Submitted:
09 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Statistical Analysis
3. Results
3.1. Baseline a Post Intervention Parameters
3.2. Principal Component Analyses
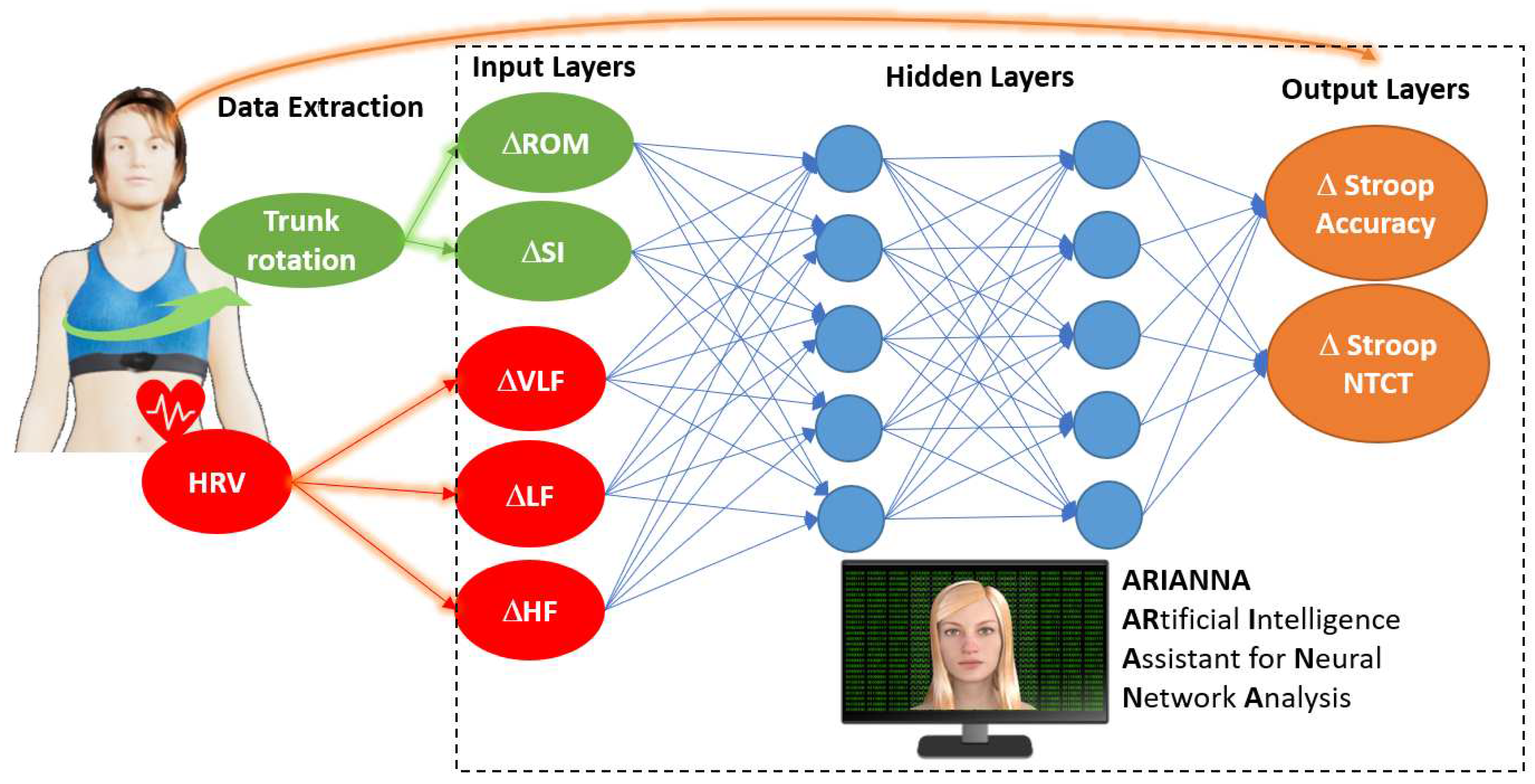
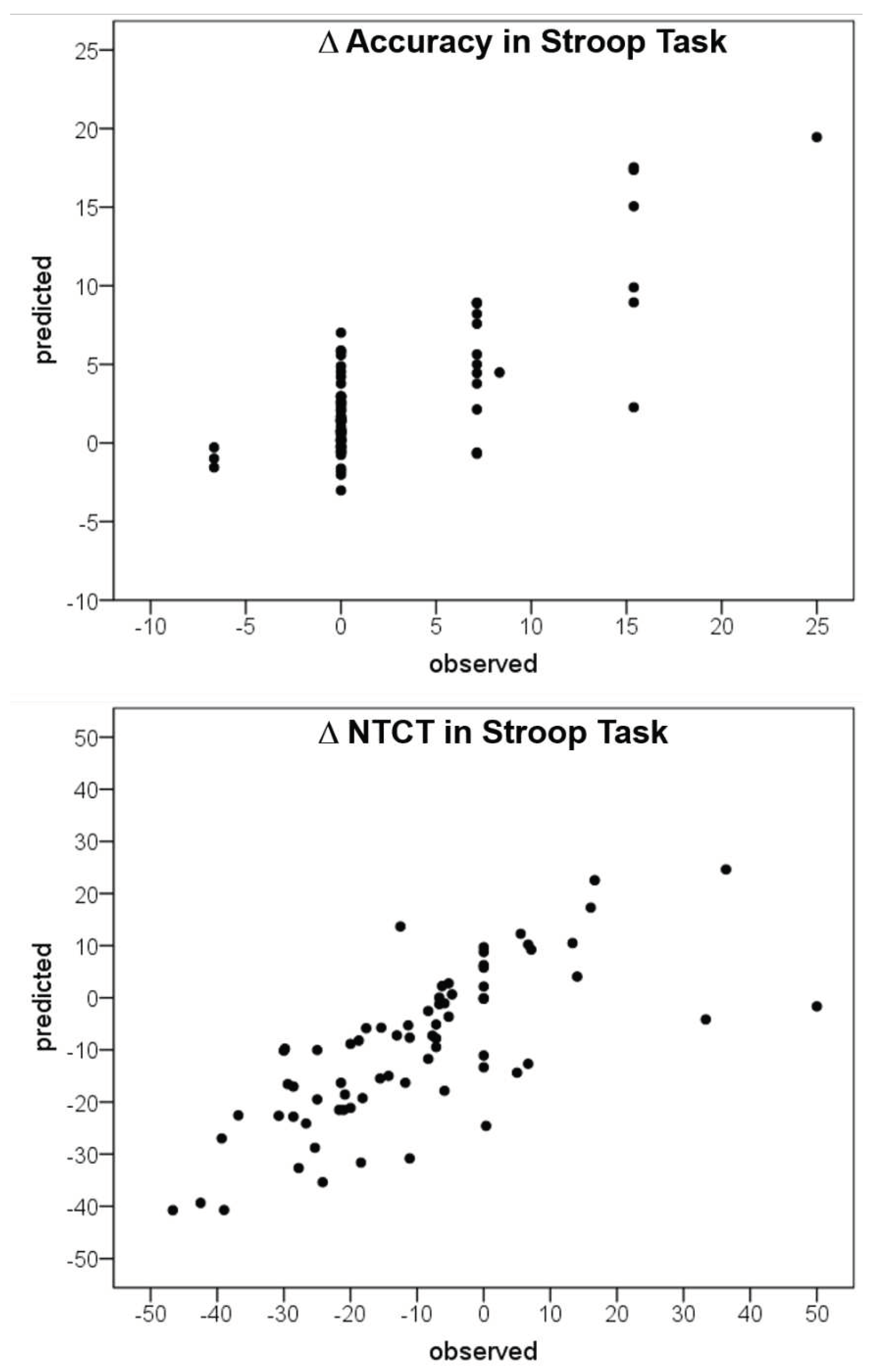
3.3. Artificial Neural Network Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Thayer, J.F.; Sternberg, E. Beyond heart rate variability: Vagal regulation of allostatic systems. Ann. New York Acad. Sci. 2006, 1088, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Gedeon, T. Objective measures, sensors and computational techniques for stress recognition and classification: A survey. Comput. Methods Programs Biomed. 2012, 108, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Xie, L.; Yu, X.; Li, M.; Zhang, J. Cerebral and neural regulation of cardiovascular activity during mental stress. Biomed. Eng. Online 2016, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Liao, S.C.; Gau, S.S. Association between Stroop tasks and heart rate variability features in patients with somatic symptom disorder. J. Psychiatr. Res. 2021, 136, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Lansbergen, M.M.; Kenemans, J.L.; van Engeland, H. Stroop interference and attention-deficit/hyperactivity disorder: A review and meta-analysis. Neuropsychology 2007, 21, 251–262. [Google Scholar] [CrossRef]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert. Rev. Med. Devices. 2016, 13, 641–59. [Google Scholar] [CrossRef]
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert. Rev. Med. Devices 2021, 18 (Suppl. S1), 79–94. [Google Scholar] [CrossRef]
- De Bartolo, D.; De Giorgi, C.; Compagnucci, L.; Betti, V.; Antonucci, G.; Morone, G.; Paolucci, S.; Iosa, M. Effects of cognitive workload on heart and locomotor rhythms coupling. Neurosci Lett. 2021, 762, 136140. [Google Scholar] [CrossRef]
- Kannape, O.A.; Barré, A.; Aminian, K.; Blanke, O. Cognitive loading affects motor awareness and movement kinematics but not locomotor trajectories during goal-directed walking in a virtual reality environment. PLoS ONE 2014, 9, e85560. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: Systematic review. JMIR mHealth uHealth 2020, 8, e18694. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, B.; Tseng, P. Embodied cognition and the perception-action link. Phys. Life Rev. 2011, 8, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Mazzà, C.; Iosa, M.; Picerno, P.; Cappozzo, A. Gender differences in the control of the upper body accelerations during level walking. Gait Posture 2009, 29, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Mekarski, J.E.; Cutmore, T.R.; Suboski, W. Gender differences during processing of the Stroop task. Percept. Mot. Skills. 1996, 83, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ciancarelli, I.; Morone, G.; Tozzi Ciancarelli, M.G.; Paolucci, S.; Tonin, P.; Cerasa, A.; Iosa, M. Identification of Determinants of Biofeedback Treatment’s Efficacy in Treating Migraine and Oxidative Stress by ARIANNA (ARtificial Intelligent Assistant for Neural Network Analysis). Healthcare 2022, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Tjolleng, A.; Jung, K.; Hong, W.; Lee, W.; Lee, B.; You, H.; Son, J.; Park, S. Classification of a Driver’s cognitive workload levels using artificial neural network on ECG signals. Appl. Ergon. 2017, 59 Pt A, 326–332. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Yagi, S.; Yana, K.; Nozawa, M.; Ono, T. State classification of heart rate variability by an artificial neural network in frequency domain. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1401–1404. [Google Scholar]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding, Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Mahony, R.; Hamel, T.; Pflimlin, J.M. Nonlinear Complementary Filters on the Special Orthogonal Group. IEEE Trans. Autom. Control. 2008, 53, 1203–1218. [Google Scholar] [CrossRef]
- De Bartolo, D.; D’amico, I.; Iosa, M.; Aloise, F.; Morone, G.; Marinozzi, F.; Bini, F.; Paolucci, S.; Spadini, E. Validation of SuPerSense, a Sensorized Surface for the Evaluation of Posture Perception in Supine Position. Sensors 2022, 23, 424. [Google Scholar] [CrossRef]
- Chakraborty, S.; Suryavanshi, C.A.; Nayak, K.R. Cognitive function and heart rate variability in open and closed skill sports. Ann. Med. 2023, 55, 2267588. [Google Scholar] [CrossRef] [PubMed]
- Pyne, J.M.; Constans, J.I.; Wiederhold, B.K.; Jegley, S.; Rabalais, A.; Hu, B.; Weber, M.C.; Hinkson, K.D. Jr, Wiederhold, M.D. Predicting Post-Traumatic Stress Disorder Treatment Response Using Heart Rate Variability to Virtual Reality Environment and Modified Stroop Task: An Exploratory Study. Cyberpsychol Behav. Soc. Netw. 2023, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yu, J.; Kang, S.; Lee, H.; Dong, S.Y. Effect of Daily Stress on Heart-Rate Variability during Stroop Color Word Task. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 5976–5979. [Google Scholar]
- Edwards, D.J.; Young, H.; Cutis, A.; Johnston, R. The Immediate Effect of Therapeutic Touch and Deep Touch Pressure on Range of Motion, Interoceptive Accuracy and Heart Rate Variability: A Randomized Controlled Trial With Moderation Analysis. Front. Integr. Neurosci. 2018, 12, 41. [Google Scholar] [CrossRef]
- Mathewson, K.J.; Jetha, M.K.; Drmic, I.E.; Bryson, S.E.; Goldberg, J.O.; Hall, G.B.; Santesso, D.L.; Segalowitz, S.J.; Schmidt, L.A. Autonomic predictors of Stroop performance in young and middle-aged adults. Int. J. Psychophysiol. 2010, 76, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R. Descartes’ Error: Emotion, Reason and the Human Brain; G. P. Putnam’s Sons: New York, NY, USA, 1994. [Google Scholar]
- Clark, A. Being There: Putting Brain, Body, and World Together Again; MIT Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Berthoz, A. Le Sens du Mouvement; Editions Odile Jacob: Paris, France, 1997. [Google Scholar]
- Carney, J. Thinking avant la lettre: A Review of 4E Cognition. Evol. Stud. Imaginative Cult. 2020, 4, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, K.J.; Jetha, M.K.; Drmic, I.E.; Bryson, S.E.; Goldberg, J.O.; Hall, G.B.; Santesso, D.L.; Segalowitz, S.J.; Schmidt, L.A. Autonomic predictors of Stroop performance in young and middle-aged adults. Int. J. Psychophysiol. 2010, 76, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Capodaglio, E.; Pelà, S.; Persechino, B.; Morone, G.; Antonucci, G.; Paolucci, S.; Panigazzi, M. Artificial Neural Network Analyzing Wearable Device Gait Data for Identifying Patients With Stroke Unable to Return to Work. Front. Neurol. 2021, 12, 650542. [Google Scholar] [CrossRef]
- Iosa, M.; Benedetti, M.G.; Antonucci, G.; Paolucci, S.; Morone, G. Artificial Neural Network Detects Hip Muscle Forces as Determinant for Harmonic Walking in People after Stroke. Sensors 2022, 22, 1374. [Google Scholar] [CrossRef]
- Iosa, M.; Paolucci, S.; Morone, G. The Future of Neurorehabilitation: Putting the Brain and Body Together Again. Brain Sci. 2023, 13, 1617. [Google Scholar] [CrossRef]
- Iosa, M.; Zoccolillo, L.; Montesi, M.; Morelli, D.; Paolucci, S.; Fusco, A. The brain’s sense of walking: A study on the intertwine between locomotor imagery and internal locomotor models in healthy adults, typically developing children and children with cerebral palsy. Front. Hum. Neurosci. 2014, 8, 859. [Google Scholar] [CrossRef] [PubMed]
- Di Vita, A.; Cinelli, M.C.; Raimo, S.; Boccia, M.; Buratin, S.; Gentili, P.; Inzitari, M.T.; Iona, T.; Iosa, M.; Morelli, D.; et al. Body Representations in Children with Cerebral Palsy. Brain Sci. 2020, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Padial, E.; Mercado, F. In exogenous attention, time is the clue: Brain and heart interactions to survive threatening stimuli. PLoS ONE 2021, 16, e0243117. [Google Scholar] [CrossRef] [PubMed]
- Grässler, B.; Herold, F.; Dordevic, M.; Gujar, T.A.; Darius, S.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Multimodal measurement approach to identify individuals with mild cognitive impairment: Study protocol for a cross-sectional trial. BMJ Open 2021, 11, e046879. [Google Scholar] [CrossRef] [PubMed]
- Wijsen, L.D.; Borsboom, D.; Alexandrova, A. Values in Psychometrics. Perspect. Psychol. Sci. 2022, 17, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Fusco, A.; Morone, G.; Paolucci, S. Walking there: Environmental influence on walking-distance estimation. Behav. Brain Res. 2012, 226, 124–132. [Google Scholar] [CrossRef]
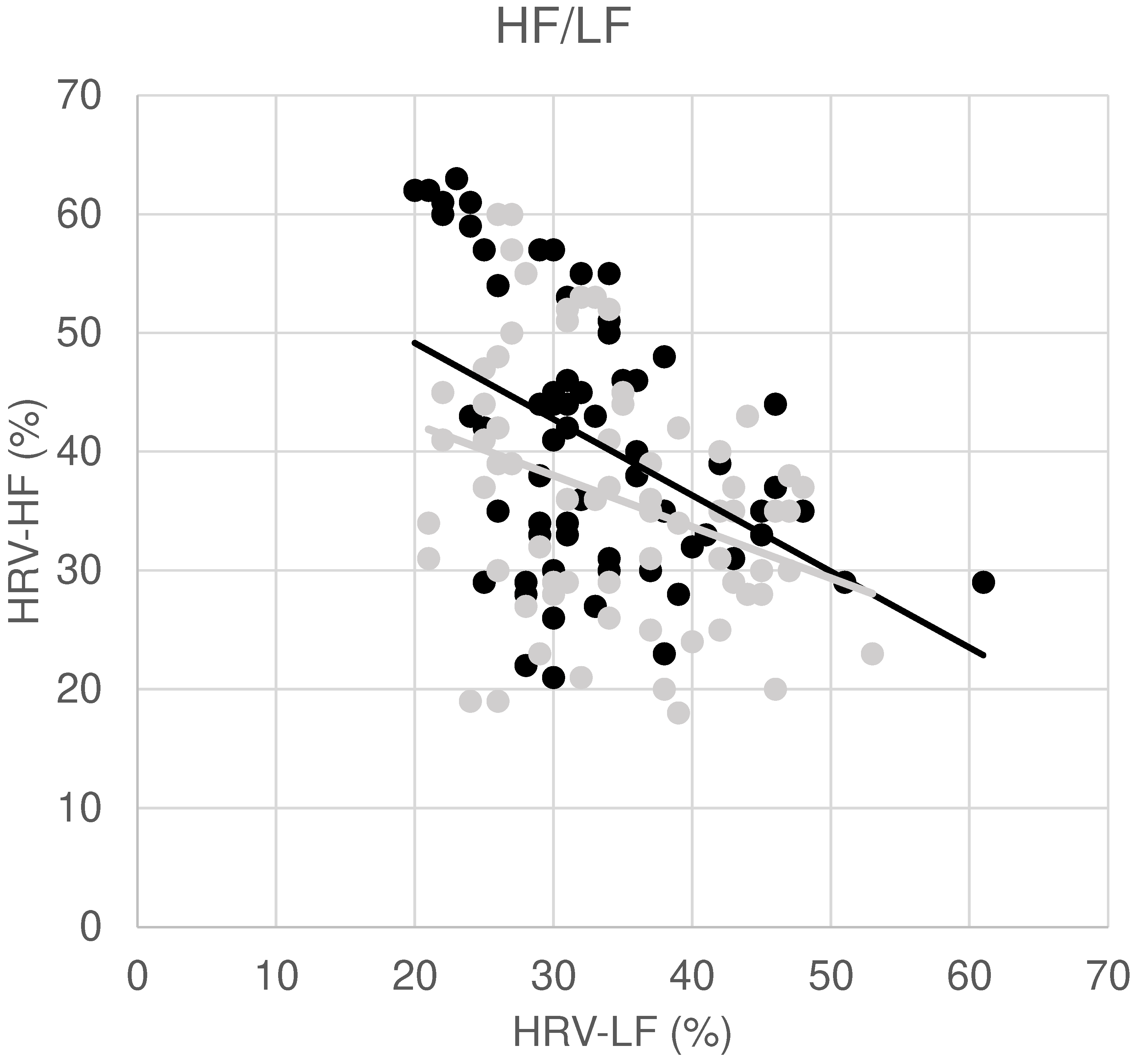
| Variable | Pre | Post | p-value |
|---|---|---|---|
| HRV-VLF (%) | 26.3±10.2 | 29.2±10.7 | 0.096 |
| HRV-LF (%) | 33.1±7.5 | 34.2±7.9 | 0.522 |
| HRV-HF (%) | 40.8±11.1 | 36.2±10.5 | 0.002 |
| HF/LF | 1.3±0.6 | 1.0±0.5 | 0.001 |
| Stroop task NTCT (s) | 18.8±5.2 | 16.4±3.9 | <0.001 |
| Stroop task ACC | 96.6±5.2 | 98.9±2.7 | <0.001 |
| Trunk ROM (deg) | 110.0±21.5 | 127.0±28.0 | <0.001 |
| Trunk SI (%) | 87.4±9.1 | 87.9±10.0 | 0.743 |
| Variable | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| HRV-VLF | -0.20 | 0.84 | -0.19 |
| HRV-LF | 0.34 | 0.36 | 0.43 |
| HRV-HF | -0.03 | -0.99 | -0.06 |
| Stroop task NTCT | 0.87 | -0.11 | -0.07 |
| Stroop task ACC | -0.83 | 0.04 | -0.02 |
| Trunk ROM | 0.12 | -0.09 | 0.72 |
| Trunk SI | -0.13 | -0.02 | 0.77 |
| Variable | Component 1 | Component 2 |
|---|---|---|
| HRV-VLF | -0.24 | 0.92 |
| HRV-LF | 0.62 | -0.01 |
| HRV-HF | -0.19 | -0.94 |
| Stroop task NTCT | 0.80 | 0.03 |
| Stroop task ACC | -0.53 | 0.05 |
| Trunk ROM | -0.27 | 0.14 |
| Trunk SI | 0.35 | 0.04 |
| Input layer parameters | Importance of the input layer in the output prediction | ||
|---|---|---|---|
| Raw Weight | Relative | Normalized | |
| ΔHRV-VLF | 0.191 | 19.1% | 81.9% |
| ΔHRV-LF | 0.214 | 21.4% | 91.8% |
| ΔHRV-HF | 0.166 | 16.6% | 71.4% |
| ΔTrunk ROM | 0.233 | 23.3% | 100% |
| ΔTrunk SI | 0.196 | 19.6% | 84.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




