1. Introduction
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is a positive-stranded ssRNA(+) virus that can cause coronavirus disease 2019 (COVID-19) including severe respiratory syndrome in humans. The outbreak of COVID-19 led to a severe pandemic that claimed more than seven million lives worldwide and 772 million confirmed cases according to the latest epidemiological update from the World Health Organization (WHO) [
1]. In Chile, a total of 5,330,856 confirmed cases of COVID-19 and 62,249 deaths including confirmed, suspected, and probable cases have been reported [
2].
In general, this disease affects all age ranges regardless of gender or condition [
3]. Adults experience respiratory symptoms of varying severity; older adults and those with comorbidities such as chronic respiratory diseases, chronic diseases, cardiovascular diseases, hypertension, diabetes, obesity or cancer develop a severe form of the disease [
4]. Those individuals are at substantially increased risk of developing COVID-19-associated acute respiratory distress syndrome (ARDS) with high letality(5). However, the age range of individuals most affected by the disease can differ from one country to another [
6,
7].
The clinical manifestations of SARS-CoV-2 infection in children and adolescents are different from those in adults. They rarely present severe respiratory symptoms and often remain asymptomatic [
8]. However, they can develop the life-threatening multisystem inflammatory syndrome MIS-C, which shows similarities to Kawasaki disease in certain inflammatory features and cardiovascular involvement, while generally lacking severe respiratory symptoms [
9,
10,
11,
12]. Pediatric individuals with previous SARS-CoV-2 infection exhibit in general higher titers of antibodies against the viral spike glycoprotein than adults [
13,
14]. Additionally, children under 5 years of age are found to have the highest prevalence of infection with the Beta coronavirus HCoV-OC43, which is the common cold coronavirus that is most closely related to SARS-CoV-2, which may in part explain the high number of asymptomatic cases [
15,
16,
17].
The spike glycoprotein is the dominant exposed antigen on enveloped viruses, and hence includes hot spots for mutations occurring in new variants, like Omicron lineages [
18]. The spike protein includes the S1 subunit spanning the receptor binding domain (RBD) and the N- terminal domain (NTD), and more conserved S2 membrane fusion subunit [
19,
20]. RBD interacts with the human ACE2 receptor (hACE2), which is responsible for entry into target cells in the lung [
21]. NTD is important for interaction with L-SIGN and DC-SIGN, and both domains are important targets for viral neutralization [
22]. Spike can trigger a series of adaptive immune responses mediated by three major cell types: B cells (humoral immunity) and CD4+ and CD8+ T cells (cell-mediated immunity) [
23,
24]. While the RBD and NTD domains are exposed on the tip of S1 and are the target of most neutralizing antibodies [
25], the less exposed S2 spike subunit is more conserved and includes cross-reactive epitopes with seasonal Beta coronaviruses [
15].
Wide vaccination coverage is a way to alleviate health systems, essential aspects to avoid collapsing medical care, and gain control over a pandemic. For COVID-19, cases continue to increase despite global vaccination campaigns [
26,
27]. Those include numerous safe and effective vaccines which have been developed to effectively reduce the risk of infection, severe disease, and death. Most vaccine strategies employ the spike protein as target including BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), among others [
28,
29]. On the other hand, vaccines based on whole-inactivated virus such as CoronaVac (Sinovac) also include the spike protein in addition to other viral structural proteins such as the nucleoprotein [
30]. Several studies have shown that antibody responses from the first massive vaccination against COVID-19 in early 2021 have decreased over the following six months after vaccination, likely contributing to an increase in breakthrough infections [
31,
32,
33].
The emergence of numerous SARS-CoV-2 variants is one of the most important developments in the COVID-19 pandemic [
34]. Yet, diverse variants of concern, interest and under monitoring have different levels of increased transmissibility and resistance to existing immunity and have emerged sequentially [
35]. Furthermore, these variants have been widespread and have evolved over time since the start of the pandemic [
36,
37,
38].
To our knowledge, the virological and immunological characteristics associated with the viral main lineages is key to inform health policies, including booster and vaccination schedules, and also inform the development of potential specific variants or pan-coronavirus vaccines. Important issues include whether the different variants escape vaccination-induced immune responses. In adults, mRNA-based vaccines recognize early variants of SARS-CoV-2, while significant overall decreases in humoral and cellular responses to Omicron lineages were recorded [
39]. In addition, booster doses in adults induce neutralizing immunity against Omicron BA.1 infection [
40]. A low effectiveness of the BNT162b2 and CoronaVac vaccines against several Omicron variants of SAR-CoV-2 has been described, reporting low levels of neutralizing antibodies in those vaccinated with BNT162b2 and lost of antibody reactivity in those vaccinated with CoronaVac [
41,
42].
Yet, scarce information on the immune responses in the pediatric population vaccinated with vaccination schemes based on CoronaVac and heterologous BTN162b2 booster doses is available [
43,
44]. Furthermore, their immune status against Omicron lineages has not been explored. Therefore, studying and analyzing the humoral and cellular immune response in vaccinated children and adolescents is important to understand the heterologous vaccination-stimulated immune response against SARS-CoV-2 Omicron variants. In this study we analyzed the humoral and cellular immune response against SARS-CoV-2 Omicron BA.1 variant in adolescents between 10 and 16 years of age vaccinated with at least two doses of CoronaVac, triple BNT162b2 or heterologous application of these vaccines.
2. Materials and Methods
2.1. Study design and population
We recruited teenager (10 – 16 years old) donors immunized with two doses of CoronaVac [CoronaVac (2x)], two doses of CoronaVac plus one dose of BNT162b2 [CoronaVac (2x) + BNT162b2 (1x)], two doses of CoronaVac plus two of BNT162b2 [CoronaVac (2x) + BNT162b2 (2x)], or three doses of BNT162b2 [(BNT162b2 (3x)]. The sample collection was performed in schools from the Metropolitan Region of Chile during the second semester of the year 2022 when the SARS-CoV-2 Omicron variants BA.1, BA.2, BA.4, BA.5 and BQ.1 were circulating predominantly [
46].
The inclusion and exclusion criteria were applied to all groups of vaccinated teenagers aged 10 to 16, who must have the complete vaccination schedule against SARS-CoV-2 with at least 30 days since their last immunization, with no history of previous SARS-CoV-2 infection. Also, the participants had no symptoms associated with COVID-19. It is important to note that these vaccination schemes were developed against the original strain of SARS-CoV-2 (Wuhan-Hu-1) when administered in Chile. The main inclusion criteria for donors were individuals between 10 and 16 years old, and no history of SARS-CoV-2 infection at least 8 months from the date of sampling. The main exclusion criteria included individuals under 10 or over 17 years old, pregnant women, individuals with morbid obesity, immunosuppression, or symptoms associated with COVID-19. Individuals who had a history of reinfection by COVID-19 were also excluded.
The vaccinated group included individuals who had completed their immunization schedule at least 3 months before sample collection and no more than 18 months before the collection. A total of 99 donors were included in the study. After applying a documentary filter, 88 donors were analyzed in this study; 18 received [CoronaVac (2x)], 35 received [CoronaVac (2x) + BNT162b2 (1x)], 21 received [CoronaVac (2x) + BNT162b2 (2x)], and 14 received triple BTN162b2 vaccination [BNT162b2 (3x)].
Samples were collected with authorization from the local ethics committee, and all participants and their respective parents or guardians provided informed consent by declaring and signing it.
2.2. Serum and PBMCs preparation.
Venous blood was used to obtain serum via a vacuum blood collection tube with clot activation and separating gel (BD Vacutainer). The tube was stored at room temperature for at least 30 minutes to allow the blood to clot and then centrifuged at 2,000 rpm for 10 min at 4°C; 2 mL of supernatant serum was recovered and liquated into two cryotubes at 1 mL each and stored at -20°C until further analysis. Then, another two Vacutainer tubes (Heparin/Lithium) were used for the isolation of peripheral blood mononuclear cells (PBMC) by Ficoll gradient.
2.3. Antibody detection
Immune response analysis was performed as described by Díaz-Dinamarca et al(47). Immunoglobulin G against SARS-CoV-2 nucleoprotein N (Snibe Diagnostic, cat. 130219015M), receptor binding domain from the spike glycoprotein S1 (S1, Wuhan-Hu-1) (Snibe Diagnostic, cat. 130219017M) and hACE2-RBD inhibition assay method (Snibe Diagnostic, cat. 130219027M), all based on the ancestral Wuhan-Hu-1 SARS-CoV-2 sequences, were detected using a chemiluminescent immunoassay (CLIA) using the SNIBE commercial kits. Briefly, 300 µL of serum was aliquoted into cryotubes and then analyzed using the Maglumi X8 CLIA detector according to the manufacturer’s instructions.
Immunoglobulin A was detected using the enzyme-linked immunosorbent assay (ELISA) commercial kit COVID-19 human IgA Spike-RBD and nucleoprotein of SARS-CoV-2 from Raybiotech (Cat: IEQ-CoVSN-IgA-1), following the manufacturer’s instructions. The samples were measured using a Biotek EPOCH 2 plate reader.
2.4. Generation of rVSV-SARS2-S-BA.1
Recombinant vesicular stomatitis virus carrying the spike protein of SARS-CoV-2 (rVSV-SARS2-S) has been shown to correlate well in viral neutralization with convalescent serum compared to the authentic SARS-CoV-2 [
48,
49,
50]. This systems allows for rapid quantification, it enters cells through pathways of SARS-CoV-2, and does not require high biosafety containment [
48]. Given that this virus contains the spike of the ancestral Wuhan-Hu-1 SARS-CoV-2 strain, we prepared rVSV-SARS2-S containing the spike of the Omicron BA.1 variant. Therefore, the SARS-CoV-2 genome sequences corresponding to the sequencing of samples from individuals infected with the Omicron BA.1 variant in different countries were obtained (GISAID accession ID: EPI_ISL_7373598; EPI_ISL_7373061; EPI_ISL_7371749; EPI_ISL_7370181; EPI_ISL_7368223; EPI_ISL_7358093; EPI_ISL_7358079; EPI_ISL_7358076; EPI_ISL_7358069; EPI_ISL_7356256; EPI_ISL_7355546; EPI_ISL_7354594; EPI_ISL_7352907; EPI_ISL_7350365; EPI_ISL_7350042; EPI_ISL_7349764; EPI_ISL_7348417; EPI_ISL_7346862; EPI_ISL_7345322; EPI_ISL_7337515), performing a multiple alignment of the open reading frames corresponding to spike glycoprotein in the Clustal W program and subsequently the generation of the consensus sequence with the EMBOSS Cons tool [
51] which was compared with the sequence previously described for omicron BA.1 [
52] to confirm the characteristic mutations of the variant. The consensus sequence was next synthesized (GenScript) and cloned into the VSV-SARS-CoV-2 spike antigenome plasmid [
48] (kindly provided by Dr. Kartik Chandran, Albert Einstein College of Medicine, NY, USA), replacing the sequence of the Wuhan-Hu-1 spike. This plasmid also encodes an eGFP reporter gene as an independent transcriptional unit. Recombinant virus rescue was carried out as previously described [
48]. Briefly, HEK293FT cells were transfected using lipofectamine 2000 (ThermoFisher) with the VSV antigenome plasmid encoding SARS-CoV-2 Omicron spike BA.1 together with auxiliar plasmids expressing T7 polymerase and VSV N, P, M, G and L proteins. Supernatants from transfected cells were transferred to Vero E6 hACE2 cells (previously generated [
49]) every day until the appearance of eGFP-positive cells examined under an inverted microscope (IX71; Olympus) and pictures taken (ProgRes C5; Jenoptik) for subsequent analyses (
Figure S1). RNA from the virus in infection passage 9 was sequenced (Macrogen) to corroborate that the coding fragment corresponded to the spike of Omicron BA.1, including the additional non-silent mutations K145E y F372S, due to serial passaging. All experiments for the generation of rVSV-SARS2-S-BA.1 and use of rVSV-SARS2-S viruses in the microneutralization assays were carried out at biosafety level 2.
2.5. rVSV-SARS2-S microneutralization assays
Microneutralization assays were performed with rVSV-SARS2-S as described previously[
49]. Briefly, the serum samples were treated at 56˚C for 30 min prior to use. Serial dilutions were then made for each sample in MEM supplemented with 2% of fetal bovine serum. Identical volumes of rVSV-SARS2-S-Wuhan-Hu-1 [
48], (kindly provided by Dr. Kartik Chandran, Albert Einstein College of Medicine, NY, USA) or rVSV-SARS2-S-BA.1 viruses were added to the previous dilutions to each well with an MOI of 0.25 and incubated for 1 h at 37˚C. The serum-virus inoculum was then added to Vero E6 hACE2 cells seeded the previous day in 96-well optical bottom plates (Thermo Scientific) at 80% confluency. To stop the infection, the cells were fixed with 4% formaldehyde (Pierce) and stained for 5 min with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Total fluorescence for GFP was measured on a Synergy plate reader (BioTek) (DAPI excitation at 360 nm and emission at 460 nm; GFP excitation at 485 nm and emission at 526 nm) and normalized against DAPI fluorescence. The half-maximal inhibitory concentration (IC50) of the sera was calculated using non-linear regression analysis based on data obtained from technical replicates.
2.6. Detection of T lymphocytes activated against SARS-CoV-2 by flow cytometry.
Activation-induced marker (AIM) detection was performed according to the method described by Grifoni et al., 2020 [
53]. Briefly, PBMCs were thawed in RPMI-1640 medium supplemented with 5% human serum, penicillin/streptomycin, L-glutamine. Subsequently, 1×106 PBMCs were stimulated with SARS-CoV-2 variant peptide combinations (Wuhan-Hu-1 and Omicron BA.1; 1 μg/ml) in a 96-well U-bottom plate at 37 °C for 24 hours. The peptides were overlapping 15-mers by 10 aminoacid spanning the spike protein corresponding to ancestral or BA.1 Omicron sublineage as previously described [
39]. Cells were further stimulated with an equimolar concentration of DMSO (negative control). After stimulation, cells were analyzed by flow cytometry as described below. Then, cells were stained with the following antibodies: anti-CD69-PerCP, anti-CD4-V510, anti-CD8-allophycocyanin (APC)-H7, anti-CD134-phycoerythrin (PE)-Cy7, anti-CD137-BV421, anti–CCR7– fluorescein isothiocyanate, anti–CD45RA-PE. FVS660 was included in cell staining (1:1000, APC; BD) and samples was processed on FACSVerse (BD Biosciences). SARS-CoV-2-specific T cells were detected by co-expression of AIM on CD4+ (OX40 and CD137) or CD8+ (CD69 and CD137) T cells. The DMSO stimulated sample was used to establish the cutoff for the activation markers. 30,000 events were acquired in the pool of live cells per sample.
2.7. Statistical analysis
In this study, the geometric mean and 95% confidence interval (CI) were estimated for each group of immunized individuals. To compare whether there was a statistically significant difference between antibodies titers, neutralizing antibodies, and AIM T cells from different vaccine combinations, an ANOVA test was used after logarithmic transformation of antibody data and Kruskal-Wallis test for AIM T cells. To assess the differences in responses between Omicron-BA.1 and Wuhan-Hu-1 within each scheme, a Wilcoxon test was applied.
If a statistically significant result was obtained from ANOVA test, post hoc pairwise comparisons were performed using Tukey’s Honest Significant Difference (HSD) method, which uses the Studentized range distribution to estimate confidence intervals of factor level mean differences. For Kruskal-Wallis test Mann-Whitney was applied.
The fold-change (FC) was calculated as the Omicron.BA-1/Wuhan-Hu-1 ratio of T cells in individuals vaccinated with each scheme, and comparisons of the geometric mean values (GMV) of these fold-changes were conducted within and between different vaccination schemes. For this purpose, after logarithmic transformation of the FC, T-test (by T-test compared with hypothetical mean of 1) and ANOVA with Tukey’s post-hoc were applied, respectively.
Statistical tests were evaluated with a 5% significance level. The statistical analyses and charts performed were obtained using R (v.4.2.3) and RStudio (v.2022.12.0.353).
3. Results
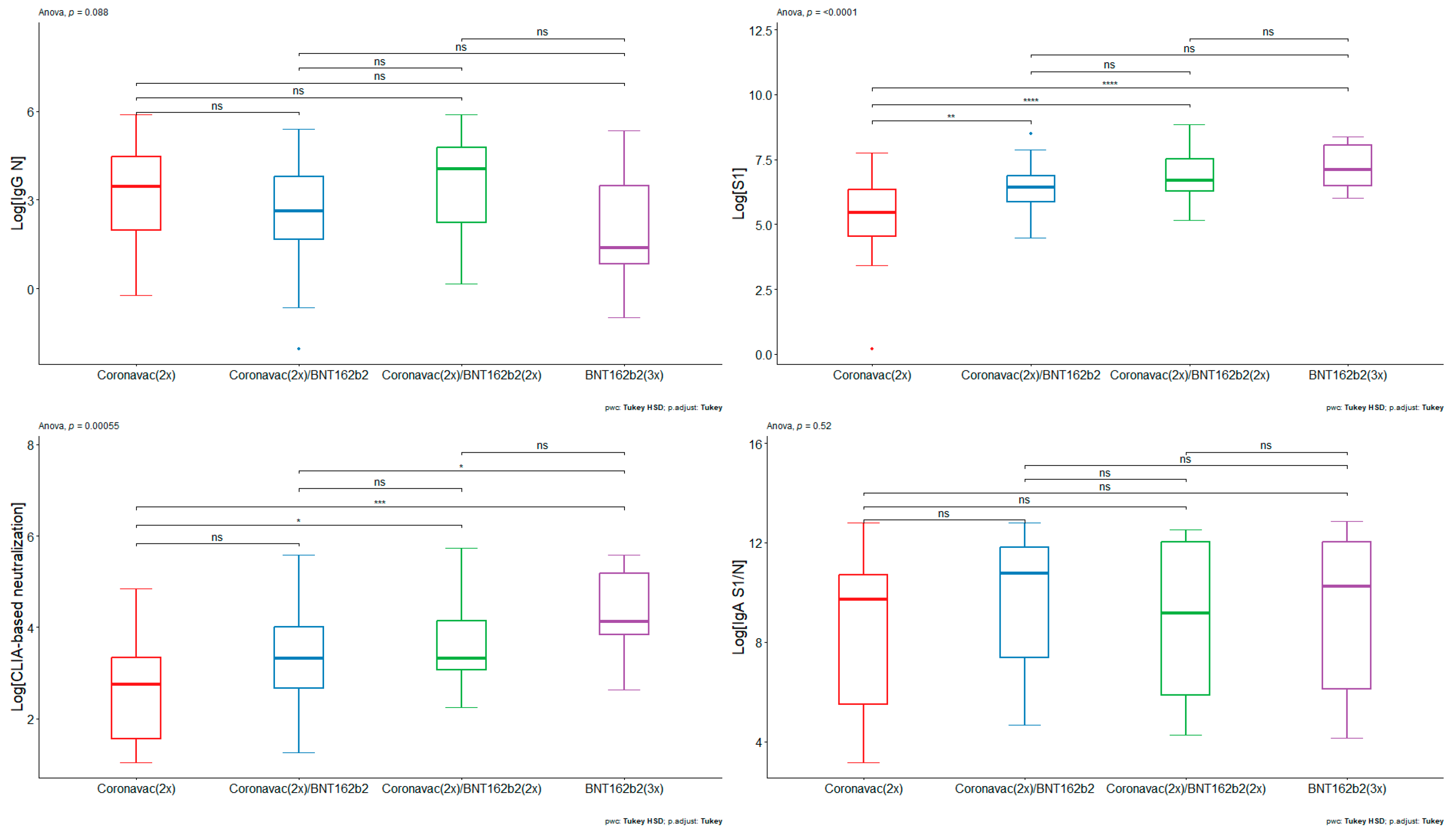
We decided to analyze humoral immune response against different viral antigens. Therefore we first quantified antibodies against SARS-CoV-2 nucleoprotein (N) (
Figure 1A). The volunteers who had received different vaccination schemes had similar ranges in the titers of antibodies against the N protein from SARS-CoV-2. CoronaVac (2x) had a GMV of 24.58 A.U. (95% CI: 9.92 – 60.95), whereas [CoronaVac(2x) + BNT152b2 (1x)] and [CoronaVac(2x) + BNT152b2 (2x)] had GMV of 13.14 A.U. (95% CI: 7.09 – 24.34) and 33.50 A.U. (95% CI:13.22 – 84.88), respectively. For the [BNT152b2 (3x)] scheme, the GMV was 6.13 A.U. (95% CI: 1.31 – 28.68). The results suggest that the pediatric population analyzed have antibodies against the N protein of SARS-CoV-2. Given that the nucleoprotein of coronaviruses is highly conserved [
54], the presence of anti-N reactivity in all vaccinee groups may imply either cross-reactivity derived from previous infections with different coronaviruses, or may be derived from previous asymptomatic infections with SARS-CoV-2.
Next, we analyzed antibodies against S1 (Wuhan-Hu-1). Contrary to the results of antibodies against nucleoprotein, there was a lower titer of antibodies against the S1 protein in pediatrics immunized with [CoronaVac(2x)] compared with all formulations and boosters based on BNT162b2 (
Figure 1B). The results indicate that the GMV of IgG against S1 concentration for individuals vaccinated with [CoronaVac (2x)] serum was 191.47 A.U. (95% CI: 82.92 – 442.14 A.U.), whereas groups vaccinated with [CoronaVac (2x) + BNT162b2 (1x)] and [CoronaVac (2x) + BNT162b2 (2x)] had a GMV of 612.20 A.U. (95% CI: 453.62– 826.22 A.U.) and 1,000.51 A.U. (95% CI: 650.21– 1,539.53 A.U.), respectively. The group of individuals who had received [BNT162b2 (3x)] had a GMV of 1,299.91 A.U. (95% CI: 783.37 – 2,157.04 A.U.). The ANOVA test yielded a statistically significant result (p-value < 0.0001), where pairwise comparisons showed statistically significant differences between [CoronaVac (2x)] and [CoronaVac (2x) + BNT162b2 (1x)] (p-value < 0.01), [CoronaVac (2x)] and [CoronaVac (2x) + BNT162b2 (2x)] (p-value < 0.0001), and BNT162b2 (3x) (p-value < 0.0001) (
Figure 1B). There were no statistically significant differences between schemes comprising BNT162b2. The data suggest that BNT162b2-based formulations promote an increase in antibodies against S1 in pediatrics.
Next, a CLIA-based hACE2 protein competition assay was performed as correlation estimate of neutralizing antibodies against SARS-CoV-2(47,55). The [BNT162b2 (3x)] vaccination scheme generated a higher level of CLIA-based neutralization compared to the [CoronaVac (2x)] and [CoronaVac (2x) + BNT162b2 (1x)] scheme (
Figure 1C). The results indicate that the GMV of CLIA-based neutralization antibodies for [CoronaVac (2x)] serum was 13.73 A.U. (95% CI: 7.62 – 24.73 A.U.), whereas the [CoronaVac (2x) + BNT162b2 (1x)] and [CoronaVac (2x) + BNT162b2 (2x)] had a GMV of 26.19 A.U. (95% CI: 18.10 – 37.89 A.U.) and 39.46 A.U. (95% CI: 25.57 – 60.91 A.U.), respectively. [BNT162b2 (3x)] had a GMV of 66.69 A.U. (95% CI: 36.84 – 120.74 A.U.) The ANOVA test yielded a statistically significant result (p-value < 0.001), where pairwise comparisons showed statistically significant differences between [CoronaVac (2x)] and [CoronaVac (2x) + BNT162b2 (2x)] (p-value < 0.05), [CoronaVac (2x)] and [BNT162b2 (3x)] (p-value < 0.001), and [CoronaVac (2x) + BNT162b2 (1x)] and [BNT162b2 (3x)] (p-value < 0.05) (
Figure 1C). No differences were found between [CoronaVac (2x)] and [CoronaVac (2x) + BNT162b2 (1)]; [CoronaVac (2x) + BNT162b2 (2x)] and [BNT162b2 (3x)]; and [CoronaVac (2x) + BNT162b2 (1x)] and [CoronaVac (2x) + BNT162b2 (2x)]. The hACE2 protein competition data suggest that BNT162b2-based formulations promote an increase in CLIA-based neutralizing antibodies in pediatric individuals. Furthermore, the data indicate that the double booster with BNT162b2 leads to a rise in CLIA-based neutralizing antibodies against SARS-CoV-2, particularly beneficial for adolescents previously immunized with CoronaVac.
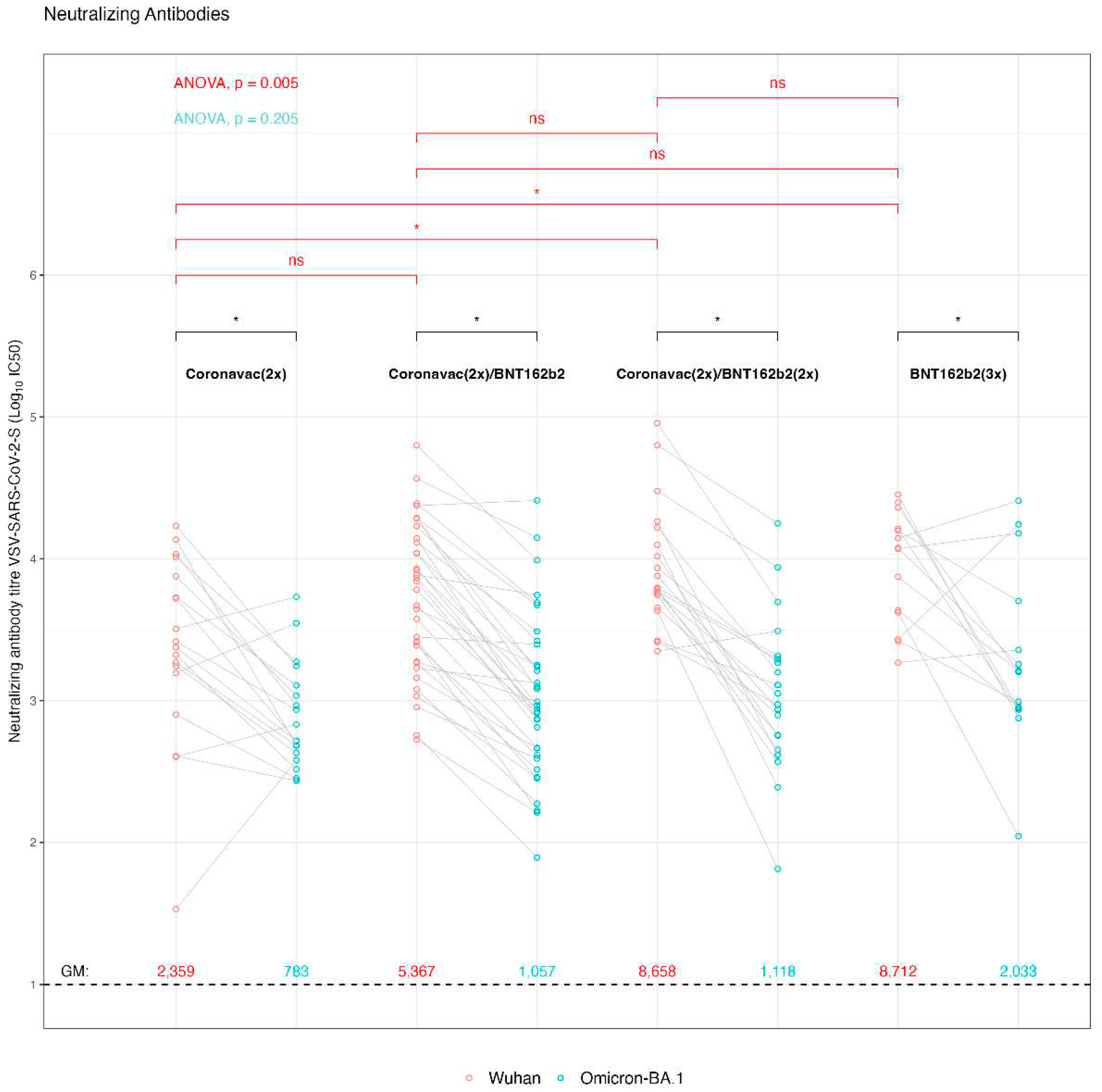
Given the relevance of SARS-CoV-2 variants that were circulating during the sample collection, where Omicron lineages BA.1, BA.2, BA.4, BA.5 y BQ.1 were prevailing in Chile, we decided to analyze the pediatric samples in terms of the presence of neutralizing antibodies against the ancestral SARS-CoV-2 Wuhan-Hu-1 strain on which the vaccine formulations were based, and also against the Omicron BA.1 variant. For this, we used a viral system based on rVSV decorated with the SARS-CoV-2 spike from the Wuhan-Hu-1 or Omicron BA.1 strains (
Figure 2). This viral system has been shown to correlate well with the authentic SARS-CoV-2 in terms of viral cell entry and sera neutralization; at the same time it does not require biocontainment measures and is easy to quantitate [
48,
49,
50]. The resulting virus neutralization data indicate that the GMV for the titrated IC50 were for [CoronaVac (2x)] 2,358 IC50 (95% IC: 1,100-5,054 IC50) for the Wuhan-Hu-1 rVSV-SARS2-S virus and 782.52 IC50 (95% IC: 511-1196 IC50) for the rVSV-SARS2-S Omicron BA.1 virus, corresponding to a 3.01-fold decrease Wuhan-Hu-1/Omicron BA.1 in reactivity. In a similar way, the GMV [IC50] Wuhan-Hu-1/Omicron BA.1 was 5.07-fold decreased for the [CoronaVac (2x) + BNT162b2 (1x)] scheme, showing GMV [IC50] f 5,367 IC50 (95% IC: 3,493-8,245 IC50) and 1,057 IC50 (95% IC: 665-1,680 IC50), for the Wuhan-Hu-1 and Omicron BA.1 rVSV-SARS2-S virus, respectively. The sera from pediatrics vaccinated with [CoronaVac (2x) + BNT162b2 (2x)] revealed a GMV [IC50] of neutralizing antibodies of 8,658 IC50 (95% IC: 5,442-13,774 IC50) and 1,118 IC50 (95% IC: 616-2,029 IC50), against the rVSV-SARS-2-S Wuhan-Hu-1 and Omicron BA.1 virus, respectively. Hence, while showing a high neutralizing antibody titer against the Wuhan-Hu-1 strain, the 7.74-fold decrease against Omicron BA-1 strain generates GMVs that are similar to those observed with the vaccination group who had received only one booster dose of BNT162b2 [CoronaVac (2x) + BNT162b2 (1x)]. Finally, the group who had received [BNT162b2 (3x)] showed a 4.28-fold decrease of the GMV [IC50] neutralizing antibodies against Wuhan-Hu-1/Omicron BA.1 virus with a GMV of 8,712 IC50 (95% IC: 5,140-14,765 IC50) and 2,032 IC50 (95% IC: 869-4750 IC50), respectively. Overall, all vaccination schedules had a decrease in neutralizing antibodies between Wuhan-Hu-1 and Omicron BA.1 rVSV-SARS-S virus (p-value < 0.01). It is important to notice that despite this neutralizing antibody titer decrease of each vaccination group against the Omicron BA-1 strainWuhan-Hu-1, the sera from some individuals of the different vaccination groups showed an increase. This may be indicative of an asymptomatic previous infection with one of the Omicron lineages. This is also in line with the observation that the IgG titers against nucleoprotein and IgA titers against S1/nucleoprotein did also not show significant difference between the various immunization schemes.
An asymptomatic previous infection may also explain, why no statistically significant difference was found between the different groups against the Omicron BA-1 strain, except for the group [CoronaVac (2x)] compared to the triple BNT162b2 [BNT162b2 (3x)] scheme (p-value < 0.05). When comparing the response against the Wuhan-Hu-1 strain, the [BNT162b2 (3x)], and [CoronaVac (2x) + BNT162b2 (2x)], generated a significant increase in neutralizing antibodies with respect to the [CoronaVac (2x)] group (p-value = 0.017), while the comparison of the neutralizing antibodies between [CoronaVac (2x)], and [CoronaVac (2x) + BNT162b2 (1x)], showed no significant differences. Together, these data suggest that the BNT162b2 double booster [CoronaVac (2x) + BNT162b2 (2x)], and the triple BNT162b2 vaccine [BNT162b2 (3x)], generated an increase in the protective humoral immune response.
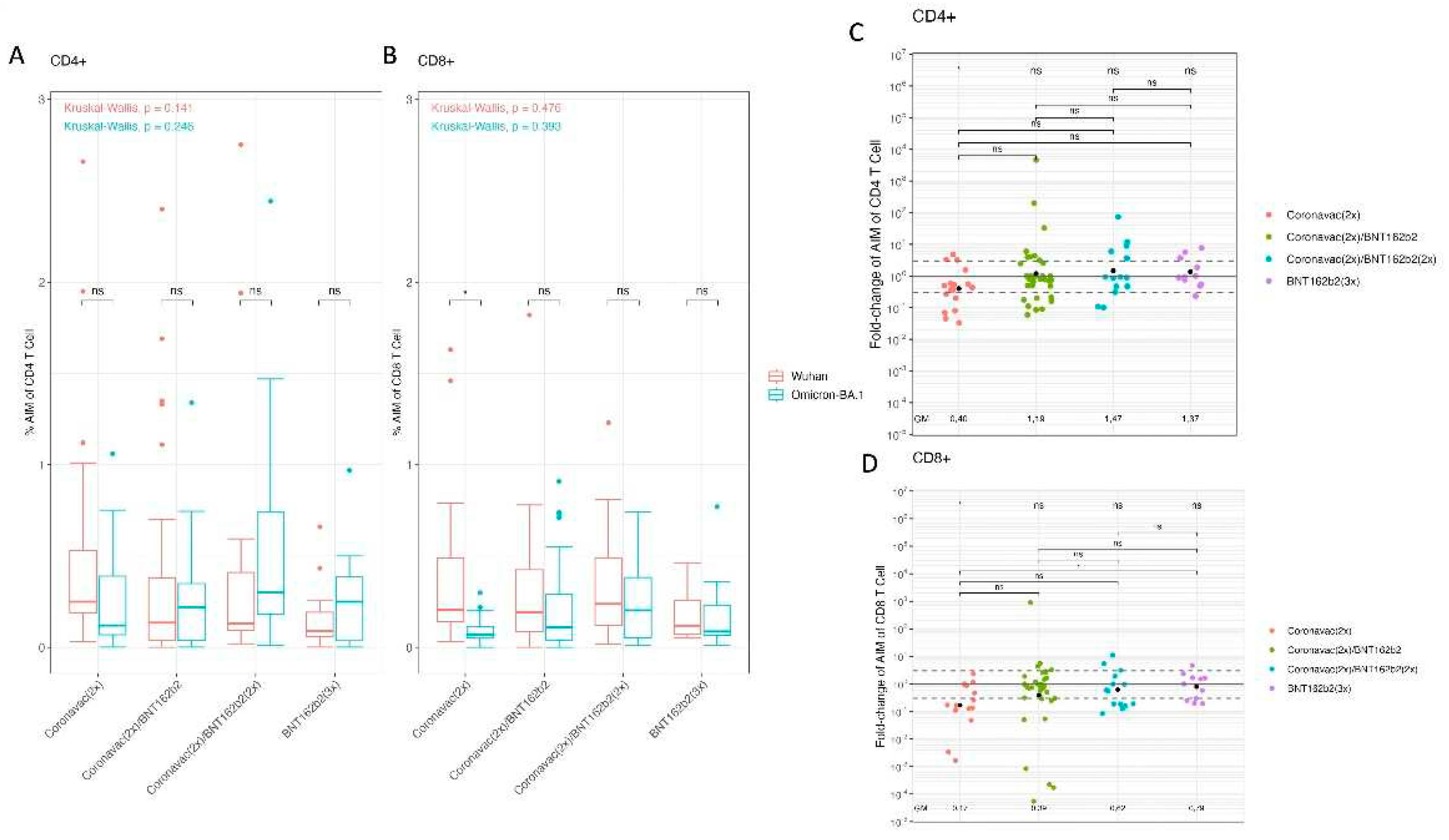
The cellular immune response has previously been described to be of great importance against SARS-CoV-2 infection [
56]. Despite the large number of spike protein mutations in the Omicron lineages, T cell recognition is extensively cross-reactive against different SARS-CoV-2 variants [
57]. In this context, an AIM T cell assay was carried out with the sera samples from pediatrics immunized with the different vaccination schemes. The T cell responses were analyzed by the activation of CD4+ T lymphocytes through Wuhan-Hu-1 or Omicron BA.1 strain peptide combinations (
Figure 3A). The processed data showed that each vaccination scheme presented similar CD4+ T cell activation levels by Wuhan-Hu-1 and Omicron BA.1 spike peptides. In addition, when comparing vaccination schemes, there were no significant differences in activation for Wuhan-Hu-1 and Omicron BA.1. Then, the T cell response was analyzed in the activation of CD8+ T lymphocytes by Wuhan-Hu-1 or Omicron BA.1 variant peptide combinations for the different vaccination schemes (
Figure 3B). Within the aforementioned tests, the [CoronaVac (2x)] vaccination scheme with spike peptides from Wuhan-Hu-1 versus Omicron BA.1 was the only one to show significant differences. In this case, Omicron BA.1 spike peptides generated less activation compared to Wuhan-Hu-1 peptides in the scheme based on [CoronaVac(2x)] (p-value = 0.004).
A more exhaustive analysis on the activation of T lymphocytes was performed by calculating a GMV fold-change for the activation of CD4+ and CD8+ T cells by Wuhan-Hu-1 and Omicron BA.1 peptides (
Figure 3C,D). The resulting data indicate that the GMV of fold-change of CD4+ T cells AIM ratio (Omicron BA.1/Wuhan-Hu-1) for [CoronaVac (2x)] was as low as 0.401 (95% CI: 0.189-0.854), whereas the [CoronaVac (2x) + BNT162b2 (1x)], [CoronaVac (2x) + BNT162b2 (2x)] and [BNT162b2 (3x)] had a GMV fold-change of the AIM ratio of (Omicron BA.1/Wuhan-Hu-1) 1.186 (95% CI: 0.536-2.625), 1.466 (95% CI: 0.534-4.027), and 1.371 (95% CI: 0.688-2.732), respectively. T-test achieved statistically significant results showing a decrease in GMV Omicron BA.1/Wuhan-Hu-1 in [CoronaVac (2x)] (p-value < 0.01). The comparisons between schemes with ANOVA test did not lead to significant differences (
Figure 3C). Furthermore, the GMV of fold-change of AIM CD8+ T cells of Omicron BA.1/Wuhan-Hu-1 for CoronaVac (2x) was 0.168 (95% CI: 0.058- 0.486), whereas the [CoronaVac (2x) + BNT162b2 (1x)] and [CoronaVac (2x) + BNT162b2 (2x)] had a GMV of 0.388 (95% CI: 0.130-1.157) and 0.618 (95% CI: 0.256-1.487), respectively. In the [BNT162b2 (3x)] had a GMV of 0.791 (95% CI: 0.406-1.542). T-test provided statistically significant results between GMV Fold-change of [CoronaVac (2x)] (p-value < 0.001). Between schemes an ANOVA test was applied showing significant differences (p-value = 0.0292) performing a Tukey post-hoc lead to meaningful results between [CoronaVac (2x)] and [BNT162b2 (3x)] (p-value = 0.042) (
Figure 3D). Our results suggest that the booster with BNT162b2 in the pediatric population vaccinated with [CoronaVac (2x)] improves the cellular immune response of CD4+ and CD8+ T lymphocytes against the Omicron spike.
4. Discussion
Children and adolescents can be infected with SARS-CoV-2, but most pediatric cases with laboratory-confirmed SARS-CoV-2 infection are mild. Unlike adults who can become infected with asymptomatic, mild, moderate or severe illness that can even lead to death, severe illness from COVID-19 in pediatric patients is rare [
8,
58]. Yet, several COVID-19 variants have been shown to evade immune responses from previously infected and vaccinated individuals [
59]. The analysis of the immune response in children and adolescents is of special interest since it has been described that vaccination against COVID-19 in children aged 5–11 years showed lower effectiveness in preventing SARS-CoV-2 infection and severe COVID-19 than in individuals aged 12 years and older [
60]. Moreover, the vaccinated pediatric age group was more susceptible to reinfections due to Omicron variants than previous variants [
61]. However, three doses of BNT162b2 or three doses of CoronaVac were effective in preventing COVID-19, hospitalizations, and severe outcomes among the pediatric population during Omicron-dominant pandemic, which was further enhanced after a booster dose [
62,
63]. In this study, we characterize the humoral and cellular immune response against Omicron BA.1 in adolescents attending schools in Santiago, Chile, suggesting that booster doses are necessary to improve the immune response against SARS-CoV-2 variants.
CoronaVac is an inactivated whole-virus vaccine that has been associated with weaker neutralization titers yet comparable or higher T cell responses than mRNA and other vaccine platforms [
64]. Clinical phase 1/2 studies with CoronaVac have shown that two-immunization schedule (3 μg dose) induced humoral immune response and neutralizing antibodies in children and adolescents aged 3–17 years [
65]. T cell responses are elicited by the inactivated whole-virus vaccines, which are directed against all structural proteins of SARS-CoV-2 and not the spike protein alone. It has been further described that T cell responses against SARS-CoV-2 S, N, and M proteins are conserved for Omicron BA.1 [
66].
The BNT162b2 mRNA vaccine based on the ancestral Wuhan-Hu-1 spike has been shown to target SARS-CoV-2 variants in children [
67], and reduces the risks of Omicron infection and COVID-19–related hospitalization among children 5-11 years of age [
68]. Our study describes that in adolescents with 10-17 years of age the immunization with [BNT162b2 (3x)] or double BNT162b2 booster vaccination after two doses of CoronaVac [CoronaVac (2x) + BNT162b2 (2x)] promote humoral immunity and cellular immune response against Omicron BA.1.
Neutralizing antibody responses are crucial to reduce COVID-19 disease severity [
59]. However, significant decreases have been observed for memory B cells and neutralizing antibodies over time and in dependence of the SARS-2 variants. In our study, we observed higher IC50 titers in the pediatric cohort compared to adults, which significantly decreased with the Omicron BA.1 strain compared with the ancestral virus. Interestingly, studies have shown that prior infection with SARS-CoV-2 can boost and broaden immunity related to neutralizing antibodies and T cell responses [
39]. Regarding this point, the pediatric population characterized here had anti-nucleoprotein antibodies, and some individuals showed increased neutralizing antibody titers against Omicron BA.1 compared to the ancestral Wuhan-Hu-1 strain, coinciding with the period of sample collection and suggesting a previous SARS-CoV-2 infection independent from vaccination. Such a possible former infection would be asymptomatic according to the inclusion criteria of our study. Interestingly, children of 3 to 5 years of age who had been vaccinated only with two doses of CoronaVac ([CoronaVac (2x)] have been shown to be more susceptible to asymptomatic infections SARS-CoV-2(69). Hence, in this cohort study, asymptomatic infections may have increased the neutralizing antibody titers independent from vaccination. In any case, even in the presence of possible previous infections, the booster with BNT162b2 promotes anti-spike antibodies, increased neutralization and evoke immunity against BA.1 in the [CoronaVac (2x) + BNT162b2 (2x)] and [BNT162b2 (3x)] vaccination scheme groups. Such a heterologous prime-boost vaccination with CoronaVac followed by BNT162b2 has also been reported by others to induce high neutralizing titer against SARS-CoV-2 Omicron strains in the pediatric population [
70].
SARS-CoV-2 vaccines of different platforms including mRNA, adenoviral vector and inactivated vaccines have been shown to produce T cell responses and very high effectiveness against hospitalization [
71,
72]. mRNA-based vaccines have been reported to induce memory T lymphocytes that do not affect their response against other variants [
39]. Furthermore, regardless of the AIM assay, responses against Omicron variants have been described to be highly conserved in CD4+ and CD8+ T cells [
39,
73]. In this context, CoronaVac can stimulate CD4+ T cell responses against spike peptides [
73]. Our data presented here provides additional evidence that the booster with BNT162b2 generates an increase in the AIM assay of CD4+ and CD8+ T lymphocytes specific to Omicron BA.1 spike protein in the pediatric population immunized with [CoronaVac (2x)].
As a whole, our study has limitations in two principal aspects; i) the pediatric cohort may have developed immune responses by asymptomatic infections in addition to the vaccination schemes, and ii) the cellular immune responses were only analyzed against the entire spike glycoprotein of SARS-CoV-2 and not against S1 and S2 subdomains separately and other viral proteins.
Finally, our findings indicate that vaccination campaigns in the pediatric population implying booster doses, such as BNT162b2 and heterologous schemes exhibit an enhanced cellular and humoral immune response against Omicron lineages. Currently, there is apprehension regarding the potential of SARS-CoV-2 emerging variants to evade the immune response elicited by approved vaccines. Vaccine boosters are essential to prevent viral immune evasion and severe forms of disease in the pediatric population.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Generation of recombinant vesicular stomatitis virus decorated with the SARS-CoV-2 spike glycoprotein of the BA.1 omicron variant (rVSV-SARS-S-BA.1).
Author Contributions
DDD, GB, DE, PD, and AEV performed the Collection and processing of samples. DDD, GB, RP, NS assisted in the volunteers’ identification, enrollment, collection of epidemiological and clinical data. GB, PD, MDS, performed SARS-CoV-2-specific antibody ELISAs and hACE2 competition assays. SCC, NAM, NDT developed the rVSV-SARS2-S-Omicron-BA.1 virus and performed and analyzed the viral microneutralization assays. DDD performed AIM T cell activation. AG and AS provided peptide reagents. RP and NS statistical analysis. DDD, RP, NS, SCC, NAM, NDT and AEV writing and editing of the manuscript. DDD, CC, JD, HGE, and AEV planning and logistics. All authors declare that they have reviewed and agree with the final manuscript.
Funding
This study was financed and supported by Public Health Institute of Chile, and Ministry of Science, technology, knowledge an innovation. This project has also been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93021C00016 to A.G. and A.S. and Contract No. 75N93019C00065. NDT, NAM and SCC received funding from the Agencia Nacional de Inovación y Desarrollo (ANID), Chile through grants FONDECYT 1221811 and Centro Ciencia & Vida, CCTE Basal FB210008.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Scientific Ethics Committee of the Scientific Ethics Committee from the Southeast Metropolitan Health Service, Santiago, Chile.
Informed Consent Statement
The studies involving human participants were reviewed and approved by Scientific Ethics Committee of the Scientific Ethics Committee from the Southeast Metropolitan Health Service, Santiago, Chile of May 19, 2022. The parents of patients/participants provided their written informed consent to participate in this study. In addition, all the participants signed an informed assent.
Data Availability Statement
The original contributions presented in the study are included in the article/
Supplementary Material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
We thank the Schools of the Metropolitan Region for providing support in the recruitment of pediatric volunteers. To Carolina Saez and Lilian Donoso who participated in the management and procurement processes and logistic assistance. To all staff of the National Agency for Medical Devices, Innovation and Development (ANDID) and all logistics assistance of the Public Health Institute of Chile. We also acknowledge Dr. Kartik Chandran (Albert Einstein College of Medicine, NY, USA) for providing the rVSV-SARS2-S antigenome plasmid and virus.
Conflicts of Interest
Alessandro Sette is a consultant for Gritstone Bio, Flow Pharma, Moderna, AstraZeneca, Qiagen, Fortress, Gilead, Sanofi, Merck, RiverVest, MedaCorp, Turnstone, NA Vaccine Institute, Emervax, Gerson Lehrman Group and Guggenheim. Alba Grifoni is a consultant for Sanofi and Pfizer. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work.
References
- COVID-19 epidemiological update – 22 December 2023 [Internet]. [cited 2024 Feb 1]. Available from:. 22 December 2023. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---22-december-2023.
- Gobierno de Chile [Internet]. [cited 2024 Feb 1]. Cifras Oficiales - Gob.cl. Available online: https://www.gob.cl/pasoapaso/cifrasoficiales/.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shi, Z.L. The First Disease X is Caused by a Highly Transmissible Acute Respiratory Syndrome Coronavirus. Virol Sin. 2020, 35, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gillies, C.L.; Singh, R.; Singh, A.; Chudasama, Y.; Coles, B.; et al. Prevalence of co-morbidities and their association with mortality in patients with COVID -19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020, 22, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.W.; Zachariah, P.; Gorelik, M.; Boneparth, A.; Kernie, S.G.; Orange, J.S.; et al. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 2020, 324, 294. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020, 324, 259. [Google Scholar] [CrossRef]
- Karron, R.A.; Garcia Quesada, M.; Schappell, E.A.; Schmidt, S.D.; Deloria Knoll, M.; Hetrich, M.K.; et al. Binding and neutralizing antibody responses to SARS-CoV-2 in very young children exceed those in adults. JCI Insight. 2022, 7, e157963. [Google Scholar] [CrossRef] [PubMed]
- Renk, H.; Dulovic, A.; Seidel, A.; Becker, M.; Fabricius, D.; Zernickel, M.; et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat Commun. 2022, 13, 128. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Ho, A.; Marques, D.F.P.; McMenamin, J.; Gunson, R.N.; Murcia, P.R. Epidemiology of Seasonal Coronaviruses: Establishing the Context for the Emergence of Coronavirus Disease 2019. J Infect Dis. 2020, 222, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, E.R.; Hardie, A.; Claas, E.C.J.; Simmonds, P.; Templeton, K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010, 48, 2940–2947. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Sun, Z.; Zhu, X.; Marti, M.M.; Srivastava, S.S.; et al. SARS-CoV-2 variants of concern: spike protein mutational analysis and epitope for broad neutralization. Nat Commun. 2022, 13, 4696. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Soh, W.T. The N-terminal domain of spike glycoprotein mediates SARS-CoV-2 infection by associating with L-SIGN and DC-SIGN.
- Zheng, M.; Song, L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell Mol Immunol. 2020, 17, 536–538. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Montgomerie, I.; Bird, T.W.; Palmer, O.R.; Mason, N.C.; Pankhurst, T.E.; Lawley, B.; et al. Incorporation of SARS-CoV-2 spike NTD to RBD protein vaccine improves immunity against viral variants. iScience. 2023, 26, 106256. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022, eabn8014. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; De Araújo Oliveira, V.; Paixão, E.S.; et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Ash, N.; et al. Protection and waning of natural and hybrid COVID-19 immunity [Internet]. Epidemiology; 2021 Dec [cited 2022 Feb 4]. Available from:. /: [cited 2022 Feb 4]. Available from: http. Available online: http://medrxiv.org/lookup/doi/10.1101/2021.12.04.21267114.
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B. 1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021, 70, 1163–1166. [Google Scholar]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med [Internet]. 2022 Jan 19 [cited 2022 Feb 4]; Available from:. /: 19 [cited 2022 Feb 4]; Available from: https. Available online: https://www.nature.com/articles/s41591-021-01676-0.
- [Internet]. European Centre for Disease Prevention and Control An agency of the European Union. SARS-CoV-2 variants of concern as of 2 February 2024. 2 February. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern.
- Bates, T.A.; Leier, H.C.; Lyski, Z.L.; McBride, S.K.; Coulter, F.J.; Weinstein, J.B.; et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021, 12, 5135. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021, 27, 717–726. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022, S0092867422000733. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St. Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mok, B.W.Y.; Chen, L.L.; Chan, J.M.C.; Tsang OTY, Lam BHS, et al.; et al. Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant by Sera From BNT162b2 or CoronaVac Vaccine Recipients. Clin Infect Dis. 2022, 75, e822–6. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.; Ding, X.; Cao, Y.; Yang, D.; Duan, Y. Effectiveness of COVID-19 vaccine in children and adolescents with the Omicron variant: A systematic review and meta-analysis. J Infect. 2023, 86, e64–6. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Rosa Duque, J.S.; Yip, K.M.; So, H.K.; Wong, W.H.S.; Lau, Y.L. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during Omicron, B.A.2 wave in Hong Kong. Commun Med. 2023, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Melo-González, F.; Gutierrez-Vera, C.; Schultz, B.M.; Berríos-Rojas, R.V.; Rivera-Pérez, D.; et al. Inactivated Vaccine-Induced SARS-CoV-2 Variant-Specific Immunity in Children. mBio. 2022, 13, e0131122. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.S.; McElrath, M.J. COVID-19 and the Path to Immunity. JAMA. 2020, 324, 1279. [Google Scholar] [CrossRef] [PubMed]
- INFORME EPIDEMIOLÓGICO N°46 VIGILANCIA GENÓMICA DE SARS-CoV-2 (COVID-19) [Internet]. Chile: MINSAL; 2022 Dec. Available online: https://www.minsal.cl/wp-content/uploads/2023/01/Informe_Epidemiologico_-N%C2%B0-46_Vigilancia-Geno%CC%81mica_de-SARS-CoV-2.pdf.
- Diaz-Dinamarca, D.; Diaz, P.; Barra, G.; Puentes, R.; Arata, L.; Grossolli, J.; et al. Humoral immunity against SARS-CoV-2 evoked by heterologous vaccination groups using the CoronaVac (Sinovac) and BNT162b2 (Pfizer/BioNTech) vaccines in Chile. Front Public Health. 2023. [CrossRef]
- Dieterle, M.E.; Haslwanter, D.; Bortz, R.H.; Wirchnianski, A.S.; Lasso, G.; Vergnolle, O.; et al. A Replication-Competent Vesicular Stomatitis Virus for Studies of SARS-CoV-2 Spike-Mediated Cell Entry and Its Inhibition. Cell Host Microbe. 2020, 1–11. [Google Scholar] [CrossRef]
- Muena, N.A.; García-Salum, T.; Pardo-Roa, C.; Avendaño, M.J.; Serrano, E.F.; Levican, J.; et al. Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals. eBioMedicine. 2022, 78, 103972. [Google Scholar] [CrossRef]
- Aguilera, X.; Hormazábal, J.; Vial, C.; Cortes, L.J.; González, C.; Rubilar, P.; et al. SARS-CoV-2 Neutralizing Antibodies in Chile after a Vaccination Campaign with Five Different Schemes. Vaccines. 2022, 10, 1051. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet TIG. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Hertanto, D.M.; Sutanto, H.; Lusida, M.I.; Kuntaman, K.; Santoso, D. The genomic and clinical features of the COVID-19 Omicron variant: a narrative review [Internet]. F1000Research; 2022 [cited 2024 Feb 5]. Available online: https://f1000research.com/articles/11-353.
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe. 2020, 27, 671–680. [Google Scholar] [CrossRef]
- Liu, B.; Su, X.; Yu, G.; Yang, S.; Wang, F.; Huang, T.; et al. An automated chemiluminescent immunoassay (CLIA) detects SARS-CoV-2 neutralizing antibody levels in COVID-19 patients and vaccinees. Int J Infect Dis. 2022, 115, 116–125. [Google Scholar] [CrossRef]
- Moga, E.; Lynton-Pons, E.; Domingo, P. The Robustness of Cellular Immunity Determines the Fate of SARS-CoV-2 Infection. Front Immunol [Internet]. 2022 [cited 2024 Feb 7];13. /: 2024 Feb 7];13. Available from: https. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.904686.
- Wang, L.; Nicols, A.; Turtle, L.; Richter, A.; Duncan, C.J.; Dunachie, S.J.; et al. T cell immune memory after covid-19 and vaccination. BMJ Med. 2023, 2, e000468. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, H.; Guo, W. Clinical Characteristics of Children With COVID-19: A Meta-Analysis. Front Pediatr. 2020, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.V.; Pinsky, B.A.; Suthar, M.S.; Lai, L.; Davis-Gardner, M.E.; Floyd, K.; et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N Engl J Med. 2021, 385, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Del Manso, M.; Mateo-Urdiales, A.; Rota, M.C.; Petrone, D.; Riccardo, F.; et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January–April, 2022. The Lancet. 2022, 400, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Chua, G.T.; Lu, L.; Chan BPC, Wong JSC, Chow CCK, et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerg Microbes Infect. 2022, 11, 543–547. [Google Scholar] [CrossRef]
- Yan, V.K.C.; Cheng, F.W.T.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Li, X.; et al. Effectiveness of BNT162b2 and CoronaVac vaccines in preventing SARS-CoV-2 Omicron infections, hospitalizations, and severe complications in the pediatric population in Hong Kong: a case-control study. Emerg Microbes Infect. 2023, 12, 2185455. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Mok, A.H.Y.; Yan, V.K.C. ffectiveness of BNT162b2 and CoronaVac vaccinations against SARS-CoV-2 omicron infection in people aged 60 years or above: a case-control study. J Travel Med. 2022, taac119. [Google Scholar] [CrossRef]
- Rosa Duque, J.S.; Wang, X.; Leung, D.; Cheng, S.M.S.; Cohen, C.A.; Mu, X.; et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat Commun. 2022, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Rosa Duque, J.S.; Leung, D.; Yip, K.M.; Lee, D.H.L.; So H kwan Wong, W.H.S.; et al. COVID-19 vaccines versus pediatric hospitalization. Cell Rep Med. 2023, 4, 100936. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Chen, J.W.; Kang, J.; Burns, M.D.; St Denis, K.J.; Sheehan, M.L.; et al. BNT162b2 induces robust cross-variant SARS-CoV-2 immunity in children. Npj Vaccines. 2022, 7, 158. [Google Scholar] [CrossRef]
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N Engl J Med. 2022, 387, 525–532. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Acevedo, J.; Pizarro, A.; et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef]
- Puthanakit, T.; Nantanee, R.; Jaru-Ampornpan, P.; Chantasrisawad, N.; Sophonphan, J.; Meepuksom, T.; et al. Heterologous Prime-boost of SARS-CoV-2 inactivated vaccine and mRNA BNT162b2 among Healthy Thai Adolescents. Vaccine, X. 2022, 12, 100211. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.P.; Cohen, C.A.; Cheng, S.M.S.; Chen, C.; Kwok, K.O.; Yiu, K.; et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirol Carlton Vic. 2022, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Melo-González, F.; Soto, J.A.; González, L.A.; Fernández, J.; Duarte, L.F.; Schultz, B.M.; et al. Recognition of Variants of Concern by Antibodies and T Cells Induced by a SARS-CoV-2 Inactivated Vaccine. Front Immunol. 2021, 12, 747830. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).