1. Introduction
Although the number of patients with gastric cancer is expected to decrease in the future due to the decline in the number of people infected with
Helicobacter pylori (H. pylori) and the spread of H. pylori eradication therapy, incidence of gastric cancer still ranks fifth among all cancer types (1,089,103) and the fourth largest number of deaths (768,793) in 2020 in the world [
1]. With the spread of esophagogastroduodenoscopy (EGD) in daily practice, early detection of gastric cancer became possible. Further combined with the birth of endoscopic submucosal dissection (ESD) in the late 1990s [
2], the era has arrived in which early-stage gastric cancer without lymph node metastasis can be cured with endoluminal surgery alone. This review outlines the current status of endoscopic diagnosis and treatment for gastric cancer as well as the future prospects in this field.
2. The Role of Endoscopy in Gastric Cancer Detection
Japan is one of the few countries where gastric cancer screening has been used as a population-based mass-screening for a long time. X-ray examination with barium meal had been the only recommended mass-screening method till 2016; however, it has been fraught with various problems such as a low participating rate (less than 50% of target generations) and radiation exposure. In the "Gastric Cancer Screening Guidelines Based on Efficacy Evaluation" published by the National Cancer Center 2014 edition in Japanese Screening Guidelines, endoscopy was recommended as a population-based mass-screening for the first time, along with X-ray examination, because there is sufficient evidence that it has a mortality reduction effect.
According to the Screening Guidelines, endoscopy once every 2~3 years is recommended for individuals over 50 years old, who have an increased risk of gastric cancer. However, it is not practical to perform EGD on every individual over the age of 50 in terms of cost-effectiveness and manpower of endoscopists. Since H. pylori infection is involved in the development of gastric cancer in more than 95% of cases, and it is known that the risk of developing gastric cancer increases as atrophy of the gastric mucosa and then intestinal metaplasia due to H. pylori infection progress, stratified screening according to the risk of gastric cancer should be explored.
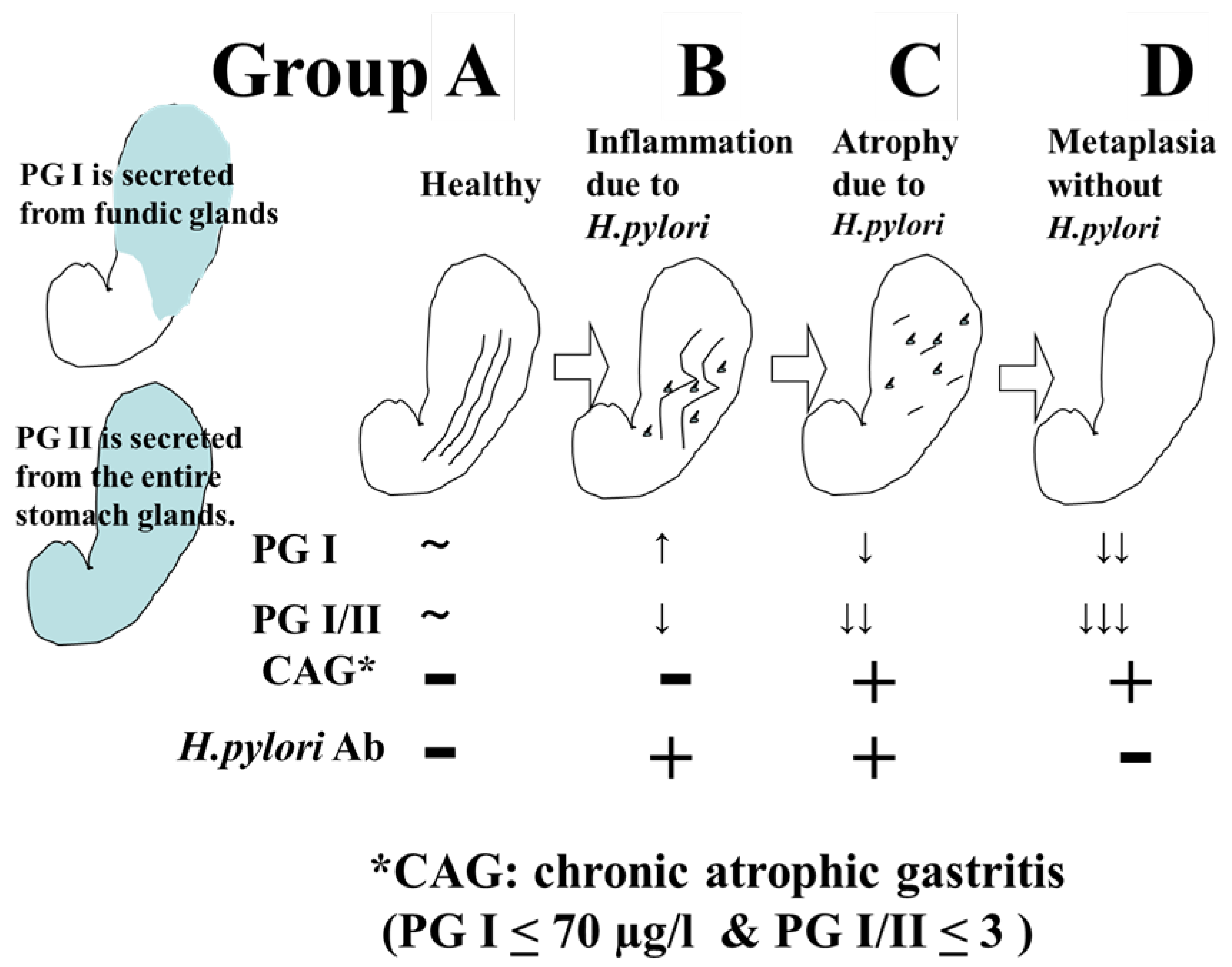
Gastric cancer screening using ABC classification is a screening method that stratifies the risk of developing gastric cancer by combining serum pepsinogen (PG) values, which are serum markers of atrophic gastritis, and anti-H. pylori IgG antibody (HP) values. When PGI≦70 ng/ml and PGI/II ratio≦3.0 are defined as PG-positive (with atrophic gastritis), the risk of developing gastric cancer in Group A (HP-negative PG-negative), Group B (HP-positive PG-negative), Group C (HP-positive PG-positive), and Group D (HP-negative PG-positive) is reported to be 0.016%, 0.14%, 0.30%, and 1.1%, respectively [
3] (
Figure 1). Based on the said report, it has been proposed that Group A should be excluded from the target of endoscopy (or once every 5 years), Group B should be examined once every 3 years, group C should be examined once every 2 years, and group D should be performed annually.
However, with the spread of H. pylori eradication therapy, there are not a few H. pylori eradication cases in Group A; therefore, it is not appropriate to uniformly remove Group A from the target of endoscopy. It is necessary to take relevant measures such as listening to the history of H. pylori eradication and performing endoscopy once every 2-3 years for eradication cases. Subgroup analysis has also pointed out that Group B includes a group with a high incidence of gastric cancer; thus more optimal risk stratification and appropriate endoscopic intervals should be explored through further examination [
3].
When a patient is presumed to be uninfected with H. pylori from the endoscopic findings of no atrophy and existence of regular arrangements of collecting venules (RAC) in the gastric angle, the risk of cancer is extremely low and thus endoscopy can be completed in a short time. On the other hand, when it is judged, with respect to H. pylori-infected or -eradicated patient, that the risk of cancer is high from the endoscopic findings such as progression of open-type atrophy, presence of intestinal metaplasia, enlarged folds of the gastric body, and nodular gastritis in the gastric antrum, a more detailed examination is required. The latter two findings are important factors to be classified as a high-risk group for undifferentiated gastric cancer [
4,
5].
3. Endoscopic Diagnosis of Early Gastric Cancer
Elevated and protruded cancers, those are principally characterized as the area with irregularities and whitish color, are usually picked up by white light observation. When mucosal surface structure is different from the surroundings, differentiation has to be made whether such lesions are non-neoplastic lesions such as fundus gland polyps, hyperplastic polyps, and intestinal metaplasia or neoplastic lesions such as adenomas and cancer. Those with remarkable irregularities with large nodules, noticeable redness in some parts, and >2 cm in size are likely to be cancer.
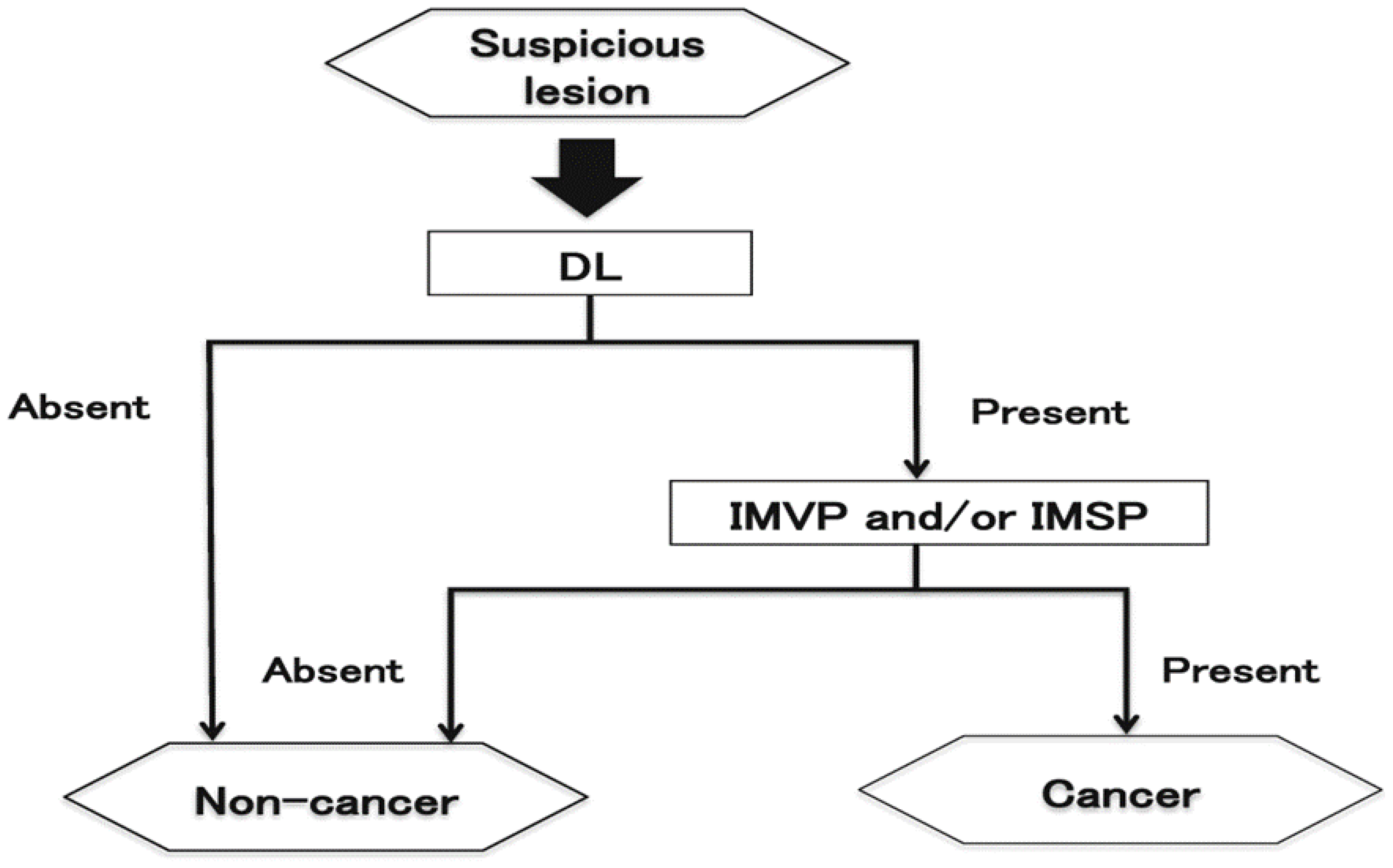
Flat and depressed cancers, those are characterized as the area with irregularities (stellate shape) and reddish or whitish color, are usually picked up by white light observation. Although it is often difficult to distinguish between cancer and non-cancer, new technologies are helping to overcome such difficulties. Detailed observation by using magnifying endoscopy with band-limited light such as narrow band imaging (NBI) reveals irregular microsurface and/or irregular microvascular pattern with demarcation lines in case of cancerous lesions. This new algorithm is known as the magnifying endoscopy simple diagnostic algorithm for gastric cancer (MESDA-G;
Figure 2) [
6] based on the vessels plus surface (VS) classification system [
7]. Although the progress of endoscopic diagnostic technology has been remarkable as described above, histological diagnosis with biopsy has be conducted to confirm whether a lesion is surely a cancer.
4. Guidelines for Endoscopic Resection for Early Gastric Cancers [8,9].
5. Indications of Endoscopic Resection for Early Gastric Cancer
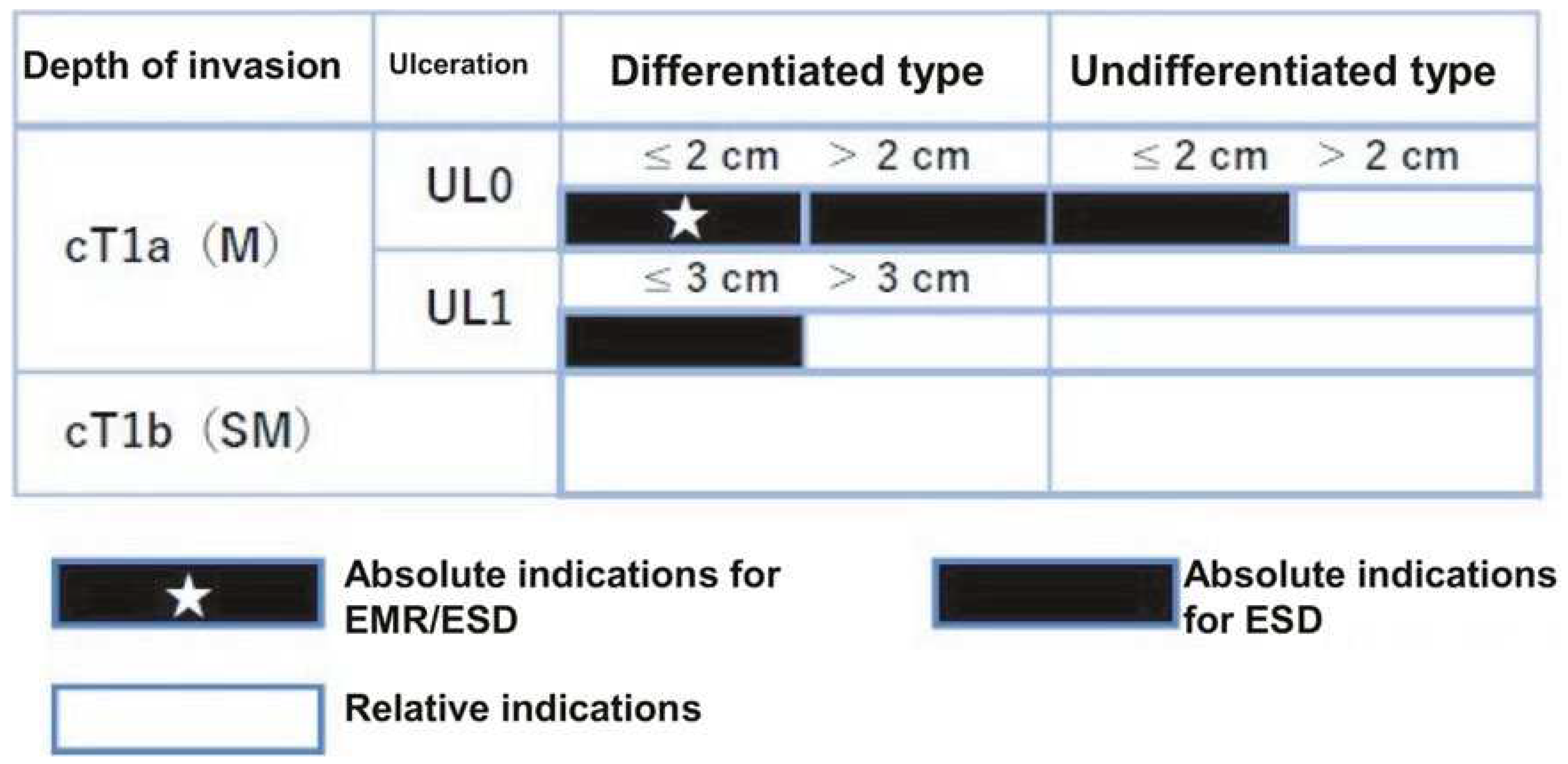
Two aspects must be confirmed to determine the indication of endoscopic resection: (i) the possibility of lymph node metastasis is extremely low, and (ii) the lesions can be endoscopically resected in an en bloc fashion.
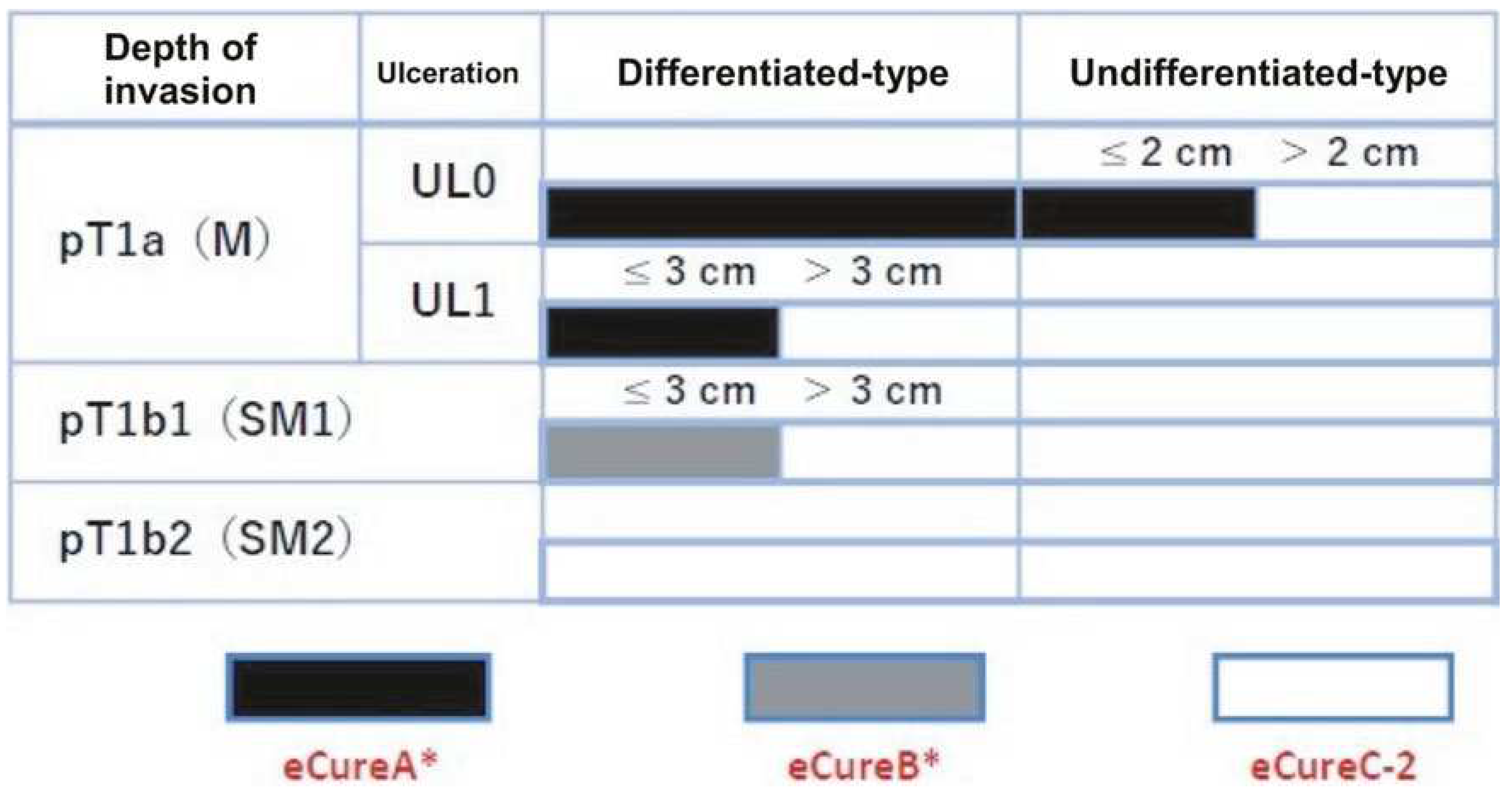
“Absolute indication” lesions are defined as those where a risk of lymph node metastasis is estimated to be <1%, and long-term results equivalent to surgical gastrectomy are proven. “Expanded indication” lesions are defined as those where a risk of lymph node metastasis is estimated to be <1%, but there is little evidence of long-term results. Furthermore, endoscopic resection for early-stage gastric cancer without lymph node metastasis, which would usually be treated by gastrectomy but surgery may not be recommended due to various clinical circumstances such as advanced age and an substantial underlining disease, is categorized as “relative indication”.
Specifically, “absolute indication” lesions are divided into EMR/ESD-adapted lesions and ESD-adapted lesions. The former is clinically differentiated intramucosal carcinoma (cT1a), ≦2 cm, and no ulcer findings (UL0). The latter is undifferentiated cT1a, ≦2 cm, and UL0; differentiated cT1a, >2 cm, and UL0; or differentiated cT1a, ≦3 cm, and ulcer findings (UL1). Lesions can be regarded as “expanded indication” for ESD, provided that the “absolute indication” lesions locally recur as differentiated cT1a after initial ESD/EMR with a C-1 grade of endoscopic curability (eCura) which is shown in detail later.
In addition, if the lesion deviates from the “absolute indication” or “expanded indication” lesion, endoscopic resection should be performed as a “relative indication” lesion only if the informed consent is obtained after sufficient explanation that the standard treatment is surgical gastrectomy and the risk of lymph node metastasis remains (
Figure 3). Therefore, in order to determine the treatment strategy, it is necessary to diagnose histological type, size, depth of invasion, and presence or absence of ulcer findings.
The histological type of cancer (differentiated carcinoma vs. undifferentiated carcinoma) is diagnosed by biopsy as described above; however, histological type can be predicted endoscopically [
10]. In general, elevated and protruded lesions are differentiated type, and among flat and depressed lesions, redness tones are often differentiated type and white tones are undifferentiated type. It has been reported that differentiated and undifferentiated types can be estimated to some extent by using band-limited light magnification observation. When endocytoscopy was applied in comparison with magnifying endoscopy in narrow band imaging, the accuracy of histological diagnosis increased from 72.2% to 78.8% [
11].
It has been pointed out that there is an error in the size measured by the endoscopic observation method. Therefore, on the premise that the size will be determined after the histological findings of the resected specimen are finally known, the size is diagnosed with reference to the biopsy forceps diameter (usually 2.5 mm in the closed state and 8 mm in the open state). Ulcer findings are diagnosed with obvious active ulcers or ulcer scars in the lesion. An ulcer is a defect of the mucosal layer or deeper, and an active ulcer refers to an open ulcer with a certain depth of white moss, excluding shallow erosion. In addition, ulcers in the healing or scar phase are diagnosed as having ulcer findings when there is obvious mucosal fold convergence at one point.
Although endoscopic ultrasound is useful as an auxiliary depth diagnosis for early gastric cancer, the additional effect is low. Moreover, from the point of view of labor and medical cost, it is rarely performed in actual clinical practice; it is usually estimated by white light observation. In the case of elevated and protruded lesions, if it exceeds 2 cm, there is a possibility of submucosal invasive carcinoma (T1b), but if the surface is smooth and regular, and erosion and redness are not observed, it is likely to be T1a regardless of size. In the case of flat and depressed lesions, the deep depression, the hardness, the unstructured surface, the nodules of unequal size in the depression, and significant redness are findings that suspect T1b.
When endoscopic resection is planned, it is necessary to accurately diagnose the horizontal cancer extent to determine the resection area. In this case, chromoendoscopy with indigo carmine is basically performed, but in recent years, it has been reported that almost the same diagnosis can be obtained by using band-limited light magnification observation [
12]. Diagnosis of horizontal cancer extent, especially in relation to some moderately to poorly differentiated carcinoma and signet ring cell carcinoma, may be difficult only by endoscopic observation, and it is desirable to take a biopsy from the unclear boundaries to confirm cancer margins.
6. Types of Endoscopic Resection for Early Gastric Cancer
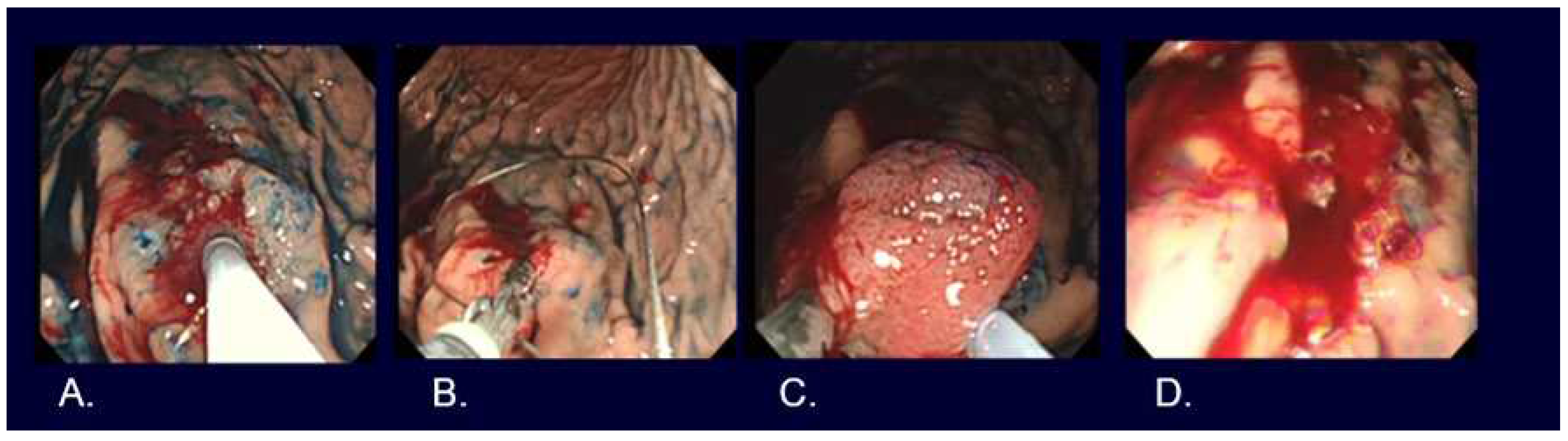
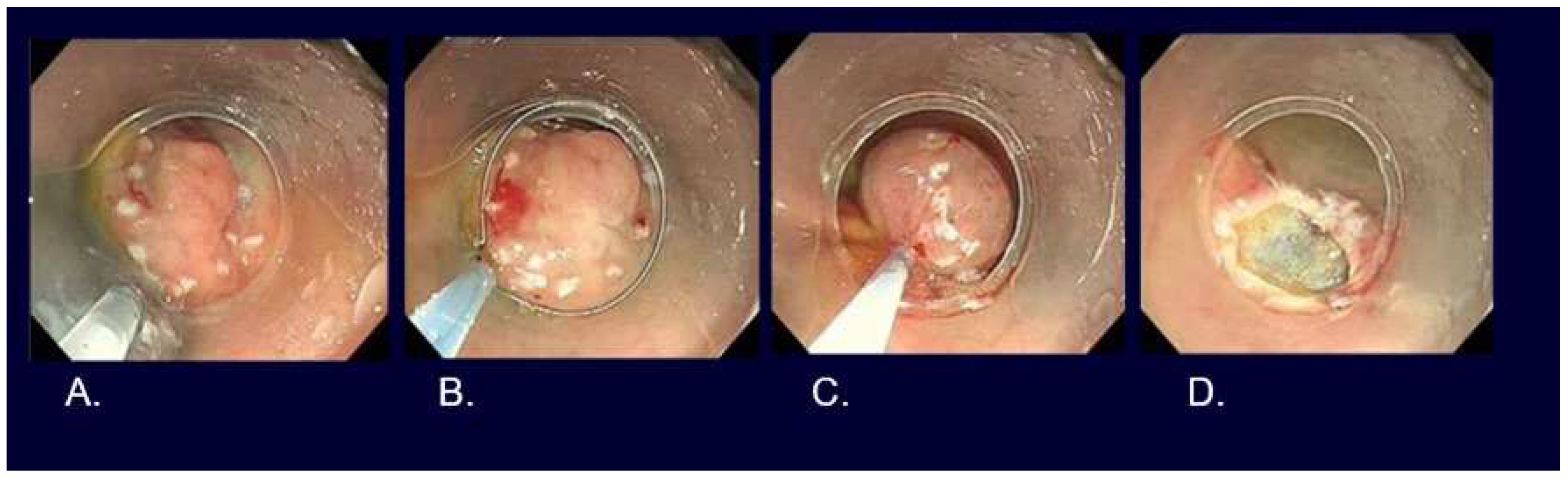
EMR, such as strip biopsy and Cap-EMR, is a method whereby the lesion is elevated, placed in a metal wire snare, and resected using high-frequency diathermy. ESD is a method whereby the mucosa surrounding the lesion is excised using a high-frequency diathermy device, followed by dissection of the submucosa beneath the lesion (
Figure 4,
Figure 5,
Figure 6).
Although no studies have examined whether treatment outcomes differ between EMR and ESD in randomized controlled trials, meta-analyses mainly in retrospective studies have shown that ESD generally provides a better en bloc resection rate (Odds ratio of 9.69) and non-local recurrence rate (Odds ratio 0.10) than EMR [
13]. According to the Treatment Guidelines Treatment Guidelines, either EMR or ESD is acceptable for lesions that are judged to be ≦2 cm, cT1a, differentiated carcinoma, and UL0; however, it has been reported that the rate of en bloc resection in EMR is significantly lower than that of ESD when the tumor size exceeds 1 cm [
14].
Multi-piece resection not only increases the rate of local recurrence but may also disable adequate pathologic evaluation of resected specimens; thus, ESD is currently the choice for most lesions. In addition, though various electrocautery devices for ESD have been developed, all of them have advantages and disadvantages; therefore, at present, it is important to use the most suitable electrocautery devices as required under relevant clinical circumstances according to the preference of the endoscopists and the location and/or nature of the lesion for the sake of safe and reliable ESD.
7. Evaluation of Curability after Endoscopic Resection
The curability of EMR and ESD is determined by two factors, the completeness of local cancer excision and the likelihood of lymph node metastasis (Figure 5).
Figure 5.
Evaluation of curability for early gastric cancer. (cited from Ono H, et al. Dig Endosc. 2021 Jan;33(1):4-20.). * Confined to en bloc resection and HM0, VM0, Ly0, and V0. pT1a (M), intramucosal cancer (histopathological diagnosis); pT1b (SM), submucosally invasive cancer (histopathological diagnosis). UL, finding of ulceration (or ulcer scar); UL0, absence of ulceration or ulcer scar; UL1, presence of ulceration or ulcer scar.
Figure 5.
Evaluation of curability for early gastric cancer. (cited from Ono H, et al. Dig Endosc. 2021 Jan;33(1):4-20.). * Confined to en bloc resection and HM0, VM0, Ly0, and V0. pT1a (M), intramucosal cancer (histopathological diagnosis); pT1b (SM), submucosally invasive cancer (histopathological diagnosis). UL, finding of ulceration (or ulcer scar); UL0, absence of ulceration or ulcer scar; UL1, presence of ulceration or ulcer scar.
When the lesion is resected en bloc, the following conditions need to be fulfilled in order to be classified as endoscopic curability A (eCuraA): (i) predominantly differentiated type, pT1a, UL0, horizontal stump negative for cancer (HM0), vertical stump negative for cancer (VM0), no lymphatic vessel infiltration (Ly0), and no venous infiltration (V0), regardless of size; (ii) long diameter ≦2 cm, predominantly undifferentiated type, pT1a, UL0, HM0, VM0, Ly0, and V0; or (iii) long diameter ≦3 cm, predominantly differentiated type, pT1a, UL1, HM0, VM0, Ly0, and V0.
When the lesion is resected en bloc and the following conditions are fulfilled, such resection is classified as endoscopic curability B (eCuraB): ≦3 cm in long diameter, predominantly of the differentiated type, pT1b1(SM1) (within <500 μm from the muscularis mucosae), HM0, VM0, Ly0, and V0.
When a lesion meets neither of the above-mentioned eCuraA nor B conditions, it is considered endoscopic cure C (eCuraC), which has a likelihood of remnant tumor. When eCuraC lesions are differentiated type lesions and fulfill other criteria to be classified into either eCuraA or eCuraB but were either not resected en bloc or had positive HM, they are considered eCuraC-1. All other eCuraC lesions are considered eCuraC-2.
The determination of curability is directly related to the subsequent patient management policy. In the case of eCuraA, follow-up is performed by endoscopy once to twice a year, and in the case of eCuraB, in addition to endoscopy once or twice a year, abdominal ultrasonography and CT examination are used to check for metastasis.
In the case of eCuraC-1, as the risk of metastasis is low, appropriate methods, among re-ESD, additional surgical resection, careful follow-up in anticipation of the burn effect at the time of resection, and cauterization (laser, argon plasma coagulation, etc.) are selected, according to the policy of the institution, after obtaining sufficient informed consent from the patient.
In the case of eCuraC-2, in principle, additional surgical resection is performed. If surgical gastrectomy is not selected for some reason, such as age or comorbidity, it is necessary to obtain the patient's full understanding and consent after explaining the risk of lymph node metastasis, the possibility of local recurrence or distant metastasis, and the difficulty of curing and poor prognosis in the event of recurrence.
When following up, it is recommended to test for H. pylori infection in patients with unknown status of H.pylori infection, and then to eradicate H. pylori in H. pylori positive patients. However, it is necessary to pay attention to the occurrence of metachronous multiple gastric cancer even after H. pylori eradication. At present, there is no evidence to change the surveillance method after endoscopic treatment due to differences in H. pylori infection status.
8. Endoscopic Resection Results for Early-Stage Gastric Cancer
Based on the Japanese case series for 10,821 lesions in 9,616 patients, ESD, EMR, and hot biopsy were performed in 10,756 lesions (99.4%), 64 lesions (0.6%), and one lesion (0.01%), respectively. Median procedure time with inter quartile range was 76 (49–120) minutes, and en bloc and R0 resections were obtained in 10,739 lesions (99.2%) and 9,914 lesions (91.6%), respectively. In terms of complications, postoperative bleeding, Intraoperative perforation, and delayed perforation were encountered in 426 lesions (4.4%), 218 lesions (2.3%), 40 lesions (0.4%), respectively. It was necessary to be rescued by blood transfusion and emergency surgery in 69 lesions (0.7%) and 23 lesions (0.2%), respectively [
15].
If perforation occurs during endoscopic resection, endoscopic clip closure should be tried, in the first place. If it is successful, successive conservative managements such as administration of antibiotics with abstinence from eating and drinking for a few days are permittable. On the other hand, if the perforation cannot be closed, or if peritonitis is suspected even if it can be closed, it is necessary to consult a surgeon and consider surgical indications.
Intraoperative bleeding, including minor ones, is almost inevitable in ESD. Since there is no established definition of bleeding, it should be noted that there are slight differences in the definition of bleeding rate described above by researchers; some researchers may count the case, for example, when hemoglobin drops by ≧2 g/dl before and after endoscopic resection, when blood transfusion is required, when vomiting blood is observed after endoscopic resection, and/or when gastric blood retention or bleeding from postoperative ulcer is observed during emergency endoscopy. For bleeding during ESD, coagulation hemostasis with hemostatic forceps that does not prevent the continuation of resection after hemostasis is desirable, but depending on the situation, the clip method and local injection method are also options. In order to prevent postoperative bleeding, proton pump inhibitors, H2 blockers, or potassium-competitive acid blockers are administered, but the preventive effect is not sufficient. In recent years, new attempts have been made such as covering the mucosal defect with PGA sheet and fibrin glue and stitching of the mucosal defect with an indwelling snare and clip [
16,
17].
Recently, long term outcomes of Japanese case series described above have been elucidated. The overall 5-year OS was 89.0% (95% CI, 88.3%-89.6%). A multivariate analysis revealed no significant differences in the hazard ratios of mortality of eCuraA subgroup A2 ((i) with >2 cm and eCuraA (iii)) (1.03 [95% CI, 0.87-1.21]), eCuraA subgroup A3 (ii)(1.18 [95% CI, 0.68–2.07]), and eCuraB (1.09 [95% CI, 0.80-1.49]), compared with that of eCuraA subgroup A1((i) with ≦2 cm). On the other hands, the hazard ratio of mortality of eCuraC (1.41 [95% CI, 1.21-1.65]) was significantly higher than that of eCuraA subgroup A1 ((i) with ≦2 cm) [
18].
9. New Developments for Personalized Medicine in Early Gastric Cancer
In 2014, the Cancer Genome Atlas (TCGA) network reported that gastric cancer can be classified into four different molecular subtypes [
19]. It was the first step to classify gastric cancer based on specific genomic abnormalities, rather than considering gastric cancer as a single disease, and showed the possibility of developing personalized medicine.
Under the said classification, EB virus-positive gastric cancer was classified as a subtype that accounted for 9% of the total, along with microsatellite instability gastric cancer, genomic stability gastric cancer, and chromosomal instability gastric cancer. The most distinctive feature of EB virus-positive gastric cancer is suppression of gene expression by hypermethylation of extensive genomic DNA. Although EB virus-positive gastric cancer has been known to have a good prognosis clinically, recent studies have suggested that EB virus-positive gastric cancer has an extremely low risk of lymph node metastasis even with T1b and thus may be cured by local resection without lymph node dissection, provided there is no vascular invasion [
20].
In 2010, the concept of gastric cancers with funding gland type was newly proposed, which category in TGCA classification has not been elucidated so far. Gastric cancers in this type have a good prognosis, suggesting that T1b, same as EB virus-positive gastric cancer, may be cured by local resection without lymph node dissection [
21]. In some gastric cancers, it is possible to evaluate curability different from the uniform curability standard stipulated in the Treatment Guidelines. In the future, ESD or technology that applies ESD may be widely applied to deep submucosal invasive carcinoma. It is expected to establish technology that can reliably perform batch excision of deep submucosal infiltrating cancers with a negative vertical stump as well as technology that enables endoscopic full-thickness resection of the gastric wall, and to develop endoscopic equipment that enables the said resection. Currently, prior to such breakthrough, as a new method of full-thickness resection of the stomach wall, the concept of laparoscopic and endoscopic cooperative surgery (LECS) for gastric submucosal tumors (mainly gastrointestinal stromal tumors (GIST)), in which an endoscope is used in combination with laparoscopic surgery, has been established and widely practiced [
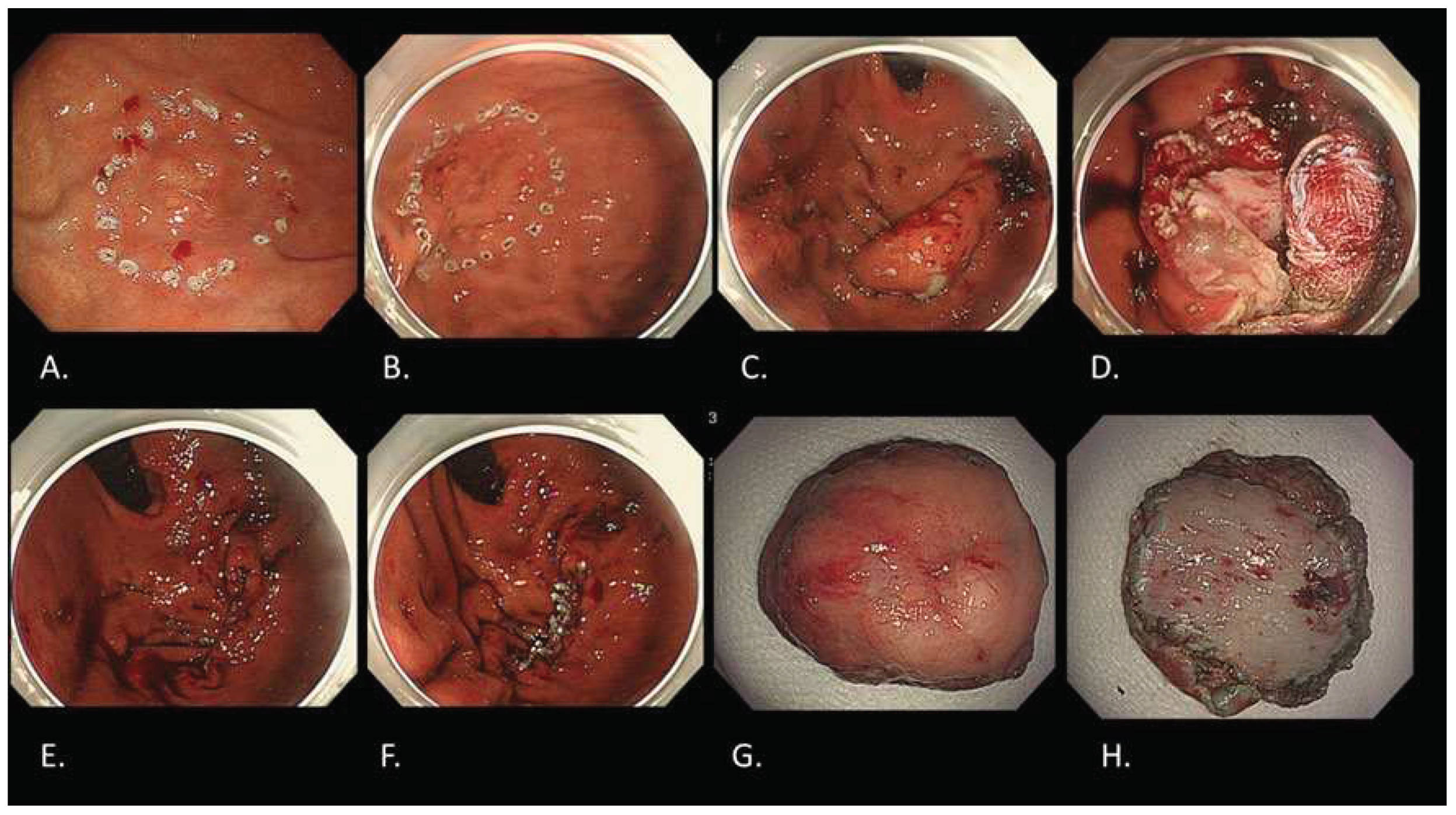
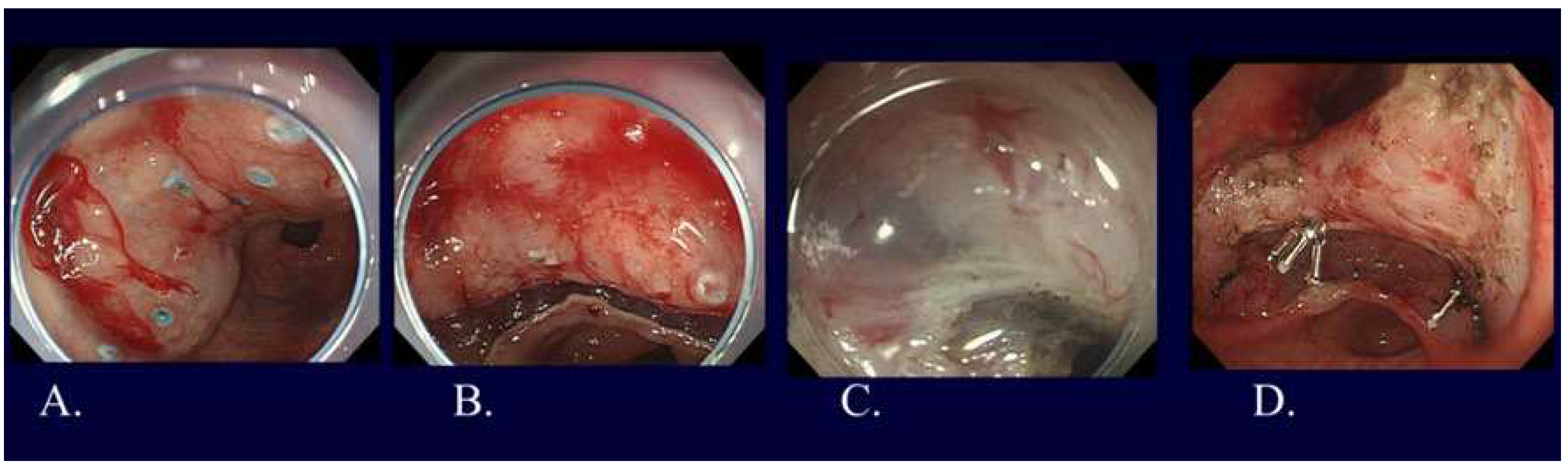
22]. As one of the LECS procedures, non-exposed endoscopic wall inversion surgery (NEWS) was developed (Figure 6) [
23]. Compared to other LECS procedures, NEWS has two distinct characteristics: possible to set the resection range more accurately and no need to worry about intra-abdominal infection or tumor seeding since there is no open connection between the stomach and the abdominal cavity. Future development of NEWS is greatly expected.
Figure 6.
NEWS for a gastric cancer with fundic gland type. A. Marking around the lesion located in gastric fundus on the day before NEWS by gastroscopy. B. Additional markings around the previous markings during NEWS by gastroscopy. C. Seromuscular incision with complete defect closure by laparoscopy. D. Mucosubmucosal incision by gastroscopy like ESD. E. Complete removal with full thickness resection by gastroscopy. F. Complete mucosal closure with endoscopic clipping by gastroscopy. G. Resected specimen observed from mucosal side. H. Resected specimen observed from serosal side. Final histological diagnosis is adenocarcinoma(tub2>tub1), Type 0-IIc, 15x10mm, pT1b2 (540 um), UL(-), ly0, v0.
Figure 6.
NEWS for a gastric cancer with fundic gland type. A. Marking around the lesion located in gastric fundus on the day before NEWS by gastroscopy. B. Additional markings around the previous markings during NEWS by gastroscopy. C. Seromuscular incision with complete defect closure by laparoscopy. D. Mucosubmucosal incision by gastroscopy like ESD. E. Complete removal with full thickness resection by gastroscopy. F. Complete mucosal closure with endoscopic clipping by gastroscopy. G. Resected specimen observed from mucosal side. H. Resected specimen observed from serosal side. Final histological diagnosis is adenocarcinoma(tub2>tub1), Type 0-IIc, 15x10mm, pT1b2 (540 um), UL(-), ly0, v0.
10. Conclusions
The current status and future prospects of endoscopic treatment for gastric cancer were outlined pursuant to the latest guidelines. We are entering an era in which tailor-made medical care can be provided in the field of endoscopy, according to the patient's situation, as can be seen from the achievements of endoscopic equipment and technology as well as the deepening of knowledge about gastric cancer.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fujishiro, M. Endoscopic Submucosal Dissection for Stomach Neoplasms. World J Gastroenterol 2006, 12, 5108–5112. [Google Scholar] [CrossRef]
- Yoshida, T.; Kato, J.; Inoue, I.; Yoshimura, N.; Deguchi, H.; Mukoubayashi, C.; Oka, M.; Watanabe, M.; Enomoto, S.; Niwa, T.; et al. Cancer Development Based on Chronic Active Gastritis and Resulting Gastric Atrophy as Assessed by Serum Levels of Pepsinogen and Helicobacter Pylori Antibody Titer. Int J Cancer 2014, 134, 1445–1457. [Google Scholar] [CrossRef]
- Nishibayashi, H.; Kanayama, S.; Kiyohara, T.; Yamamoto, K.; Miyazaki, Y.; Yasunaga, Y.; Shinomura, Y.; Takeshita, T.; Takeuchi, T.; Morimoto, K.; et al. Helicobacter Pylori-Induced Enlarged-Fold Gastritis Is Associated with Increased Mutagenicity of Gastric Juice, Increased Oxidative DNA Damage, and an Increased Risk of Gastric Carcinoma. J Gastroenterol Hepatol 2003, 18, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tanaka, A.; Yamanaka, Y.; Manabe, N.; Kusunoki, H.; Miyamoto, M.; Tanaka, S.; Hata, J.; Chayama, K.; Haruma, K. Nodular Gastritis With Helicobacter Pylori Infection Is Strongly Associated With Diffuse-type Gastric Cancer In Young Patients. Dig Endosc 2007, 19, 180–184. [Google Scholar] [CrossRef]
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying Endoscopy Simple Diagnostic Algorithm for Early Gastric Cancer (MESDA-G). Dig Endosc 2016, 28, 379–393. [Google Scholar] [CrossRef]
- Yao, K.; Anagnostopoulos, G.K.; Ragunath, K. Magnifying Endoscopy for Diagnosing and Delineating Early Gastric Cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Early Gastric Cancer (Second Edition). Dig Endosc 2021, 33, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association Japanese Gastric Cancer Treatment Guidelines 2021 (6th Edition). Gastric Cancer 2023, 26, 1–25. [CrossRef]
- Nakayoshi, T.; Tajiri, H.; Matsuda, K.; Kaise, M.; Ikegami, M.; Sasaki, H. Magnifying Endoscopy Combined with Narrow Band Imaging System for Early Gastric Cancer: Correlation of Vascular Pattern with Histopathology (Including Video). Endoscopy 2004, 36, 1080–1084. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Hirasawa, T.; Ishizuka, N.; Hatamori, H.; Ikenoyama, Y.; Tokura, J.; Ishioka, M.; Tokai, Y.; Namikawa, K.; Yoshimizu, S.; et al. Diagnostic Performance in Gastric Cancer Is Higher Using Endocytoscopy with Narrow-Band Imaging than Using Magnifying Endoscopy with Narrow-Band Imaging. Gastric Cancer 2021, 24, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Asada-Hirayama, I.; Kodashima, S.; Sakaguchi, Y.; Ono, S.; Niimi, K.; Mochizuki, S.; Tsuji, Y.; Minatsuki, C.; Shichijo, S.; Matsuzaka, K.; et al. Magnifying Endoscopy with Narrow-Band Imaging Is More Accurate for Determination of Horizontal Extent of Early Gastric Cancers than Chromoendoscopy. Endosc Int Open 2016, 4, E690–698. [Google Scholar] [CrossRef]
- Lian, J.; Chen, S.; Zhang, Y.; Qiu, F. A Meta-Analysis of Endoscopic Submucosal Dissection and EMR for Early Gastric Cancer. Gastrointest Endosc 2012, 76, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ogata, S.; Kawazoe, S.; Watanabe, K.; Koyama, T.; Kajiwara, T.; Shimoda, Y.; Takase, Y.; Irie, K.; Mizuguchi, M.; et al. Clinical Outcomes of EMR for Gastric Tumors: Historical Pilot Evaluation between Endoscopic Submucosal Dissection and Conventional Mucosal Resection. Gastrointest Endosc 2006, 63, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ono, H.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Toya, Y.; Nakagawa, M.; et al. Long-Term Survival After Endoscopic Resection For Gastric Cancer: Real-World Evidence From a Multicenter Prospective Cohort. Clin Gastroenterol Hepatol 2023, 21, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Tsuji, Y.; Hirasawa, K.; Takimoto, K.; Wada, T.; Mochizuki, S.; Ohata, K.; Sakaguchi, Y.; Niimi, K.; Ono, S.; et al. Endoscopic Tissue Shielding to Prevent Bleeding after Endoscopic Submucosal Dissection: A Prospective Multicenter Randomized Controlled Trial. Endoscopy 2019, 51, 619–627. [Google Scholar] [CrossRef]
- Goto, O.; Oyama, T.; Ono, H.; Takahashi, A.; Fujishiro, M.; Saito, Y.; Abe, S.; Kaise, M.; Iwakiri, K.; Yahagi, N. Endoscopic Hand-Suturing Is Feasible, Safe, and May Reduce Bleeding Risk after Gastric Endoscopic Submucosal Dissection: A Multicenter Pilot Study (with Video). Gastrointest Endosc 2020, 91, 1195–1202. [Google Scholar] [CrossRef]
- Suzuki, H.; Takizawa, K.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Endo, M.; Nakagawa, M.; et al. Short-Term Outcomes of Multicenter Prospective Cohort Study of Gastric Endoscopic Resection: “Real-World Evidence” in Japan. Dig Endosc 2019, 31, 30–39. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tsuji, Y.; Ushiku, T.; Shinozaki, T.; Yamashita, H.; Seto, Y.; Fukayama, M.; Fujishiro, M.; Oda, I.; Katai, H.; Taniguchi, H.; et al. Risk for Lymph Node Metastasis in Epstein-Barr Virus-Associated Gastric Carcinoma with Submucosal Invasion. Dig Endosc 2021, 33, 592–597. [Google Scholar] [CrossRef]
- Ueyama, H.; Yao, T.; Nakashima, Y.; Hirakawa, K.; Oshiro, Y.; Hirahashi, M.; Iwashita, A.; Watanabe, S. Gastric Adenocarcinoma of Fundic Gland Type (Chief Cell Predominant Type): Proposal for a New Entity of Gastric Adenocarcinoma. Am J Surg Pathol 2010, 34, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Hiki, N.; Yamamoto, Y.; Fukunaga, T.; Yamaguchi, T.; Nunobe, S.; Tokunaga, M.; Miki, A.; Ohyama, S.; Seto, Y. Laparoscopic and Endoscopic Cooperative Surgery for Gastrointestinal Stromal Tumor Dissection. Surg Endosc 2008, 22, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, M.; Furukawa, K.; Yamamura, T.; Nakamura, M.; Honda, T.; Maeda, O.; Ishigami, M.; Kawashima, H. Nonexposed Wall-Inversion Surgery as a Novel Local Resection Method for Neoplasms in the Gastrointestinal Tract. Nagoya J Med Sci 2020, 82, 175–182. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).