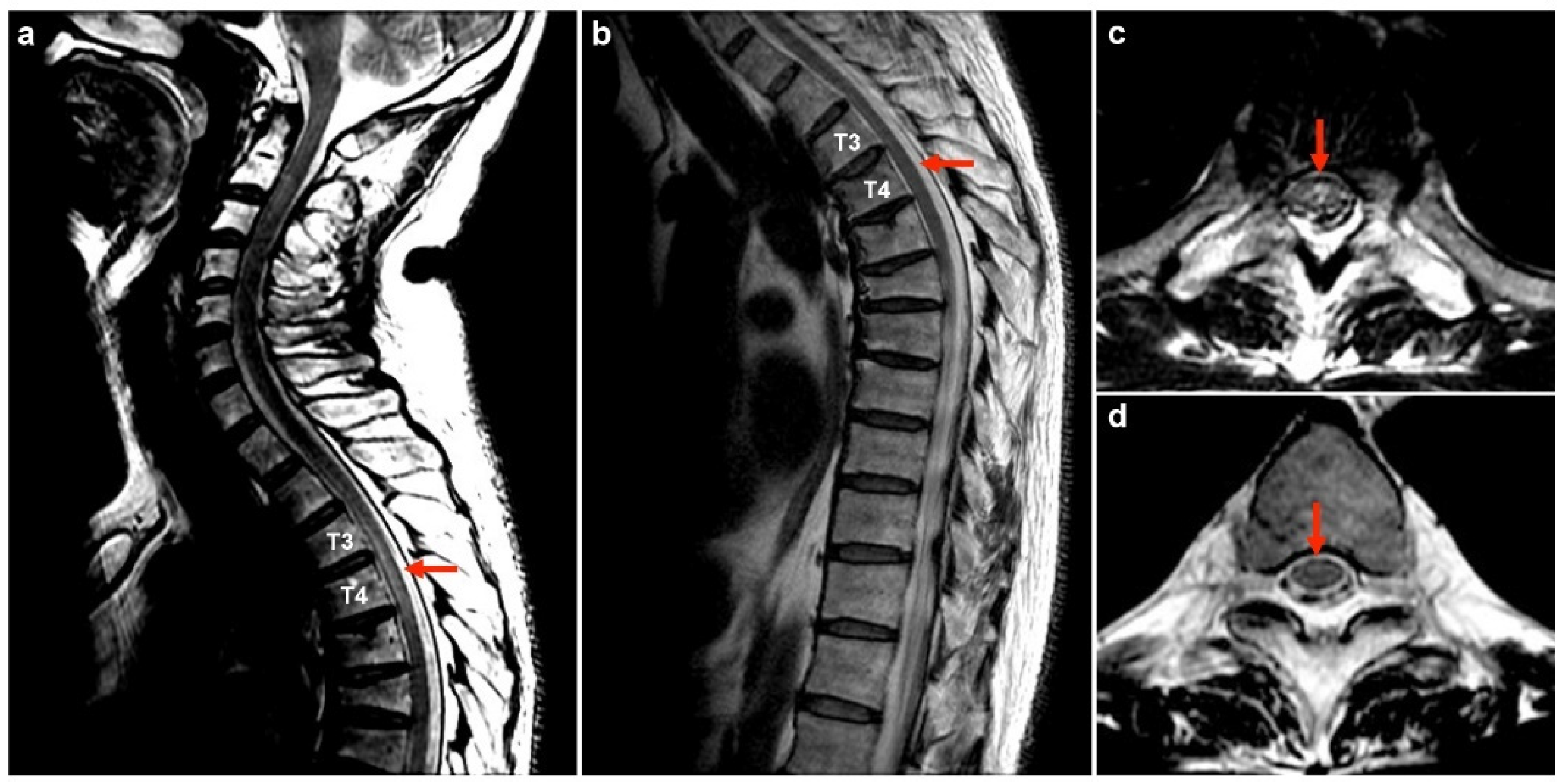
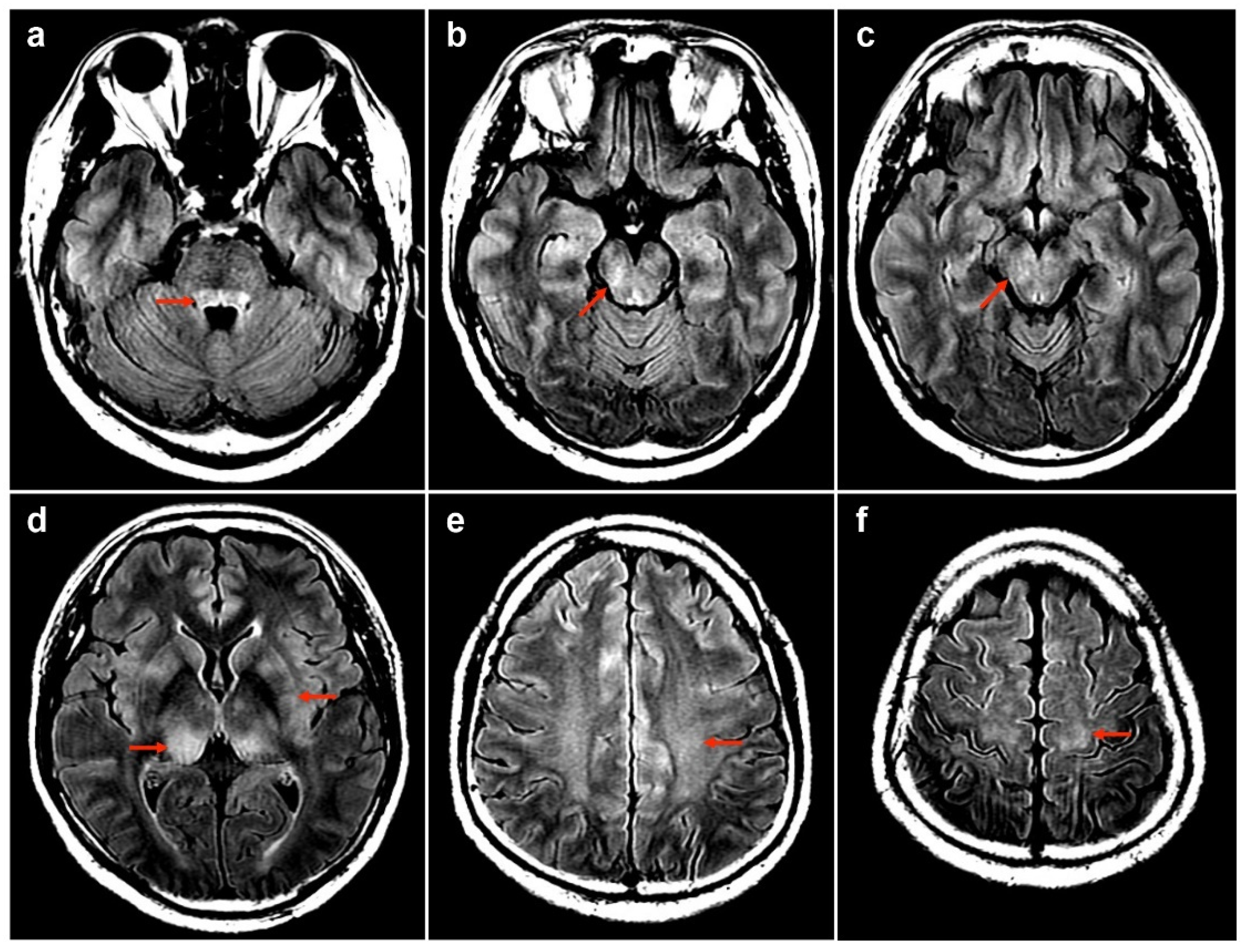
| 1 | 50M/HBV carrier | 1st AZ/13 | MOGAD | - | + | - | 175 | 99 | 0 | 78.1 | 0.47 | NA | Bil thalami, pu, subcortical WM, brainstem | T3-T4 | PT | R | Index case |
| 2 | 45M | 1st AZ/12 | ADEM+ SSM | - | - | NA | 44 | P | NA | N | NA | + | Pons, R MCP, R thalamus; C+ | Cervical, thoracic and conus medullaris;C+ | PT | R | [21] |
| 3 | 63M/DM, HL, IHD, Af | 1st AZ/12 | ADEM | - | - | NA | 2 | NA | NA | 69 | N | + | Bil WM, bil CC, L thalamus, IC, L midbrain, lower pons, R MCP | N | PT + PP | E(D20) | [22] |
| 4 | 45M/atopic dermatitis | 1st AZ/8 | LETM | - | - | NA | 481 | NA | 67 | 140 | 0.43 | - | N | C3-T2 | PT | I | [23] |
| 5 | 25F | 1st AZ/12 | LETM | - | - | NA | NA | NA | NA | 54.6 | 0.55 | - | N | T3-T5, T7-T8, T11-L1 ;C+ in T7-T8 | PT | I | [24] |
| 6 | 58M/DM, pulmonary sarcoidosis | 1st AZ/7 | LETM | - | - | + | 11 | 100 | 0 | 162 | 0.54 | + | NA | C1-T10; C+ in T3-T4, T9-T10 | PT + PE | I | [25] |
| 7 | 41M/DM | 1st AZ/14 | LETM | - | NA | NA | 11 | 100 | 0 | 44.3 | NA | NA | N | T1-T6; C+ | PT | R | [26] |
| 8 | 44F | 1st AZ/4 | SSM | - | - | NA | ↑ | P | NA | 76.7 | NA | - | N | T7-T8, T10-T11; C+ in T7-T8 | PT | R | [27] |
| 9 | 36M | 1st AZ/8 | SSM | - | - | NA | NA | NA | NA | 54 | NA | NA | N | C6-C7; C+ | PT | R | [28] |
| 10 | 44F | 1st Janssen/10 | LETM | - | NA | NA | 227 | 96 | 0 | 43 | N | - | N | C2 to upper thoracic | PT + PE | R | [29] |
| 11 | 34M | 2nd Sputnik V/21 | NMOSD | + | - | NA | ↑ | P | NA | ↑ | NA | - | Lateral, 3rd and 4th ventricles, thalamus, CC, optic chiasma | N | PP | I | [30] |
| 12 | 88F/DM, AD | 2nd Pfizer/29 | ADEM | NA | NA | NA | NA | NA | NA | NA | NA | - | Bil MCPs | NA | PT | R | [31] |
| 13 | 56F/Post-infectious rhombencephalitis | 1st Pfizer/14 | ADEM-like | - | - | - | N | NA | NA | N | N | - | L cerebellar peduncle and L centrum semiovale | NA | Oral steroid | R | [32] |
| 14 | 64M | 1st Pfizer/18 | NMOSD | + | NA | NA | N | NA | NA | N | N | - | CC, L frontal and parietal WM; C- | Cervical to the conus; C+ | PT + PE + RTX | I | [33] |
| 15 | 38M | 1st Pfizer/2 | SSM | NA | NA | NA | NA | NA | NA | 62.1 | N | NA | NA | T11-T12; C+ | NA | NA | [34] |
| 16 | 69F/cervical ca, HL, hypothyroidism | 1st Pfizer/2 | LETM | - | - | + | N | NA | NA | N | N | + | N | C3-C4 to T2-T3 | PT | I | [35] |
| 17 | 19F/atopic dermatitis, depression | 1st Moderna/14 | ADEM+ LETM |
- | - | - | 294 | 91 | 1 | 64.8 | NA | - | Bil hemispheres, pons, medulla, cerebellum; C+ | Medulla to T11; C+ | PT + PE | R | [36] |
| 18 | 46F/c | 1st Moderna/2 | NMOSD | - | NA | NA | NA | NA | NA | NA | NA | NA | N | C6-T2 | PT | R | [37] |
| 19 | 76F/HTN, vit B12 deficiency | 1st Moderna/2 | LETM | - | NA | NA | 15 | NA | 73 | 57.2 | NA | - | N | C2-C5; C+ in C3 | PT | I | [38] |
| 20 | 67F/CAD, CKD, neuropathy | 1st Moderna/1 | LETM | - | - | + | 2 | NA | NA | 56 | 0.61 | + | Nonspecific WM | C1-C3; C+ | PT + PP | I | [39] |
| 21 | 63M | 2nd Moderna/1 | SSM | - | - | NA | 3 | NA | NA | 37 | N | NA | Nonspecific bil corona radiata | Conus medullaris; C+ | IVIG + PT | I | [40] |
| 22 | 46F/Hashimoto’s thyroiditis | 2nd Sinovac/30 | ADEM-like | - | - | NA | 0 | 0 | 0 | 45 | NA | - | L thalamus, bil corona radiata, L diencephalon, R parietal cortex | NA | PT | R | [41] |
| 23 | 78F/DM, HTN, breast ca | 2nd Sinovac/21 | LETM | - | - | NA | 2 | NA | NA | 56 | 0.69 | - | N | C1-T3 | PT | I | [42] |
| 24 | 24F | 1st Sinopharm/14 | ADEM | - | - | - | 51 | NA | NA | NA | NA | - | Bil temporal | NA | IVIG | I | [43] |
| 25 | 71M/DM, HTN, IHD | 1st Sinopharm/5 | LETM | - | - | NA | 0 | 0 | 0 | N | N | - | N | Cervico-medullary junction to C3 | PT | I | [44] |
| 26 | 50F | 1st inactivated/3 | NMOSD | + | - | NA | 31 | NA | NA | N | N | - | Area postrema , bil hypothalamus | N | PT | I | [45] |
| 27 | 65M | 1st AZ/8 | LETM | - | - | NA | N | NA | NA | 70 | NA | - | NA | C4-C6 | PT | R | [52] |
| 28 | 68F/HTN, pancreatic ca | 2nd Moderna/14 | MOGAD | - | + | NA | 0 | 0 | 0 | 32 | NA | + | R lateral pons, trigeminal nerve, MCP | NA | PT | I | [53] |
| 29 | 59M | 1st AZ/14 | MOGAD | - | + | NA | 110 | NA | NA | 625 | N | + | N | T7-L1 | PT + PE | I | [54] |
| 30 | 45M/allergic asthma | 1st AZ/7 | MOGAD | - | + | NA | 43 | NA | NA | 40.6 | N | - | Bil subcortical and gray-white matter | T10-conus | PT | I | [55] |
| 31 | 26M | 1st AZ/20 | MOGAD | - | + | NA | 184 | NA | NA | 88 | NA | - | Bil MCPs, pons | C3-C6 | PT | I | [56] |
| 32 | 56F/HTN | 1st AZ/2 | ADEM-like | NA | NA | NA | NA | NA | NA | NA | NA | NA | L parietal WM, body of CC | NA | Oral steroid | I | [57] |
| 33 | 81M | 1st Moderna/13 | ADEM | NA | - | NA | 69 | 83% | NA | 52 | N | NA | R dorsal medulla, L pons, midbrain, thalami | NA | PT + IVIG + PP | E(D26) | [58] |
| 34 | 63F/HL, hypothyroidism | 1st Pfizer/7 | NMOSD | + | - | NA | 33 | 91% | NA | 57 | NA | - | L thalamus | T6-T12 | PT + PP | R | |
| 35 | 54F/ITP | 2nd Moderna/3 | NMOSD | + | - | NA | 26 | 86% | NA | 71 | NA | - | N | T2-T9 | PT | I | |
| 36 | 55M | 1st mRNA/21 | ADEM | NA | NA | NA | 200 | 95% | NA | 75 | N | NA | Bil WM | NA | PT | R | [59] |
| 37 | 36F | 1st AZ/14 | ADEM | - | - | + | 59 | NA | NA | 40 | N | + | Subcortical WM, PIC, pons and L MCP | N | PT | R | [60] |
| 38 | 37M | 1st Sinopharm/30 | ADEM | NA | NA | NA | 2 | NA | NA | 56 | 0.61 | - | L cerebral peduncle, bil pons, medulla | N | PT | R | [61] |
| 39 | 27F/Rectovaginal fistula | 2nd Pfizer/4 | LETM | - | - | NA | 7 | NA | NA | 43 | N | - | N | C5-C7 | PT | I | [62] |
| 40 | 61F/HTN, anxiety | 1st Pfizer/5 | ADEM | NA | - | NA | N | NA | NA | 61 | N | - | Deep WM, brainstem, cerebellum | N | PT + IVIG | I | [63] |
| 41 | 64M | 1st AZ/10 | ADEM | - | - | NA | 25 | P | NA | NA | N | NA | Bil mesial temporal, hippocampus, MCPs | NA | PT + PP + RTX | R | [64] |
| 42 | 64M | 2nd AZ/20 | ADEM+ SSM | - | - | NA | N | NA | NA | N | N | NA | Bil perirolandic cortex, corona radiata | T8-T9 dorsal | PT + IVIG + RTX | I | |
| 43 | 46M | 1st AZ/5 | ADEM + LETM | - | - | NA | 63 | NA | NA | 52 | N | NA | Bil MCP, pontine tegmentum, R paramedian medulla, L thalamocapsular | LETM | PT + PE | I | |
| 44 | 42F | 1st AZ/5 | ADEM-like | - | - | NA | N | NA | NA | N | N | NA | R temporal | NA | Oral steroid | R | |
| 45 | 56F | 1st AZ/10 | ADEM-like | NA | NA | NA | 1 | 16% | 20% | ↑ | N | NA | Subcortical WM, basal ganglia | NA | PT | R | [65] |
| 46 | 44F/HL, hypothyroidism, renal stone, anxiety | 1st mRNA/6 | ADEM+ LETM |
- | NA | NA | 105 | NA | NA | 98 | N | - | Multifocal PV lesions; C+ in L frontal WM | C3-C4 to thoracic with sparing C5-C6; C+ in T7-T8 | PT + PP | I | [66] |
| 47 | 70F | 3rd Sinovac/7 | NMOSD | + | NA | NA | N | N | N | N | N | - | NA | C1-C7 and T1-T3 | PT + PE + CP | E(M2) | [67] |
| 48 | 26F | 1st Sinovac/10 | NMOSD | + | NA | NA | N | N | N | N | N | - | N | C4-C5 | PT + PE + RTX | I | [68] |
| 49 | 46F | 1st AZ/10 | NMOSD | + | NA | NA | N | N | N | N | N | - | R lateral medulla, PV | C2-C3 | PT +AZT | I | |
| 50 | 80M | 2nd Pfizer/2 | NMOSD | + | + | NA | 39 | 93% | NA | N | N | - | N | T3-T10 | PT + PE + MMF | I | [69] |
| 51 | 43F | 2nd Pfizer/1 | NMOSD | + | - | NA | 6 | NA | NA | 40.1 | N | + | R ON, R periatrium, L crus cerebri | C1 to mid-thoracic | PT + PE + RTX | R | [70] |
| 52 | 29F | 1st AZ/11 | MOGAD | NA | + | NA | 0 | NA | NA | 18 | N | - | Long intraorbital segment of R ON | NA | PT + PP | NA | [71] |
| 53 | 26F | 1st Covaxin/11 | LETM | - | - | NA | 207 | NA | P | 95.8 | N | NA | NA | C2-L1 | PT + PP | NA | |
| 54 | 54F | 1st AZ/14 | ADEM-like | - | - | NA | 8 | P | NA | 77 | N | NA | CC, PV, subcortical WM, infratentorial | NA | PT + PP | NA | |
| 55 | 44M | 1st AZ/7 | MOGAD | NA | + | NA | 130 | P | NA | 38 | N | NA | NA | Cervical and dorsal cord, conus | PT + PP | NA | |
| 56 | 50F | 1st AZ/28 | SSM | - | - | NA | 2 | P | NA | 28 | N | NA | NA | C6 | PT | NA | |
| 57 | 39M | 1st AZ/14 | MOGAD | NA | + | NA | NA | NA | NA | NA | NA | NA | Long intraorbital segment of R ON | NA | PT | NA | |
| 58 | 54M | 1st AZ/14 | MOGAD | NA | + | NA | NA | NA | NA | NA | NA | NA | R pons | N | PT | NA | |
| 59 | 34M | 1st AZ/1 | ON | - | - | NA | 2 | P | NA | 26 | N | NA | R ON | NA | PT | NA | |
| 60 | 35M | 1st AZ/9 | MOGAD | NA | + | NA | 58 | P | NA | 47.4 | N | NA | Midbrain, pons, L MCP, PICs, thalamus, bil centrum semiovale | Cervical to conus | PT | NA | |
| 61 | 20F | 1st AZ/3 | ADEM-like | - | - | NA | NA | NA | NA | NA | NA | NA | Pericallosal, callososeptal, PV, fronto-parietal | NA | PT | NA | |
| 62 | 31M | 1st AZ/14 | LETM | - | - | NA | 370 | NA | P | 174 | N | NA | NA | Cervico-dorsal long segment | PT + PP + RTX | NA | |
| 63 | 20F | 1st Covaxin/1 | ADEM+ SSM |
- | - | NA | 8 | P | NA | 24.9 | N | - | Juxtacortical | C5 | PT + PP | NA | |
| 64 | 45F | 1st AZ/21 | MOGAD | NA | + | NA | 2 | P | NA | 52.3 | N | + | Bil ON | N | PT + PP | NA | |
| 65 | 33F | 1st AZ/14 | MOGAD | NA | + | NA | 105 | P | NA | 28.12 | N | NA | Bil fronto-parietal | NA | PT | NA | |
| 66 | 53F | 2nd AZ/1 | ADEM+ LETM |
- | - | NA | 6 | P | NA | 54.2 | N | NA | Bil subcortical, PV WM, insular, cerebellum, brainstem | C5-C7 and T6-T7 | PT | NA | |
| 67 | 38M | 2nd AZ/6 | ADEM-like | - | - | NA | 6 | NA | NA | 67.8 | N | NA | L MCP, R corona radiata | NA | PT | NA | |
| 68 | 30M | 1st AZ/14 | ADEM+ ON | - | - | NA | 4 | 50 | NA | 26.8 | N | + | Bil subcortical lesions, bil ON | NA | PT + PP + RTX | NA | |
| 69 | 30F | 1st AZ/15 | ADEM+ SSM |
- | - | NA | 4 | NA | NA | 36 | N | + | CC | C3 | PT + PP + MMF | NA | |
| 70 | 36M | 2nd AZ/32 | MOGAD | NA | + | NA | 720 | 80 | NA | 144.4 | N | NA | Bil trigeminal n, pons | Obex to conus | PT + PP | NA | |
| 71 | 27F | 1st AZ/8 | ADEM-like | - | - | NA | Clear | NA | NA | 27.7 | N | NA | Bil PV WM | N | PT | NA | |
| 72 | 60M | 2nd AZ/14 | ADEM | - | - | NA | 9 | 90 | NA | 68.3 | N | - | R pons, midbrain, temporal, parietal, CC | NA | PT + MMF | NA | |
| 73 | 23F | 2nd AZ/7 | ADEM+ LETM |
- | - | NA | NA | NA | NA | NA | NA | - | R frontal horn and bil lateral ventricles | C2-C5 and T4 myelitis | PT | NA | |
| 74 | 40M | 1st AZ/10 | MOGAD | NA | + | NA | 8 | 100 | 0 | 32 | N | + | Pons, bil thalami and R frontal cortex | C4-T3 | PT + MMF | NA | |
| 75 | 45M | 1st AZ/10 | MOGAD | - | + | NA | 44 | 44 | NA | 90.9 | N | NA | Brainstem, supratentorial | Cervicodorsal cord | PT + PP | NA | |
| 76 | 34F | 2nd AZ/36 | NMOSD | + | - | NA | 1 | NA | NA | 15.3 | N | - | Dorsal aspect of medulla | NA | PT + PP + RTX | NA | |
| 77 | 31M | 1st AZ/42 | ADEM+ LETM |
- | - | NA | 32 | 100 | 0 | 49.2 | N | NA | Cervico-medullary junction, R frontal subcortical | C2-C5 | PT + PP + MMF | NA | |
| 78 | 52F | 1st AZ/35 | ADEM-like | - | - | NA | 2 | NA | NA | 40.5 | N | NA | L frontal, insular, midbrain | NA | PT + PP + RTX | NA | |
| 79 | 65F | 1st AZ/42 | NMOSD | + | - | NA | 17 | NA | NA | 49 | N | NA | Frontal subcortical WM | T2-T11 | PT + PP + MMF | NA | |
| Abbreviations: Af: atrial fibrillation. AD: Alzheimer’s disease. AZT: azathioprine. C+: with contrast enhancement. CC: corpus callosum. CKD: chronic kidney disease. CP: cyclophosphamide. DM: diabetes mellitus. E: expired. HBV: hepatitis B virus. HL: hyperlipidemia. HTN: hypertension. I: improvement. IC: internal capsule. IHD: ischemic heart disease. ITP: immune thrombocytopenic purpura. MCP: middle cerebellar peduncle. MMF: Mycophenolate mofetil. N: normal. NA: not available. ON: optic neuritis. P: predominant. PIC: posterior limb of internal capsule. PP: plasmapheresis. PT: steroid pulse therapy. PU: putamen. PV: periventricular. R: complete recovery. RTX: rituximab. WM: white matter. a Oxford-AstraZeneca, marketed as Covishield. A kind of viral vector (chimpanzee adenovirus) vaccine. b Johnson & Johnson’s Janssen, a kind of viral vector (human adenovirus) vaccine. c A kind of viral vector (human adenovirus) vaccine. d Pfizer-BioNTech, marketed as Comirnaty. A kind of messenger RNA vaccine. e A kind of messenger RNA vaccine. f Marketed as CoronaVac. A kind of inactivated vaccine. g Also known as BBIBP-CorV. A kind of inactivated vaccine. h Clinical and MRI features are compatible with ADEM, but without presentation of encephalopathy | |||||||||||||||||
| Vaccine types | ||||
| Viral vector (n=49) | mRNA (n=20) | Inactivated(n=10) | P value | |
| Sex, n (%) | 0.027* | |||
| Male | 28 (57) | 6 (30) | 2 (20) | |
| Female | 21 (43) | 14 (70) | 8 (80) | |
| Mean age of onset (S.D.) | 44.3 (12.2) | 58.1 (17.9) | 44.8 (21.8) | 0.002* |
| Doses (1st/2nd/3rd) | 41/8/0 | 13/7/0 | 7/2/1 | 0.042* |
| Post-vaccination onset time (days) | 13.6 | 8.1 | 13.6 | 0.086 |
| Clinical presentations, n (%) | 0.200 | |||
| ADEM | 15 (31) | 6 (30) | 3 (30) | |
| ADEM with myelitis | 14 (29) | 2 (10) | 1 (10) | |
| Pure myelitis | 12 (24) | 6 (30) | 3 (30) | |
| ON | 4 (8) | 0 (0) | 0 (0) | |
| NMOSD | 4 (8) | 6 (30) | 3 (30) | |
| Serum Autoantibodies , n | 0.044* | |||
| Negative | 31 | 14 | 7 | |
| MOG | 14 | 1 | 0 | |
| AQP4 | 4 | 4 | 3 | |
| MOG+AQP4 | 0 | 1 | 0 | |
| CSF, n | ||||
| WBC count | 74.2 | 57.1 | 30.1 | 0.605 |
| Lym predominant (yes/no) | 23/3 | 6/2 | 1/4 | 0.004* |
| Elevated total protein (yes/no)@ | 24/19 | 10/8 | 4/5 | 0.817 |
| CSF/serum glu ratio (<0.6/>0.6) | 4/33 | 0/13 | 0/8 | 0.295 |
| Oligoclonal bands (+/-) | 9/13 | 4/11 | 0/9 | 0.071 |
| Brain MRI lesions, n | ||||
| Cortex (+/-) | 7/33 | 1/16 | 2/5 | 0.329 |
| Deep grey matters (+/-) | 8/32 | 2/15 | 2/5 | 0.598 |
| Subcortical white matters (+/-) | 18/22 | 5/12 | 2/5 | 0.454 |
| Periventricular white matters (+/-) | 16/24 | 6/11 | 1/6 | 0.424 |
| Periaqueductal white matters (+/-) | 3/37 | 0/17 | 0/7 | 0.389 |
| Brainstem (+/-) | 20/20 | 7/10 | 2/5 | 0.532 |
| Gadolinium enhanced (+/-) | 1/9 | 2/9 | 0/5 | 0.562 |
| Spine MRI, n | ||||
| Segments of cord lesions (≥ 3/< 3) | 20/8 | 12/2 | 4/2 | 0.143 |
| Gadolinium enhanced (+/-) | 6/6 | 7/1 | 0/4 | 0.015* |
| Treatment, n | 0.754 | |||
| 1st line immunotherapy | 43 | 17 | 8 | |
| 1st and 2nd line immunotherapy | 6 | 2 | 2 | |
| Outcome, n | 0.762 | |||
| Recovery | 11 | 7 | 2 | |
| Improved | 11 | 11 | 5 | |
| Expired | 1 | 1 | 1 | |
|
*Pearson chi-squared test, p<0.05 # Independent t test, p<0.05 @ Elevated total protein defined as total protein > 45 mg/dL | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





