1. Introduction
Urinary tract infections are one of the most prevalent forms of infection in humans, and currently rank second among the most common infections [1]. It is defined as the presence of a microorganism in the kidneys, ureters, bladder or urethra (in this case, some presence of normal flora is admitted in circumstances considered normal).
In females, considering the anatomy, namely the proximity to the vagina and anus and the shorter urethra, the probability of urinary infections is much higher than in males, as well as asymptomatic colonization itself, which may represent between 1 and 5% of total positive urine cultures [2]. There are other factors and conditions (definitive or transitory) that can contribute to an increased likelihood of developing urinary infections, such as genetic factors (reduced presence of CXCR1 – interleukin 8 receptor; kidney damage with genetic origin), diabetes mellitus, pregnancy, history of early urinary tract infection, circumcision, neurogenic bladder, kidney transplant, menopause, prostatism, catheterization, urinary tract obstruction and dysfunctional elimination [3,4].
The recurrence of infections is closely associated with the fact that the urinary system has direct communication with the outside, through the urethra [5].
Urinary infections can occur via the hematogenous route, particularly in newborns, constituting a condition of urosepsis, via the lymphatic route, which, although described, is very rare and is associated with cases of severe intestinal infection or retroperitoneal abscess, and via the ascending route, which It is the genesis of most urinary infections in humans. However, the Human Being has defense mechanisms that allow him some protection, such as urination, the normal flora of the distal part of the urethra, the composition of urine (low osmolarity and high concentration of urea and organic acids) and anti-inflammatory properties. adherence and antimicrobials of the bladder mucosa, along with the immune system [6–8].
Timely diagnosis is crucial, as it helps to reduce the likelihood of complicated urinary infections, with associated kidney damage and consequent kidney scarring. Usually, for the diagnosis of a urinary infection, an initial combination of characteristic clinical manifestations on the part of the patient (burning/pain when urinating, pollakiuria) and a positive summary urine analysis (type II urine) are necessary, complementing the definitive diagnosis with microbiological testing (identification of the strain and respective antibiogram). These tests can be complemented with other clinical analyzes that may indicate positivity for infection, such as blood count and C-reactive protein, among others9. However, at an early stage there is no evidence that the determination of other more specific infectious markers, such as procalcitonin and even blood culture can contribute decisively to the diagnosis [10].
Globally and across different continents, the most prevalent bacteria in community-acquired urinary infections is Escherichia coli, with great emphasis, followed by Staphylococcus saprophyticus, species of Proteus and Klebsiella and Enterococcus faecalis. If we analyze the urinary infections most associated with individuals in hospital, although Escherichia coli continues to dominate, there is an increase in other strains, namely Proteus sp, Pseudomonas aeruginosa, Klebsiella and some fungi, with emphasis on Candida sp. [11–13].
Ideally, treatment should only begin after the identification of the microorganism and its behavior against the antibiotics tested, either for greater effectiveness or to avoid the development of resistance or in specific clinical situations and the effectiveness is proven, such as urinary infections. recurrent in women [14]. Although we are witnessing a development in the use of PCR (polymerase chain reaction) detection, microbiological tests are often time-consuming and require between 24 and 48 hours to obtain concrete and effective answers, therefore in many situations , particularly if associated with symptoms or clinical severity, empirical therapy begins, which is based on the accumulated knowledge that exists about this type of infections, namely the knowledge of which strains are most common and which antibiotics are most effective for these strains . This knowledge is acquired through the study and analysis of previous infections, which is clearly the scope of the work presented here, which is intended to contribute decisively to increasing knowledge on this topic [15,16].
The objectives of this study are: To study the sociodemographic characteristics of patients who had a positive urine culture between 2018 and 2022. Analyze the main strains associated with positive urine cultures between 2018 and 2022. Examine the response of the main microorganisms identified to the antibiotics tested.
2. Materials and Methods
A retrospective observational study was carried out on all positive urine cultures between January 2018 and December 2022 at a Hospital Center in the Center of Portugal, totaling 8340 samples. Data were collected with computer support, including sex (male or female), age, origin (emergency, hospitalization, consultation or day hospital), pregnancy (yes or no), previous antibiotic therapy (yes or no), catheterization (yes or no), bacteria isolated (strain) and antibiotics tested (sensitive or resistant).
Statistical analysis was performed using IBM SPSS Statistics software, version 29.0.1 for Mac IOS. Descriptive statistics and inferential statistics were used to compare groups, using the t test for independent samples (normal distribution) or the Mann-Whitney U test (non-normal distribution).
This work was approved by the Ethics Committee and the Data Protection Officer at the University of Beira Interior, and all ethical precepts were scrupulously respected by the Researchers. Informed consent was waived given the retrospective nature and the fact that no user identifying data was used.
This work is part of an ITUCIP study (Urinary Tract Infections in the Interior Center of Portugal).
3. Results
8340 urine cultures were analyzed, corresponding to all urine samples positive for bacteria entered at the Hospital Center between January 2018 and December 2022. The majority belonged to female individuals (68.3%) and the remaining 2644 to men. Regarding age, there is a variation between one month and 99 years, with the most frequent ages being 87 years (3.5%), 85 years (3.4%), 83 and 86 years (3%) and 84 and 89 years old (2.9%), The average was 63.4 years old. By age groups, it was observed that 9.7% of urine cultures were from individuals aged 18 years or less, 64.2% from people aged between 18 and 65 years and 26.1% were from individuals aged over 65 years old.
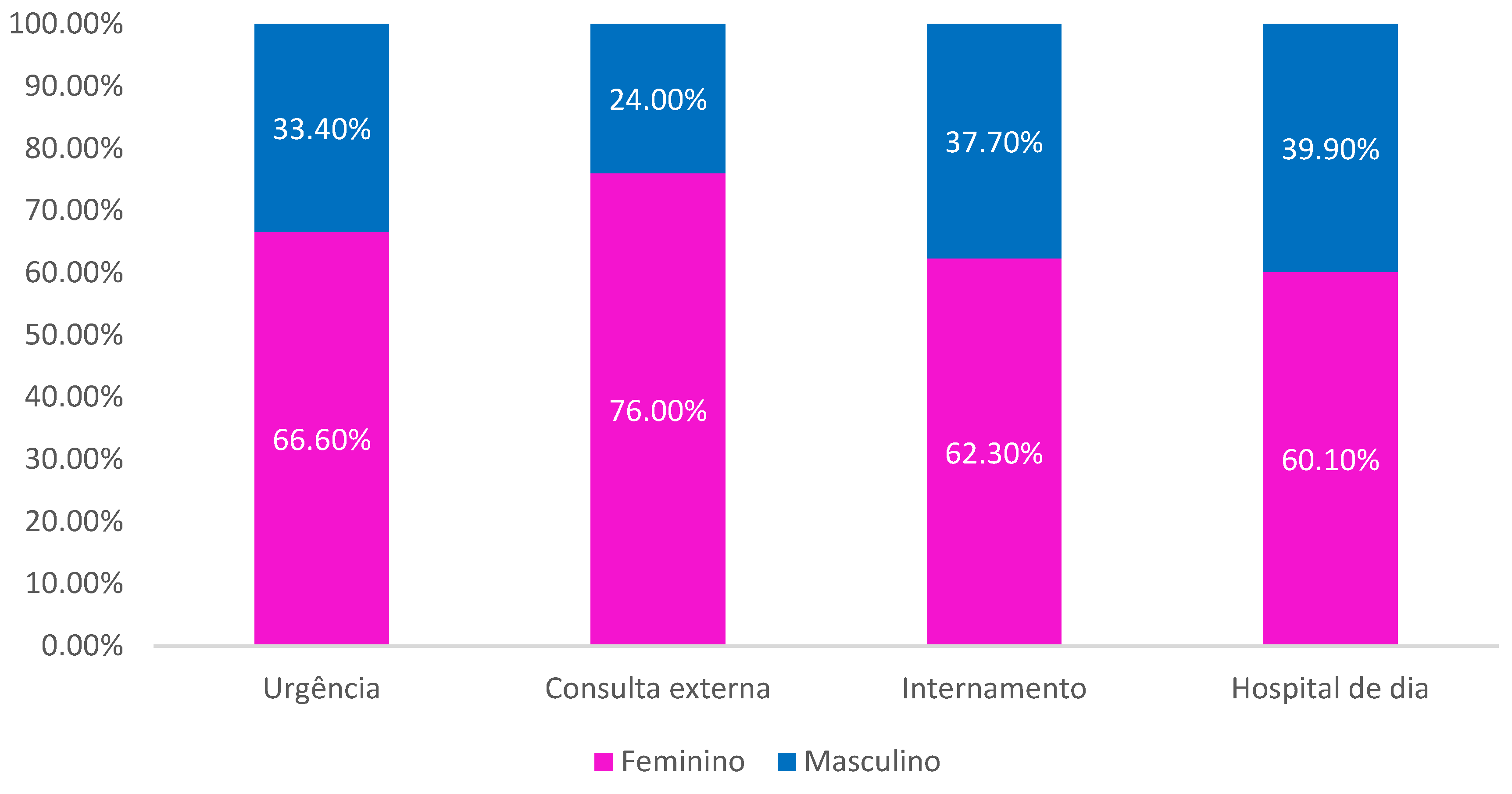
Regarding origin, it is observed that the majority were patients originating in the Emergency Department (43.2%), followed by Inpatient (31%), Outpatient Consultation (25.5%) and Day Hospital (0.3% ). It is observed that the female sex was always the most prevalent of all origins (
Figure 1).
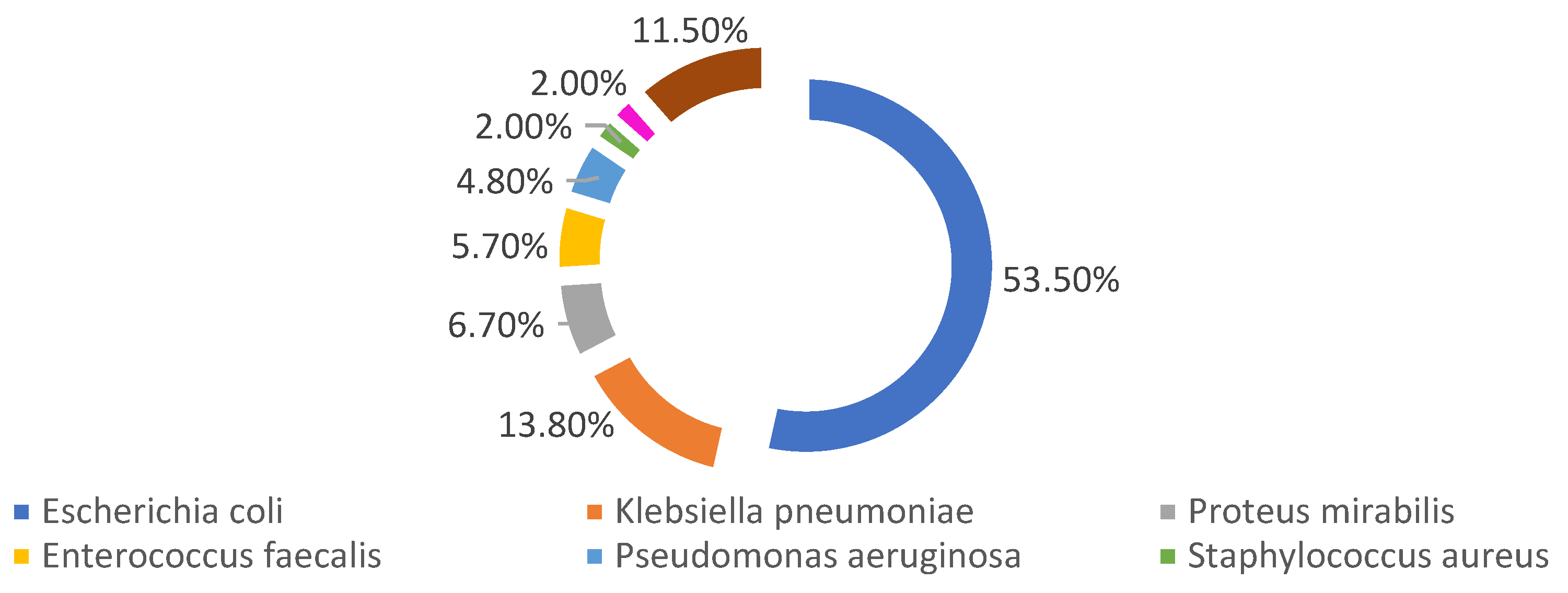
Among the female members of the sample, 3% were pregnant, with 17.5% in the first trimester, 31.3% in the second trimester and 51.2% in the third trimester of pregnancy. 13.5% of the individuals in the sample were catheterized at the time of urine collection for urine culture, 56% of whom were women. 4.1% of the individuals in the sample were taking antibiotics at the time of urine collection for urine culture, with 50% having catheters. 59.4% of people taking antibiotics were women. Regarding the most identified strains, there is a clear dominance of
Escherichia coli (53,5%), followed by
Klebsiella pneumoniae (13,8%) and Proteus mirabilis (6,7%). A further 58 different bacteria were identified, however with little expression each, representing, together, less than 11,5% of the sample, being represented as “others”. (
Figure 2).
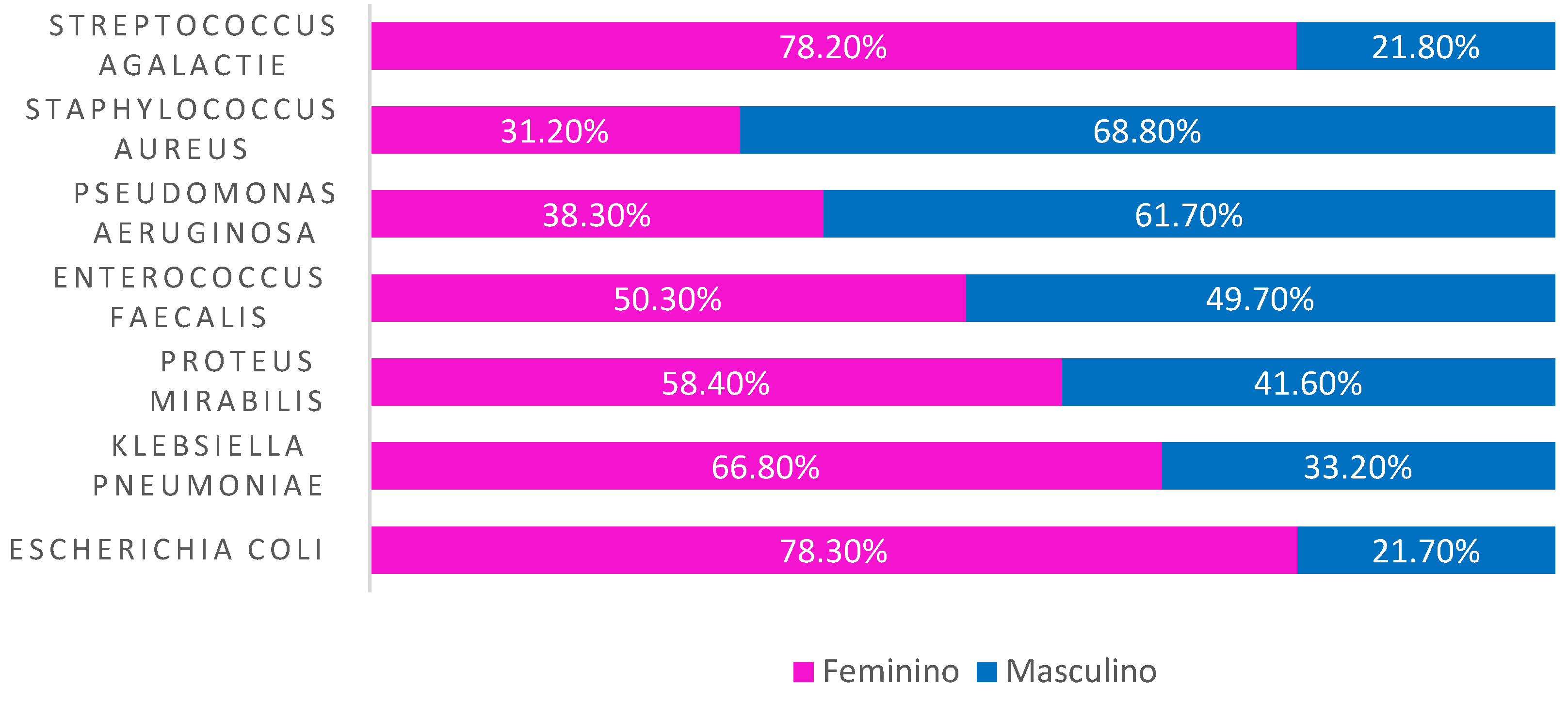
Analyzing by sex, we observed that Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis and Streptococcus agalactiae had a higher incidence in women, while Pseudomonas aeruginosa and Staphylococcus aureus appeared more in men (
Figure 3).
The majority of those infected by Escherichia coli originated in the emergency room (47,3%), similar to what occurred with those infected by Klebsiella pneumoniae (40,6%) and Proteus mirabillis (49,5%). Regarding those infected with Enteroccus faecalis, Pseudomonas aeruginosa and Staphylococcus aureus, the majority came from hospitalization (47,6% and 53,4% and 45.3%, respectively). The majority of those infected with Streptococcus agalactiae came from external consultations.
Of the emergency patients, the majority (58,5%) were identified with Escherichia coli, 12,9% with Klebsiella pneumoniae and 7,7% with Proteus mirabillis. Of those who were hospitalized, 39,9% were diagnosed with Escherichia coli, 16,9% with Klebsiella pneumoniae and 8,2% with Pseudomonas aeruginosa. Of the day hospital patients, the majority (65.2%) had Escherichia coli and 8.7% had Pseudomonas aeruginosa. In outpatients, 60.4% had Escherichia coli, 11.3% Klebsiella pneumoniae and 5.9% Enterococcus faecalis.
Among the patients who were taking antibiotics at the time of urine collection for urine culture, 37,4% were identified with Escherichia coli, 15,9% with Klebsiella pneumoniae, 12,2% with Acinetobacter baumanni, 10,3 % with Pseudomonas aeruginosa, 8,5% Enterococcus faecalis and 7,1% Enterococcus faecium, with the remaining identifications in marginal percentage values. Analyzing the origin of the patients, we observed that 18.8% were from Emergency, 19,1% from Consultation, 0,3% from Day Hospital and 61,8% from Inpatient.
Among the individuals who were catheterized at the time of urine collection, it was observed that in 36.3% Escherichia coli was identified, in 19.8% Klebsiella pneumoniae, in 9,2% Pseudomonas aeruginosa and in 3,5% Acinetobacter baumanni, with the remaining identifications divided into another 12 species, but in residual values.
19.1% came from the emergency department, 7,2% from the outpatient consultation, 73,5% from the inpatient setting and 0,2% from the day hospital.
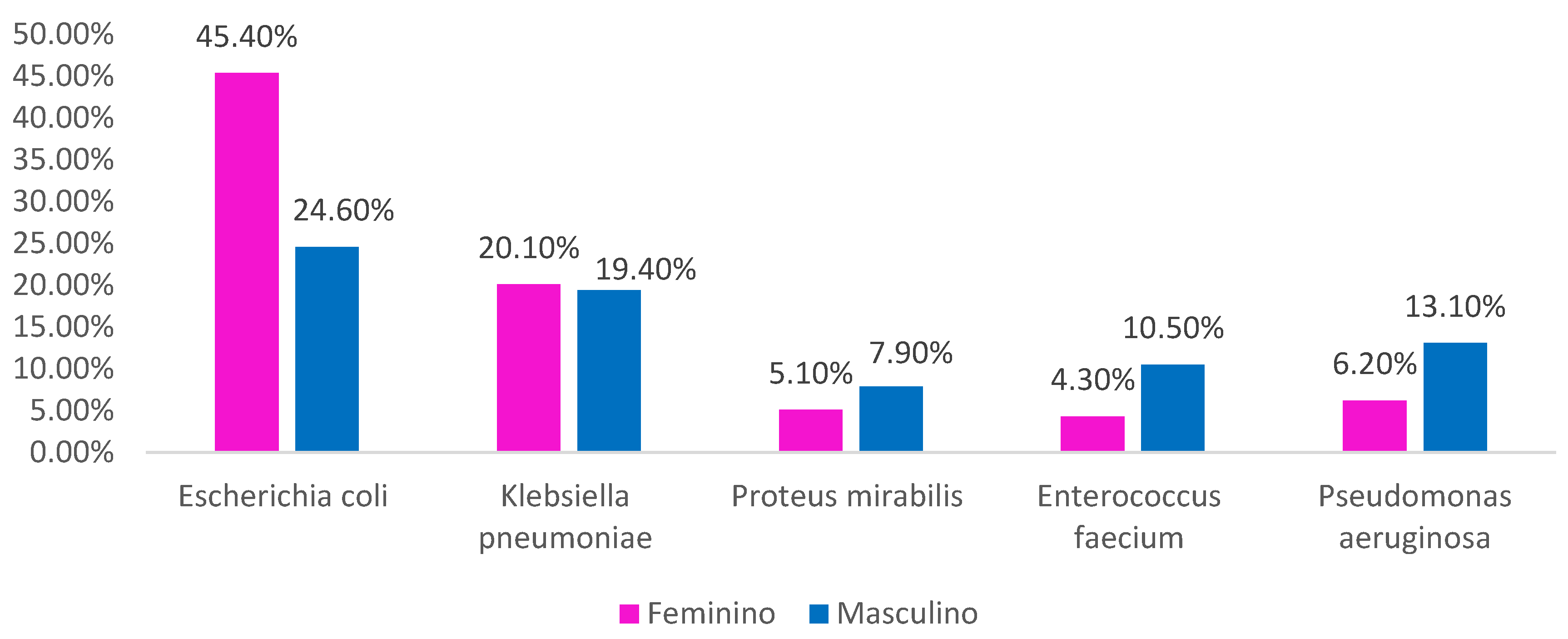
Crossing sex and galliation, we always observed
Escherichia coli as the most dominant bacteria, although in different proportions between men and women. We also found that Proteus mirabilis, Enterococcus faecium and
Pseudomonas aeruginosa are those that affect men more compared to women (
Figure 4).
Analyzing the most prevalent strains in pregnant women, we observed that in all trimesters, Escherichia coli is always the predominant bacteria (65,7% in trimester 1, 42,9% in trimester 2 and 46,6% in trimester 3). A highlight is the 15.9% of pregnant women in the second trimester infected with Klebsiella pneumoniae, 15,9% with Enterococcus faecalis and 14% infected with Streptococcus agalactiae. In the third trimester, 19,4% of pregnant women were infected with Streptococcus agalactiae and 15,9% with Enterococcus faecalis.
Analyzing the behavior of the two most prevalent bacteria in relation to the antibiotics tested, specifically the percentage of resistant strains per year, we observe the results obtained in
Table 1 and
Table 2.
4. Discussion
In the work presented here, it was possible to fully collect the results of positive urine cultures that were admitted to the Hospital center between January 2018, and December 2022. It thus presents itself as a faithful portrait of the panorama for this microbiological analysis, allowing us to understand the profile of urinary infections around influence of this health institution.
The characterization of patients in terms of age group is in line with the results of the 2021 Census [17], with the predominance of the age group between 18 and 65 years old. Women are much more susceptible to developing urinary infections, particularly due to anatomy, and in the sample in question here they also constituted the majority, like other studies [18]. In fact, 1 in 2 women will have at least one episode of urinary tract infection throughout their lives and 1 in 3 will have an episode before the age of 24 [19]. In addition to anatomical interference, pregnancy is also identified as a preponderant factor that can contribute to these higher numbers, as it is the most prevalent infection during this period and can contribute to complications in the fetus and mother [20]. 3% of the women in the sample under analysis were pregnant. The risk of pre-eclampsia increases in pregnant women who develop a urinary tract infection during the first trimester, and in our study 17,5% of pregnant women were in the first trimester [21] and special attention should therefore be given to these women. In the study under analysis here, it was detected that it is in the third trimester that more pregnant women developed urinary infections, as opposed to the literature that points to the first trimester as more prevalent [22]. Although Escherichia coli is always the most isolated bacteria in all trimesters of pregnancy among the women in the sample, in the second trimester 15,9% of pregnant women were infected with Klebsiella pneumoniae and 15.9% were infected with Enterococcus faecallis and in the third trimester 19. 4% with Streptococcus agalactie, these values agreeing with those of other studies on similar samples [23,24].
Most samples came from the Emergency Department, thus inferring that they were from patients from outside the hospital, as well as patients from external consultations, who constituted the third most prevalent group. There is a similarity between the values obtained for Escherichia coli and Klebisella pneumoniae in patients from these two origins, with the difference being that the third most identified bacteria in the Emergency Department was Proteus mirabillis and in the Outpatient Clinic it was Enterococcus faecalis. It is known that Proteus mirabillis may be associated with catheterization [25] and there are more catheterized patients coming from the Emergency Department than from the Consultation, which could be one of the differences, along with the age group, which is higher in patients in the Urgency. It is also common knowledge that many people who go to the Emergency Room in the older age groups come from care institutions and are often recurring episodes. It is observed that whatever the patient's origin, Escherichia coli is always the most isolated bacteria, but in different proportions, and in samples from the Emergency Room, Day Hospital and Outpatient Clinic the proportion varies between 58,5% and 65,2%, while in hospitalization, Escherichia coli continued to be the most prevalent bacteria, but with much lower values when compared to outpatients, which is also justified mainly by the greater diversity of bacteria that circulate in hospitals, for procedures that hospitalized patients may be undergoing (catheterization, broad-spectrum antibiotic therapy, serious concomitant infections, surgical procedures…) and that lead to healthcare-associated infections (HAI), in which urinary tract infection plays a role prominent.
In the study under analysis here, at the time of collection, 13,5% were fitted, with a predominance of women (56%). We cannot, however, analyze whether some of the infections may result from previous catheterization, as we do not have data on the subject. Between 70 and 80% of urinary infections associated with healthcare are directly related to individuals with catheters [26]. 73,5% of the insulated patients who took part in this study came from Hospitalization and 61.8% of those who were undergoing antibiotic therapy at the time of collection also came from Hospitalization, which may be one of the indicators for the existence of tract infections. more complicated urinary tract infections and with more aggressive strains, as well as a wider range of strains. It should also be noted that among patients coming from inpatient care and undergoing hospitalization, 61,8% were being treated with at least one antibiotic at the time of urine collection. There has been a huge effort on the part of Health Professionals to implement behaviors and protocols that contribute to reducing urinary infections associated with HAI, although the reductions are not always significant27,28.
4.1% of the individuals in the sample were taking antibiotics at the time of collecting urine for urine culture, which goes against good microbiological practices, as the presence of the drug in the body leads to changes in the flora and leads to difficulties in identification microbiology at laboratory level. It may also be a factor that leads to the inability to identify the microorganism present in urine, requiring repeat collections and new laboratory procedures, with a consequent increase in diagnostic time and costs [29]. However, considering that more than 60% of these people were hospitalized, antibiotics will be clinically advised, even possibly for other pathologies, and the urinary infection may have been subsequent and, as we saw previously, even resulted from a procedure associated with healthcare. However, the importance of performing a urine culture remains, especially because it may contribute to an adjustment in therapy, particularly in terms of the antibiogram [30,31]. There are benefits in carrying out a urine culture prior to the administration of antibiotic therapy, since the study of the microorganism's sensitivity to antibiotics will allow the clinician to decide on the best alternative, but the justifiable clinical need for treatment (with the reasons we have already explored previously) must always be based on existing practical evidence in that geographic area, which is only achieved through retrospective analysis studies3 [2,33,34].
In this work, 65 different strains were identified, many of which had only one or two incidences (positive urine cultures) and were always associated with individuals from hospitalization. It is normal for the flora in healthcare institutions to be very diverse, which is why associated infections are also very diverse and it is usually in these patients that urinary infections are more complicated and difficult to treat [35]. In fact, 53,4% of urine cultures that identified Pseudomonas aeruginosa came from hospitalized patients, as well as 45,3% of those that identified Staphylococcus aureus, in line with other similar articles [36,37].
We carried out an analysis of the resistance of Escherichia coli and Klebsiella pneumoniae, as they are the two most isolated bacteria in this study, carrying out an evaluation over five or three years (depending on the tests carried out), of the resistance of the bacteria to the antibiotics tested through the antibiogram.
It is observed that Escherichia coli decreased resistance to four antibiotics over a period of 5 years (Amoxicillin/Clavulanic Acid; Cefuroxime Axetil; Trimethoprim/Sulfamethoxazole and Ertapenem) and decreased resistance to seven antibiotics over a period of 3 years (Ciprofloxacin; Ampicillin; Cefotaxime; Ceftazidime; Cefepime; Gentamicin; Meropenem). According to a 2019 study, in Europe there has been an increase in resistance of Escherichia coli to Trimethoprim/Sulfamethoxazole, however in our study in 2018 we observed 27,7% resistance and in 2022 this value dropped to 26,7% [38]. Also with regard to amoxicilia/clavulanic acid, although there is a decrease in resistance between the years 2018 and 2022 (variation of -3,6%), the values presented are much higher than those found in Germany (5.3%), and similar to those found in France (37.6%)38. Escherichia coli also showed a decrease in resistance to ciprofloxacin (change of -2,5% in three years), resulting in 20,6% resistance, values very similar to those found in a study carried out in Brazil [39]. Ampicillin was another of the antibiotics to which the bacteria showed a decrease in resistance between the years studied, despite, however, being one of the antibiotics that maintains one of the highest resistance values (46.1% of E. coli are resistant). It has been common for this antibiotic to present these high values for several years [40,41] .No Escherichia coli in 2020 showed resistance to Meropenem (last year of antibiogram available) and in 2022 none showed resistance to Ertapenem. These are very positive values, however they are not in line with what appears in other studies [42,43]. In a study published in 2023, precisely the opposite was observed with regard to Cefepime and Amoxicillin/Clavulanic Acid, as they found an increase in resistance to both antibiotics [41].
On the other hand, this bacterium increased, over a period of five years, its resistance to Nitrofurantoin and over a period of three years to Colistin, Amikacin and Fosfomycin. Nitrofurantoin showed an increase of 23.6%, with the big jump in resistance being in the years 2021 and 2022. Until 2020 the values were relatively low and in line with what was happening at the time, with resistances between 0,8% and 2,0% [44], respectively. There is even an article from 2008 that points out that Escherichia coli resistant to nitrofurantoin had an intrinsic defect that made them less capable of developing pathology45. This antibiotic has been widely used to treat urinary infections, especially because the guidelines place it as one of those included in the first line of administration [46].
Amikacin was also one of the antibiotics to which Escherichia coli increased its resistance, although it was only 0,4% in 2020 compared to 0% in 2018. However, there are studies that demonstrate a positive association in the ability to overcome urinary infections caused by Escherichia coli in the association between amikacin and nitrofurantoin [47].
In 2018, colistin presented only 0,1% of Escherichia coli as resistant and in 2020 this number rose to 1,5%. These values are of great concern to the scientific community and several studies have recently been carried out to fully understand the resistance mechanisms associated with this phenomenon, namely plasmids and chromosomal mediation [48,49]. A very positive highlight is the fact that none of the antibiotics tested in 2022 show 100% resistance, with the highest resistance value belonging to Amoxicillin/Clavulanic Acid. If we analyze the year 2018, we observe that the fact remains that no antibiotic presents resistance greater than 50%, with the highest value obtained being in Ampicillin (48.7%).
With regard to Klebsiella pneumoniae, we found that in a period of three years resistance decreased against three antibiotics (Piperacillin/Tazobactam, Cefepime and Meropenem) and in a period of five years it decreased compared to four antibiotics (Amoxicillin/Clavulanic Acid, Cefuroxime Axetil, Fosfomycin and Trimethoprim/Sulfamethoxazole). It is essential, however, to highlight that only in the case of Fosfomycin this decrease was high (variation of -11.4%), as in the other antibiotics it was very discreet and maintained resistance levels always higher than 19%, with emphasis on Amoxicillin/Acid Clavulanic and Meropenem with values greater than 30%. Fosfomycin has been a widely analyzed antibiotic with good results, as shown by some studies, both from the point of view of oral50 and intravenous use [51].
This bacterium increased resistance to five antibiotics in three years (Ampicilia, Cefotaxime, Gentamicin, Ciprofloxacin and Colistin) and to four antibiotics in five years (Ceftazidime, Amikacin, Nitrofurantoin and Ertapenem). A very important highlight is the existence of three antibiotics to which none of Klebsiella pneumoniae was sensitive (Ceftazidime, Amikacin and Metronidazole). However, in the bibliography, there are situations in which Amikacin may prove to be an antibiotic worth considering52. Some studies show that this resistance can be associated with a plasmid, which simultaneously contributes to resistance to both Amikacin and Ampicillin simultaneously. In our study we observed that there were 35.8% of Klebsiela pneumoniae strains resistant to Ampicillin in 2020 and only 1.3% resistant to Amikacin. The great leap in resistance to this antibiotic happened precisely in the following year (2021), where all strains became resistant and remained so in 2022. The increase in resistance to Ciprofloxacin was also validated in another recent study [41], however, in this same study, an increase compared to Amoxicillin/Clavulanic Acid was also identified to values of 36.63%. In our study, a decrease in the resistance of Amoxicillin/Clavulanic Acid was observed compared to 2018, however the value obtained (35.8%) is very similar to that in the work of López Sampedro [41].
A very important highlight is the fact that all Klebsiella pneumoniae identified in 2022 were resistant to Amoxicillin, Ceftazidime, Clotrimazole and Amikacin, while in 2018 none of the strains found were resistant to all antibiotics, with the highest value obtained being in resistance to Amoxicillin/Clavulanic Acid (41.0%) and Meropen (40.0%), which demonstrates a very significant increase in resistance to antibiotics within a period of 5 years.
All strains in this work come from urine and are, according to the bibliography, those that tend to present higher levels of resistance to antibiotics [53].
Urinary infections are those that contribute most to the consumption of antibiotics, therefore they are directly related to increases in bacterial resistance, which can have several recurrences and therefore require the individual to take prolonged antibiotics. All prescriptions must be made based on microbiological evidence [54].
5. Conclusion
By carrying out this work, it was possible to analyze all positive urine cultures that were carried out in a Health Institution between the years 2018 and 2022, thus constituting an important source of information. Females were the most prevalent and the average age was close to 63 years. The most prevalent strain was always Escherichia coli. In females, strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabillis and Streptococcus agalactiae dominated, and in males, Staphylococcus aureus and Pseudomonas aeruginosa. Many urine cultures came from the Emergency Department. Most individuals were not taking antibiotics at the time of sample collection and less than one sixth were catheterized. In the time periods analyzed, Escherichia coli decreased its resistance against 11 antibiotics and increased compared to 5 antibiotics, while Klebsiella pneumoniae decreased compared to 7 and increased compared to 7, with emphasis on the presence of 3 antibiotics with a rate of 100% resistance to all Klebsiella pneumoniae strains identified by 2022.
It was not possible to understand the resistance mechanisms used by the bacteria, nor whether these bacteria were associated with recurrent urinary infections.
Author Contributions
MCB: conception and design of the study; data collection; review and approval of the final manuscript. PC: data analysis; review and approval of the final manuscript. FR: conception and design of the study; data collection; review and approval of the final manuscript.
Ethical Considerations
This work obtained a positive opinion from the Ethics Committee and the Data Protection Officer of the University of Beira Interior (CE-UBI-Pj-2023-020-ID1643), with all ethical precepts being taken into account during its course.
Financing Statement
This work did not receive any type of funding.
Acknowledgments
To the Laboratory Team who provided support in the sample collection process.
Conflicts of Interests
The authors declare that they have no conflict of interest in relation to this work.
References
- Mlugu EM, Mohamedi JA, Sangeda RZ, Mwambete KD. Prevalence of urinary tract infection and antimicrobial resistance patterns of uropathogens with biofilm forming capacity among outpatients in morogoro, Tanzania: a cross-sectional study. BMC Infect Dis, 2023;23(1):660. [CrossRef] [PubMed]
- Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am, 2014 Mar;28(1):1-13. [CrossRef] [PubMed]
- Kaur R, Kaur R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad Med J, 2021 Dec;97(1154):803-812. [CrossRef] [PubMed]
- Sobel JD, Kaye D. Host factors in the pathogenesis of urinary tract infections. Am J Med, 1984 May 15;76(5A):122-30. [CrossRef] [PubMed]
- Murray BO, Flores C, Williams C. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front Cell Infect Microbiol, 2021;11:691210. [CrossRef] [PubMed]
- Chu CM, Lowder JL. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol, 2018;219(1):40-51. [CrossRef] [PubMed]
- Lacerda Mariano L, Ingersoll MA. The immune response to infection in the bladder. Nat Rev Urol, 2020;17(8):439-458. [CrossRef] [PubMed]
- Clemens JQ. Infection and Inflammation of the Genitourinary Tract. J Urol, 2022;208(3):716-717. [CrossRef]
- Best J, Kitlowski AD, Ou D, Bedolla J. Diagnosis and management of urinary tract infections in the emergency department. Emerg Med Pract, 2014;16(7):1-24.
- Covino M, Manno A, Merra G, et al. Reduced utility of early procalcitonin and blood culture determination in patients with febrile urinary tract infections in the emergency department. Intern Emerg Med, 2020;15(1):119-125. [CrossRef]
- Guermazi-Toumi S, Boujlel S, Assoudi M, Issaoui R, Tlili S, Hlaiem ME. Susceptibility profiles of bacteria causing urinary tract infections in Southern Tunisia. J Glob Antimicrob Resist, 2018;12:48-52. [CrossRef]
- Jean SS, Coombs G, Ling T, et al. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-Pacific region: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010-2013. Int J Antimicrob Agents, 2016;47(4):328-334. [CrossRef] [PubMed]
- Gravey F, Loggia G, de La Blanchardière A, Cattoir V. Bacterial epidemiology and antimicrobial resistance profiles of urinary specimens of the elderly. Med Mal Infect, 2017;47(4):271-278. [CrossRef] [PubMed]
- Gormley EA. Recurrent urinary tract infection in women: emerging concepts regarding etiology and treatment considerations. Curr Urol Rep, 2003;4(5):399-403. [CrossRef]
- Ramírez F, Exeni A, Alconcher L, et al. Guía para el diagnóstico, estudio y tratamiento de la infección urinaria: actualización 2022 [Clinical practice guideline for the diagnosis and management of urinary tract infections: 2022 update]. Arch Argent Pediatr, 2022;120(5):S69-S87. [PubMed]
- Gonzalez CM, Schaeffer AJ. Treatment of urinary tract infection: what's old, what's new, and what works. World J Urol, 1999;17(6):372-382. [CrossRef] [PubMed]
- 17 –. https://www.pordata.pt/censos/resultados/emdestaque-beira+baixa-538.
- Hansen MA, Valentine-King M, Zoorob R, et al. Prevalence and predictors of urine culture contamination in primary care: A cross-sectional study. Int J Nurs Stud. 2022;134:104325. [CrossRef]
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49(2):53-70. [CrossRef] [PubMed]
- Ansaldi Y, Martinez de Tejada Weber B. Urinary tract infections in pregnancy. Clin Microbiol Infect. 2023;29(10):1249-1253. [CrossRef]
- Taghavi Zahedkalaei A, Kazemi M, Zolfaghari P, Rashidan M, Sohrabi MB. Association Between Urinary Tract Infection in the First Trimester and Risk of Preeclampsia: A Case-Control Study. Int J Womens Health. 2020;12:521-526. [CrossRef]
- Laari JL, Anab M, Jabong DP, Abdulai K, Alhassan AR. Maternal Age and Stage of Pregnancy as Determinants of UTI in Pregnancy: A Case of Tamale, Ghana. Infect Dis Obstet Gynecol. 2022;2022:3616028. [CrossRef]
- Rosana Y, Ocviyanti D, Halim M, et al. Urinary Tract Infections among Indonesian Pregnant Women and Its Susceptibility Pattern. Infect Dis Obstet Gynecol. 2020;2020:9681632. [CrossRef]
- Dube R, Al-Zuheiri STS, Syed M, Harilal L, Zuhaira DAL, Kar SS. Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women. Antibiotics (Basel). 2022;12(1):33. [CrossRef] [PubMed]
- Sabbuba NA, Mahenthiralingam E, Stickler DJ. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol. 2003;41(11):4961-4965. [CrossRef] [PubMed]
- Clarke K, Hall CL, Wiley Z, et al. Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention. J Hosp Med. 2020;15(9):552-556. [CrossRef] [PubMed]
- Gauron G, Bigand T. Implementation of evidence-based strategies to reduce catheter-associated urinary tract infections among hospitalized, post-surgical adults. Am J Infect Control. 2021;49(6):843-845. [CrossRef] [PubMed]
- Fink R, Gilmartin H, Richard A, Capezuti E, Boltz M, Wald H. Indwelling urinary catheter management and catheter-associated urinary tract infection prevention practices in Nurses Improving Care for Healthsystem Elders hospitals. Am J Infect Control. 2012;40(8):715-720. [CrossRef]
- Foong KS, Munigala S, Jackups R Jr, Yarbrough ML, Burnham CA, Warren DK. Incidence and Diagnostic Yield of Repeat Urine Culture in Hospitalized Patients: an Opportunity for Diagnostic Stewardship. J Clin Microbiol. 2019;57(10):e00910-19. [CrossRef]
- Ganzeboom KMJ, Uijen AA, Teunissen DTAM, et al. Urine cultures and antibiotics for urinary tract infections in Dutch general practice. Prim Health Care Res Dev. 2018;20:e41. [CrossRef]
- Shimoni Z, Avdiaev R, Froom P. Urine Cultures in Hospitalized Geriatric Patients Presenting With Fever. Am J Med Sci. 2017;353(1):17-21. [CrossRef] [PubMed]
- Piñeiro Pérez R, Cilleruelo Ortega MJ, Ares Álvarez J, et al. Recomendaciones sobre el diagnóstico y tratamiento de la infección urinaria [Recommendations on the diagnosis and treatment of urinary tract infection]. An Pediatr (Engl Ed). 2019;90(6):400.e1-400.e9. [CrossRef]
- Al-Mendalawi MD. Antibiotic resistance pattern and empirical therapy for urinary tract infections in children. Saudi Med J. 2008;29(10):1520.
- Gökçe İ, Çiçek N, Güven S, et al. Changes in Bacterial Resistance Patterns of Pediatric Urinary Tract Infections and Rationale for Empirical Antibiotic Therapy. Balkan Med J. 2017;34(5):432-435. [CrossRef]
- Bhowmik D, Singh S. Complicated urinary tract infections. J Indian Med Assoc. 2013;111(8):545-549. [PubMed]
- Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101-111. [CrossRef]
- Muder RR, Brennen C, Rihs JD, et al. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis. 2006;42(1):46-50. [CrossRef] [PubMed]
- Kot B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol J Microbiol. 2019;68(4):403-415. [CrossRef]
- Moreira da Silva RCR, de Oliveira Martins Júnior P, Gonçalves LF, et al. Ciprofloxacin resistance in uropathogenic Escherichia coli isolates causing community-acquired urinary infections in Brasília, Brazil. J Glob Antimicrob Resist. 2017;9:61-67. [CrossRef]
- Gobernado M, Valdés L, Alós JI, et al. Antimicrobial susceptibility of clinical Escherichia coli isolates from uncomplicated cystitis in women over a 1-year period in Spain. Rev Esp Quimioter. 2007;20(1):68-76. Rev Esp Quimioter.
- López-Sampedro I, Hernández-Chico I, Gómez-Vicente E, Expósito-Ruiz M, Navarro-Marí JM, Gutiérrez-Fernández J. Evolution of Antibiotic Resistance in Escherichia coli and Klebsiella pneumoniae from Urine Cultures. Arch Esp Urol. 2023;76(3):203-214. [CrossRef]
- Oteo J, Delgado-Iribarren A, Vega D, et al. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int J Antimicrob Agents. 2008;32(6):534-537. [CrossRef]
- Hayakawa K, Matsumura Y, Uemura K, et al. Effectiveness of cefmetazole versus meropenem for invasive urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2023;67(10):e0051023. [CrossRef]
- Zhang X, Zhang Y, Wang F, et al. Unravelling mechanisms of nitrofurantoin resistance and epidemiological characteristics among Escherichia coli clinical isolates. Int J Antimicrob Agents. 2018;52(2):226-232. [CrossRef]
- Sandegren L, Lindqvist A, Kahlmeter G, Andersson DI. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother. 2008;62(3):495-503. [CrossRef]
- Mahdizade Ari M, Dashtbin S, Ghasemi F, et al. Nitrofurantoin: properties and potential in treatment of urinary tract infection: a narrative review. Front Cell Infect Microbiol. 2023;13:1148603. Published 2023 Jul 27. [CrossRef]
- Zhong ZX, Cui ZH, Li XJ, et al. Nitrofurantoin Combined With Amikacin: A Promising Alternative Strategy for Combating MDR Uropathogenic Escherichia coli. Front Cell Infect Microbiol. 2020;10:608547. Published 2020 Dec 21. [CrossRef]
- Abavisani M, Bostanghadiri N, Ghahramanpour H, et al. Colistin resistance mechanisms in Gram-negative bacteria: a Focus on Escherichia coli. Lett Appl Microbiol. 2023;76(2):ovad023. [CrossRef]
- Anyanwu MU, Jaja IF, Okpala COR, Njoga EO, Okafor NA, Oguttu JW. Mobile Colistin Resistance (mcr) Gene-Containing Organisms in Poultry Sector in Low- and Middle-Income Countries: Epidemiology, Characteristics, and One Health Control Strategies. Antibiotics (Basel). 2023;12(7):1117. [CrossRef]
- Hatlen TJ, Flor R, Nguyen MH, Lee GH, Miller LG. Oral fosfomycin use for pyelonephritis and complicated urinary tract infections: a 1 year review of outcomes and prescribing habits in a large municipal healthcare system. J Antimicrob Chemother. 2020;75(7):1993-1997. [CrossRef]
- Burgos RM, Rodvold KA. ZTI-01 (fosfomycin for injection) in the treatment of hospitalized patients with complicated urinary tract infections. Future Microbiol. 2019;14:461-475. [CrossRef]
- Rodrigues D, Baldissera GS, Mathos D, Sartori A, Zavascki AP, Rigatto MH. Amikacin for the treatment of carbapenem-resistant Klebsiella pneumoniae infections: clinical efficacy and toxicity. Braz J Microbiol. 2021;52(4):1913-1919. [CrossRef] [PubMed]
- Ballén V, Gabasa Y, Ratia C, Ortega R, Tejero M, Soto S. Antibiotic Resistance and Virulence Profiles of Klebsiella pneumoniae Strains Isolated From Different Clinical Sources. Front Cell Infect Microbiol. 2021;11:738223. [CrossRef] [PubMed]
- Urbaniec J, Getino M, McEwan TB, et al. Anti-persister efficacy of colistin and meropenem against uropathogenic Escherichia coli is dependent on environmental conditions. Microbiology (Reading). 2023;169(11). [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).