Introduction
The distribution, is the geographical area that occupies a species in a certain time and space. It is a key concept in ecology and biogeography, and is influenced by a variety of factors, such as climate, topography, resource availability, competition with other species, and the dispersal ability of each organism (Chuine 2010; Kissling et al. 2018; Bakx et al. 2019). Understanding species distribution according to these factors is fundamental for biodiversity conservation, as it helps to identify priority areas for ecosystem protection and management (Da Silva et al. 2020; Piccolo et al. 2020; Srinivasulu et al. 2021). Thus, studies associating these environmental factors can provide valuable information on the ecological mechanisms governing the spatio-temporal the spatial distribution of threatened species.
Species distribution models (SDM) are tools and approaches used in biodiversity conservation to predict and understand the potential spatial distribution of species as a function of environmental and geographic variables (Peterson and Soberón 2012; Piccolo et al. 2020; Srinivasulu et al. 2021). These models are based on the idea that the presence or absence of a particular species within a given region is closely linked to specific environmental variables (Phillips and Dudík 2008). However, acquiring such direct data in the field can pose logistical problems due to access to remote areas and ethical implications for endangered species. Consequently, SDM allows leveraging existing data, such as observational records, to extrapolate and make inferences about distribution in areas where direct information may be limited (Gaubert et al. 2002; Fois et al. 2018; Meza-joya et al. 2018; Da Silva et al. 2020).
The Long-tailed Rattlesnake
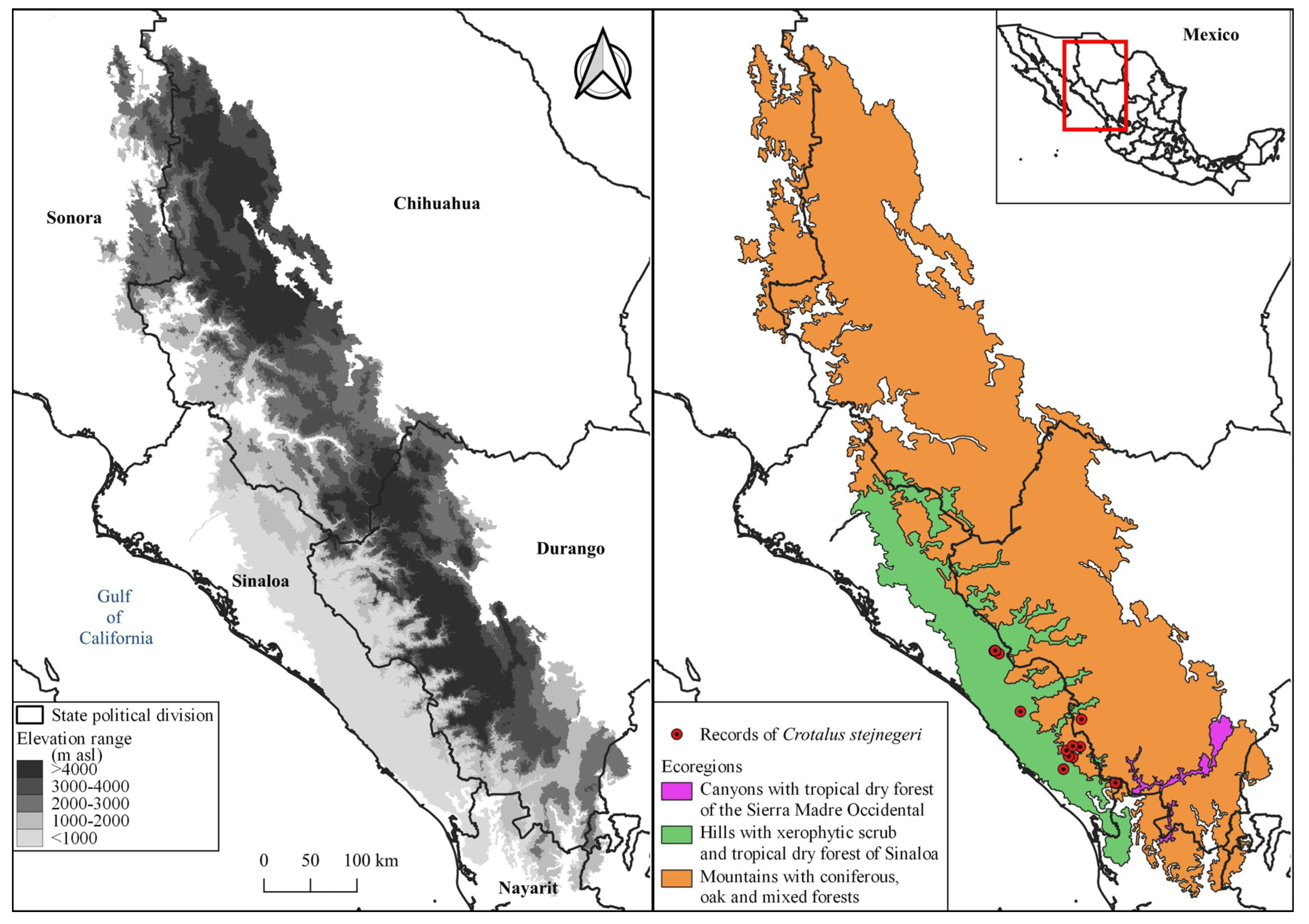
Crotalus stejnegeri (Dunn 1919) is a species endemic to Mexico (
Figure 1). It is classified as vulnerable (VU) by the IUCN (Mendoza-Quijano 2007) and threatened (A) by the Mexican species protection standard (SEMARNAT 2010). This species is considered rare because it has not been collected since 1976 and has a restricted distribution (Armstrong and Murphy 1979). Van der Heiden and Flores-Villela (2013) provided a geographic review of
C. stejnegeri collections to clarify its distribution in Sinaloa and Durango states, where records of the species have been reported. The authors acknowledge that
C. stejnegeri is present in southern Sinaloa and southwestern Durango (only in the locality of Ventanas). However, they dispute the record in Durango’s Yamoriba area, at 1780 m asl, deemed too high for the species, as noted by Robert Meidinger (pers. comm., 31 July 2011 in Uetz et al. 2023). Interestingly, Van der Heiden and Flores-Villela (2013) did not address the historical records of the species in Nayarit. In the herpetofauna listing of Nayarit, Woolrich-Piña et al. (2016) omitted
C. stejnegeri due to a lack of photographic evidence and documentation supporting its presence in the state. It is noteworthy that they also failed to mention that the ENCB-IPN 8307 collection record near San Blas was a juvenile
Crotalus basiliscus (pers. comm., Jesús Alberto Loc Barragán, after examining collection records from Nayarit).
Reyes-Velasco et al. (2010) proposed that the range of C. stejnegeri may be much larger than currently known. The authors provide several explanations supporting this assertion, including the existence of illicit operations and the limited availability of passable routes, particularly challenging to traverse during the wet season in these regions. Additionally, anthropogenic factors with the potential to impact its habitat, such as agriculture, livestock grazing, deforestation, and mining, are identified (Castro-Bastidas and Serrano 2022; Jacobo-González et al. 2023; unpublished data; HACB). The presence of C. stejnegeri in southern Sinaloa and the neighboring states of Durango and Nayarit is believed to be probably greatly underestimated (Van der Heiden and Flores-Villela 2013).
Presently, there is a 70% increase in C. stejnegeri records, facilitated by the contributions of citizen science in the state of Sinaloa (45 records from GBIF 2024, compared to 13 collections in Van der Heiden and Flores-Villela 2013). Additionally, recent information on the biology and ecology of the species is now available (Van der Heiden 2019, 2021). However, information on its natural history and population structure remains limited. The main objective of this study is to develop a species distribution model (SDM) for the long-tailed rattlesnake (C. stejnegeri) in order to map its environmental suitability, estimate its potential distribution, and identify environmental factors limiting its geographic range.
Despite previous efforts to delineate its distribution in specific regions such as Sinaloa and Durango, the available information presents significant gaps and disagreements about the extent of its range. In addition, anthropogenic factors such as deforestation, agriculture and mining pose additional threats to its habitat, further complicating conservation efforts. Given this context, by predicting the potential distribution of C. stejnegeri, I hope that provide valuable information for ecosystem management and conservation decision-making, identifying priority areas for the protection and restoration of its habitat. This research will not only expand our understanding of the distribution of C. stejnegeri, but will also exemplify how species distribution models can overcome the difficulties of obtaining direct data in the field, particularly for species with limited or inconsistent information.
Methodology
Data source. Records of C. stejnegeri were obtained after a review of records in scientific collection databases (Global Biodiversity Information Facility: GBIF 2024; Vertnet Networks: Vertnet 2016), citizen science observations (iNaturalist) and literature records (Van der Heiden and Flores-Villela 2013; Van der Heiden 2019). Each record underwent verification, and those lacking coordinates were assigned one if a reference locality was provided via Google Earth. Records without coordinates or a reference locality were excluded. Nearest neighbor analysis was conducted to mitigate any spatial bias present in the C. stejnegeri distribution data. The expected mean distance was 0.145, indicating the average distance that would be expected between records if they were randomly distributed. However, the resulting index of 22042.762 and the observed mean distance of 3209.109 suggest significant clustering of records, which could indicate the presence of spatial biases in the data. Duplicate records were eliminated because clustering of the data was obtained (Abdelaal et al. 2019). Records from Yamoriba in Durango and San Blas in Nayarit were not considered due to geographic and misidentification concerns associated with these records. Of the 45 presence records, 27 records of C. stejnegeri were used for model generation, which can be considered a moderate amount despite the scarce sampling of the species for several years.
On the other hand, the selection of environmental variables was obtained from the climatological database CHELSA v2.1 (Brun et al. 2022). This database encompasses 19 traditional bioclimatic variables with a 30-second resolution (~1 km
2), and its data range from 1980 to 2018 (Appendix). These variables were chosen primarily because the majority of
C. stejnegeri records are post-2000. Additionally, an elevation raster file (
Figure 2) with the same resolution was integrated into the model generation (INEGI 2023). Moreover, Mexico's ecoregions at level IV (CONABIO 2020) served as the geographic boundary for the model (Soberón and Nakamura 2009). These ecoregions share similar ecological and biogeographic characteristics, including various vegetation types that may influence the distribution of
C. stejnegeri (Bakx et al. 2019). The raster files were cropped according to the ecoregions that intersected with nearby records of the species: Canyons with tropical dry forest of the Sierra Madre Occidental (SMO), hills with xerophytic scrub and tropical dry forest of Sinaloa, and mountains with coniferous, oak, and mixed forests (
Figure 2).
Data analysis and model validation. Initially, multicollinearity was addressed and the most relevant predictors that showed the greatest contribution to the model were selected. Variance inflation factors (VIF) of all environmental variables were evaluated. This analysis was performed with the "sdm" package in R ver.1.0.46 (R Core Team 2016), where the VIF index was obtained for each variable (Naimi and Araújo 2016). A VIF index > 5 is considered to indicate high multicollinearity among the variables, so it is recommended to discard them for model generation (Abdalaal et al. 2019). Of the five bioclimatic variables obtained, Bio9 (3,002), Bio11 (6,500), Bio14 (2,650), Bio15 (6,300) and Bio19 (1,488) presented a VIF index < 10. However, the variables Bio11 and Bio15 showed a high multicollinearity index (Appendix) and a positive linear correlation coefficient (0.813). After a reassessment of multicollinearity, the variable Bio11 was excluded, but Bio15 was retained due to its representation of seasonality data (see Appendix). Despite its high multicollinearity, it was decided not to exclude this variable because Sinaloa has pronounced seasonality (Serrano et al. 2014) and contains the majority of C. stejnegeri records. In this second analysis, none of the variables showed a significant VIF index (< 3) and there was no evidence of a positive linear correlation coefficient ≤ 0.5 between them.
I utilized the MaxEnt v3.4.4 software (Phillips et al. 2006) to generate the SMD due to its efficiency in yielding acceptable results even with limited data (Abdalaal et al. 2019; Da Silva et al. 2020). Automation of linear L, quadratic Q, and P product features it allows capturing complex relationships, increasing model flexibility, simplifying model response interpretation, and minimizing selection bias, resulting in a more accurate and reliable representation of species distribution. To enhance model robustness, we employed a bootstrap-type model fitting, with randomization of 40% of the test records while using the remaining data for training, provides an effective way to improve stability, reduce selection bias, enhance model generalization, and control estimation error. A total of 27 replicates were generated, corresponding to the number of records of C. stejnegeri, with a maximum of 5,000 iterations (Warren and Seifert 2011; Dai et al. 2022). This choice was based on the intention to use the model with average data from these replicas, aiming to mitigate the impact of any inherent variability in the data and obtain a more robust estimation of the potential distribution of C. stejnegeri. The test of equality between sensitivity and specificity served as the threshold rule. Furthermore, the jackknife test, recognized as the best index for small sample sizes (Phillips et al. 2006), was employed to evaluate the percentage contribution of each variable.
To assess the accuracy of the resulting models, we calculated the area under the curve (AUC) of the Receiver Operating Characteristic Curve (ROC). The AUC score stands out as a key metric for measuring model performance, primarily due to its independence from the choice of thresholds (Abdalaal et al. 2019; Dai et al. 2022). A higher AUC value, closer to 1, indicates superior model performance (Phillips et al. 2006). The AUC plot is generated by plotting true positive predictions (sensitivity) against false positive predictions (1-specificity) (Fielding and Bell 1997). Due to the moderate sample size (27 records) and the simplicity of this approach to model replication generation, the use of AUC as the primary evaluation metric is widely accepted and utilized, making it particularly useful for comparing models and assessing their performance.
Finally, the output from MaxEnt was in logistic format, representing a habitat suitability map for the species with values ranging from 0 (unsuitable) to 1 (optimal). For additional analysis, the MaxEnt results were imported into QGIS 3.34.3 (QGIS 2022), where three classes of potential habitats were defined according to the suitability for the presence of C. stejnegeri: low potential (0–0.30), moderate potential (0.31-0.69), and high potential (≥ 0.77–1).
Results and Discussion
The MaxEnt model with the average value of the replicates showed an AUC of 0.879, with a standard deviation of 0.065. Since some of the individual models could have been influenced by unrealistic or atypical data, the average was considered a better option to obtain a more balanced and reliable estimation of the species' distribution. For this reason, the estimation of the environmental suitability can be considered to have a good degree of reliability (Phillips et al. 2006).
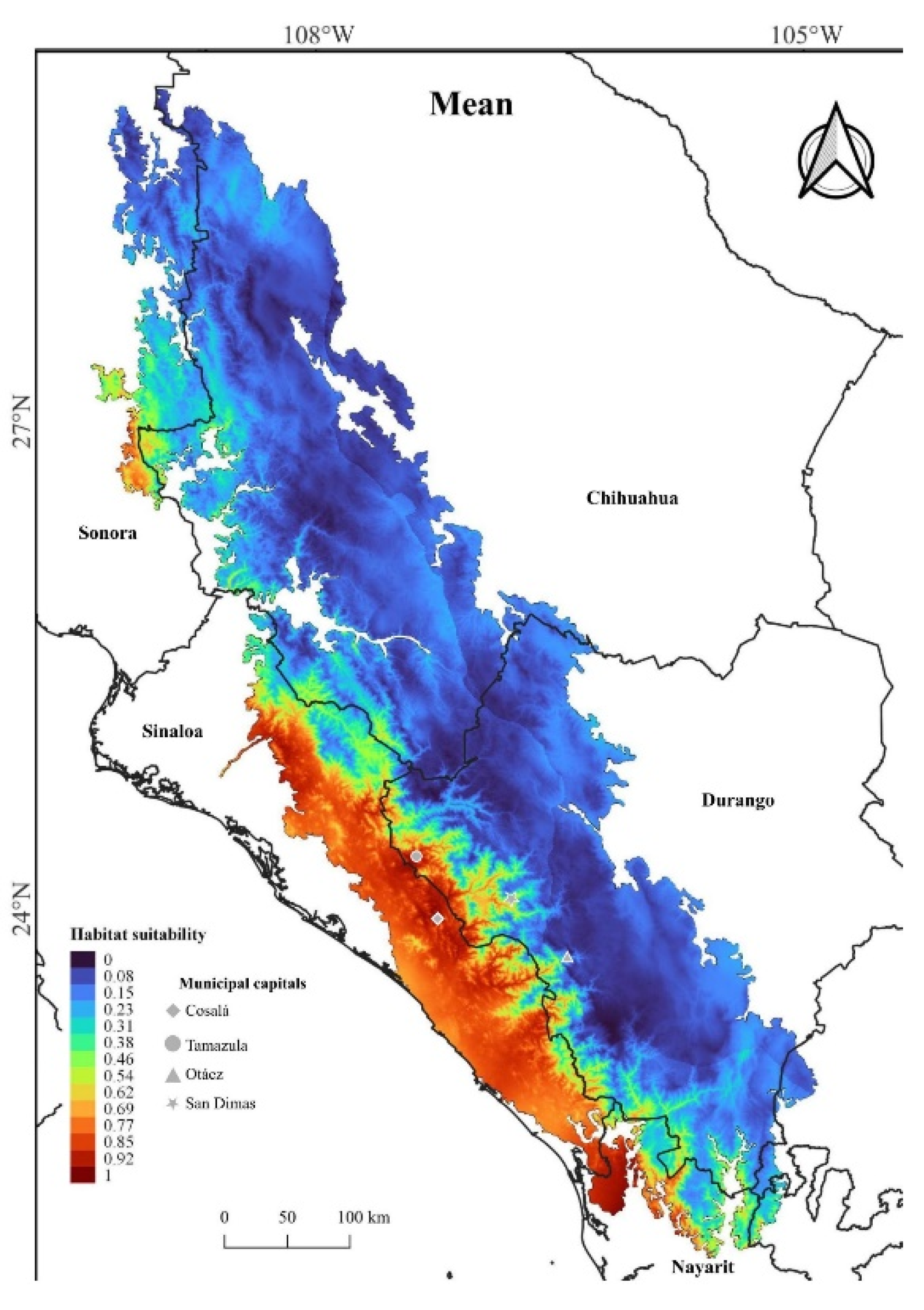
Figure 3 shows the potential distribution for
C. stejnegeri. Low probability zones of species presence are represented by blue shades, while reddish colors indicate high habitat suitability, predominantly concentrated in the foothills of the SMO spanning from Sinaloa to the northern part of Nayarit. These regions exhibit high habitat suitability possibly due to the dominance of tropical dry forest (Serrano et al. 2014; Woolrich-Piña et al. 2016), the prevalent vegetation type where the majority of the species’ records have been documented (
Figure 2B; GBIF 2024). These results suggest that
C. stejnegeri has no affinity for low elevation zones, contrary to the information presented by Campbell and Lamar (2024) for Nayarit. In the state of Durango, elevated levels of habitat suitability for
C. stejnegeri are observed in the SMO, particularly in the municipalities of Tamazula and Otáez, adjacent to the municipality of Cosalá in Sinaloa. This heightened suitability may be attributed to the proximity of these Durango municipalities to Cosalá in Sinaloa, where a significant number of
C. stejnegeri records have been documented.
In the same region, particularly in the municipality of San Dimas in Durango, a small area with high habitat suitability for
C. stejnegeri is depicted in
Figure 3. This observation aligns with Robert Meidinger’s comment (in Uetz et al. 2023), questioning the altitudinal range of 1,780 m asl as being too high for
C. stejnegeri, as evident by the low habitat suitability around Yamiroba in the municipality of San Dimas, Durango (
Figure 2 and
Figure 3). This is consistent with the altitudinal preferences of rattlesnake species (Lara-Galván et al. 2020; Serna-Lagunes et al. 2020), highly adapted to their elevation ranges to develop their physiological activities. On the other hand, Reyes-Velasco et al. (2013) suggested that
C. stejnegeri could also be found in Nayarit, Sonora, and Chihuahua, although
Figure 3 shows a high suitability of the habitat for the species in the north of Nayarit and southwest of Sonora, the state of Chihuahua does not present a marked suitability environment for the species. This discrepancy might be attributed to
C. stejnegeri’s neotropical biogeographic affinity, since northeast Sinaloa may be the northernmost limit for neotropical flora and fauna (Gentry 1946; Morrone et al. 2017; Castro-Bastidas 2022; Pío-León et al. 2023; Castro-Bastidas et al. 2024). Furthermore, this could also apply to southwest Sonora, although
Figure 3 shows an area of high suitability, this result is probably due to the fact that this is a transitional region from the Sonoran Desert to the dry forests at the foot of the SMO (Bezy et al. 2017; unpublished data, HACB).
The significant influence of air temperature during the driest quarter (Bio9), particularly in April, May, and June, on the habitat suitability model for C. stejnegeri, suggests that alterations in this variable have a substantial impact on the potential distribution of the species, accounting for a considerable 74.5% of the model's influence. This underscores the importance of temperature conditions during the reproductive periods of rattlesnake species, aligning with their heightened activity levels during mid-spring to late summer (Armstrong and Murphy 1979; Schuett et al. 2002).
Additionally, precipitation seasonality (Bio15) plays a significant role, albeit to a lesser extent compared to temperature, representing 15.3% of the model's influence. This highlights the importance of variations in the amount and distribution of precipitation throughout the year in shaping the potential distribution of C. stejnegeri. Similarly, the amount of precipitation during the driest month (Bio14) in April is also a relevant factor, contributing 9.4% to the model. This emphasizes the significance of water availability during the driest period in the modeling process.
The observed distribution patterns of C. stejnegeri may align with the typical reproductive behavior of rattlesnake species in North America, as well as the warm and humid conditions characteristic of the south-central region of Sinaloa's tropical dry forest (Serrano et al. 2014). These factors likely contribute to shaping the distribution of the species.
On the other hand, monthly precipitation during the coldest quarter (Bio19) has the smallest contribution to the model at 0.8%. This minimal influence may be attributed to the variations observed between replicates, suggesting that other factors play a more significant role in determining habitat suitability for C. stejnegeri during the colder months.
These results are consistent with those obtained for other rattlesnake species where temperature and precipitation ranges are important environmental factors in their distribution (Sunny et al., 2019; Lara-Galván et al. 2020). Although it has also been suggested that these factors go beyond influencing physiological aspects of the species such as reproductive activity, they also influence other aspects related to their survival such as densities of predators, prey and competitors, weed cover and relative humidity (Yañez-Arenas et al. 2020).
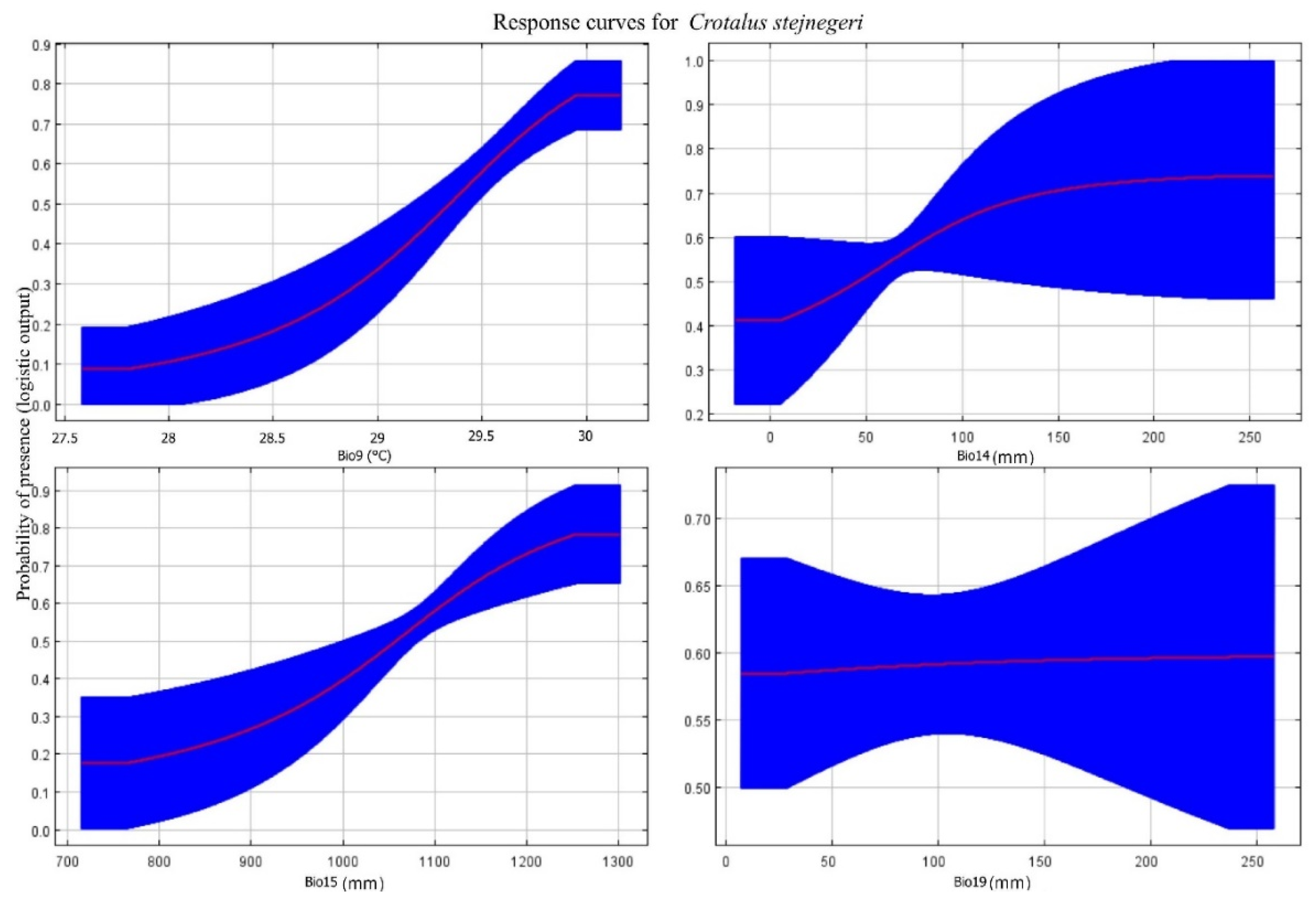
For a more in depth analysis that integrates both the potential distribution and the biological and ecological aspects of
C. stejnegeri,
Figure 4 shows the response curves of key climatic variables. We observe that
C. stejnegeri exhibits a higher probability of occurrence in areas with air temperatures between 29–30 °C during the driest quarter (Bio9) (April, May and June), suggesting a preference for specific thermal conditions for its activity and reproduction. In addition, the species shows ranges of probability of occurrence related to the amount of precipitation (between 25–100 mm) during the driest month (Bio14) (April) and the seasonality of precipitation (Bio15) between 1,000–1,200 mm, reflecting its dependence on water during critical periods such as breeding and foraging.
On the other hand, the low probability of occurrence in areas with monthly precipitation during the coldest quarter between 50–150 mm (Bio19) (December, January and February) suggests a physiological response of adaptation to more adverse climatic conditions. However, the significant variability in this relationship is less consistent or more variable in specific areas. The results obtained by Lara-Galván et al. (2020) for C. basiliscus in the Mexican state of Zacatecas are similar to those presented here for C. stejnegeri, but differ in the annual range temperature (Bio7) for C. basiliscus and mean temperature of the driest quarter (Bio9) for C. stejnegeri. This suggests that C. stejnegeri may be more adapted to warm and dry climates, C. basiliscus may show a preference for cooler and wetter climatic conditions. In addition, C. stejnegeri may be more influenced by temperature and water availability during its breeding period, whereas C. basiliscus may be more dependent on precipitation to maintain its habitat and find prey throughout the year.
These findings not only provide information on the potential distribution of the species, but also offer valuable insights into its biology and ecology. The identification of critical climatic variables associated with the presence of C. stejnegeri may help guide more effective conservation and management strategies, while highlighting the importance of considering uncertainty in model predictions, which may indicate areas for future research or improvements in data collection.
Conclusions
The MaxEnt model delivers a reliable estimate of habitat suitability for C. stejnegeri. The potential distribution of the species is prominently observed in the foothills region of the SMO, particularly in the municipalities of Sinaloa and Durango, characterized by a prevalence of tropical dry forest. These results align with previous observations regarding the species’ preference for specific altitudes. The absence of distinct environmental suitability in Chihuahua may be linked to the neotropical biogeographic affinity of the species. Bioclimatic variables, notably the temperature of the driest quarter (April, May, and June) and precipitation seasonality, play crucial roles in the modeling, with water availability during the driest month (April) identified as a key factor. However, the variability highlighted by the blue standard deviation bands in the response curves, particularly in precipitation during the coldest quarter (December, January, and February), underscores the necessity of considering uncertainty in model predictions. Additionally, it directs attention to specific areas that warrant further research and efforts to enhance records of the species’ presence.
Acknowledgments
To the curators of the herpetological collection at the Natural History Museum, London for the availability of the photograph of C. stejnegeri (NHMUK: 1883.4.16.64). In addition, to Jesús Alberto Loc Barragán for providing information on the alleged records of the species in Nayarit, Mexico. Also, to the anonymous reviewers who contributed substantially to the improvement of this manuscript.
|
Appendix. Bioclimatic variables from the CHELSEA climatological database and Variance Inflation Factors (VIF) analysis of the best predictor variables for the model. The asterisks show the highly correlated variables in the first analysis. |
| Code |
Unit |
Variable |
First multicollinearity analysis (VIF) |
Second multicollinearity analysis (VIF) |
| Bio1 |
C° |
mean annual air temperature |
Infinite |
— |
| Bio2 |
C° |
mean diurnal air temperature range |
Infinite |
— |
| Bio3 |
C° |
isothermality |
Infinite |
— |
| Bio4 |
C° |
temperature seasonality |
Infinite |
— |
| Bio5 |
C° |
mean daily maximum air temperature of the warmest month |
Infinite |
— |
| Bio6 |
C° |
mean daily minimum air temperature of the coldest month |
Infinite |
— |
| Bio7 |
C° |
annual range of air temperature |
Infinite |
— |
| Bio8 |
C° |
mean daily mean air temperatures of the wettest quarter |
Infinite |
— |
| Bio9 |
C° |
mean daily mean air temperatures of the driest quarter |
3.002 |
1.421 |
| Bio10 |
C° |
mean daily mean air temperatures of the warmest quarter |
Infinite |
— |
| Bio11 |
C° |
mean daily mean air temperatures of the coldest quarter |
6.500* |
— |
| Bio12 |
mm |
annual precipitation amount |
Infinite |
— |
| Bio13 |
mm |
precipitation amount of the wettest month |
Infinite |
— |
| Bio14 |
mm |
precipitation amount of the driest month |
2.650 |
2.296 |
| Bio15 |
mm |
precipitation seasonality |
6.300* |
1.442 |
| Bio16 |
mm |
mean monthly precipitation amount of the wettest quarter |
Infinite |
— |
| Bio17 |
mm |
mean monthly precipitation amount of the driest quarter |
Infinite |
— |
| Bio18 |
mm |
mean monthly precipitation amount of the warmest quarter |
Infinite |
— |
| Bio19 |
mm |
mean monthly precipitation amount of the coldest quarter |
1.488 |
1.462 |
| DEM |
m snm |
Elevation |
Infinite |
— |
Literature Cited
- Abdelaal, M., M. Fois, G. Fenu, and G. Bacchetta. 2019. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecological Informatics 50: 68–75. [CrossRef]
- Armstrong, B.L. and J.B. Murphy. 1979. The Natural History of Mexican Rattlesnakes. University of Kansas, Museum of Natural History, University of Kansas, Lawrence.
- Bakx, T.R.M., Z. Koma, A.C. Seijmonsbergen, and W.D. Kissling. 2019. Use and categorization of Light Detection and Ranging vegetation metrics in avian diversity and species distribution research. Diversity and Distributions 25: 1045–1059. [CrossRef]
- Bezy, R.L., P.C. Rosen, T.R. Van Devender, and E.F. Enderson. 2017. Southern distributional limits of the Sonoran Desert herpetofauna along the mainland coast of northwestern Mexico. Mesoamerican Herpetology 4: 138–167.
- Brun, P., N.E. Zimmermann, C. Hari, L. Pellisier and D.N. Karger. 2022. Global climate-related predictors at kilometre resolution for the past and future. Earth System Science Data 14: 5573-5603. [CrossRef]
- Campbell, J.A. and W.W. Lamar. 2004. The venomous reptiles of the western hemisphere. Comstock, Cornell University Press.
- Castro-Bastidas, H.A. and J.M. Serrano. 2022. La plataforma naturalista como herramienta de ciencia ciudadana para documentar la diversidad de anfibios en el estado de Sinaloa, México. Revista Latinoamericana de Herpetología 5: 156–178. [CrossRef]
- Castro-Bastidas, H.A. 2022. Nuevos registros del sapo chihuahuense Incilius mccoyi (Anura: Bufonidae) y ampliación de distribución de la rana esmeralda Exerodonta smaragdina (Anura: Hylidae) para Sinaloa, México. Revista Latinoamericana de Herpetología 5: 15–19. [CrossRef]
- Castro-Bastidas, H.A., H. Velarde-Urías, J.D. Jacobo-González, and J.M. Serrano. 2024. The amphibians and reptiles of Sierra Surutato, Sinaloa, Mexico. Sonoran Herpetologist 37(1): 40–48.
- CONABIO. 2020. Ecorregiones terrestres de México. <http://www.biodiversidad.gob.mx/region/ecorregiones>.
- Chuine, I. 2010. Why does phenology drive species distribution? Philosophical Transactions of the Royal Society B-Biological Sciences 365: 3149–3160. [CrossRef]
- Da Silva, F.P., H. Fernandes-Ferreira, M.A. Montes, and L.G. da Silva. 2020. Distribution modeling applied to deficient data species assessment: A case study with Pithecopus nordestinus (Anura, Phyllomedusidae). Neotropical Biology and Conservation 15: 165–175. [CrossRef]
- Dai, X., W. Wu, L. Ji, S. Tian, B. Yang, B. Guan and D. Wu. 2022. MaxEnt model-based prediction of potential distributions of Parnassia wightiana (Celastraceae) in China. Biodiversity Data Journal 10: e81073. [CrossRef]
- Fielding, H.A. and J.F. Bell. 1997. A review of methods for the assessment of prediction error in conservation presence/absence models. Environment Conservation 24:38–39.
- Fois, M., A. Cuena-Lombraña, G. Fenu, and G. Bacchetta. 2018. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecological Modelling 385: 124–132. [CrossRef]
- Gaubert, P., G. Veron, M. Colyn, A. Dunham, S. Shul and M. Tranier. 2002. A reassessment of the distributional range of the rare Genetta johnstoni (Viverridae, Carnivora), with some newly discovered specimens. Mammal Review 32: 132–144. [CrossRef]
- GBIF. 2024. GBIF Occurrence Download: Crotalus stejnegeri. [CrossRef]
- Gentry, H.S. 1946. Notes on the vegetation of Sierra Surotato in northern Sinaloa. Bulletin of the Torrey Botanical Club 73: 451–462.
- INEGI. 2023. <https://www.inegi.org.mx/app/geo2/elevacionesmex/>.
- Jacobo-González, J.D., D.S. Chan-Chon, A. Razo-Pérez, A. Leal-Orduño, E. Centenero-Alcalá, and R.A. Lara-Resendiz. 2023. Herpetofauna of the “El mineral de nuestra señora de la candelaria” reserve: a biological treasure in Sinaloa. Revista Latinoamericana de Herpetología 6: e801. [CrossRef]
- Kissling, W.D., J.A. Ahumada, A. Bowser, M. Fernandez, N. Fernández, E.A. García et al. 2018. Building essential biodiversity variables (EBVs) of species distribution and abundance at a global scale. Biological Reviews 93: 600–625. [CrossRef]
- Lara-Galván, J.L, J.F. Martínez-Montoya, J.J. Sigala-Rodríguez, C.E. Esparza-Estrada, O.C. Rosas-Rosas, L. Ávila-Herrera, and A.M. Barbosa. 2020. Rattlesnake (Crotalus spp.) distribution and diversity in Zacatecas, Mexico. ZooKeys 1005: 103–132. [CrossRef]
- Mendoza-Quijano, F. 2007. Crotalus stejnegeri. The IUCN Red List of Threatened Species 2007: e.T64333A12771355. [CrossRef]
- Meza-Joya, F.L., E. Ramos, F. Cediel, V. Martínez-Arias, J. Colmenares, and D. Cardona. 2018. Predicted distributions of two poorly known small carnivores in Colombia: the Greater Grison and Striped Hog-nosed Skunk. Mastozoología Neotropical 25: 89–105. [CrossRef]
- Morrone, J.J., T. Escalante, and G. Rodríguez-Tapia. 2017. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 4277: 277–279. [CrossRef]
- Naimi, B. and M. Araújo. 2016. Sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 39(4): 368–375. [CrossRef]
- Peterson, A.T. and J. Soberón. 2012. Species distribution modeling and ecological niche modeling: getting the concepts right. Natureza and Conservação 10: 102–107.
- Phillips, S.J., R.P. Anderson, and R.E. Chapire. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [CrossRef]
- Phillips, S.J. and M. Dudík. 2008. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161–175. [CrossRef]
- Piccolo, R.L., J. Warnken, A.L.M. Chauvenet, and J.G. Castley. 2020. Location biases in ecological research on Australian terrestrial reptiles. Scientific Reports 10: 9691. [CrossRef]
- Pío-León, J.F., M. González-Elizondo, R. Vega-Aviña, M.S. González-Elizondo, J.G. González-Gallegos, B. Salomón-Montijo, M.G. Millán-Otero, and C.A. Lim-Vega. 2023. Las plantas vasculares endémicas del estado de Sinaloa, México. Botanical Sciences 101: 243–269. [CrossRef]
- QGIS Development Team. 2022. QGIS 3.34.3. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
- R Core Team. 2016. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
- Reyes-Velasco, J., C.I. Grünwald, J.M. Jones, and G.N. Weatherman. 2010. Rediscovery of the rare Autlán longtailed rattlesnake, Crotalus lannomi. Herpetological Review 41: 19–25.
- Reyes-Velasco, J., J.M. Meik, E.N. Smith, and T.A. Castoe. 2013. Phylogenetic relationships of the enigmatic longtailed rattlesnakes (Crotalus ericsmithi, C. lannomi, and C. stejnegeri). Molecular Phylogenetics and Evolution 69: 524–534. [CrossRef]
- Schuett, G.W., S.L. Carlisle, A.T. Holycross, J.K. O´Leile, D.L. Hardy, E.A. van Kirk, and W.J. Murdoch. 2002. Mating system of male Mojave rattlesnakes (Crotalus scutulatus): seasonal timing of mating, agonistic behavior, spermatogenesis, sexual segment of the kidney, and plasma sex steroids, pp. 515–532. In: G.W. Schuett, M. Höggren, M.E. Douglas, and H.W. Greene (eds.), Biology of the Vipers. Eagle Mountain Publishing. USA.
- SEMARNAT. 2010. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, para la Protección ambiental-Especies nativas de México de flora y fauna silvestre-Categoría de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, México.
- Serna-Lagunes, R., G.B. Torres-Cantú, P. Andrés-Meza, N. Mora-Collado, R.C. Llarena-Hernández, and O.R. Leyva-Ovalle. 2020. Altitudinal adaptation of Crotalus intermedius Troschel, 1865 in a natural protected area from Mexico (Squamata: Viperidae). Herpetology Notes 13: 883–889.
- Serrano, J.M., C.A. Berlanga-Robles, and A. Ruiz-Luna. 2014. High amphibian diversity related to unexpected values in a biogeographic transitional area in north-western Mexico. Contributions to Zoology 83: 151–166. [CrossRef]
- Soberón, J. and M. Nakamura. 2009. Niches and distributional areas: Concepts, methods, and assumptions. Proceedings of the National Academy of Sciences 106(2): 19644–19650. [CrossRef]
- Srinivasulu, A., B. Srinivasulu, and C. Srinivasulu. 2021. Ecological niche modelling for the conservation of endemic threatened squamates (lizards and snakes) in the Western Ghats. Global Ecology and Conservation 28: e01700. [CrossRef]
- Sunny, A., F.J. Gandarilla-Aizpuro, O. Monroy-Vilchis, and M.M. Zarco-Gonzalez. 2019. Potential distribution and habitat connectivity of Crotalus triseriatus in Central Mexico. Herpetozoa 32: 139–-148. [CrossRef]
- Uetz, P., P. Freed, R. Aguilar, F. Reyes, and J. Hošek (eds.). 2023. The Reptile Database. <http://www.reptile-database.org>.
- Van der Heiden, A.M. and O.A. Flores-Villela. 2013. New records of the rare Sinaloan Long-tailed Rattlesnake, Crotalus stejnegeri, from southern Sinaloa, Mexico. Revista Mexicana de Biodiversidad 84: 1343–1348. [CrossRef]
- Van der Heiden, A.M. 2019. Crotalus stejnegeri (Sinaloan Long-tailed Rattlesnake). Reproduction in captivity. Herpetological Review 50: 742–743.
- Van der Heiden, A.M. 2021. Crotalus stejnegeri (Sinaloan Long-tailed Rattlesnake). Arboreal behavior. Herpetological Review 52: 868–869.
- Vertnet [Vertebrate Networks] (2016) Database: Crotalus stejnegeri. https:www.vertnet.org.
- Warren, D.L. and S.N. Seifert. 2011. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications 21: 335–342. [CrossRef]
- Woolrich-Piña, G.A, P. Ponce-Campos, J.A. Loc-Barragán, J.P. Ramírez-Silva, V. Mata-Silva, J.D. Johnson, E. García-Padilla and L.D. Wilson. 2016. The herpetofauna of Nayarit, Mexico: composition, distribution, and conservation. Mesoamerican Herpetology 3: 376–448.
- Yañez-Arenas, C., S. Castaño-Quintero, R. Rioja-Nieto, K. Rodríguez-Medina, and X. Chiappa-Carrara. 2020. Assessing the relative role of environmental factors that limit the distribution of the Yucatan rattlesnake (Crotalus tzabcan). Journal of Herpetology 54(2): 216–224. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).