1. Introduction
Alcoholic beverages containing ethanol (ethyl alcohol, EtOH) have been legally consumed by adults in most countries. Chronic EtOH consumption is associated with many diseases including liver cirrhosis, pancreatitis, memory impairment, colorectal cancer, hypertensive heart disease and infertility [
1]. Particularly, EtOH adversely affects histology and functions of male reproductive tissues in both men and experimental animals. In chronic alcoholic men, their sex hormonal levels such as follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone are decreased [
2]. The same decreases have also been observed in animal models [
3,
4]. Histopathology in the seminiferous tubules have been reported in patients with chronic alcoholism and animals with prolonged EtOH consumption. This includes depletion and dissociation of testicular germ cells in the seminiferous tubule epithelium together with their sloughing into the lumen. These abnormalities result in the decreases in the tubule diameter [
5,
6,
7,
8]. Apoptosis is also increased in testicular germ cells [
7,
8] and lipid accumulation at the base of Sertoli cells is noted in EtOH consuming rats [
9]. All of this histopathology is closely associated with a decreased rate of spermatogenesis [
5,
6,
10] and in some men with heavy EtOH consumption, they could have the Sertoli cell-only syndrome (i.e., Sertoli cells are the main cells in the seminiferous tubules [
6]).
Impairment observed in the testis of males with prolonged EtOH consumption could be from decreased levels of FSH, LH and testosterone. However, EtOH is also metabolized into harmful agents. Acetaldehyde is generated from EtOH through the activity of aldehyde dehydrogenase or CYP2E1 [
11], both present in the testis [
12,
13]. In fact, CYP2E1 expression is induced by EtOH and its catalysis of EtOH generates not only acetaldehyde but also reactive oxygen species (ROS) [
14], which in turn causes peroxidation of polyunsaturated fatty acids (PUFA) [
11]. This lipid peroxidation results in the production of lipid aldehydes such as malondialdehyde (MDA) and other lipid peroxidation products such as hydroxynoenol (HNE). Acetaldehyde, lipid aldehydes (e.g., MDA) and lipid enols (e.g., HNE) are very reactive compounds. They form adducts with DNA and proteins and this could lead to cell apoptosis [
11,
15]. In fact, rats, which consume EtOH for a prolonged period, showed increased levels of MDA in the testis [
8,
16] and a higher degree of testicular germ cell apoptosis [
7,
8,
16,
17,
18,
19].
In certain studies, spermatogenesis is still ongoing in rats prolongedly consuming EtOH. Close to normal organization of testicular germ cells and Sertoli cells in the seminiferous tubules is described in these EtOH rats, although a slight decrease in sperm production is noted. However, only 50% of caudal epididymal sperm are viable and a number of them have abnormal morphology especially in the head region [
20,
21,
22]. The much less severe disruption on spermatogenesis in these studies could be due to lower doses of EtOH in the rat feeding regimens and/or lower susceptibility of these rats to EtOH. Nonetheless, these results suggest that the adverse effects of EtOH may be initially targeted to the post-meiotic events in the seminiferous tubules and/or post-testicular processes in other reproductive organs, such as the epididymis and seminal vesicle. The cauda epididymis is the site where testicular sperm migrate to and become mature. The final sperm chromatin condensation due to an increase in the disulfide bonds of protamines, which allows compact head shape formation, occurs during sperm migration from the caput to cauda epididymis [
23]. Furthermore, caudal epididymal epithelial cells secrete proteins and small metabolites essential for sperm maturation [
24,
25,
26,
27]. Carnitine and glycerophosphocholine (GPC) are metabolites, present at millimolar concentration in the caudal epididymal lumen, which are important for sperm metabolism, motility and survival during their epididymal residence [
28,
29,
30,
31,
32,
33,
34]. In addition to the epididymis, the seminal vesicle is important for sperm survival and acquisition to further fertilizing competence in both male and female reproductive tracts [
35,
36]. It secretes fructose, an energy substrate for sperm, and citrate, a buffering component of the semen [
37,
38,
39,
40]. Although both the epididymis and seminal vesicle are androgen target tissues [
41,
42,
43] and their normal structure and function depend on this hormone, they can also be directly affected by harmful EtOH metabolites, which would result in both structural and functional impairment. In fact, damage induced by prolonged EtOH consumption has been described in the epididymis and in the seminal vesicle. Tangsrisakda and Iamsaard [
20] have described the presence of vacuoles in the epithelial cell layer of the cauda epididymis in EtOH rats as well as round cells in the epididymal lumen. In the caput epididymis of EtOH consuming rats, Pereira et al. [
44] have demonstrated marked accumulation of lipid droplets in the principal and clear cells, whereas Sadeghzadeh et al. [
45] have shown a massive increase of collagen fibers in the spaces surrounding the epididymal duct - an indication of fibrosis. In the seminal vesicle, electron microscopy indicates a decreased number of vacuoles containing secretory granules in the glandular epithelial cells, implicating subfunctionality of the glandular secretion [
46,
47].
In this report, using rats as an experimental model and a prolonged EtOH consumption regimen that still allowed ongoing spermatogenesis, we comprehensively investigated whether the cauda epididymis and seminal vesicle were selectively affected by EtOH on their histology, cellular viability, lipid integrity and metabolite secretion.
2. Materials and Methods
2.1. Animals
Male Wistar rats (10 weeks old; 380-400 g) purchased from Nomura Siam International the Co., Ltd. (Bangkok, Thailand) were boarded in a temperature-controlled room (23 ± 2°C) with a photoperiod of 12 h dark/12 h light. Use and handling of rats were approved by the Institutional Animal Care and Use Committee of Khon Kaen University (protocol: IACUC-KKU-100/64), following instructions of the Ethics of Animal Experimentation of National Research Council of Thailand and ARRIVE checklists and guidelines.
2.2. Administration of Ethanol into Rats
Adult rats (n = 6) were administered 40% (v/v) ethanol (RCI Labscan Ltd., Bangkok, Thailand) of a various volume so that they received 3 g of EtOH per kg body weight. This volume was calculated from the density of EtOH of 0.79 g/ml. The administration was by oral gavage for 56 consecutive days, while control animals (n = 9) were administered water in parallel. The EtOH-administered rats were called EtOH animals.
2.3. Assessment of Overall Behavior and Appearance of the Animals, Body Weight and Weight of Selected Organs
Overall behavior (eating/drinking and movement) and appearance of control rats and EtOH rats were daily assessed. Both control and EtOH animals were weighed daily until the last EtOH/water administration. Then, all animals were euthanized and their various body parts were opened for tissue collection and weighing. These tissues included reproductive organs: the testis, the epididymis and the paired seminal vesicles, and somatic organs: the brain, the paired submandibular glands, the pancreas, the stomach, the liver, the kidney, the spleen and the adrenal gland.
2.4. Histological Analyses of the Epididymis and Seminal Vesicle
The right side of the epididymides and seminal vesicles were rapidly fixed in 10% formalin at room temperature (RT) for 48 h, whereas the left organs were subjected to fluid collection and protein extraction. The fixed cauda epididymis and seminal vesicle were cut in the mid-transverse plane. All tissues were processed by standard methods for the preparation of paraffin-embedded tissue blocks from which sections (5 μm thick) were generated. Sections were randomly taken from 5 control and 5 EtOH rats for staining with hematoxylin and eosin (H/E; Bio-Optica, Milan, Italy), or Masson’s trichrome kit (Abcam, Cambridge, UK) specifically detecting collagen fibers, and viewed under an Axio Imager A2 light microscope (ZEISS, Oberkochen, Germany). Microscopic images on slides were photographed with an AxioCam ICc 5 digital camera and used for morphometric analyses on the following parameters: (1) height of the epithelial cells, (2) distribution of collagen fibers and (3) thickness of smooth muscle layer surrounding the epididymal duct or the seminal vesicle wall. Measurement of these parameters were done in 10 different fields (2 each from each control or EtOH rat) and viewed with a 40X objective lens. At least 100 areas were measured for each parameter. Measurements and processing of the data were performed using ImageJ software (version 1.53k, National Institutes of Health, Bethesda, Maryland, USA). The remaining paraffin sections were used for TUNEL assay and immunofluorescence of caspase enzymes.
2.5. TUNEL Assay
For detection of apoptotic cells on tissue sections, the TUNEL assay kit (Abcam) was used. Sections of the cauda epididymis and seminal vesicle, randomly selected from 5 control and 5 EtOH rats, were deparaffinized and rehydrated and then permeabilized with proteinase K (20 min, RT) following the manufacturer’s protocol. After washing with Tris-buffered saline (TBS, pH 7.4), the endogenous peroxidase on tissue sections was inactivated by incubating with 3% H2O2 for 5 min. Labeling reactions solutions, containing biotin-labeled deoxynucleotide triphosphate and terminal deoxynucleotidyl transferase, were gently dropped onto the sections to react with the exposed 3’OH end of the fragmented DNA strands in apoptotic nuclei, and the sections were incubated (90 min, RT) in a moisture chamber. To probe the incorporated biotinylated nucleotides at the site of fragmented DNA, the sections were further incubated (30 min, RT) with the streptavidin-horseradish peroxidase (HRP) conjugate solution, and then with diaminobenzidine solution (15 min, RT). The TUNEL-positive apoptotic cells were revealed as brown color in the nuclei, and the section was counterstained with methyl green for 3 min and photographed under the Axio ImagerA2 light microscope.
2.6. Immunofluorescence of Caspase Enzymes
Sections of the cauda epididymis and seminal vesicle from 5 control and 5 EtOH rats, were deparaffinized and rehydrated. They were then immersed in the citrate buffer (10 mM citric acid and 0.05% Tween 20, pH 6.0) and heated in a microwave at 560 Watt (5 min, 3 times) for antigen retrieval. After washing with phosphate-buffered saline (PBS, pH 7.4), the sections were permeabilized with 0.2% Triton X-100 (Honeywell, Inc., Charlotte, North Carolina, USA) for 10 min in a moisture chamber. Non-specific binding of proteins was blocked with 3% bovine serum albumin (Merck, Darmstadt, Germany) in PBS for 1 h. Sections were incubated with mouse anti-caspase 9 or mouse-anti caspase 3 antibody (1:100 dilution; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) overnight at 4°C. Following washing out unbound primary antibody with PBS, the sections were incubated (90 min) with the secondary antibody - goat anti-mouse IgG (H+L) conjugated with Alexa Flour 488 (1:300 dilution; Thermo Fisher Scientific, Inc., Waltham, Massachusetts, USA) in a dark moisture chamber. The sections were also incubated (10 min) with Hoechst 33342 (1:10,000 dilution; Abcam) to specifically stain the nuclei. All procedural steps were performed at RT unless mentioned otherwise. After mounting with glycerol, the sections were viewed under a Nikon ECLIPSE 80i epifluorescence microscope (Nikon, Tokyo, Japan) using fluorescein and Hoechst filters.
2.7. Processing of the Cauda Epididymis and Seminal Vesicle for Luminal Fluid Collection and Protein Extraction
These procedures were carried out on the left side of the freshly collected epididymides and seminal vesicles. Since the epididymal duct is very long and highly convoluted with a diameter less than 1 mm, the luminal fluid had to be released during tissue mincing. Briefly, the cauda epididymis (100 mg) of control and EtOH rats was first immersed in 1,000 μl of PBS and minced into small pieces with sterile surgical scissors. This was followed by centrifugation at 14,000 g, at 4°C, for 10 min to sediment the tissue minces. The supernatant was the diluted fluid mainly from the epididymal duct lumen and called the “caudal epididymal fluid (CEF)”, although it would also contain a small portion of fluid from the interstitial spaces. The lysate was then prepared from the sedimented epididymis tissue minces by treatment on ice with 1,000 μl of RIPA buffer (Cell Signaling Technology, Inc., Danvers, Massachusetts, USA) supplemented with protease inhibitor cocktails (Merck). The treated tissue suspension was homogenized with a tissue grinder and then sonicated with an ultrasonic processor (Cole-Parmer, Vernon Hills, Illinois, USA) at 20 kHz and 10 times for a few seconds each time on ice. Subsequently, the sonicated suspension was centrifuged at 14,000 g, at 4°C, for 10 min to pellet cellular particulates. The supernatant containing extracted proteins from the cauda epididymis was then stored at -20°C until use for biochemical work.
The seminal vesicle is a sacculated glandular tissue with the lumen of a substantial volume. To collect fluid from the seminal vesicle lumen, the proximal end of the organ was held with a pair of forceps. By squeezing the tissue towards the distal end using a pair of non-tooth forceps, the luminal fluid was released into a tube containing 1,000 μl of PBS. The fluid suspension was then centrifuged (14,000 g, 4°C, 10 min) and the supernatant obtained was called the “seminal vesicle fluid (SVF)”. The sedimented seminal vesicle tissue was then weighed and 100 mg was taken for further protein extraction. This was started with rinsing the fluid-voided tissue in a beaker containing PBS followed by removing the tissue from this PBS and placing it in another tube. The tissue was then added with 1,000 μl of RIPA buffer supplemented with protease inhibitor cocktails before mincing the tissue into small pieces, and the suspension was homogenized and sonicated as described above for the epididymis tissue minces. Following centrifugation (14,000 g, 4°C, 10 min) to pellet cellular particulates, the supernatant containing extracted proteins from the fluid-voided seminal vesicle was stored at -20°C until use for biochemical work.
2.8. Protein Quantification
Proteins in the CEF and SVF and those extracted from the cauda epididymis and seminal vesicle tissue minces were quantified on a NaNoDrop spectrophotometer (Thermo Fisher Scientific, Inc.) at A280, assuming the mass extinction coefficient (1%) of 10.
2.9. Immunoblotting of Caspase Enzymes
Total proteins (50 μg) extracted from the cauda epididymis and seminal vesicle from the same 5 control and 5 EtOH rats, selected for caspase immunofluorescence, were separated on 12% SDS-PAGE [
48] and electrotransferred onto nitrocellulose membrane [
49]. Non-specific protein binding to the membrane was blocked with 5% skim milk dissolved in TBST (TBS containing 0.1% Tween, pH 7.4). The membrane was incubated (4°C, overnight) with mouse anti-caspase 9 IgG or mouse anti-caspase 3 IgG antibodies (1:1,000 dilution; Santo Cruz Biotechnology, Inc.) and then with the secondary antibody - rabbit anti-mouse IgG conjugated with HRP (1:5,000 dilution; Merck). The membrane was also probed for the housekeeping protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using the primary antibody - mouse anti-GAPDH IgG (1:10,000 dilution; Abcam) and the secondary antibody - rabbit anti-mouse IgG conjugated with HRP (1:5,000 dilution; Merck). TBST was used to wash the membrane between each incubation step. The antigen-antibody complexes on the membrane were detected using an enhanced chemiluminescence (GE Healthcare, Chicago, Illinois, USA) system and visualized under a Gel Documentation 4 (GE Healthcare). The relative intensity of caspase protein (both the proprotein and cleaved form) to that of GAPDH was analyzed using the ImageJ software (National Institutes of Health). The average intensity of the enzyme bands of control rats was normalized to 1. Therefore, the average expression of the enzymes in EtOH rats was relative to the average control values of 1.
2.10. Quantification of Malondialdehyde (MDA)
The CEF and SVF samples along with the RIPA extracts of the minced cauda epididymis and seminal vesicle were from 5 randomly selected control and 5 EtOH rats. Levels of MDA in the fluid and tissue extract were measured as previously described [
21]. Briefly, samples were mixed with thiobarbituric acid (TBA) solution containing 8.1% SDS (Bio-Rad Laboratories, Inc., Hercules, California, USA), 0.8% TBA (Merck), and 20% acetic acid (RCI Labscan Ltd.). The mixtures were then heated (95°C, 60 min) to allow formation of the pink MDA-TBA adduct. After cooling down, the mixtures were added with
n-butanol and pyridine (15:1 dilution; RCI Labscan Ltd.) and centrifuged (4,000
g, 10 min). The collected pink supernatant was measured for its absorbance at 543 nm. A standard curve of 1,1,3,3-tetramethoxypropane (Merck) ranging from 0-16 nmol/ml was constructed with R
2 close to 1 and used for reading MDA levels in the caudal epididymal and seminal vesicle samples. Data were expressed as ng MDA/mg extracted protein.
2.11. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
The caudal epididymal or seminal vesicle fluid (CEF and SVF, 300 μl each) from 5 randomly selected control and 5 EtOH rats was mixed with 300 μl of NMR buffer (0.075 M Na
2HPO
4, 2 nM NaN
3, 0.08% trimethylsilylpropanoic acid (TSP), pH 7.4) and centrifuged (20,000
g, 4°C, 15 min). The supernatant (550 μl) of each sample was transferred into a 400 MHz NMR spectrometer (Bruker, Billerica, Massachusetts, USA). All
1H NMR spectra obtained were adjusted with the MATLAB R2015a software (MathWorks, Inc., Natick, Massachusetts, USA). The water peak (4.5 to 5.0 ppm) and TSP peak (-1 to 0.551 ppm) regions were removed. Identification of metabolites in both fluid samples was performed using Statistical Total Correlation Spectroscopy (STOCSY) available in the MATLAB software. Then, identified metabolites were confirmed with the online database, Chenomx NMR Suite software (Chenomx, Inc., Edmonton, Alberta, Canada) and Human Metabolome Database (HMDB,
https://hmdb.ca), based on the correlation of their specific chemical shifts. To quantify the metabolites in each fluid, the maximum intensity obtained from individual peaks identified in
1H NMR spectra was calculated for the concentration by referencing to the known TSP concentration [
50]. Because the fluid sample was diluted twofold with the NMR buffer for the analysis, the obtained concentration of each metabolite was multiplied by 2 to obtain its concentration in CEF and SVF. Metabolomics profiling of all fluid samples was conducted at Khon Kaen University Phenome Centre, Khon Kaen, Thailand.
2.12. Statistical Analysis
Except for body weight data, which were processed by two-way ANOVA with Tukey’s multiple comparison, all data were subjected to unpaired t test (if data were normally distributed) or Mann-Whitney test (if data were abnormally distributed) to compare the difference between control and EtOH-treated groups. Statistical analyses were carried out using GraphPad Prism 9 (GraphPad Software, Inc., Boston, Massachusetts, USA). Statistically significant differences were considered when P was <0.05. Data were expressed as mean ± standard deviation (SD). For metabolomics analysis, the orthogonal partial least squares-discriminant analysis (OPLS-DA) was performed using SIMCA software (Sartorius, Göttingen, Germany). The validity of all OPLS-DA models was determined by R2 and Q2 values. To indicate the validation of these models, they were further assessed by analysis of variance testing of cross-validated predictive residuals (CV-ANOVA) and considered when permutation P was <0.05.
4. Discussion
In this report, we demonstrated several damage features of the cauda epididymis and seminal vesicle of rats with prolonged EtOH consumption following the regimen that still allows ongoing spermatogenesis [
20,
21]. The presumption that spermatogenesis still continued in our experiment was supported by our findings that the weight of the testis of EtOH rats was not different from that of control rats (
Figure 1) and the regular size of the sperm mass still existed in >95% of the caudal epididymal lumen in the tissue cross sections of EtOH rats. The damage features in the cauda epididymis and seminal vesicle included decreases in size (weight), histopathology, increases in lipid peroxidation (revealed by elevated MDA levels) and rises in apoptosis in the two tissues, relative to the corresponding parameters in control-untreated animals.
Histopathology in the cauda epididymis included swelling of epididymal basal cells and dislocalization of epididymal epithelial cell nuclei together with the reduced height of the epithelial cells. It was likely that the adverse effects of EtOH on this tissue manifested through the topical direct action of harmful EtOH metabolites rather than systemic consequences of the markedly reduced levels of male reproductive hormones (FSH, LH and androgen) [
2,
3,
4], since by the time that these hormones were much decreased, spermatogenesis would have been arrested. Nonetheless, we cannot exclude the possibility that the effect of EtOH on the spleen and adrenal gland as seen by their weight changes in EtOH rats (
Figure 1A) might have consequences on histopathology of the epididymis and seminal vesicle. For the mechanisms involving direct action of EtOH metabolites, ROS produced during EtOH conversion to acetaldehyde by CYP2E1 [
14] would induce peroxidation of PUFA, thus generating various lipid aldehydes including MDA (from
n-3 PUFA) and other lipid aldehydes [
11], as well as other lipid peroxidation products [
11]. In fact, CYP2E1 is present in the epididymis [
13] and its expression is enhanced by EtOH [
11,
51]. MDA, other lipid aldehydes and lipid peroxidation products would form adducts with proteins and DNA and this could cause apoptosis and cellular damage [
52]. Notably, increases in MDA levels were observed in the cauda epididymis of EtOH rats (
Figure 4). Acetaldehyde, a small lipid aldehyde, was generated from EtOH by not only the enzyme CYP2E1 [
14] but also aldehyde dehydrogenase present in the epididymis [
12,
53]. Acetaldehyde can also form adducts with DNA and proteins, thus adding to the induction of apoptosis (
Figure 5) and cellular damages, as revealed in the histopathology (
Figure 2) in the cauda epididymis of EtOH rats.
Histopathology was also observed in the seminal vesicle of EtOH rats. This included decreases in mucosal folding and epithelial cell height, disorganization of some areas of the epithelial cell layer and appearance of pyknotic nuclei (
Figure 3). Although CYP2E1 has not been described in the seminal vesicle, the increase in MDA levels in this tissue of EtOH rats suggested its existence (
Figure 4), and the increase in apoptosis (
Figure 6) further implicated that DNA and protein adduct formation may have occurred in the seminal vesicle of EtOH rats likely through similar mechanisms as described above for the epididymis.
Interestingly, the muscle layer of both the epididymis and seminal vesicle in EtOH rats was enlarged with increased distribution of the collagen fibers (
Figure 2 and
Figure 3). There was also increased amounts of the collagen fibers in the interstitial space of the cauda epididymis in EtOH rats (
Figure 2). Our finding agrees with the previous report on the increase of the collagen fibers in the same area in the caput epididymis [
45]. The abnormally higher amounts of collagen would lead to fibrosis and stiffness of the muscle layer [
54] of the epididymis and seminal vesicle. Consequently, the secretion of metabolites into the lumen of these two organs may be defective due to abnormality of the muscle contraction.
The epididymis and seminal vesicle are male reproductive organs, which support maturation, survival and signaling/development of sperm, so that they gain fertilizing ability, prior to their encountering with mature ovulated eggs [
24,
25,
26,
27,
30,
35,
36,
55,
56]. Metabolites secreted by these organs are important for these processes. In EtOH rats, the levels of a number of metabolites in the epididymis and seminal vesicle, important for sperm metabolism/maturation/survival were decreased. In untreated animals, the epididymal lumen contains carnitine, GPC and myo-inositol at very high levels, as well as fructose, fructose 2,6-bisphosphate and alanine at substantial levels [
38,
57]. Carnitine shuttles fatty acyl chains by forming acylcarnitine through the mitochondrial membrane for β-oxidation, which generates energy [
31]. Carnitine also converts acetyl-CoA into acetylcarnitine. This lowers the mitochondrial concentration of acetyl-CoA, which would otherwise inhibit pyruvate dehydrogenase, an integral enzyme in the tricarboxylic acid (TCA) cycle, and subsequently energy production [
31]. Acetylcarnitine also directly increases the sperm motility rates [
32]. GPC and myo-inositol play a role in osmotic regulation of the epididymal fluid [
33,
34,
58]. In addition, myo-inositol can be converted to be derivatives, including phosphatidylinositol phosphate (PIP), PIP3, IP3 and IP4, all important for sperm membrane modifications, motility and signal activation [
58]. Fructose present in both the epididymal and seminal vesicle lumen enters sperm to provide energy through the glycolytic pathway [
37], whereas fructose 2,6-bisphosphate, a component of the epididymal fluid, enhances glycolysis through insulin action [
59]. The amino acid, alanine, present in the epididymal lumen can also be metabolized by sperm to pyruvate to enter the TCA cycle for energy production [
60]. In the seminal vesicle lumen, fructose and citrate are present at very high levels. While fructose undergoes glycolysis to provide energy to sperm as stated above, citrate gives buffering capacity to semen for its physiological pH [
38,
39,
40]. Glycerate and lactate in the seminal vesicle fluid are also energy providing metabolites, with the former entering the glycolytic pathway and the latter the TCA cycle [
37]. Finally, leucine and isoleucine (which could isomerize to leucine) in the seminal vesicle fluid can stimulate motility [
61]. All metabolites mentioned herein were significantly decreased in the fluid from the epididymis and/or seminal vesicle in EtOH rats, as shown by our
1H NMR analyses (
Table 1). It is possible that these decreases could cause the observed histological abnormality of these two tissues or vice versa. Certainly, these decreases in metabolites would lead to improper maturation and development of sperm as well as their decreased ability to survive in EtOH males and eventually this would lead to male infertility/subfertility as described previously [
10,
62,
63,
64].
In summary, our studies using rats as an experimental model suggested that initial adverse effects of EtOH after its prolonged consumption occurred at the cauda epididymis and seminal vesicle in both structural and functional aspects. Our findings herein corroborate the previous observations in EtOH rats on the decrease of caudal epididymal sperm with normal morphology concurrently with the 50% increase in non-viability of these sperm [
20,
21], and it is likely that sperm from EtOH rats have much decreased fertilizing ability. Our results in rats describing the epididymis and seminal vesicle as the initial targets of EtOH-induced male subfertility should be validated in men. While the experimental approaches on evaluating histopathology and increases in lipid peroxidation and apoptosis of the two tissues are not practical to perform in humans, the
1H NMR profiling of metabolites integral to sperm development and survival can be implemented in seminal plasma prepared from ejaculated semen. Seminal plasma together with “omics” approaches has served as a diagnostic fluid for disorder of male reproductive physiology [
65], and specifically this NMR analysis approach has been widely used to assess human male fertility/infertility [
66,
67,
68,
69,
70,
71]. Since seminal plasma contains secretion from the epididymis and seminal vesicle, metabolites that come from the epididymis (such as carnitine and GPC) and from the seminal vesicle (such as fructose) should be quantified in EtOH consuming men and compared with those in control men with no EtOH consumption. Decreased levels of these metabolites in seminal plasma of EtOH men together with low quality of their ejaculated sperm and failure to impregnate their partners would be an indication of impairment to their epididymis and/or seminal vesicle, which causes subfertility/infertility, and they should be advised to stop EtOH consumption. Since the continuation of EtOH consumption especially at high amounts will lead to damages of other organs with severe adverse effects (such as cirrhosis) in due time, cessation of EtOH consumption following the warning from the seminal plasma metabolite data will prevent the consumers’ health from delving further into an abysmal state.
Figure 1.
A: Body weight growth curve of control and EtOH male rats during the experimental period of 56 days. Means ± SD’s of the weekly body weight of each group were shown. Day 1 and Day 56 was the first and last day, respectively, of the EtOH oral administration to the EtOH rat group. The EtOH treatment regimen was 3 g of EtOH per kg body weight daily. Water of the same weight as EtOH was given instead to the control animals. There were 9 control (orally fed with water) rats and 6 EtOH rats. Two-way ANOVA showed no significant differences in the body weight increase between the control and EtOH groups during this 56-day period. B: Weight of the testis, epididymis and paired seminal vesicles of control and EtOH rats. Mean ± SD of the weight of each left and right testis and of each left and right epididymis from control males (n = 18) and EtOH counterparts (n = 16) is shown in the bar graphs. However, both seminal vesicles from each animal were removed as a pair from the animal for weighing. Therefore, mean ± SD of the weight shown was of the two seminal vesicles of each animal (n = 9 and 6 for control and EtOH animals, respectively). Note significant decreases in the weight of the epididymis and seminal vesicles of EtOH rats, compared with that of the control counterparts. C: Weight of various somatic organs (the brain, the paired submandibular salivary glands, the pancreas, the stomach, the liver, the kidney, the spleen and the adrenal gland) of control and EtOH rats. Each of the left and right kidneys and adrenal glands of each animal was weighed. Therefore, the number of weight values of these two organs was 18 for control animals and 12 for EtOH rats, whereas the number of values of other organs was 9 and 6 for control and EtOH rats, respectively. Note significant differences of the weight of the spleen and the adrenal gland between the EtOH and control rats. *, **, *** denote statistical differences with P <0.05, 0.01 and 0.001, respectively.
Figure 1.
A: Body weight growth curve of control and EtOH male rats during the experimental period of 56 days. Means ± SD’s of the weekly body weight of each group were shown. Day 1 and Day 56 was the first and last day, respectively, of the EtOH oral administration to the EtOH rat group. The EtOH treatment regimen was 3 g of EtOH per kg body weight daily. Water of the same weight as EtOH was given instead to the control animals. There were 9 control (orally fed with water) rats and 6 EtOH rats. Two-way ANOVA showed no significant differences in the body weight increase between the control and EtOH groups during this 56-day period. B: Weight of the testis, epididymis and paired seminal vesicles of control and EtOH rats. Mean ± SD of the weight of each left and right testis and of each left and right epididymis from control males (n = 18) and EtOH counterparts (n = 16) is shown in the bar graphs. However, both seminal vesicles from each animal were removed as a pair from the animal for weighing. Therefore, mean ± SD of the weight shown was of the two seminal vesicles of each animal (n = 9 and 6 for control and EtOH animals, respectively). Note significant decreases in the weight of the epididymis and seminal vesicles of EtOH rats, compared with that of the control counterparts. C: Weight of various somatic organs (the brain, the paired submandibular salivary glands, the pancreas, the stomach, the liver, the kidney, the spleen and the adrenal gland) of control and EtOH rats. Each of the left and right kidneys and adrenal glands of each animal was weighed. Therefore, the number of weight values of these two organs was 18 for control animals and 12 for EtOH rats, whereas the number of values of other organs was 9 and 6 for control and EtOH rats, respectively. Note significant differences of the weight of the spleen and the adrenal gland between the EtOH and control rats. *, **, *** denote statistical differences with P <0.05, 0.01 and 0.001, respectively.
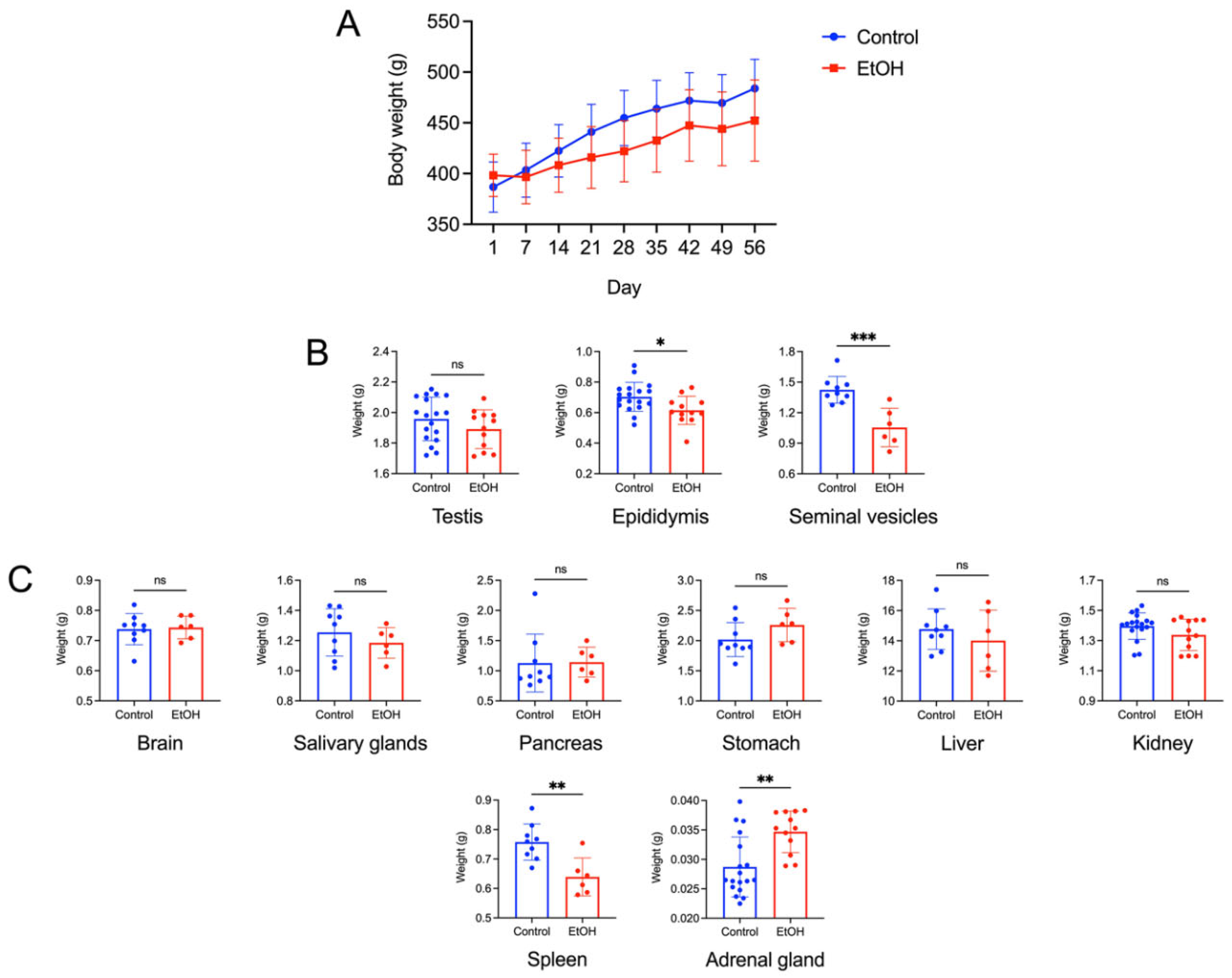
Figure 2.
Prolonged EtOH oral administration induced histological damages to the rat epididymis. Sections of the cauda epididymis were stained with H/E in A, B and C panels, and with Masson’s trichrome in D panel. Low magnification images of the epididymal duct cross sections with the sperm mass content are displayed in A panel. A smaller sperm mass was observed in about 5% of the epididymal lumen sections of EtOH rats (asterisk). Changes of the epididymal duct epithelium in EtOH rats were observed at higher magnification (B and C). Various types of epithelial cells with the well-aligned basement membrane and apical membrane were seen in the epididymal ducts of control rats. In contrast, there was a swelling of basal cells (green arrowheads) and deletion of the apical membrane surface of the principal cell (blue arrowhead) in EtOH rats. In control rats, the nearly-round shaped nuclei of principal cells were well-aligned and situated at some distance above the basement membrane with the chromatin in the diffuse state. Contrarily, a number of the principal cell nuclei (pink arrowheads) had the chromatin in the condensed state and were flattened down right above the basement membrane (B, top and bottom of the right panels). Pyknotic nuclei were also observed in some basal cells of EtOH rats (example in B, middle right panel - orange arrowhead). The height of the epididymal epithelial cells of EtOH rats was significantly lower than that of control cells (C). Using Masson’s trichrome dye to specifically stain collagen fibers (blue staining), the percentage of collagen fiber area and the thickness of the smooth muscle area (marked by a red cross-bar) were shown to be significantly increased in EtOH rats as compared with those in control animals (D). Note that there were more of collagen fibers in both the muscle layer and interstitial space of EtOH rats. The scale bar in the insets in C is 20 μm. Note that all histology images of the cauda epididymis shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for the histology studies. However, the morphometric analyses were performed from quantitative data obtained from the 5 control and 5 EtOH rats and the data were expressed as mean ± SD. *** denotes statistical differences with P <0.001.
Figure 2.
Prolonged EtOH oral administration induced histological damages to the rat epididymis. Sections of the cauda epididymis were stained with H/E in A, B and C panels, and with Masson’s trichrome in D panel. Low magnification images of the epididymal duct cross sections with the sperm mass content are displayed in A panel. A smaller sperm mass was observed in about 5% of the epididymal lumen sections of EtOH rats (asterisk). Changes of the epididymal duct epithelium in EtOH rats were observed at higher magnification (B and C). Various types of epithelial cells with the well-aligned basement membrane and apical membrane were seen in the epididymal ducts of control rats. In contrast, there was a swelling of basal cells (green arrowheads) and deletion of the apical membrane surface of the principal cell (blue arrowhead) in EtOH rats. In control rats, the nearly-round shaped nuclei of principal cells were well-aligned and situated at some distance above the basement membrane with the chromatin in the diffuse state. Contrarily, a number of the principal cell nuclei (pink arrowheads) had the chromatin in the condensed state and were flattened down right above the basement membrane (B, top and bottom of the right panels). Pyknotic nuclei were also observed in some basal cells of EtOH rats (example in B, middle right panel - orange arrowhead). The height of the epididymal epithelial cells of EtOH rats was significantly lower than that of control cells (C). Using Masson’s trichrome dye to specifically stain collagen fibers (blue staining), the percentage of collagen fiber area and the thickness of the smooth muscle area (marked by a red cross-bar) were shown to be significantly increased in EtOH rats as compared with those in control animals (D). Note that there were more of collagen fibers in both the muscle layer and interstitial space of EtOH rats. The scale bar in the insets in C is 20 μm. Note that all histology images of the cauda epididymis shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for the histology studies. However, the morphometric analyses were performed from quantitative data obtained from the 5 control and 5 EtOH rats and the data were expressed as mean ± SD. *** denotes statistical differences with P <0.001.
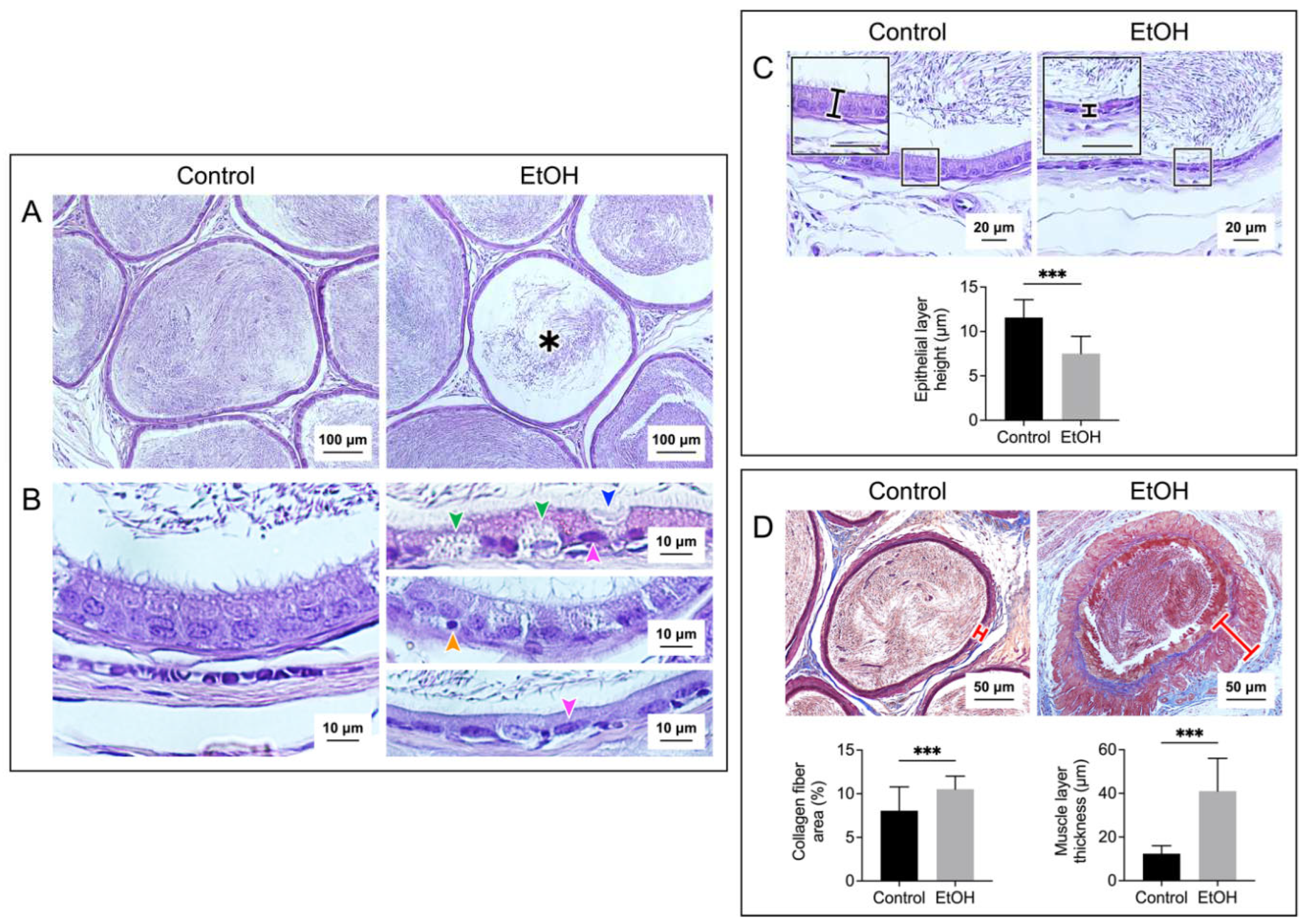
Figure 3.
Prolonged EtOH oral administration induced histological damages to the rat seminal vesicle. Sections of the seminal vesicle were stained with H/E in A, B, and C panels, and with Masson’s trichrome in D panel. The cross sections of the seminal vesicle containing the mucosal epithelium (Ep) and muscle layer (Ms) together with the lumen are displayed in A panel. Decreases in folding were observed in some areas of the mucosal epithelium of EtOH rats (asterisk). Changes of the epithelial cell layers in EtOH were observed at higher magnification (B and C). Epithelial cells with the well-aligned basement membrane and apical membrane were seen in control rats. In contrast, in some areas of the epithelial layer in EtOH rats, there existed pyknotic nuclei (red arrowhead) and cell disorganization (blue arrowheads) (B). Overall, the seminal vesicle epithelial height of EtOH rats was significantly decreased as compared with that of control counterparts (C). Using Masson’s trichrome dye to specifically stain collagen fibers (blue staining), the percentage of collagen fiber area and the thickness of the smooth muscle area (marked by a red cross-bar) were shown to be significantly increased in EtOH rats as compared with those in control animals (D). The scale bar in the insets is 20 μm. Note that all histology images of the seminal vesicle shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for the histology studies. However, the morphometric analyses were performed from quantitative data obtained from the 5 control and 5 EtOH rats. Data were expressed as mean ± SD. **, *** denote statistical differences with P <0.01 and 0.001, respectively.
Figure 3.
Prolonged EtOH oral administration induced histological damages to the rat seminal vesicle. Sections of the seminal vesicle were stained with H/E in A, B, and C panels, and with Masson’s trichrome in D panel. The cross sections of the seminal vesicle containing the mucosal epithelium (Ep) and muscle layer (Ms) together with the lumen are displayed in A panel. Decreases in folding were observed in some areas of the mucosal epithelium of EtOH rats (asterisk). Changes of the epithelial cell layers in EtOH were observed at higher magnification (B and C). Epithelial cells with the well-aligned basement membrane and apical membrane were seen in control rats. In contrast, in some areas of the epithelial layer in EtOH rats, there existed pyknotic nuclei (red arrowhead) and cell disorganization (blue arrowheads) (B). Overall, the seminal vesicle epithelial height of EtOH rats was significantly decreased as compared with that of control counterparts (C). Using Masson’s trichrome dye to specifically stain collagen fibers (blue staining), the percentage of collagen fiber area and the thickness of the smooth muscle area (marked by a red cross-bar) were shown to be significantly increased in EtOH rats as compared with those in control animals (D). The scale bar in the insets is 20 μm. Note that all histology images of the seminal vesicle shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for the histology studies. However, the morphometric analyses were performed from quantitative data obtained from the 5 control and 5 EtOH rats. Data were expressed as mean ± SD. **, *** denote statistical differences with P <0.01 and 0.001, respectively.
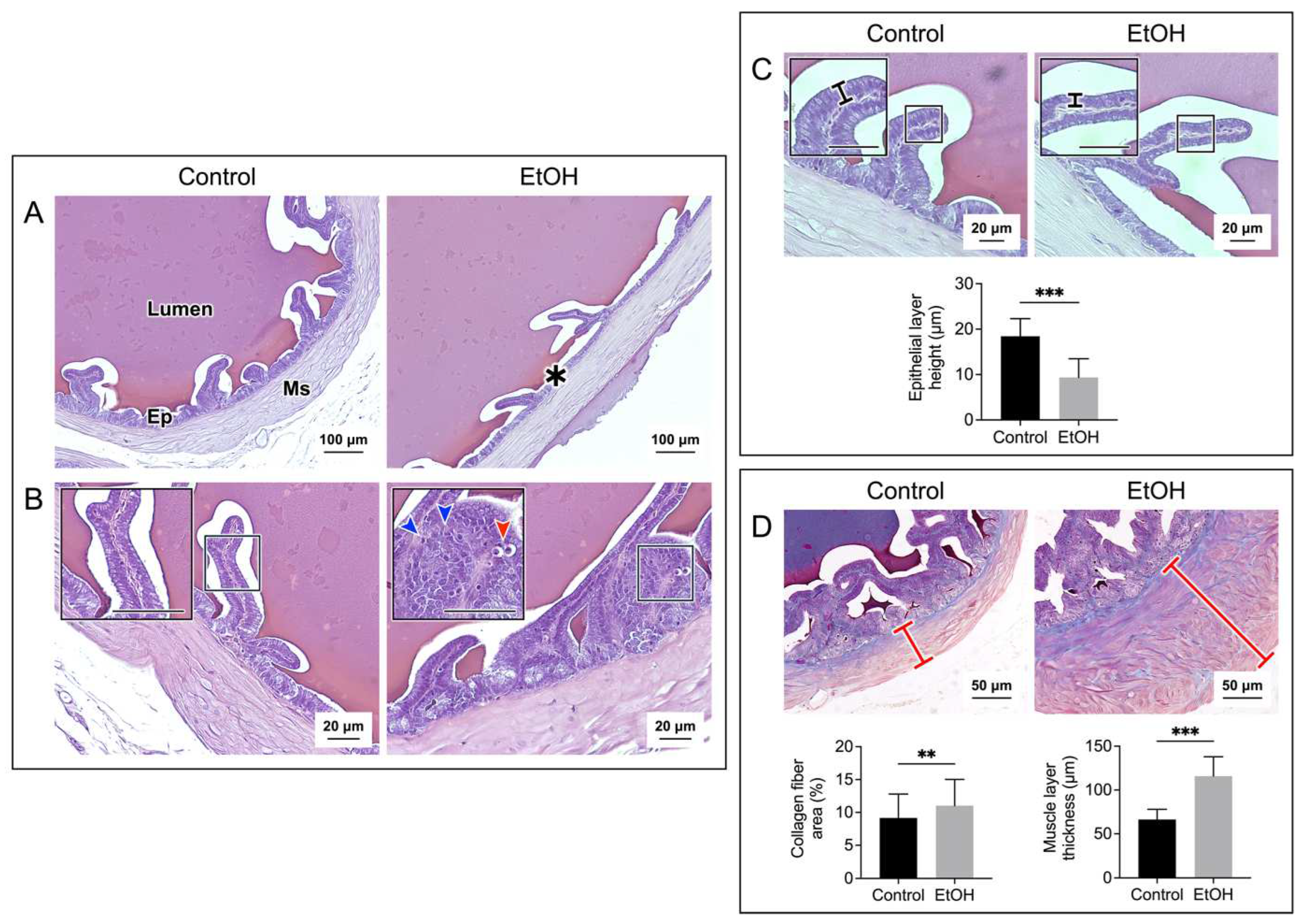
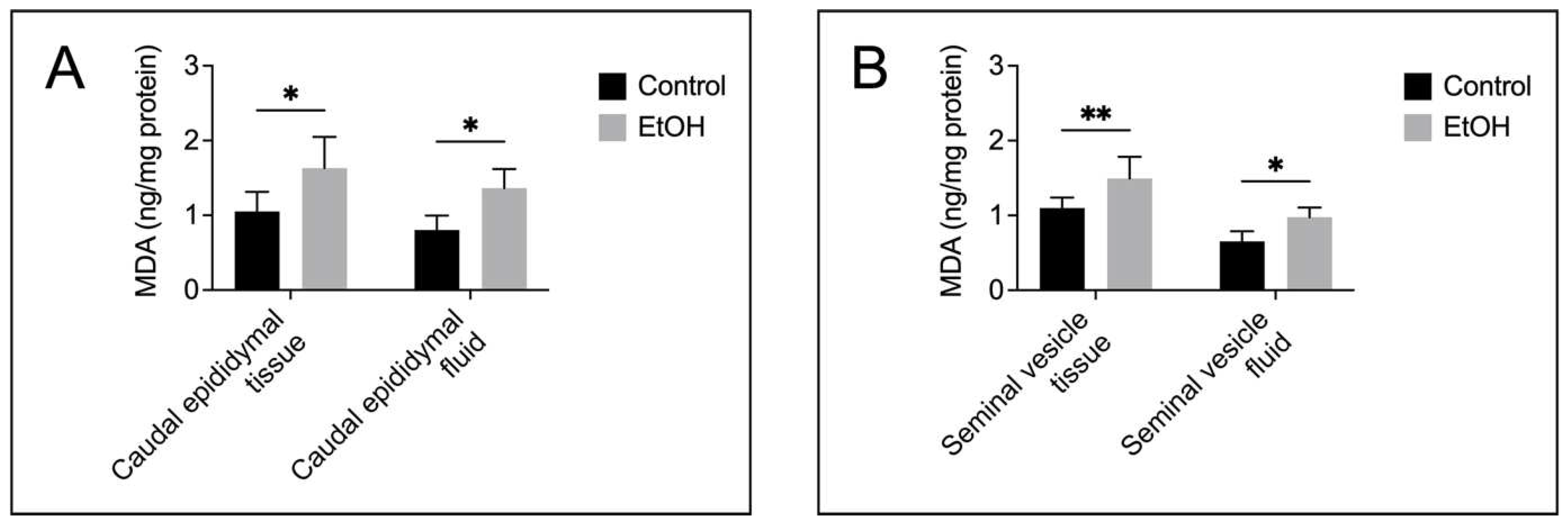
Figure 4.
Increases in lipid peroxidation as demonstrated by malondialdehyde (MDA) levels in the cauda epididymis (A) and seminal vesicle (B) of EtOH rats, compared with those in control animals. The MDA quantification was performed from samples from 5 control rats and 5 EtOH rats. Data were expressed as mean ± SD. *, ** denote statistical differences with P <0.05 and 0.01, respectively.
Figure 4.
Increases in lipid peroxidation as demonstrated by malondialdehyde (MDA) levels in the cauda epididymis (A) and seminal vesicle (B) of EtOH rats, compared with those in control animals. The MDA quantification was performed from samples from 5 control rats and 5 EtOH rats. Data were expressed as mean ± SD. *, ** denote statistical differences with P <0.05 and 0.01, respectively.
Figure 5.
Increases in apoptosis of epididymal epithelial cells of EtOH rats. Apoptosis was demonstrated by the TUNEL assay performed on the cauda epididymis sections (A). The brown signals indicated DNA breakages. The cauda epididymis sections were counterstained with methyl green. Immunofluorescence of caspase 9 (green signals, B) and caspase 3 (green signals, C) was also performed on the cauda epididymis sections. Nuclei of the epithelial cells were counterstained with Hoechst 33342 (blue fluorescence). The scale bar in the insets is 20 μm. Note that the TUNEL and immunofluorescence images of the cauda epididymis shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for these studies. Immunoblotting using anti-caspase 9 and anti-caspase 3 antibodies of the cauda epididymis tissues revealed the procaspase forms and the cleaved forms (B and C) of the enzymes and the levels of their relative expression were quantified by densitometric analyses (bar graphs). The intensity of GAPDH was used to figure out the relative intensity of both the proenzyme and cleaved forms in each cauda epididymis sample from 5 control rats and 5 EtOH rats. The average intensity of the enzyme bands of control rats was then normalized to 1 and this normalization ratio was used to figure out relative intensity of the procaspase forms and cleaved forms of all control and EtOH samples. The relative expression of the procaspase forms and cleaved forms was expressed as mean ± SD. *, *** denote statistical differences with P <0.05 and 0.001, respectively.
Figure 5.
Increases in apoptosis of epididymal epithelial cells of EtOH rats. Apoptosis was demonstrated by the TUNEL assay performed on the cauda epididymis sections (A). The brown signals indicated DNA breakages. The cauda epididymis sections were counterstained with methyl green. Immunofluorescence of caspase 9 (green signals, B) and caspase 3 (green signals, C) was also performed on the cauda epididymis sections. Nuclei of the epithelial cells were counterstained with Hoechst 33342 (blue fluorescence). The scale bar in the insets is 20 μm. Note that the TUNEL and immunofluorescence images of the cauda epididymis shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for these studies. Immunoblotting using anti-caspase 9 and anti-caspase 3 antibodies of the cauda epididymis tissues revealed the procaspase forms and the cleaved forms (B and C) of the enzymes and the levels of their relative expression were quantified by densitometric analyses (bar graphs). The intensity of GAPDH was used to figure out the relative intensity of both the proenzyme and cleaved forms in each cauda epididymis sample from 5 control rats and 5 EtOH rats. The average intensity of the enzyme bands of control rats was then normalized to 1 and this normalization ratio was used to figure out relative intensity of the procaspase forms and cleaved forms of all control and EtOH samples. The relative expression of the procaspase forms and cleaved forms was expressed as mean ± SD. *, *** denote statistical differences with P <0.05 and 0.001, respectively.
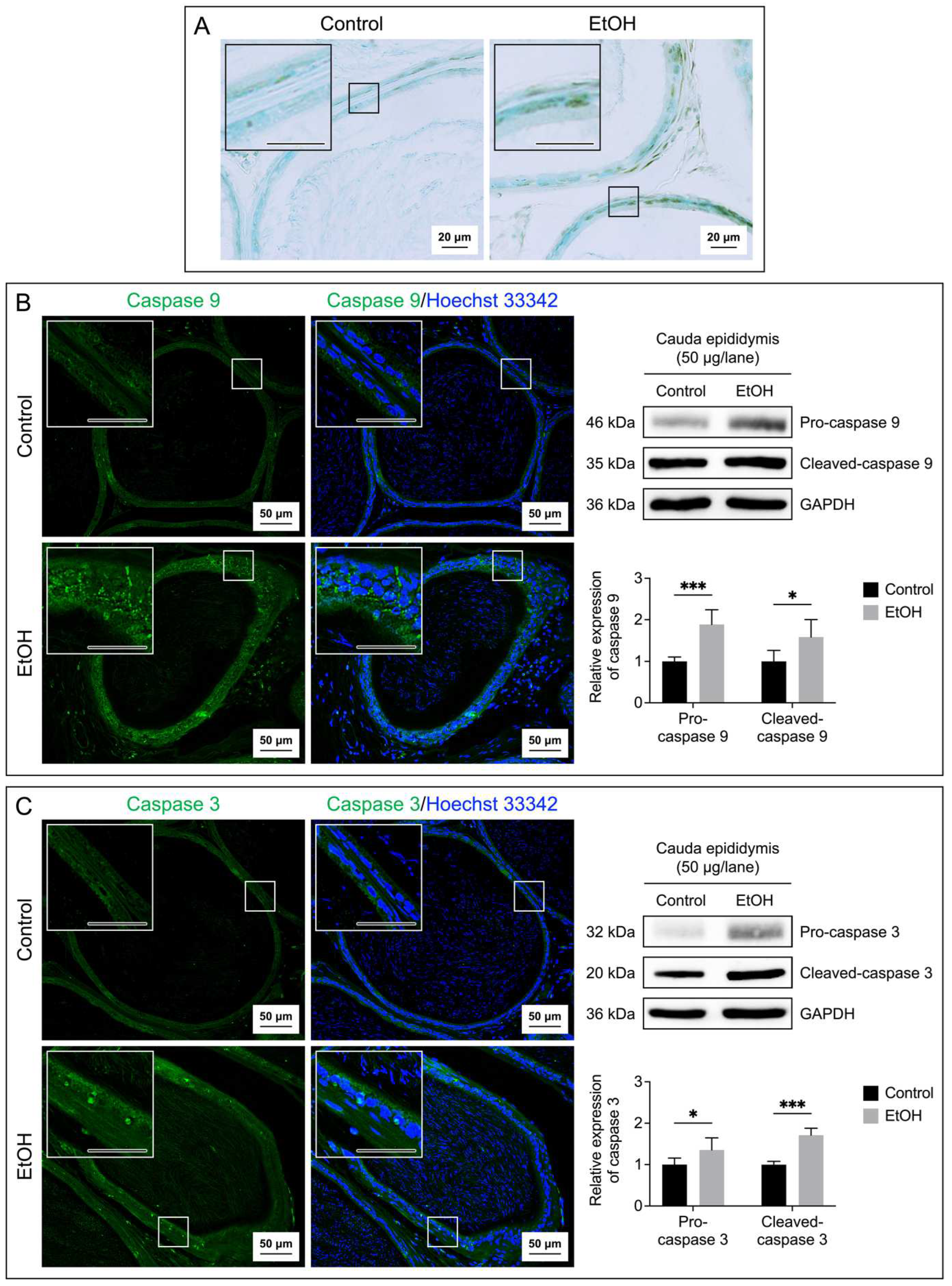
Figure 6.
Increases in apoptosis of seminal vesicle epithelial cells of EtOH rats. Apoptosis was demonstrated by the TUNEL assay performed on the seminal vesicle sections (A). The brown signals indicated DNA breakages. The seminal vesicle sections were counterstained with methyl green. Immunofluorescence of caspase 9 (green signals, B) and caspase 3 (green signals, C) was also performed on the seminal vesicle sections. Nuclei of the epithelial cells were counterstained with Hoechst 33342 (blue fluorescence). The scale bar in the insets is 20 μm. Note that the TUNEL and immunofluorescence images of the seminal vesicle shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for these studies. Immunoblotting using anti-caspase 9 and anti-caspase 3 antibodies of the seminal vesicle tissues revealed the procaspase forms and the cleaved-caspase forms (B and C) of the enzymes and the levels of their relative expression were quantified by densitometric analyses (bar graphs). The intensity of GAPDH was used to figure out the relative intensity of both the proenzyme and cleaved forms in each seminal vesicle sample from 5 control rats and 5 EtOH rats. The average intensity of the enzyme bands of control rats was then normalized to 1 and this normalization ratio was used to figure out relative intensity of the procaspase forms and cleaved forms of all control and EtOH samples. The relative expression of the procaspase forms and cleaved forms was expressed as mean ± SD. *, **, *** denote statistical differences with P <0.05, 0.01 and 0.001, respectively.
Figure 6.
Increases in apoptosis of seminal vesicle epithelial cells of EtOH rats. Apoptosis was demonstrated by the TUNEL assay performed on the seminal vesicle sections (A). The brown signals indicated DNA breakages. The seminal vesicle sections were counterstained with methyl green. Immunofluorescence of caspase 9 (green signals, B) and caspase 3 (green signals, C) was also performed on the seminal vesicle sections. Nuclei of the epithelial cells were counterstained with Hoechst 33342 (blue fluorescence). The scale bar in the insets is 20 μm. Note that the TUNEL and immunofluorescence images of the seminal vesicle shown were from one control rat and one EtOH rat, although the results were representative of the 5 control and 5 EtOH rats used for these studies. Immunoblotting using anti-caspase 9 and anti-caspase 3 antibodies of the seminal vesicle tissues revealed the procaspase forms and the cleaved-caspase forms (B and C) of the enzymes and the levels of their relative expression were quantified by densitometric analyses (bar graphs). The intensity of GAPDH was used to figure out the relative intensity of both the proenzyme and cleaved forms in each seminal vesicle sample from 5 control rats and 5 EtOH rats. The average intensity of the enzyme bands of control rats was then normalized to 1 and this normalization ratio was used to figure out relative intensity of the procaspase forms and cleaved forms of all control and EtOH samples. The relative expression of the procaspase forms and cleaved forms was expressed as mean ± SD. *, **, *** denote statistical differences with P <0.05, 0.01 and 0.001, respectively.
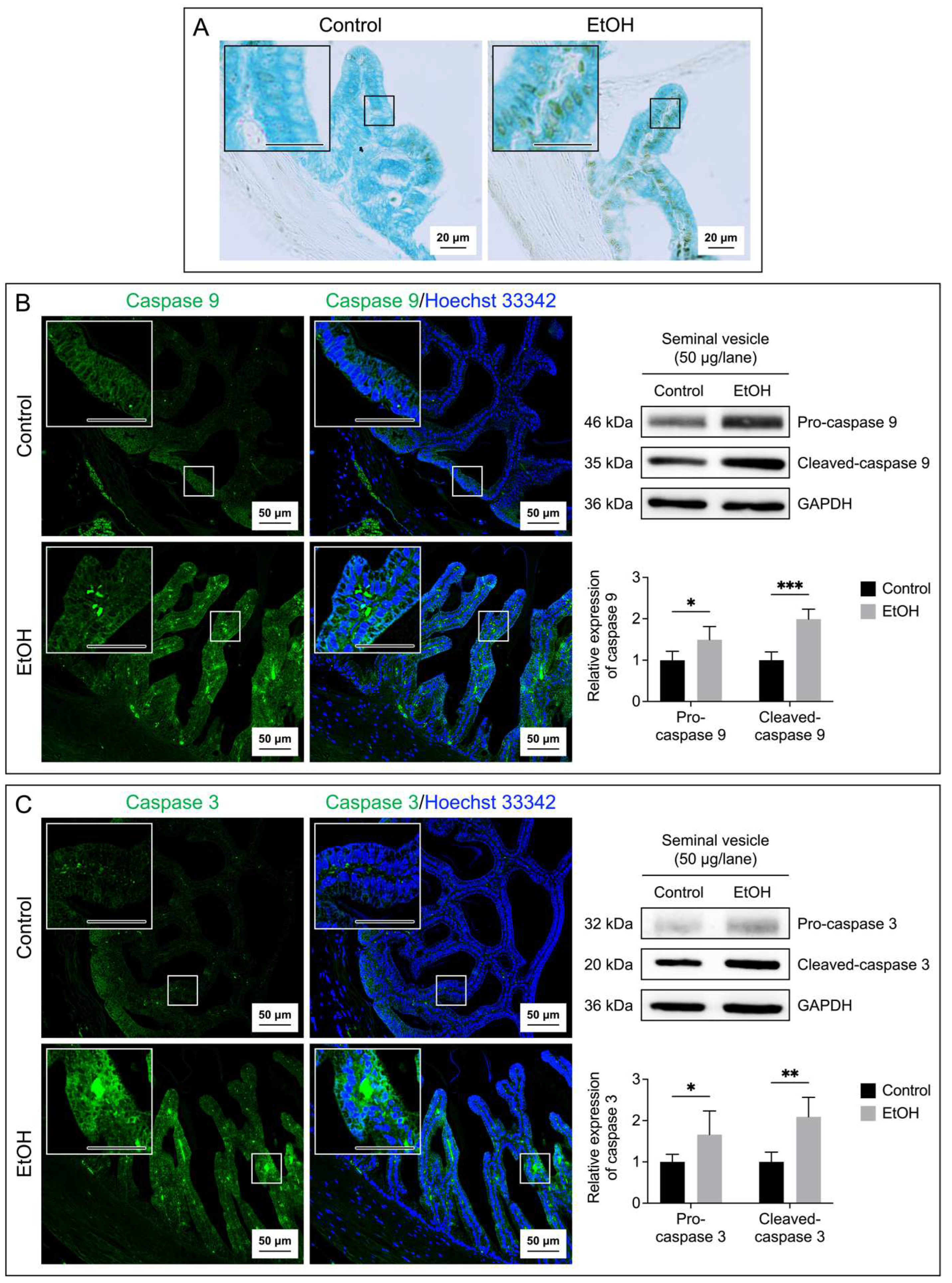
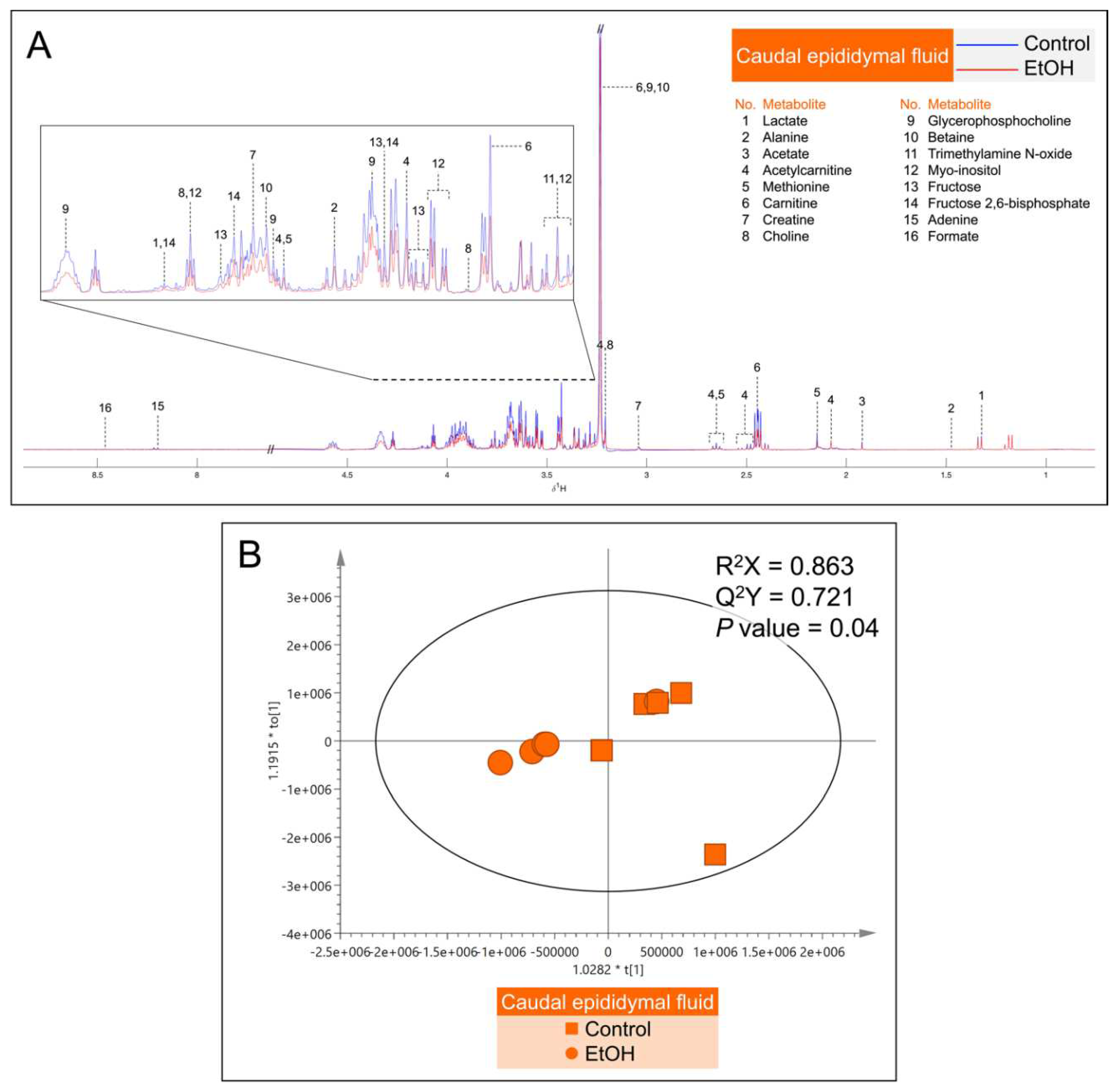
Figure 7.
A: Median
1H NMR spectra of the caudal epididymal fluid (CEF) from 5 control (blue peaks) rats and from 5 EtOH (red peaks) rats. Metabolites detected were identified by their known chemical shifts (
Table S1).
B: Orthogonal partial least squares-discriminant analysis (OPLS-DA) score plot of the
1H NMR spectral data of CEF metabolites from 5 control rats and 5 EtOH rats. Note that the data of two EtOH samples were almost 100% overlapping, making the plot of the EtOH group appearing as having only four points. Permutation
P value = 0.04 indicates the significant differences of metabolites between control and EtOH rats.
Figure 7.
A: Median
1H NMR spectra of the caudal epididymal fluid (CEF) from 5 control (blue peaks) rats and from 5 EtOH (red peaks) rats. Metabolites detected were identified by their known chemical shifts (
Table S1).
B: Orthogonal partial least squares-discriminant analysis (OPLS-DA) score plot of the
1H NMR spectral data of CEF metabolites from 5 control rats and 5 EtOH rats. Note that the data of two EtOH samples were almost 100% overlapping, making the plot of the EtOH group appearing as having only four points. Permutation
P value = 0.04 indicates the significant differences of metabolites between control and EtOH rats.
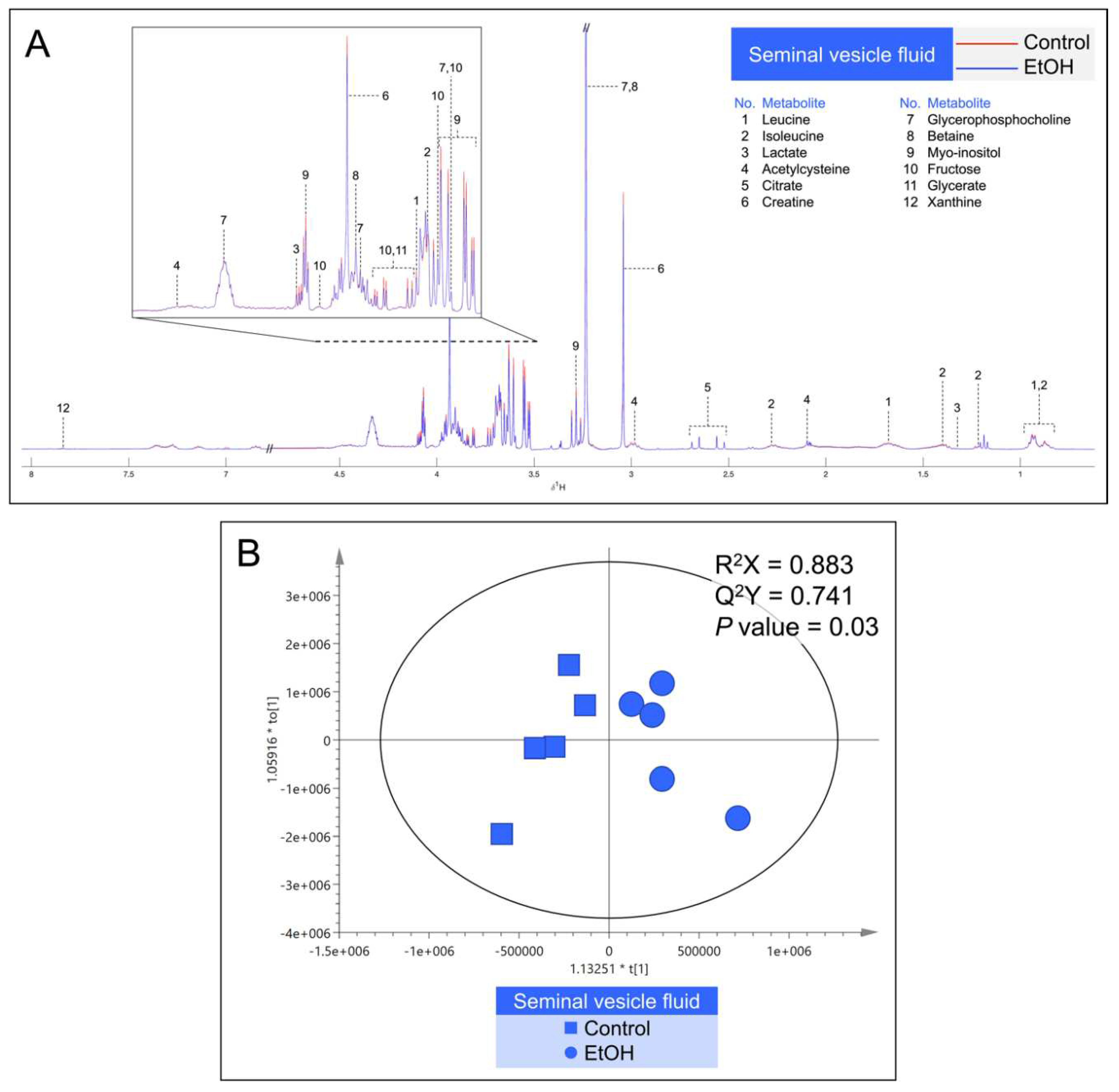
Figure 8.
A: Median
1H NMR spectra of the seminal vesicle fluid (SVF) from 5 control (red peaks) rats and from 5 EtOH (blue peaks) rats. Metabolites detected were identified by their known chemical shifts (
Table S2).
B: Orthogonal partial least squares-discriminant analysis (OPLS-DA) score plot of the
1H NMR spectral data of metabolites from SVF metabolites from 5 control rats and 5 EtOH rats. Permutation
P value = 0.03 indicates the significant differences of metabolites between control and EtOH rats.
Figure 8.
A: Median
1H NMR spectra of the seminal vesicle fluid (SVF) from 5 control (red peaks) rats and from 5 EtOH (blue peaks) rats. Metabolites detected were identified by their known chemical shifts (
Table S2).
B: Orthogonal partial least squares-discriminant analysis (OPLS-DA) score plot of the
1H NMR spectral data of metabolites from SVF metabolites from 5 control rats and 5 EtOH rats. Permutation
P value = 0.03 indicates the significant differences of metabolites between control and EtOH rats.
Table 1.
Significant changes of metabolite levels in the fluid of the cauda epididymis (CEF) and seminal vesicle (SVF) in EtOH rats as compared with those in control animals.
Table 1.
Significant changes of metabolite levels in the fluid of the cauda epididymis (CEF) and seminal vesicle (SVF) in EtOH rats as compared with those in control animals.
| |
Control
(mM)#
|
EtOH
(mM)#
|
Caudal epididymal fluid (CEF)
Alanine
Carnitine
Glycerophosphocholine (GPC)
Myo-inositol
Fructose
Fructose 2,6-bisphosphate |
0.92 ± 0.23
13.66 ± 3.15
4.55 ± 1.05
0.90 ± 0.33
1.00 ± 0.27
0.72 ± 0.13 |
0.41 ± 0.16*
6.44 ± 2.61*
2.15 ± 0.87*
0.38 ± 0.17*
0.43 ± 0.34*
0.37 ± 0.16* |
Seminal vesicle fluid (SVF)
Leucine
Isoleucine
Lactate
Citrate
Myo-inositol
Fructose
Glycerate |
3.98 ± 0.37
0.85 ± 0.12
2.55 ± 0.21
2.19 ± 0.72
6.10 ± 0.65
3.01 ± 0.37
3.04 ± 0.43 |
2.58 ± 0.64*
0.61 ± 0.17*
1.87 ± 0.41*
1.45 ± 0.28*
4.83 ± 0.81*
2.19 ± 0.42*
2.19 ± 0.46* |