Submitted:
08 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hyaluronic Acid-Vancomycin Complex
2.3. Physicochemical Characterization of HA-VAN25 in Solid State
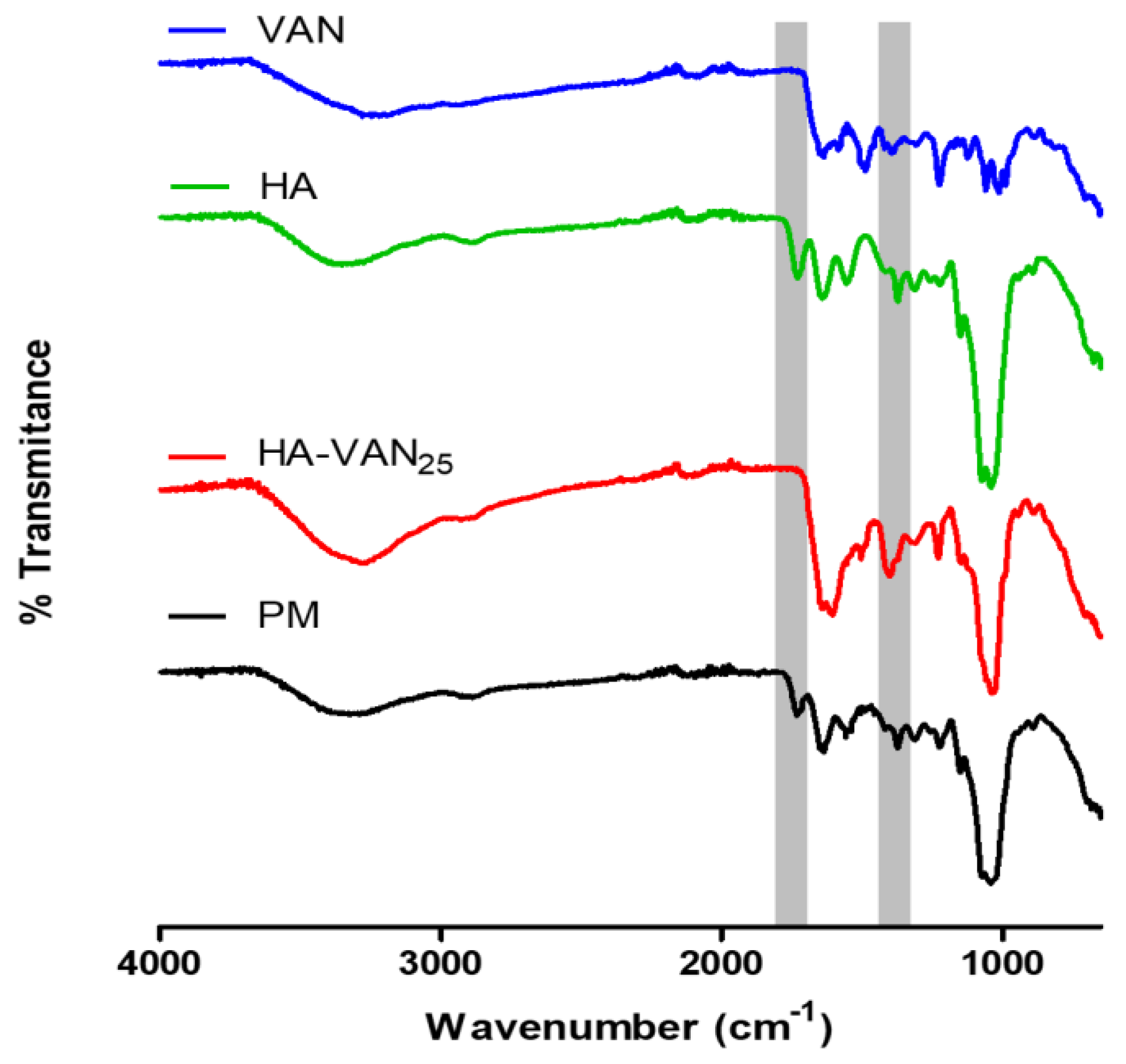
2.3.1. Infrared Spectroscopy
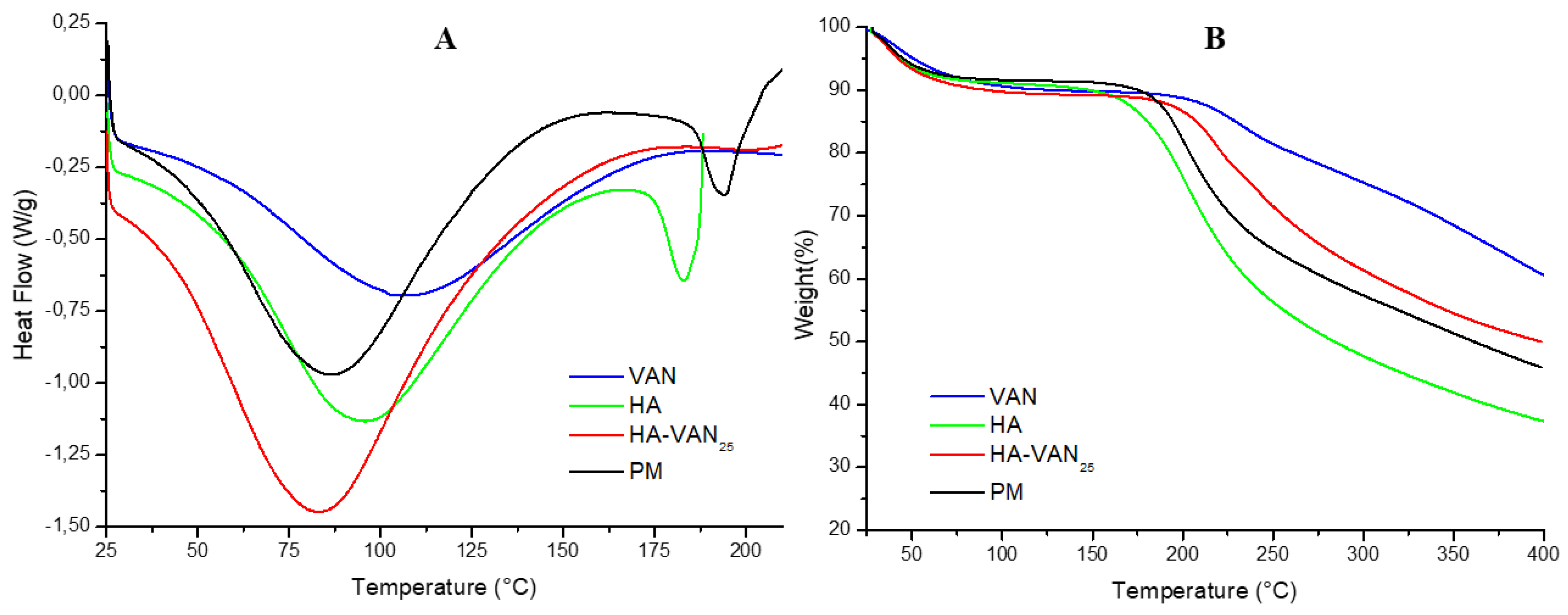
2.3.2. Thermal Analysis
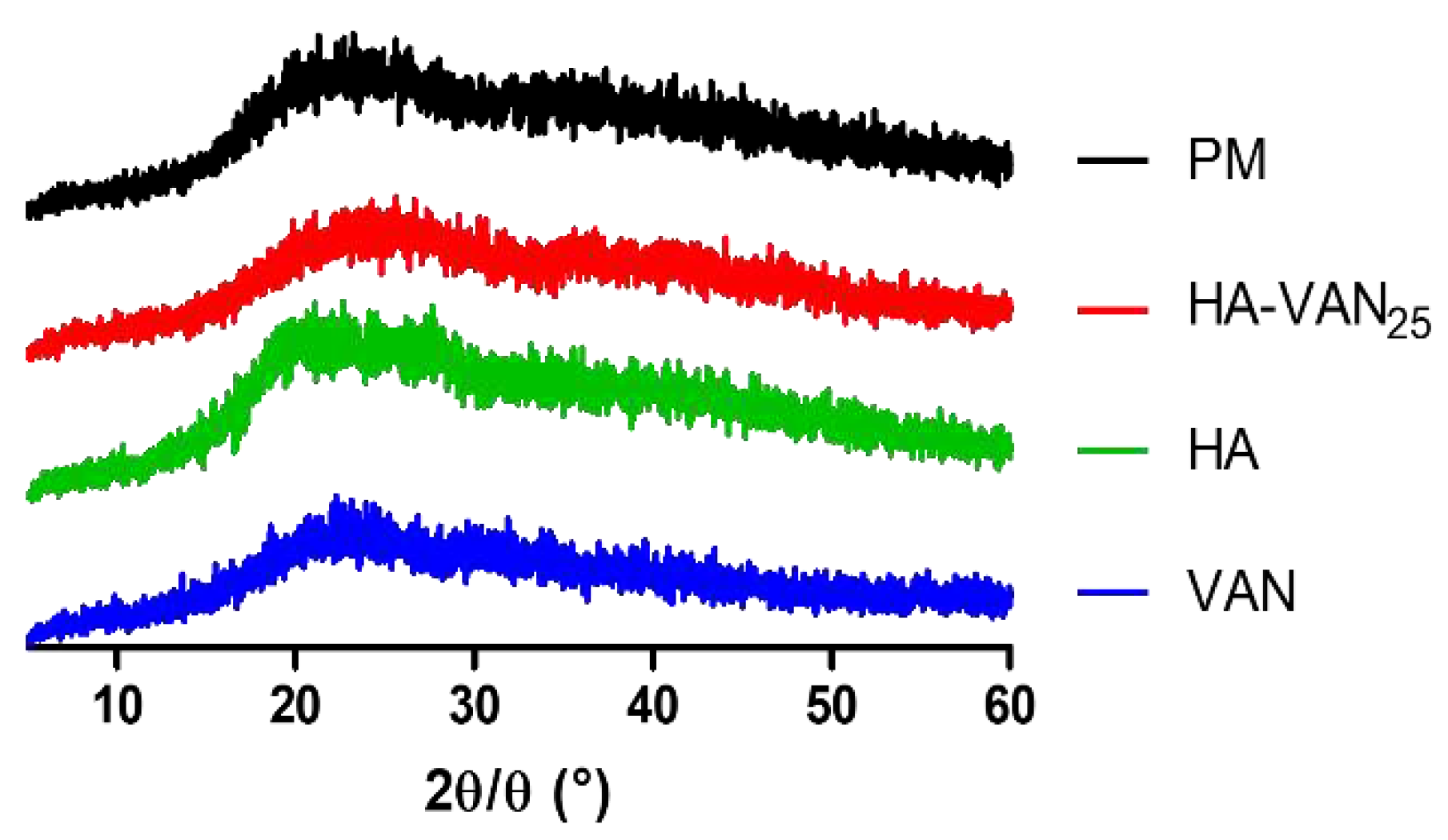
2.3.3. X-ray Diffraction
2.4. Powder Characterization
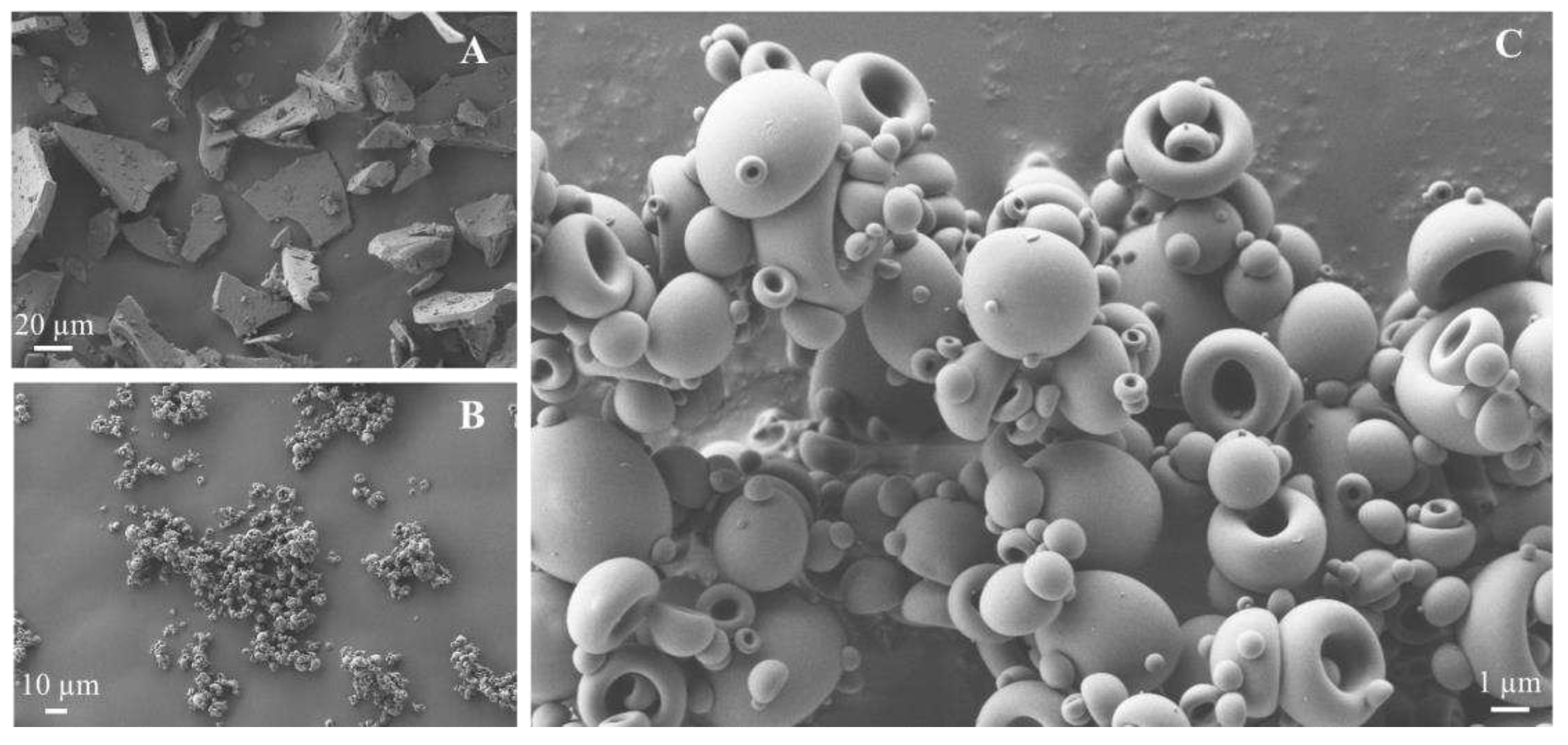
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Density Determination and Flow Properties
2.4.3. Particle Size Distribution by Laser Diffraction
2.5. In vitro Biopharmaceutical Performance of HA-VAN25 Complex
2.5.1. Aerodynamic Performance Assessment
2.5.2. Analytical Quantification of Vancomycin
2.5.3. Dissolution Study
2.5.4. Franz’ Cell Diffusion Study
2.6. Antibacterial Activity Assays
3. Results and Discussion
3.1. HA-VAN25 Complex Preparation
3.2. Physicochemical Characterization of HA-VAN25 in the Solid State
3.3. Powder Characterization
3.4. In Vitro Biopharmaceutical Performance of HA-VAN25 Complex
3.5. Antibacterial Activity Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guía de diagnóstico y tratamiento de pacientes con Fibrosis Quística: actualización. Resumen ejecutivo, Arch. Argent. Pediatr., vol. 112, no. 3, pp. 291–292, 2014.
- C. J. Dickinson KM, Cystic fibrosis, Pediatr Rev., vol. 42, no. 2, pp. 55–67, 2021.
- A. C. Blanchard and V. J. Waters, Opportunistic Pathogens in Cystic Fibrosis: Epidemiology and Pathogenesis of Lung Infection, J. Pediatric Infect. Dis. Soc., vol. 11, (suplement_2), pp. S3–S12, 2022. [CrossRef]
- Q. J. Epps, K. L. Epps, D. C. Young, and J. T. Zobell, State of the art in cystic fibrosis pharmacology optimization of antimicrobials in the treatment of cystic fibrosis pulmonay exacerbations: III. Executive summary, Pediatr. Pulmonol., vol. 56, no. 7, pp. 1825–1837, 2021.
- G. McDermott, E. Reece, and J. Renwick, Microbiology of the cystic fibrosis airway, Encycl. Microbiol., no. 2002, pp. 186–198, 2019.
- Ó. Fielbaum, Updated Treatment of Cystic Fibrosis, Rev. Medica Clin. Las Condes, vol. 28, no. 1, pp. 60–71, 2017.
- M. M. Abdelaziz, A. Hefnawy, A. Anter, M. M. Abdellatif, M. A. F. Khalil, and I. A. Khalil, Respirable spray dried vancomycin coated magnetic nanoparticles for localized lung delivery, Int. J. Pharm., vol. 611, 2022. [CrossRef]
- B. Lindley, Z. Bhakta, K. Gray, A. Watanabe, L. Leclair, and D. C. Young, Pharmacokinetics of intermittent dosed intravenous vancomycin in adult persons with cystic fibrosis, Pediatr. Pulmonol., vol. 57, no. 11, pp. 2646–2651, 2022. [CrossRef]
- G. Waterer, J. Lord, T. Hofmann, and T. Jouhikainen, Phase I, dose-escalating study of the safety and pharmacokinetics of inhaled dry-powder vancomycin (AeroVAnc) in volunteers and patients with cystic fibrosis: A New Approach to Therapy for Methicillin-Resistant Staphylococcus aureus, Antimicrob. Agents Chemother., vol. 64, no. 3, pp. 1–7, 2020. [CrossRef]
- C. J. McKinzie et al., Off-label use of intravenous antimicrobials for inhalation in patients with cystic fibrosis, Pediatr. Pulmonol., vol. 54, no. S3, pp. S27–S45, 2019. [CrossRef]
- L. Lin et al., Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation, Int. J. Pharm., vol. 533, no. 1, pp. 84–92, 2017. [CrossRef]
- F. D. Battistini, M. E. Olivera, and R. H. Manzo, Equilibrium and release properties of hyaluronic acid-drug complexes, Eur. J. Pharm. Sci., vol. 49, no. 4, pp. 588–594, 2013. [CrossRef]
- F. D. Battistini, J. Flores-Martin, M. E. Olivera, S. Genti-Raimondi, and R. H. Manzo, Hyaluronan as drug carrier. the in vitro efficacy and selectivity of Hyaluronan-Doxorubicin complexes to affect the viability of overexpressing CD44 receptor cells, Eur. J. Pharm. Sci., vol. 65, pp. 122–129, 2014.
- M. S. Ardusso, R. H. Manzo, and A. F. Jimenez-Kairuz, Comparative study of three structurally related acid polyelectrolytes as carriers of basic drugs: Carbomer, Eudragit L-100 and S-100, Supramol. Chem., vol. 22, no. 5, pp. 289–296, 2010. [CrossRef]
- V. L. Romero, R. H. Manzo, and F. L. Alovero, Enhanced bacterial uptake and bactericidal properties of ofloxacin loaded on bioadhesive hydrogels against Pseudomonas aeruginosa, J. Chemother., vol. 22, no. 5, pp. 328–334, 2010. [CrossRef]
- M. C. Palena, M. C. García, R. H. Manzo, and A. F. Jimenez-Kairuz, Self-organized drug-interpolyelectrolyte nanocomplexes loaded with anionic drugs. Characterization and in vitro release evaluation, J. Drug Deliv. Sci. Technol., vol. 30, pp. 45–53, 2015. [CrossRef]
- M.-G. M. Máiz Carro L, Use of Hyaluronic Acid (HA) in Chronic Airway Diseases., Cells., vol. 9, no. 10, p. 2210, 2020.
- M. Di Cicco, D. Peroni, M. Sepich, M. G. Tozzi, P. Comberiati, and R. Cutrera, Hyaluronic acid for the treatment of airway diseases in children: Little evidence for few indications, vol. 55, no. 8. 2020. [CrossRef]
- USP <616>, Bulk Density and Tapped Density of Powders.
- USP <1174> Powder flow.
- USP <601>. Aerosols, metered-dose inhalers and dry powder inhalers.
- N. A. Peppas, R. Gurny, E. Doelker, and P. Buri, Modelling of drug diffusion through swellable polymeric systems, J. Memb. Sci., vol. 7, no. 3, pp. 241–253, 1980. [CrossRef]
- M. P. Weinstein and J. S. Lewis, The clinical and laboratory standards institute subcommittee on Antimicrobial susceptibility testing: Background, organization, functions, and processes, vol. 58, no. 3. 2020. [CrossRef]
- N. C. Pan, H. C. B. Pereira, M. L. C. da Silva, A. F. D. Vasconcelos, and M. A. P. C. Celligoi, Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses, Appl. Biochem. Biotechnol., vol. 182, no. 1, pp. 276–293, 2017. [CrossRef]
- C. Hao et al., One-pot synthesis of vancomycin-encapsulated ZIF-8 nanoparticles as multivalent and photocatalytic antibacterial agents for selective-killing of pathogenic gram-positive bacteria, J. Mater. Sci., vol. 56, no. 15, pp. 9434–9444, 2021. [CrossRef]
- B. P. Sullivan, N. El-Gendy, C. Kuehl, and C. Berkland, Pulmonary Delivery of Vancomycin Dry Powder Aerosol to Intubated Rabbits, Mol. Pharm., vol. 12, no. 8, pp. 2665–2674, 2015.
- A. M. Vasi, M. I. Popa, M. Butnaru, G. Dodi, and L. Verestiuc, Chemical functionalization of hyaluronic acid for drug delivery applications, Mater. Sci. Eng. C, vol. 38, no. 1, pp. 177–185, 2014. [CrossRef]
- M. E. Olivera, R. H. Manzo, F. Alovero, A. F. Jimenez-Kairuz, and M. V. Ramírez-Rigo, Polyelectrolyte-drug ionic complexes as nanostructured drug carriers to design solid and liquid oral delivery systems. Elsevier Inc., 2017.
- L. Chen, T. Okuda, X. Y. Lu, and H. K. Chan, Amorphous powders for inhalation drug delivery, Adv. Drug Deliv. Rev., vol. 100, pp. 102–115, 2016. [CrossRef]
- T. Parumasivam, A. S. Ashhurst, G. Nagalingam, W. J. Britton, and H. K. Chan, Inhalation of Respirable Crystalline Rifapentine Particles Induces Pulmonary Inflammation, Mol. Pharm., vol. 14, no. 1, pp. 328–335, 2017. [CrossRef]
- L. Máiz Carro and M. Blanco-Aparicio, New Inhaled Antibiotics and Forms of Administration, Open Respir. Arch., vol. 2, no. 3, pp. 251–264, 2020.
- A. K. Goyal, T. Garg, S. Bhandari, and G. Rath, Advancement in pulmonary drug delivery systems for treatment of tuberculosis. Elsevier Inc., 2017.
- B. Chaurasiya and Y. Y. Zhao, Dry powder for pulmonary delivery: A comprehensive review, Pharmaceutics, vol. 13, no. 1, pp. 1–28, 2021. [CrossRef]
- T. Peng et al., Influence of physical properties of carrier on the performance of dry powder inhalers, Acta Pharm. Sin. B, vol. 6, no. 4, pp. 308–318, 2016. [CrossRef]
- F. Martinelli et al., Engineered sodium hyaluronate respirable dry powders for pulmonary drug delivery, Int. J. Pharm., vol. 517, no. 1–2, pp. 286–295, 2017. [CrossRef]
- Ceschan NE, Rosas MD, Olivera ME, Dugour AV, Figueroa JM, Bucalá V, Ramírez-Rigo MV., Development of a Carrier-Free Dry Powder Ofloxacin Formulation With Enhanced Aerosolization Properties, J Pharm Sci., vol. 103, no. 9, pp. 2787-2797. [CrossRef]
- J. Kozáková et al., Dry powder inhaler of colistimethate sodium for lung infections in cystic fibrosis: optimization of powder construction, Drug Dev. Ind. Pharm., vol. 45, no. 10, pp. 1664–1673, 2019. [CrossRef]
- T. M. Crowder, J. A. Rosati, J. D. Schroeter, A. J. Hickey, and T. B. Martonen, Fundamental effects of particle morphology on lung delivery: Predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development, Pharm. Res., vol. 19, no. 3, pp. 239–245, 2002. [CrossRef]
- L. Gradon and T. R. Sosnowski, Formation of particles for dry powder inhalers, Adv. Powder Technol., vol. 25, no. 1, pp. 43–55, 2014. [CrossRef]
- P. D. Hickey AJ, Mansour HM, Telko MJ, Xu Z, Smyth HD, Mulder T, McLean R, Langridge J, Physical characterization of component particles included in dry powder inhalers. II. Dynamic characteristics., J Pharm Sci., vol. 96, no. 5, pp. 1302–19, 2007.
- M. S. Hassan and R. W. M. Lau, Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties, AAPS PharmSciTech, vol. 10, no. 4, pp. 1252–1262, 2009. [CrossRef]
- M. Chougule, B. Padhi, K. Jinturkar, and A. Misra, Development of Dry Powder Inhalers, Recent Pat. Drug Deliv. Formul., vol. 1, no. 1, pp. 11–21, 2008. [CrossRef]
- B. V. Basavaraj, N. Saritha, S. Bharath, R. Deveswaran, and V. Madhavan, Vigna mungo mucilage - A natural polymer in the design of matrix based SR tablet of aceclofenac, Int. J. Pharm. Sci. Rev. Res., vol. 21, no. 2, pp. 125–130, 2013.
- S. Hamedani, S. Yaqoubi, R. Safdari, H. Hamishehkar, and A. Nokhodchi, A novel particle engineering method for the production of inhalable cromolyn sodium powders by a combination of spray drier and nebulizer, J. Drug Deliv. Sci. Technol., vol. 78, no. August, 2022. [CrossRef]
- J. Shur, H. Harris, M. D. Jones, J. S. Kaerger, and R. Price, The role of fines in the modification of the fluidization and dispersion mechanism within dry powder inhaler formulations, Pharm. Res., vol. 25, no. 7, pp. 1631–1640, 2008. [CrossRef]
- M. Malamatari, S. Somavarapu, K. Kachrimanis, M. Bloxham, K. M. G. Taylor, and G. Buckton, Preparation of theophylline inhalable microcomposite particles by wet milling and spray drying: The influence of mannitol as a co-milling agent, Int. J. Pharm., vol. 514, no. 1, pp. 200–211, 2016. [CrossRef]
- A. K. Pilcer G, Rosière R, Traina K, Sebti T, Vanderbist F, New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients., J Pharm Sci., vol. 102, no. 6, pp. 1836–1846, 2013. [CrossRef]
- M. L. Guzman, E. A. Soria, C. Laino, R. H. Manzo, and M. E. Olivera, Reduced food interaction and enhanced gastrointestinal tolerability of a new system based on risedronate complexed with Eudragit E100: Mechanistic approaches from in vitro and in vivo studies, Eur. J. Pharm. Biopharm., vol. 107, pp. 263–272, 2016. [CrossRef]
- Vancomycin monograph in “DrugBank online” Available online: https://go.drugbank.com/drugs/DB00512.
- “Mathematical models of drug release,” Strateg. to Modify Drug Release from Pharm. Syst., 1st ed.; Marcos Luciano Bruschi; 2015, pp. 63–86.
- S. K. Fridkin et al., Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001, Clin. Infect. Dis., vol. 36, no. 4, pp. 429–439, 2003.
- L. A. Canal and H. San, ¿Impacto de la concentración mínima inhibitoria de vancomicina o incorrecto manejo terapéutico en el fracaso clínico por Staphylococcus aureus resistentes a meticilina?, p. 2015, 2015.
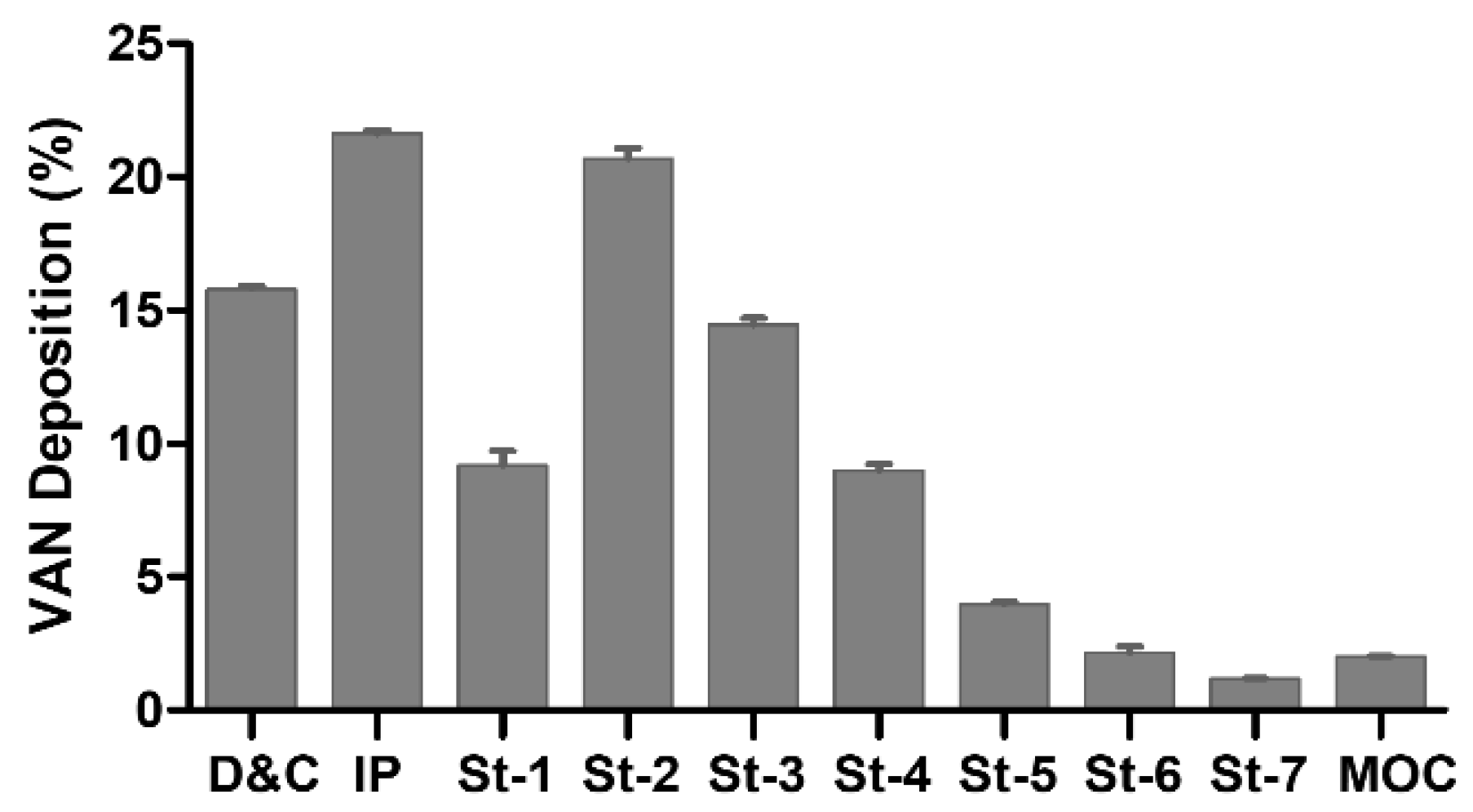
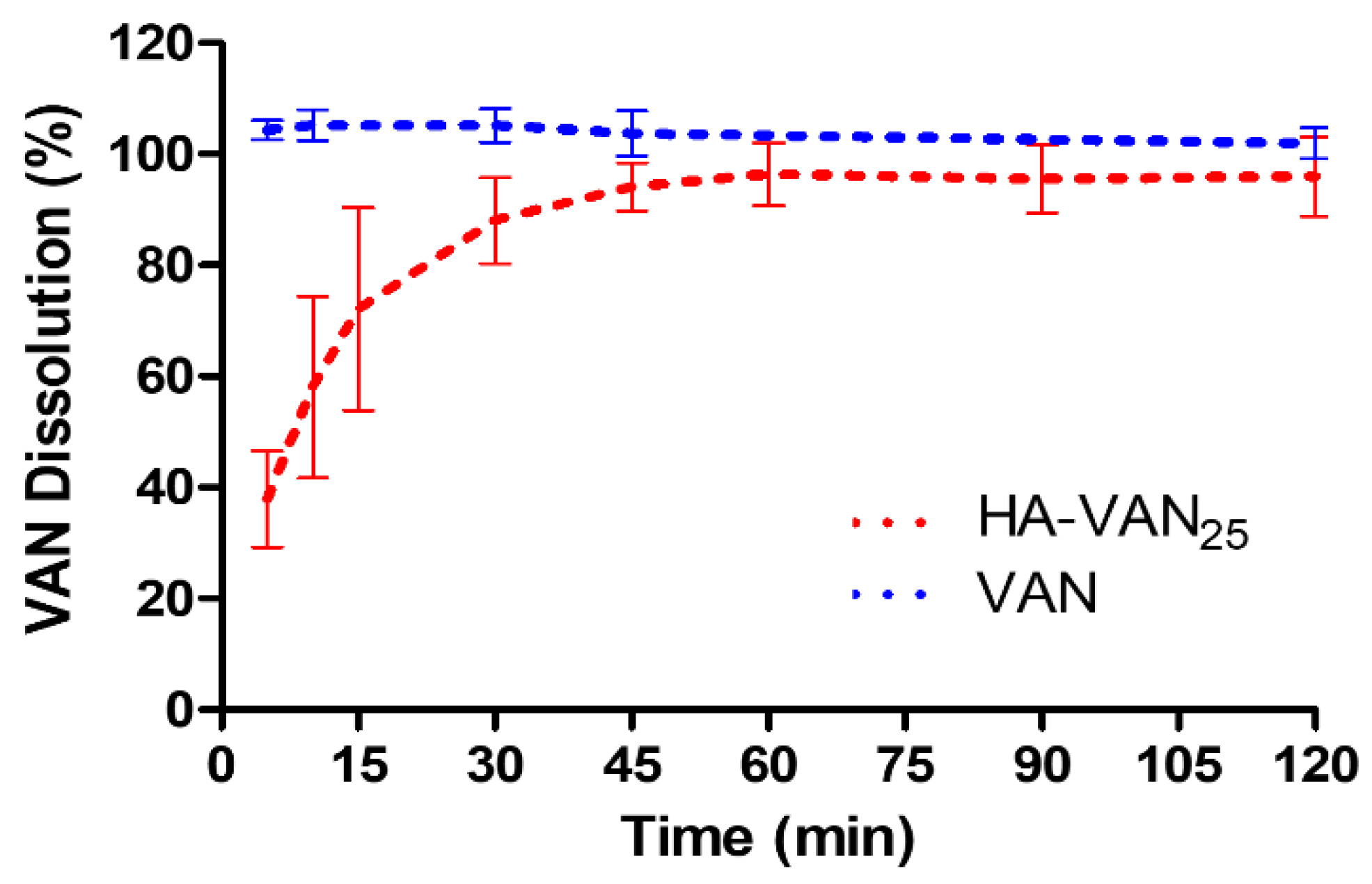
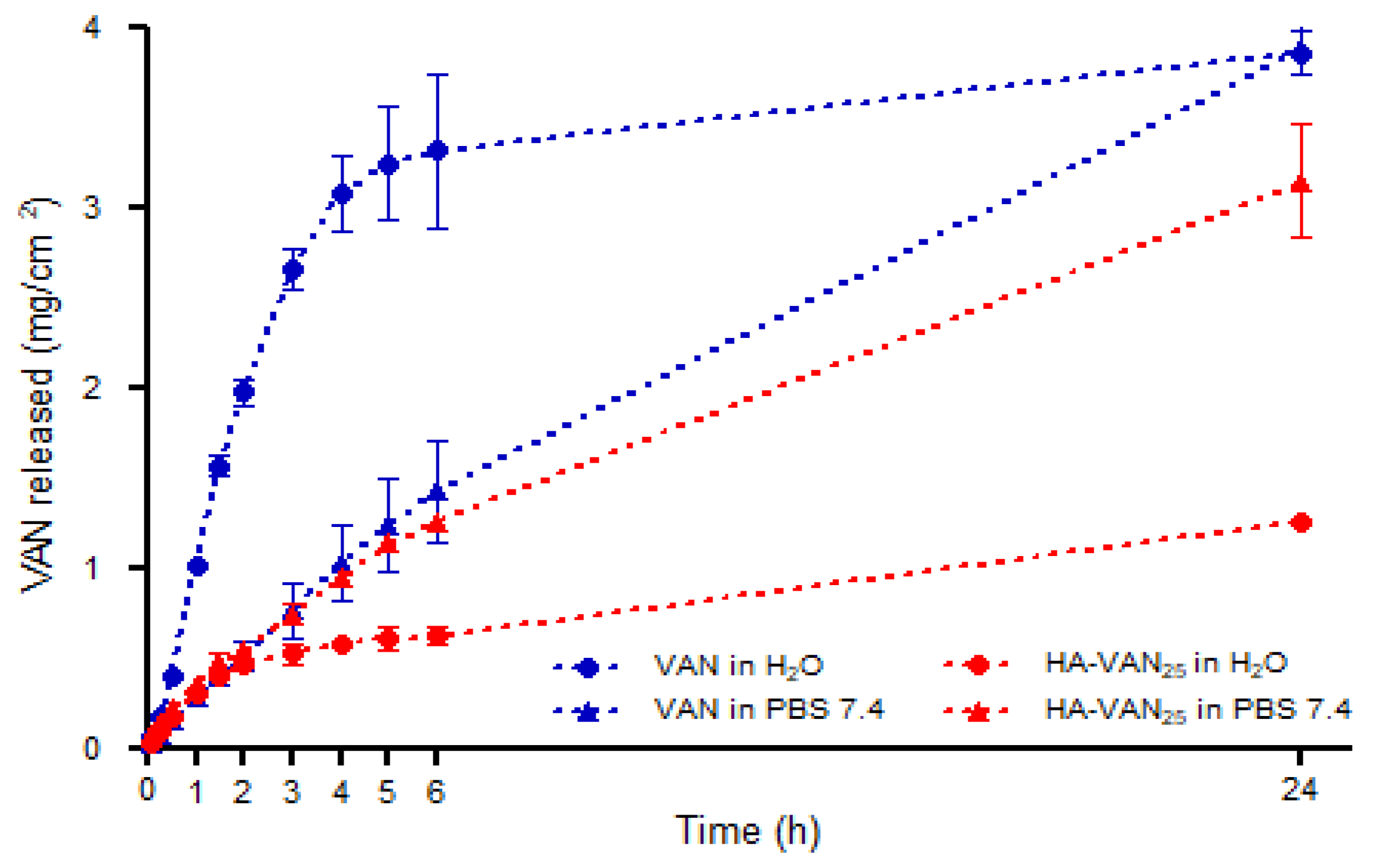
| ED (mg) | EF (%) | FPF (%) | Extra-FPF (%) | MMAD (µm) |
|---|---|---|---|---|
| 7.9 ± 0.4 | 84.2 ± 0.1 | 42.9 ± 0.2 | 25.9 ± 0.1 | 4.29 ± 0.03 |
| Receptor medium | ||||||
|---|---|---|---|---|---|---|
| H20 | PBS 7.4 | |||||
| k | n | R2 | k | n | R2 | |
| HA-VAN25 | 0.5 | 0.60 | 0.96 | 0.4 | 0.67 | 0.99 |
| Samples | 29213 | 25923 | 43300 |
|---|---|---|---|
| HA | 8 mm±0.0 | 8 mm±0.0 | 8 mm±0.0 |
| VAN | 29 mm±0.5 | 31 mm±0.5 | 30 mm±0.5 |
| HA-VAN25 | 30 mm±0.5 | 31 mm±0.5 | 31 mm±0.5 |
| 29213 | 25923 | 43300 | ||||
|---|---|---|---|---|---|---|
| Samples | MIC | MBC | MIC | MBC | MIC | MBC |
| HA | > 2500 | -- | > 2500 | -- | > 2500 | -- |
| VAN | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 |
| HA-VAN25 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





