1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first emerged in December 2019 in Wuhan (China) and spread subsequently worldwide. As of March 2023, the number of SARS-CoV-2 confirmed cases is over 761 million, according to the World Health Organization COronaVIrus Disease 19 (COVID-19) Dashboard. COVID-19 does not resolve its clinical relevance in the acute phase of the disease, instead it can trigger a nosological entity known as post-COVID syndrome, or also “long COVID”, “persistent COVID”, “long-tail COVID” and “post-acute sequelae of SARS-CoV-2” (PASC) [
1]. The National Institute for Health and Care Excellence (NICE) defined post-COVID syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis” [
2]. The US Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) adopted a mildly narrower definition, considering as long-COVID syndrome only symptoms extending beyond 4 weeks after initial infection [
3]. Either way, as post-COVID is characterized by relevant neurological involvement together with frequent respiratory and cardiovascular symptoms, the umbrella term of “neuro-long-COVID” was proposed [
4].
Whether or not neuro-long-COVID is to be considered an independent pathological entity is a matter of debate: some authors sustain that its neurological signs and symptoms are poorly characterized and lack consistency, resembling functional disorders [
5], while other authors openly argue against labelling post-COVID neurological involvement as functional [
6]. Anyway, direct SARS-CoV-2 central nervous system invasion, abnormal systemic and neurologic immunological responses, cytokine storm, glial dysfunction, virosis-induced endothelial changes and other mechanisms have been proposed as pathogenetic mechanisms underlying post-COVID neurologic symptoms [
7]. Among those, neuroinflammation emerges as a likely pathogenetic element: even before the spread of SARS-CoV-2, neuroinflammation had already been investigated as a physiopatogenetic factor in some neuropsychiatric diseases such as major depressive disorder, in which immune activation levels resulted directly correlated to cognitive-behavioral modifications [
8]. In this context, it was demonstrated that serum biomarkers of inflammation such as C-reactive peptide, D-dimer, proinflammatory cytokines, procalcitonin, Vascular Cell Adhesion Molecule-1 (sVCAM-1) and complement pathway components are significatively increased in post-COVID patients with neurological involvement [
1,
9].
For this reason, it becomes crucial to find new approaches to control the neuroinflammatory process, acting on the immune system regulation. From this perspective, a putative intervention might be represented by Palmitoylethanolamide (PEA), an endogenous molecule belonging to the N-acylethanolamines family. PEA has been found in several human tissues, including brain, and is synthetized “on demand” in response to stress factors to restore the tissue homeostasis, exerting its protective action thanks to its ability to modulate mast cells hyperactivation through the so-called autacoid local injury antagonism (ALIA) mechanism [
10]. A very large number of publications have documented PEA efficacy in various pathologies which, although with different etiologies, share the pathogenetic mechanism of non-resolving neuroinflammation. Furthermore, it has been demonstrated that the co-ultramicronization between umPEA (the ultramicronized form of PEA which increases its bioavailability and therefore its biological efficacy) [
11,
12] and specific polyphenols like polydatin or luteolin originates microcomposites with higher neuroprotective, anti-inflammatory and antioxidant properties [
13,
14,
15]. The co-ultramicronization of PEA and luteolin (co-ultraPEALut) yielded in fact promising results in preclinical trials as a neuroinflammation antagonist in diseases such as traumatic brain or spinal injuries, vascular dementia, and ischemic stroke [
16,
17,
18].
The aim of this investigation was to: i) describe through neuropsychological tests the course of the cognitive impairment in post-COVID patients treated with co-ultraPEALut, ii) compare outcomes of patients treated with and without co-ultraPEALut, and iii) compare the outcomes of patients treated with co-ultraPEALut alone and those treated with co-ultraPEALut and corticosteroids.
2. Materials and Methods
The present retrospective study involved 26 outpatients affected by subjective cognitive impairment arisen following SARS-CoV-2 infection and visited between July 2021 and December 2022 at the Neurology Unit of the University Hospital and Health Services of Trieste (Italy). Patients were treated with a commercial oral formulation composed by co-ultramicronized association of 700 mg PEA and 70 mg of Luteolin (Food for Special Medical Purposes, Glialia®, Epitech Group SpA, Saccolongo, Italy) for a period of at least two months (twice daily for 30 days and once daily for the following 30 days or more), as an add-on to their concomitant therapy for comorbidities.
Patients of both genders with a previous SARS-CoV-2 infection documented by a positive nasopharyngeal swab, and reporting subjective cognitive decline during and/or following the infection for a period of at least 12 weeks, were considered for this investigation. Patients with known cognitive or psychiatric disorders, ongoing corticosteroid therapy at the time of the first neurological evaluation, or having a more likely explanation of the neurological deficits following the diagnostic work-up, were excluded from the study.
The diagnostic work-up included clinical evaluation by a neurologist dedicated to post-COVID neurological involvement, blood tests, cerebral imaging (preferentially Magnetic Resonance Imaging, MRI) and electroencephalography. At the time of the clinical assessment, the medical history collection was combined with the neuropsychological evaluation which included:
i. Montreal cognitive assessment test (MoCA) adjusted by age and education level according to Aiello [
19,
20], which evaluates both non-instrumental (executive functioning, attention) and instrumental (language, memory, visuospatial abilities, orientation) cognitive domains. In order to prevent a learning effect, two different versions of MoCA were utilized for the two serial evaluations performed at the beginning and at the end of the study. Of note, MoCA proved to be superior to Mini-Mental State Examination (MMSE) at revealing subclinical defects in post-COVID patients [
21];
ii. Fatigue Severity Scale (FSS) (Krupp et al., 1989), a questionnaire with 9-items, each ranging from 1 (strongly disagree) to 7 (strongly agree), with a cut score >4 indicatives of significant fatigue [
22];
iii. Prospective and Retrospective Memory Questionnaire (PRMQ) [
23], a self-reported tool consisting of 16 items rating on a 5-point scale (5=very often; 1=never) which evaluates the frequency of prospective and retrospective memory failure, with higher scores indicative of higher incidence of memory errors or memory loss;
iv. Subjective assessment of health change, which allowed each patient to be categorized as worsened, stable, improved, or remitted in comparison to the previous visit.
All examinations, except for the subjective assessment which was proposed at the end of the study, were performed prior to starting treatment with co-ultraPEALut (T0) and at the re-evaluation time point (T1) after almost 10 months on average, according to the clinical practice in force in the outpatient’s clinic unit involved.
Previously collected data from neuro-long-COVID patients not receiving co-ultraPEALut (15 control patients) were used to compare MoCA, FSS, and PRMQ scores at baseline, and to FSS and PRMQ mean scores between the two groups before and after the treatment. Furthermore, an exploratory analysis was performed on a small subgroup of co-ultraPEALut-treated patients who was prescribed with corticosteroids (prednisone) administered orally at the dose of 50 mg daily for 3 days followed by 8-day décalage, in addition to the aforementioned therapies. In these patients, co-ultraPEALut and corticosteroids administration started at the same time.
Obtained data were tested for normality with Kolmogorov-Smirnov test and then compared with Student’s t-test. Values are expressed as mean ± standard deviation (SD). A p-value of less than 0.05 was considered statistically significant.
The research was conducted according to the principles of the Declaration of Helsinki and according to the Good Clinical Practice (GCP).
3. Results
3.1. Patients’ Characteristics
26 outpatients (73.08% females) treated with co-ultraPEALut were evaluated: their average age ± S.D. was 54.69 ± 11.26 years and their average years of education were 14.10 ± 3.05. Among those, 22 patients (84.62%) experienced a paucisymptomatic infection; 4 (15.38%) required hospitalization, and no one underwent intensive care unit admission. The time elapsed between the first nasal swab tested positive for SARS-CoV-2 (NS) and the first neurological evaluation (T0) was 7.33 ± 4.75 months, while the time between T0 and the re-evaluation (T1) was 9.92 ± 5.37 months (
Table 1, first section).
Data from 15 non-co-ultra-PEALut-treated patients (
Table 1, second section) were collected and used as controls: they resulted similar for demographic features, except for the female-to-male ratio which was lower in the control group. Sex comparisons were not specifically targeted in this study, as previous evidence in our clinic’s neuro-long-COVID outpatients showed no significant differences in symptoms between females and males [
4].
3.2. Outcome of co-ultraPEALut and control patients
At T0, raw and adjusted MoCA, PRMQ and FSS scores resulted comparable between co-ultraPEALut and control patients, with only slightly lower PRMQ scores in the control group (
Table 2, second section).
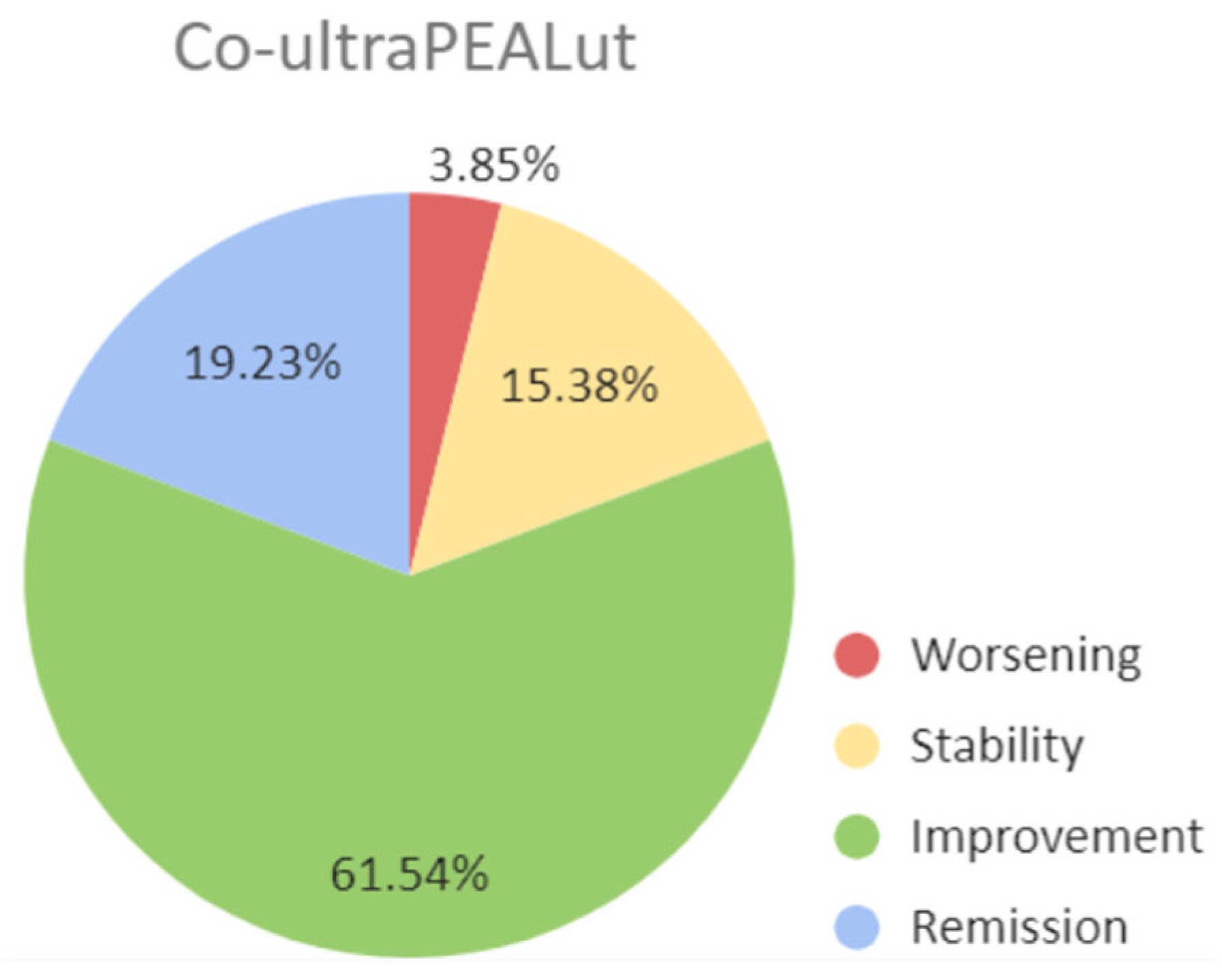
Co-ultraPEALut patients showed an improvement in all the administered tests from the first neurological evaluation to the re-evaluation time. In particular, a statistical significance was reached for PRMQ (T0: 51.94 ± 10.55, T1: 39.67 ± 13.02, p <.00001) and MoCA raw scores (T0: 25.76 ± 2.3, T1: 27,2 ± 2, p .0260), and a trend toward an improvement was seen for MoCA adjusted scores (T0: 24.34 ± 2.58, T1: 25.59 ± 2.39, p .0958). Also FSS scores underwent an improvement from T0 to T1, even though not statistically significant (T0: 5.03 ± 1.69, T1 4.52 ± 1.63, p .3188). The subjective assessment of health change over time revealed that 4 patients (15.38%) reported stability in their health status, 16 (61.54%) perceived an improvement, and 5 (19.23%) declared a complete remission of symptoms with a return to the baseline health status (
Table 2,
Figure 1), while only one patient reported a global worsening of its symptoms.
From T0 to T1 control patients showed a reduction (i.e., an improvement) of PRMQ scores (T0: 45.77 ± 13.47, T1: 42.33 ± 16.86, p .2051), as well as of FSS scores (T0: 4.95 ± 1.57, T1: 4.06 ± 1.47, p .1352), although not statistically significant in both cases.
On average, FSS scores decreased from T0 to T1 by 0.51 points in the co-ultraPEALut group and by 0.89 points in the control group (p .4762); while PRMQ scores decreased by 12.27 points and 7.97 points, respectively (p .16828).
Unfortunately, an analogue comparison analysis could not be performed for MoCA scores, due to the administration of this test to the control patients only at T0, as well as for the subjective evaluation of change, which had not been uniformly collected.
3.3. Outcome of co-ultraPEALut subgroups patients
A comparison was performed between the subgroup of patients treated with only co-ultraPEALut (19 patients) and the one treated with both co-ultraPEALut and corticosteroids (7 patients).
Cognitive impairment showed an improvement over time in both subgroups: in the co-ultraPEALut group MoCA raw score was 25.93 ± 2.52 at the first neurological evaluation time and 27.11 ± 1.82 at T1 (p .1417), whereas in co-ultraPEALut and corticosteroids-treated patients the score increased from 25.33 ± 1.21 to 27.43 ± 2.3 (p .0709); as well as MoCA adjusted score went from 24.54 ± 2.82 at T0 to 25.33 ± 2.03 at T1 (p .8488) and from 24.47 ± 2.2 to 26.27 ± 3.11 (p .2609), respectively. Similar results were obtained for FSS scores: among co-ultraPEALut patients the mean score decreased from 4.54 ± 1.54 at T0 to 4.29 ± 1.68 at T1 (p .6803), while for patients treated with both co-ultraPEALut and corticosteroids the score went from 6.8 ± 0.23 to 5.24 ± 1.32 (p .6251). Finally, the PRMQ score improvement throughout the observation period was statistically significant for patients treated with co-ultraPEALut alone (mean score of 53.38 ± 9.92 at T0 and 40.24 ± 13.3 at T1, p .0067), unlike that obtained by patients treated with co-ultraPEALut and corticosteroids (mean score of 47.25 ± 11.35 at T0 and 38.29 ± 12.88 at T1, p .2782) (
Table 3).
Moreover, for MoCA (raw and adjusted) and PRMQ scores a statistically significant difference between the two subgroups over time was not reported: from T0 to T1, MoCA raw and adjusted scores increased by 1.18 and 0.79 points in the co-ultraPEALut-treated group and by 2.1 and 1.8 points in the co-ultraPEALut plus corticosteroids-treated one (p .3081 and 0.4875, respectively), while PRMQ scores decreased by 13.14 and 8.96 points (p .3662). Only FSS score showed a significant difference between the two subgroups with a reduction of 0.25 point in the co-ultraPEALut-treated group and of 1.56 points in patients treated with co-ultraPEALut and corticosteroids (p .01754).
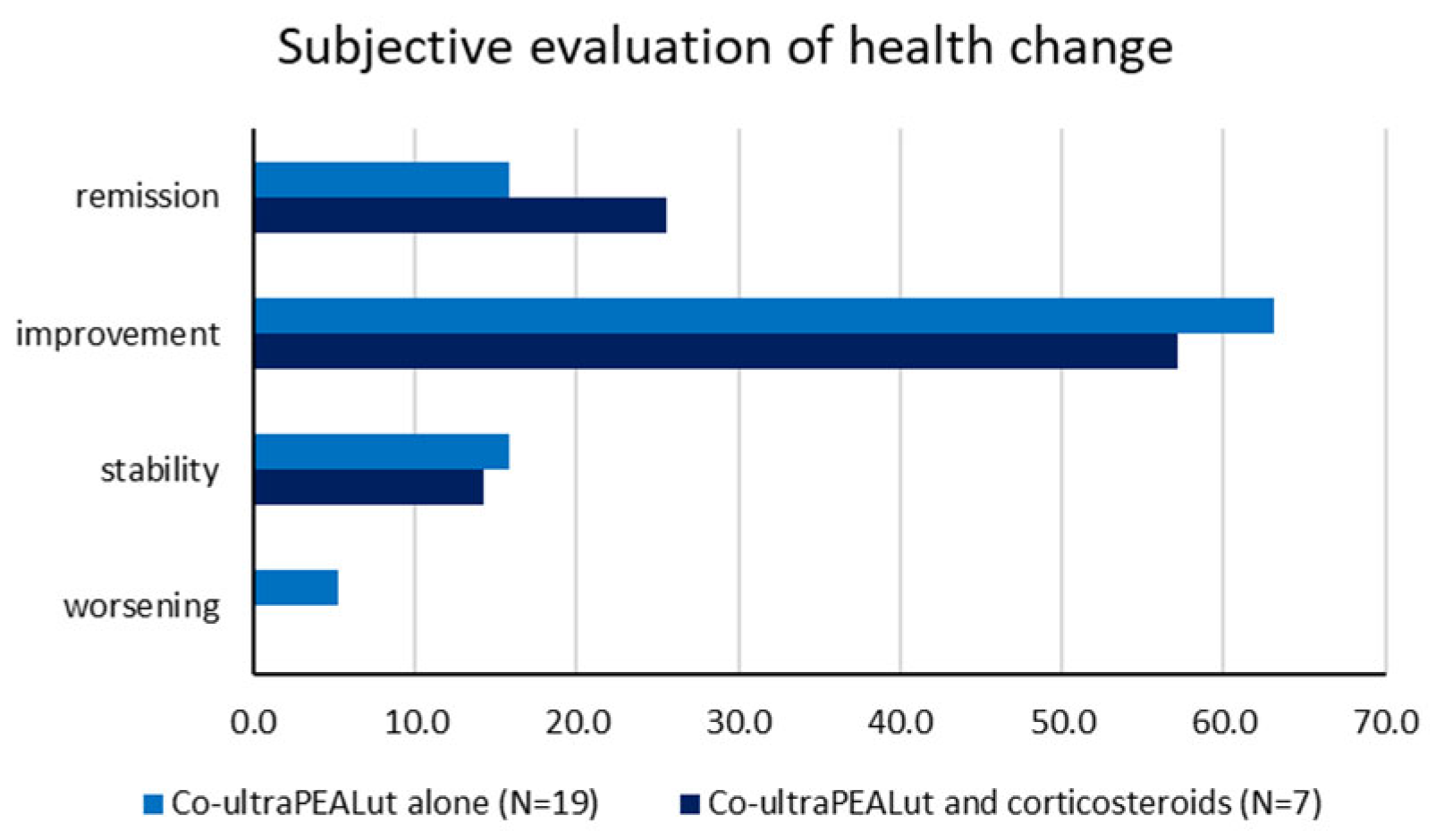
Subjective evaluation of health change over time did not differ substantially within the two subgroups: among patients receiving only co-ultraPEALut, 3 (15.79%) declared a stability in their health, 12 (63.16%) an improvement, 3 (15.79%) a complete remission of the symptoms and only one reported a worsening of its condition; as well as among patients taking corticosteroids and co-ultraPEALut, no patient declared a worsening, while stability, improvement and remission were reported by 1 (14.29%), 4 (57.14%) and 2 (28.57%) patients, respectively (
Table 3,
Figure 2).
4. Discussion
This exploratory retrospective study evaluated outpatients referring to our Neurology unit for subjective cognitive impairment after a documented SARS-CoV-2 infection. Neuropsychological tests were administered before and after treatment with co-ultraPEALut, a substance whose anti-inflammatory properties had been proved in both preclinical and clinical settings. Post-COVID neurologically involved patients treated with co-ultraPEALut and observed over an average course of almost 10 months showed, at the end of the observation period, both an objectively and subjectively cognitive improvement, with statistically significant results in both the MoCA (raw score) and PRMQ questionnaires. Control patients who were not prescribed co-ultraPEALut also showed an improvement in the cognitive functions, even though not as impactful (e.g., on PRMQ scores). Furthermore, the comparison between patients treated with co-ultraPEALut alone and those treated with co-ultraPEALut and corticosteroids revealed that, except for the severity of fatigue, the two groups did not obtain statistically different results in the assessments carried out. This may favor the proinflammatory pathophysiologic hypothesis of neuro-long-COVID and might highlight the importance of neuroinflammation modulation in this kind of neurologic issues.
COVID-19 patients are in fact subjected to an elevation in pro-inflammatory serum and cerebro-spinal fluid (CSF) biomarkers and display neuronal damage and glial activation, quantitatively correlated with neurological involvement and disease severity [
24,
25]. Among the most studied pro-inflammatory mediators in post-COVID patients, both TNF-alpha, associated with impaired synaptic plasticity, microglial activation and subsequent detrimental effect on memory [
26], and IL-6, linked in murine models with cognitive dysfunction [
27], have been associated with mood alterations and cognitive deficits [
24].
Clinically, the most frequently reported neuro-long-COVID symptoms are cognitive deficits (including brain fog), fatigue, and persistent hypo-/anosmia; together with headache, sleep disturbances, depression and anxiety, and autonomic nervous system dysfunctions such as orthostatic intolerance and sudomotor alterations [
1,
3,
4,
28]. A prospective study held in Milan (Italy) on 226 post-COVID patients reported that 78% of them had difficulties in at least one cognitive domain, especially executive functions and motor coordination, and that 36% experienced psychological-psychiatric involvement, i.e., depression symptoms, after 3 months from hospital discharge; beyond to demonstrate a positive correlation between depressive symptoms and systemic inflammation biomarkers during and after COVID-19 [
29]. Moreover, a study conducted in our clinic demonstrated that 61% of post-COVID patients reported autonomic nervous system dysregulation which appears to be connected with pro-inflammatory immune dysregulation as the sympathetic system activation induces cytokines production [
28,
30].
Neuro-long-COVID patients have also been studied with magnetic resonance imaging, finding significative cerebral perfusion alterations in the arterial spin labeling sequences (ASL-MRI): the large network connectivity seems to be disrupted as metabolism of the frontal, temporal and parietal areas appear to be altered [
31]. These observations combines with metabolism abnormalities in several brain regions found in 18-fuorodeoxyglucose positron emission tomography (18FDG-PET) studies [
32]. Moreover, in two thirds of neuro-long-COVID patients, electroencephalographic (EEG) features resulted abnormal, with slowing or epileptiform discharges [
33]. Also, inhibitory GABAergic and excitatory glutamatergic regulatory circuits appear impaired in paired-pulse transcranial magnetic stimulation (ppTMS) with the long-interval Intracortical Inhibition (LICI) and Intracortical Facilitation (ICF) which can be lower in people with neuro-long COVID, possibly reflecting a mild and often transitory encephalopathy caused by direct or indirect action of SARS-CoV-2 [
34].
This study, despite some limitations (the monocentric nature of the study, limited numerosity, lack of randomization, incomplete data, and different examinators involved in the testing of co-ultraPEALut-treated and control patients), demonstrated that co-ultaPEALut, by reducing the neuroinflammation underlying the pathology, seems to be able to improve the cognitive impairment and ameliorate the state of health perceived by neuro-post-COVID patients. Although more in-depth studies are required, the findings reported in this contribution adds some insight to the post-COVID cognitive symptoms and new approaches for their treatment.
5. Conclusions
In conclusion, our main finding is that outpatients presenting with subjective cognitive impairment following SARS-CoV-2 infection and treated with co-ultraPEALut globally improved over time, both proved by neuropsychological testing and subjectively. Its beneficial role is likely due to its anti-inflammatory properties. Further research is warranted in order to shed light on the possible beneficial effect of co-ultraPEALut, alone or in combination with anti-inflammatory drugs on neurological involvement in post-COVID.
Author Contributions
Conceptualization, V.C., G.F. and P.M.; methodology, V.C., G.F. and P.M.; formal analysis, V.C..; writing—original draft preparation, V.C..; writing—review and editing, V.C., G.F., A.M., A.L., V.P., P.M..; supervision, V.P., P.M.. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ceban F, Ling S, Lui LMW, et al. (2022) Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 101:93–135. [CrossRef]
- (2020) Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/ng188 (accessed on 26 May 2023).
- Crook H, Raza S, Nowell J, et al. (2021) Long covid—mechanisms, risk factors, and management. BMJ 374:n1648. [CrossRef]
- Michelutti M, Furlanis G, Stella AB, et al. (2022) Sex-dependent characteristics of Neuro-Long-COVID: Data from a dedicated neurology ambulatory service. J Neurol Sci 441:120355. [CrossRef]
- Teodoro T, Chen J, Gelauff J, Edwards MJ (2023) Functional neurological disorder in people with long COVID: A systematic review. Eur J Neurol 30:1505–1514. [CrossRef]
- Van der Feltz-Cornelis CM, Moriarty AS, Strain WD (2023) Neurological Dysfunction in Long COVID Should Not Be Labelled as Functional Neurological Disorder. Viruses 15:783. [CrossRef]
- Nalbandian A, Sehgal K, Gupta A, et al. (2021) Post-acute COVID-19 syndrome. Nat Med 27:601–615. [CrossRef]
- Bortolato B, Carvalho AF, Soczynska JK, et al. (2015) The Involvement of TNF-α in Cognitive Dysfunction Associated with Major Depressive Disorder: An Opportunity for Domain Specific Treatments. Curr Neuropharmacol 13:558–576. [CrossRef]
- Bulla R, Rossi L, Furlanis G, et al. (2023) A likely association between low mannan-binding lectin level and brain fog onset in long COVID patients. Front Immunol 14:1191083. [CrossRef]
- Aloe L, Leon A, Levi-Montalcini R (1993) A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 39 Spec No:C145-147. [CrossRef]
- Impellizzeri D, Bruschetta G, Cordaro M, et al. (2014) Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J Neuroinflammation 11:136. [CrossRef]
- Petrosino S, Cordaro M, Verde R, et al. (2018) Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front Pharmacol 9:249. [CrossRef]
- Di Paola R, Fusco R, Gugliandolo E, et al. (2016) Co-micronized Palmitoylethanolamide/Polydatin Treatment Causes Endometriotic Lesion Regression in a Rodent Model of Surgically Induced Endometriosis. Front Pharmacol 7:382. [CrossRef]
- Peritore AF, Siracusa R, Crupi R, Cuzzocrea S (2019) Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 11:2175. [CrossRef]
- Petrosino S, Di Marzo V (2017) The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol 174:1349–1365. [CrossRef]
- Caltagirone C, Cisari C, Schievano C, et al. (2016) Co-ultramicronized Palmitoylethanolamide/Luteolin in the Treatment of Cerebral Ischemia: from Rodent to Man. Transl Stroke Res 7:54–69. [CrossRef]
- Campolo M, Crupi R, Cordaro M, et al. (2021) Co-Ultra PEALut Enhances Endogenous Repair Response Following Moderate Traumatic Brain Injury. Int J Mol Sci 22:8717. [CrossRef]
- Siracusa R, Impellizzeri D, Cordaro M, et al. (2017) Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia. Front Neurol 8:233. [CrossRef]
- Aiello EN, Gramegna C, Esposito A, et al. (2022) The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res 34:375–382. [CrossRef]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [CrossRef]
- Aiello EN, Fiabane E, Manera MR, et al. (2022) Screening for cognitive sequelae of SARS-CoV-2 infection: a comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 43:81–84. [CrossRef]
- Anton HA, Miller WC, Townson AF (2008) Measuring Fatigue in Persons with Spinal Cord Injury. Arch Phys Med Rehabil 89:538–542. [CrossRef]
- Smith G, Della Sala S, Logie RH, Maylor EA (2000) Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Mem Hove Engl 8:311–321. [CrossRef]
- Lyra e Silva NM, Barros-Aragão FGQ, De Felice FG, Ferreira ST (2022) Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology 209:109023. [CrossRef]
- Sutter R, Hert L, De Marchis GM, et al. (2021) Serum Neurofilament Light Chain Levels in the Intensive Care Unit: Comparison between Severely Ill Patients with and without Coronavirus Disease 2019. Ann Neurol 89:610–616. [CrossRef]
- Figueiredo CP, Barros-Aragão FGQ, Neris RLS, et al. (2019) Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun 10:3890. [CrossRef]
- Burton MD, Johnson RW (2012) Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav Immun 26:732–738. [CrossRef]
- Buoite Stella A, Furlanis G, Frezza NA, et al. (2022) Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol 269:587–596. [CrossRef]
- Mazza MG, Palladini M, De Lorenzo R, et al. (2021) Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun 94:138–147. [CrossRef]
- Fudim M, Qadri YJ, Ghadimi K, et al. (2020) Implications for Neuromodulation Therapy to Control Inflammation and Related Organ Dysfunction in COVID-19. J Cardiovasc Transl Res 13:894–899. [CrossRef]
- Ajčević M, Iscra K, Furlanis G, et al. (2023) Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci Rep 13:5808. [CrossRef]
- Hosp JA, Dressing A, Blazhenets G, et al. (2021) Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain awab009. [CrossRef]
- Furlanis G, Buoite Stella A, Biaduzzini F, et al. (2023) Cognitive deficit in post-acute COVID-19: an opportunity for EEG evaluation? Neurol Sci 44:1491–1498. [CrossRef]
- Manganotti P, Michelutti M, Furlanis G, et al. (2023) Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin Neurophysiol 151:83–91. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).