1. Introduction
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. IgAN causes end-stage kidney disease (ESKD) in 20–40% of all cases within 20 years after diagnosis [
1], and reduces life expectancy by 10 years [
2].
Diagnosis is based on immunofluorescence or immunohistochemical microscopic evidence of IgA-dominant or codominant mesangial immune deposits [
3]. C3 deposits are identified in 90% of IgAN biopsies [
4]. The activation of the complement system by pathological circulating IgAs seems to play a crucial role, although the precise pathophysiology of IgA–mediated complement activation remains poorly understood. A variety of mechanisms is thought to be at play, which may act alone or in combination, i.e.: (i) the stabilization of the C3 convertase, (ii) the activation of the lectin pathway through polymeric IgAs, and (iii) inherited partial deficiencies of complement regulatory proteins due to pathogenic gene variants [
5].
Recently, the overactivation of the alternative complement pathway (ACP) has been recognized as a key driver of ESKD progression, by the means of progressive arteriolar damage and thrombotic microangiopathy (TMA) [
6,
7]. In 2012, El Karoui showed that 53% of IgAN cases presented chronic or acute TMA [
8], although the incidence of TMA in other studies was lower (2-40% of cases)[
7,
9,
10,
11,
12]. One third of TMA cases was associated with a picture of atypical hemolytic uremic syndrome (aHUS), i.e. non-immune microangiopathic hemolytic anemia (MAHA), thrombocytopenia and acute kidney injury (AKI). Renal prognosis was significantly worse in IgAN cases with TMA, and the presence of aHUS further increased the risk of ESKD [
6].
A recent study identified low serum C3 and low C3/C4 plasma ratio as negative prognostic factors in IgAN [
13]. Moreover, a low C3/C4 ratio at the time of kidney biopsy correlated with worse clinical and histological findings [
14]. Another study, which did not assess renal outcomes of hypocomplementemic patients, showed the negative prognostic role of microangiopathic lesions
per se, which were associated with a higher incidence of hypocomplementemia; glomerular C3 staining intensity also correlated with worse renal prognosis, the histological finding of TMA and the degree of interstitial fibrosis [
10]. In sum, these data suggest that progressive microangiopathic damage caused by the ACP overactivation might be a significant determinant of disease progression in IgAN. Such progressive damage would be in line with the wide spectrum of disease severity typical of IgAN, ranging from indolent to rapid progression towards ESKD.
Based on these assumptions, our study aimed at assessing whether, in patients with IgAN, low serum C3 (LowC3) and the histological evidence of TMA, which are considered indirect signs of ACP overactivation, are associated with increased risk of ESKD, and whether the relationship between LowC3 and ESKD reflects the development of TMA.
2. Materials and Methods
Study population. We enrolled all patients with biopsy-proven IgAN, followed from April 1st 2008 to March 31st 2019 by the Nephrology Unit of Parma University Hospital (Parma, Italy).
Data collection. We retrospectively followed-up patients until last visit before November 30
th 2023 or ESKD or death. We extracted clinical data at the time of renal biopsy and during follow-up from electronic medical records. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI 2009) equation [
15].
Histological diagnoses. Renal biopsy specimens were routinely assessed by light microscopy and direct immunofluorescence. For light microscopy, formalin-fixed paraffin-embedded sections were stained with methenamine silver, periodic acid-Schiff (PAS), haematoxylin & eosin (H&E) and Masson’s trichrome. For direct immunofluorescence, the specimens were embedded in OCT (Tissue Tek, Miles Inc., Elkhart, IN, USA) and stored in liquid nitrogen. Samples were cut to 3 µm sections by a cryostat (Leica CM1850, Leica Mycrosystems, Germany), placed on poly-L-lysine coated glass and stored at −80°C until use. These sections were rinsed in a 0.01 mol/L phosphate-buffered saline (PBS), pH 7.4 and incubated for 30 min at room temperature with fluorescein isothiocyanate (FITC)-conjugated rabbit antihuman IgG, IgA, IgM, kappa, lambda, C3c, C1q and fibrinogen antisera (Dako, Copenhagen,Denmark). Negative controls were processed in parallel using PBS or an equivalent concentration of non-immune rabbit or mouse serum as primary antibodies. Samples were observed using a Leitz Diaplan microscope (Leica, Milan, Italy). The deposition of each antiserum was scored as “negative” (diffuse negative, focal slightly positive), “+” (diffuse slightly positive, focal moderately positive), “++” (diffuse moderately positive, focal intensely positive) and “+++” (diffuse intensely positive). All slides were reviewed by two independent expert renal pathologists (GMR, MD), who were blinded to the patients’ clinical data, and scored using the Oxford Classification of IgAN [
16]. The presence of acute TMA (glomerular thrombi, endothelial edema, mesangiolysis, presence of fragmented red blood cells, arteriolar thrombi, arteriolar intimal edema, necrosis of myocytes and/or fibrinoid necrosis) or chronic TMA (double contours of the capillary loops and/or hyperplastic arteriolosclerosis, i.e. "onion skin" appearance of arterioles) was assessed, and scored as either present or absent, chronic or acute.
Data analysis. We performed all analyses using Stata 18 (StataCorp, College station, TX, US). We regarded two-sided P values <0.05 as statistically significant. We tested differences between two groups using Mann-Whitney test for continuous variables and Fisher's exact test for categorical variables. We estimated ESKD-free crude survival using Kaplan-Meier estimators and tested the crude difference with log-rank test. Time started from time of diagnosis until ESKD or last follow-up visit. No patient died or was lost to follow-up. We computed adjusted hazard ratios using multivariable Cox proportional hazards regression models. We adjusted the models for confounders that we selected based on background knowledge. We checked linearity of continuous variables with fractional polynomials, and proportional-hazards assumption using Schoenfeld residuals. We fitted the crude and adjusted Cox proportional hazards regression models with either TMA or low C3 (separately), but also models that included both TMA and low C3 in order to estimate what is the association with ESKD that was independent of either variable. In addition, using mediation analysis, we estimated the overall proportion mediated by TMA of the adjusted relation between low C3 and ESKD. To this purpose we used the Stata program med4way[
17]. The program med4way estimates the components of the 4-way decomposition of the total effect of Low C3 on ESKD in the presence of the mediator TMA with which the Low C3 may interact: the total effect of the Low C3 on ESKD is broke down into components due to mediation alone, to interaction alone, to both mediation and interaction, and to neither mediation nor interaction [
18]).
3. Results
Of the 56 patients included in the study, 12 (21%) presented low serum C3 (LowC3) (normal range: 90-180 mg/dl) at the time of renal biopsy; clinical and laboratory parameters at renal biopsy are reported in
Table 1.
The LowC3 group had by definition lower mean sC3 values (78.4 ± 10.9 vs 117.6 ± 20.3 mg/dL, p < 0.000), however mean sC4 values (23.8 ± 7.8 mg/dL vs 28.5 ± 6.9 mg/dL; p=0.048) were significantly lower too, although sC4 was within normal range in the entire study cohort (normal range: 10-40 mg/dl). At biopsy, we did not observe significant differences in age, eGFR, 24-hour proteinuria, and laboratory parameters related to TMA (hemoglobin, platelets, and LDH).
The use of steroids did not differ significantly between the two groups while the administration of RAASi was slightly more frequent in the non LowC3 group.
3.1. Histopathological findings
We found substantial uniformity between the two groups in terms of MEST-C parameters and immunofluorescence positivity. We identified 17/56 (30%) patients with TMA which coexisted with typical IgAN findings.
Of the 17 patients with TMA, 8 had acute TMA. The other 9 had signs of chronic TMA. Two (4%) of the 17 TMA patients had aHUS at disease onset. TMA was significantly more frequent in the LowC3 group [7/12 (58%) vs 9/44 (20%), p 0.02]. Considering other parameters such as age at onset, gender distribution, proteinuria, and eGFR, no significant differences were found between TMA-positive and TMA-negative patients. In addition, treatment approaches were similar in patients with and without TMA.
3.2. Renal outcomes
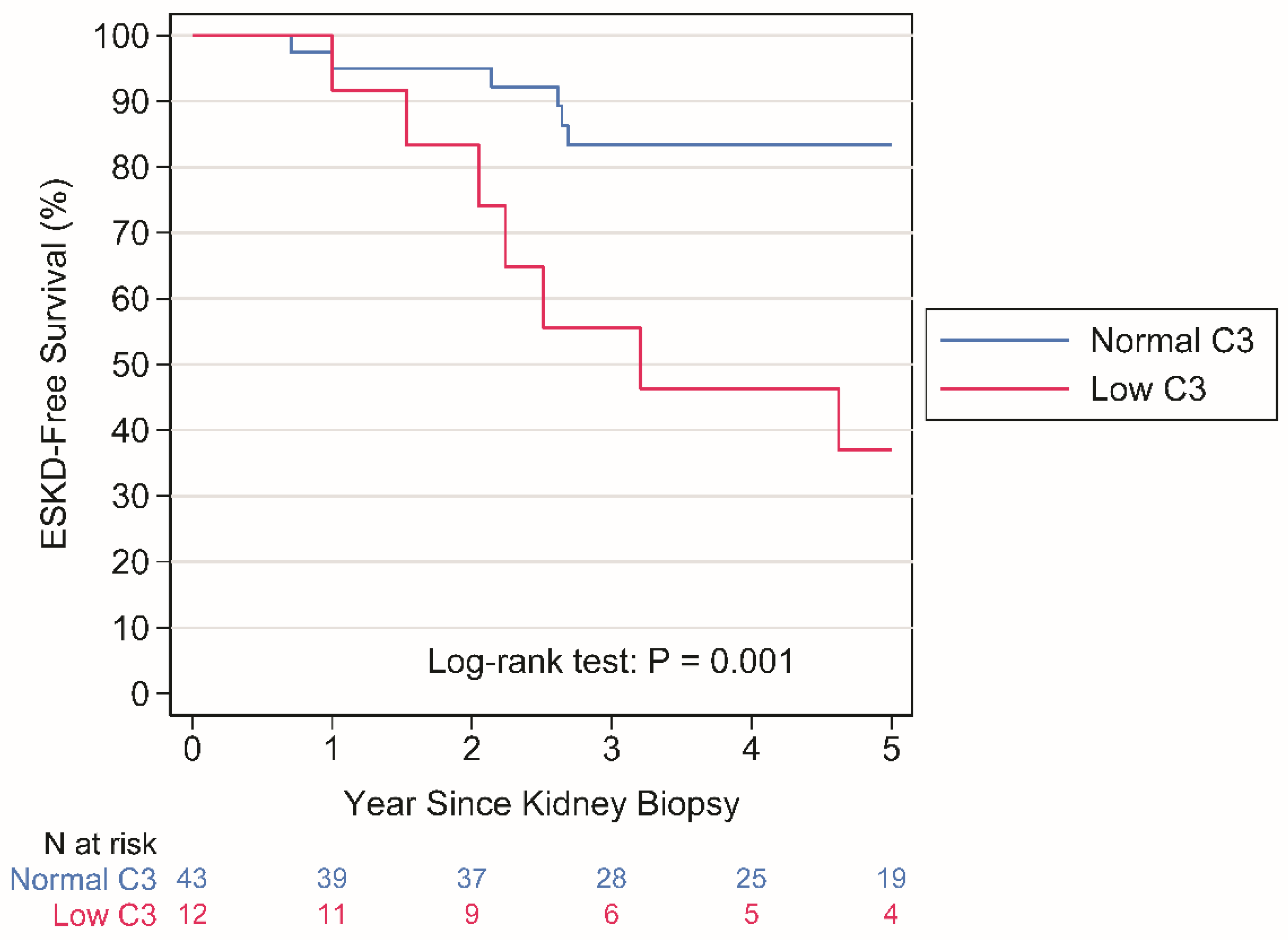
Crude analysis. ESKD occurred in 8 patients with normal C3 levels over a period of 248 person-years (py) (rate: 3.2 per 100 py) and in 8 patients with LowC3 levels over a period of 50 py (rate: 15.9 per 100 py), P = 0.001 by Log-rank test (
Figure 1).
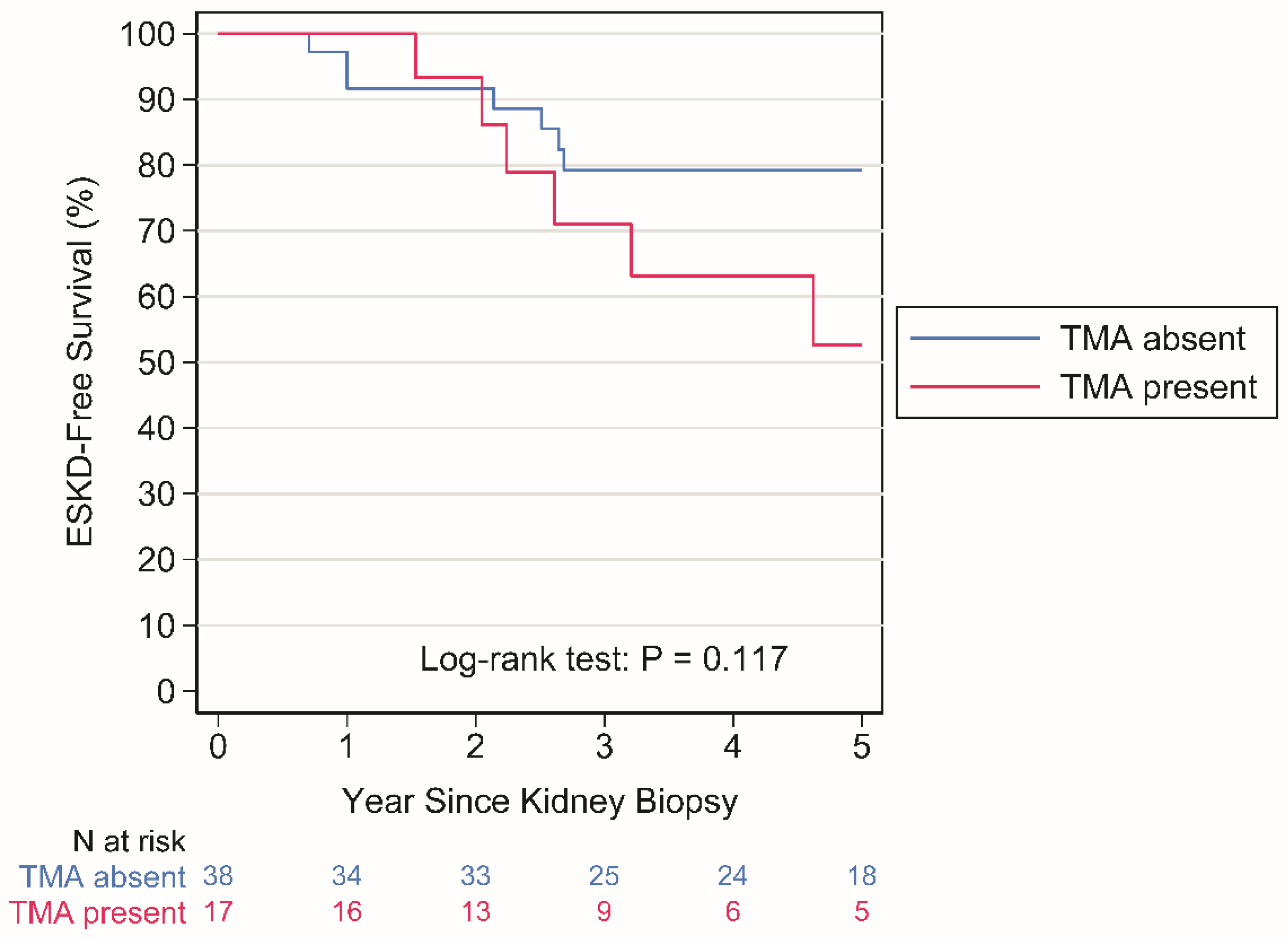
ESKD occurred in 9 patients with no TMA over a period of 224 person-years (py) (rate: 4.0 per 100 py) and in 7 patients with TMA over a period of 74 py (rate: 9.4 per 100 py), P = 0.117 by Log-rank test (
Figure 2).
Multivariable-adjusted regression analysis. Results from crude and multivariable-adjusted Cox proportional hazards regression models are reported in
Table 2. LowC3 was strongly associated with an increased hazard of ESKD (crude hazard ratio [HR]: 4.62 [95%CI: 1.71 to 12.48; P=0.003] (
Table 2).
The association was not affected after adjustment for sex, ethnicity and use of RAASi (adjusted hazard ratio [aHR]: 5.84 [95%CI; 1.69 to 20.15; P= 0.005] (
Table 2). In contrast, TMA was associated with a numerical increased hazard of ESKD which was not statistically significant [HR: 2.17 [95%CI: 0.80 to 5.87; 0.126; aHR: 2.21 [95%CI: 0.66 to 7.47; P= 0.200] (
Table 2). Most importantly, as shown in
Table 2, when LowC3 and TMA were jointly included in the Cox regression model, the hazard ratio of LowC3 was virtually unaffected whereas the point estimates of the hazard ratio of TMA became close to the null value of 1 (LowC3 HR, adjusted for TMA: 4.14 [95%CI: 1.47 to 11.68; P=0.007]; LowC3 aHR, adjusted for TMA: 5.55 [95%CI: 1.46 to 21.01; P =0.012]; TMA HR, adjusted for LowC3: 1.51 [95%CI: 0.53 to 4.30; P=0.438], TMA aHR, adjusted for LowC3: 1.15 [95%CI: 0.30 to 4.48; P= 0.836].
3.2.1. Mediation analysis
After adjustment for sex, ethnicity, and RAASi use, the estimated overall proportion of the relation between C3 and ESKD mediated by TMA was low and not statistically significant (overall proportion of the relation between LowC3 and ESKD mediated by TMA: 10% [95%CI: 0 to 62; P= 0.697]).
4. Discussion
Our study provides evidence that C3 hypocomplementemia is associated with an increased risk of ESKD with mechanisms that are largely independent from TMA. It additionally shows that the association, if any, between TMA and ESKD is entirely accounted for by C3 hypocomplementemia. This evidence might suggest that the overactivation of ACPis a key driver of disease progression in IgAN, whether it leads to TMA or not. Indeed, approximately 20% of patients in our study had low sC3 values at renal biopsy; these patients had worse renal prognosis (
Figure 1). Notably, in the LowC3 group, though sC4 was within normal limits, the latter was significantly lower and correlated strictly with sC3. Previous studies reported that about 20% of patients with IgAN had decreased sC3 levels at kidney biopsy [
14,
19], similarly to our findings; in a retrospective study [
14] patients with decreased sC3 levels had more intense deposition of C3 in the mesangium than those who had normal sC3 levels, and serum C3 levels were significantly associated with progression to kidney failure. However, the predictive value of serum C3 was lower than clinical markers such as proteinuria and eGFR. In contrast, another study of 496 patients with IgAN, of whom 22% had low levels of C3, reported that the renal survival rate of the group with decreased C3 levels was lower than that of the group with normal C3 levels. However, upon propensity score matching sC3 levels were not associated with disease progression [
19]. Others have suggested that for IgAN, C3/C4 ratio may be a better marker for disease activity and predictor of renal failure than sC3 levels alone [
13]. Our study didn’t confirm this finding (
Supplementary Materials).
In our study, on the other hand, the predictive role of ESKD of baseline C3 hypocomplementemia is prominent. As a result of not excluding patients with IgAN who had AKI or normal renal function, our study differed from that conducted by Yang X et al [
19].
Moreover, our study hints at the existence of a continuum between C3 hypocomplementemia and the appearance of TMA. The latter was significantly more frequent in the LowC3 cohort, particularly acute TMA. We hypothesize that the reduction in sC3 reflects a dysregulated activation of the ACP in IgAN, which could lead to the progression from “pure” IgAN to renal TMA, which in turn is responsible for severe hypertension [
6].
We did not observe any differences between groups in terms of MEST-C scores. This might reflect the fact that there are no appreciable differences in terms of mesangial proliferation, focal segmental glomerulosclerosis, endocapillary hypercellularity, chronicity or crescents between groups or merely that our sample size is too small to identify any actual difference in distribution of such lesions between patients with normal and LowC3.
Previous reports [
7,
8] showed that renal prognosis was strictly related to acute but also chronic microangiopathic lesions at kidney biopsy and that endothelial damage until TMA relate with tubular atrophy, proteinuria and hypertension. Based on our findings, it appears that TMA might be only an epiphenomenon of ACP overactivation and does not determine a worse renal prognosis by itself.
5. Conclusions
Our study has the major limitation of a small sample size, which warrants for larger studies. However, low sC3 at the time of biopsy emerges as a clear predictor of CKD progression, strongly supporting the crucial role of complement overactivation/dysregulation as a driver of progression towards ESKD, further highlighting the rationale of ongoing pharmacological trials of complement blockade in IgAN (20).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1: Crude and multivariable adjusted HR associated with C3 to C3/C4 ratio from Cox PH regression models. Table S2: Statistical tests that C3/C4 ratio is better than C3 alone to predict end stage kidney disease.
Author Contributions
Conceptualization, L.M.; methodology, U.M..; software, U.M:.; validation, G.M.R., M.D and I.P.; formal analysis, L.M, U.M.; investigation, F.R, I.P, E.F: resources, E.F.; data curation, F.R, I.P.; writing—original draft preparation, L.M., F.R.; writing—review and editing, G.M.R.; visualization,M.D.; supervision, L.M. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of COMITATO ETICO AREA VASTA EMILIA ROMAGNA (protocol code 945/21-08-2019)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Because of the nature of this research, participants of this study did not agree for their data to be shared publicly, sosupporting data are not available..
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol 1968, 74,694–5.
- Berthoux FC, Mohey H, Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol 2008 ,28, 4–9. [CrossRef]
- Hastings MC, Bursac Z, Julian BA, Villa Baca E, Featherston J, Woodford SY, Bailey L, Wyatt RJ. Life Expectancy for Patients From the Southeastern United States With IgA Nephropathy. Kidney Int Rep 2017,24, 99-104. [CrossRef]
- Jennette JC. The immunohistology of IgA nephropathy. Am J Kidney Dis.1988, 12,348–52. [CrossRef]
- Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol 2015, 26, 1503-12. [CrossRef]
- Manenti L, Rossi GM, Pisani I, Gentile M, Fontana F, Pilato FP, Delsante M, Ricco F, Mignani R, Mele C, Bresin E, Fiaccadori E. IgA nephropathy and atypical hemolytic uremic syndrome: a case series and a literature review. J Nephrol. 2022, 35, 1091-00. [CrossRef]
- Chua JS, Zandbergen M, Wolterbeek R, Baelde HJ, van Es LA, de Fijter JW, Bruijn JA, Bajema IM.. Complement-mediated microangiopathy in IgA nephropathy and IgA vasculitis with nephritis. Mod Pathol 2019, 32,1147-57. [CrossRef]
- El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O, Frémeaux-Bacchi V, Delahousse M, Duong Van Huyen JP, Loupy A, Bruneval P, Nochy D. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 2012, 2, :137–48. [CrossRef]
- Cai Q, Shi S, Wang S, Ren Y, Hou W, Liu L, Lv J, Haas M, Zhang H. Microangiopathic Lesions in IgA Nephropathy: A Cohort Study. Am J Kidney Dis 2019, 74, 629-39. [CrossRef]
- Neves PDMM, Souza RA, Torres FM, Reis FA, Pinheiro RB, Dias CB, Yu L, Woronik V, Furukawa LS, Cavalcante LB, de Almeida Araújo S, Wanderley DC, Malheiros DM, Jorge LB. Evidences of histologic thrombotic microangiopathy and the impact in renal outcomes of patients with IgA nephropathy. PLoS One, 2020, 15, :e0233199. [CrossRef]
- Zhang Y, Sun L, Zhou S, Xu Q, Xu Q, Liu D, Liu L, Hu R, Quan S, Xing G. Intrarenal Arterial Lesions Are Associated with Higher Blood Pressure, Reduced Renal Function and Poorer Renal Outcomes in Patients with IgA Nephropathy. Kidney Blood Press Res, 2018,4, 639-50.
- Chang A, Kowalewska J, Smith KD, Nicosia RF, Alpers CE. A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin Nephrol ,2006, 66, 397-404. [CrossRef]
- Pan M, Zhou Q, Zheng S, You X, Li D, Zhang J, Chen C, Xu F, Li Z, Zhou Z, Zhang J. Serum C3/C4 ratio is a novel predictor of renal prognosis in patients with IgA nephropathy: a retrospective study. Immunol Res, 2018, 66, 381-91. [CrossRef]
- Kim SJ, Koo HM, Lim BJ, Oh HJ, Yoo DE, Shin DH, Lee MJ, Doh FM, Park JT, Yoo TH, Kang SW, Choi KH, Jeong HJ, Han SH. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. 2012 7, e40495. [CrossRef]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009, 150, 604-12. [CrossRef]
- Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int, 2017, 91, 1014-21. [CrossRef]
- Discacciati A, Bellavia A, Lee JJ, Mazumdar M, Valeri L. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int J Epidemiol, 2018 16. Epub ahead of print. [CrossRef]
- VanderWeele T.J. A unification of mediation and interaction: a 4-way decomposition. Epidemiology, 2014 , 749-61. [CrossRef]
- Yang X, Wei RB, Wang Y, Su TY, Li QP, Yang T, Huang MJ., Li KY., Chen XM. Decreased Serum C3 Levels in Immunoglobulin A (IgA) Nephropathy with Chronic Kidney Disease: A Propensity Score Matching Study. Med Sci Monit, 2017,23, 673–681. [CrossRef]
- Caravaca-Fontán F, Gutiérrez E, Sevillano ÁM, Praga M. Targeting complement in IgA nephropathy. Clin Kidney J, 2023, 16(Suppl 2),ii28-ii39. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).