Submitted:
09 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
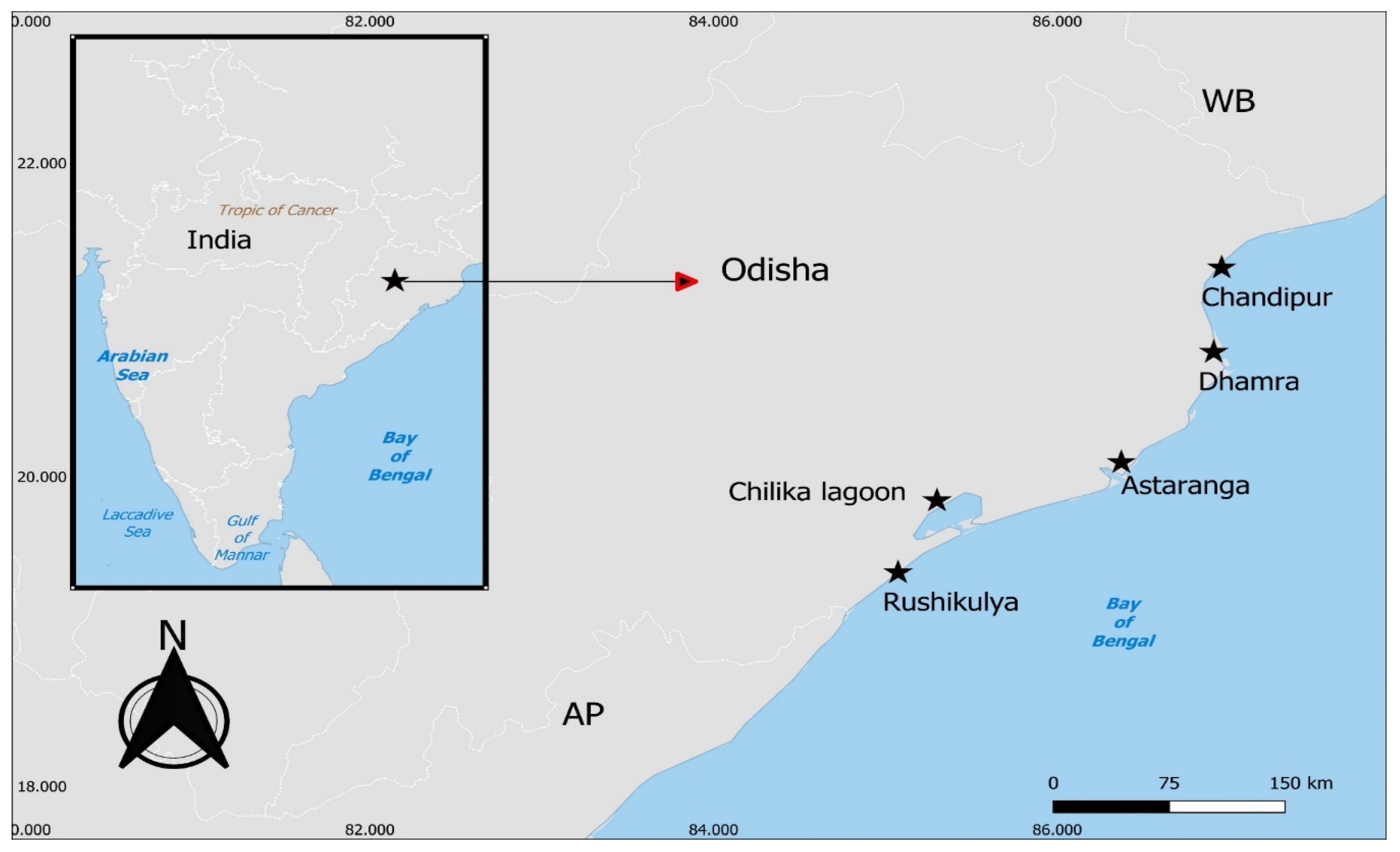
2.1. Study Area
2.2. Sediment Sampling and Analysis
2.3. Saltmarsh Plant Sampling and Analysis
2.4. Valuation of Carbon Stocks
2.6. Statistics
3. Results
3.1. Seasonal Variation in Physical Parameters
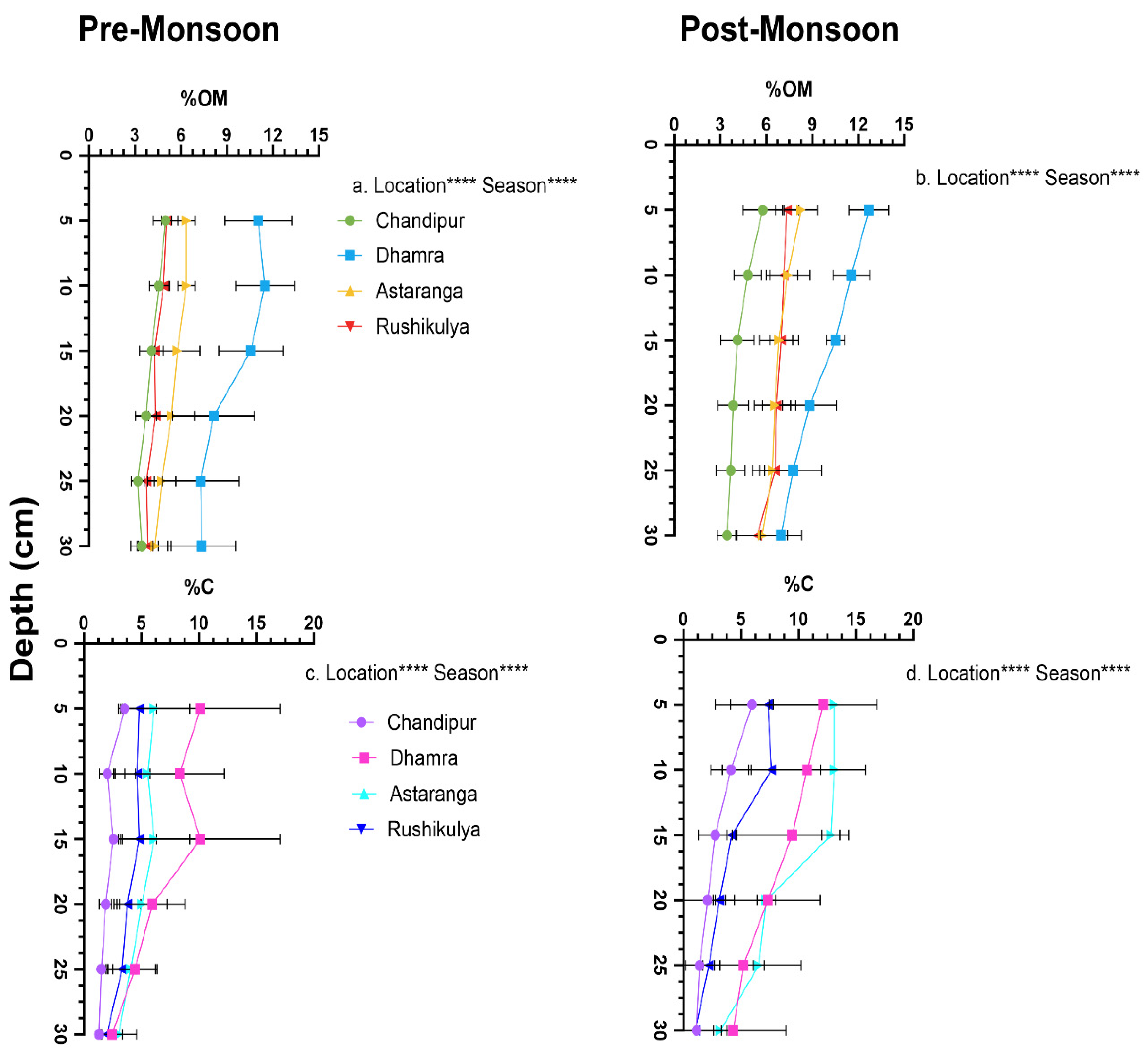
3.2. Seasonal Variation in P. coarctata Sediment Variables and Carbon Stocks
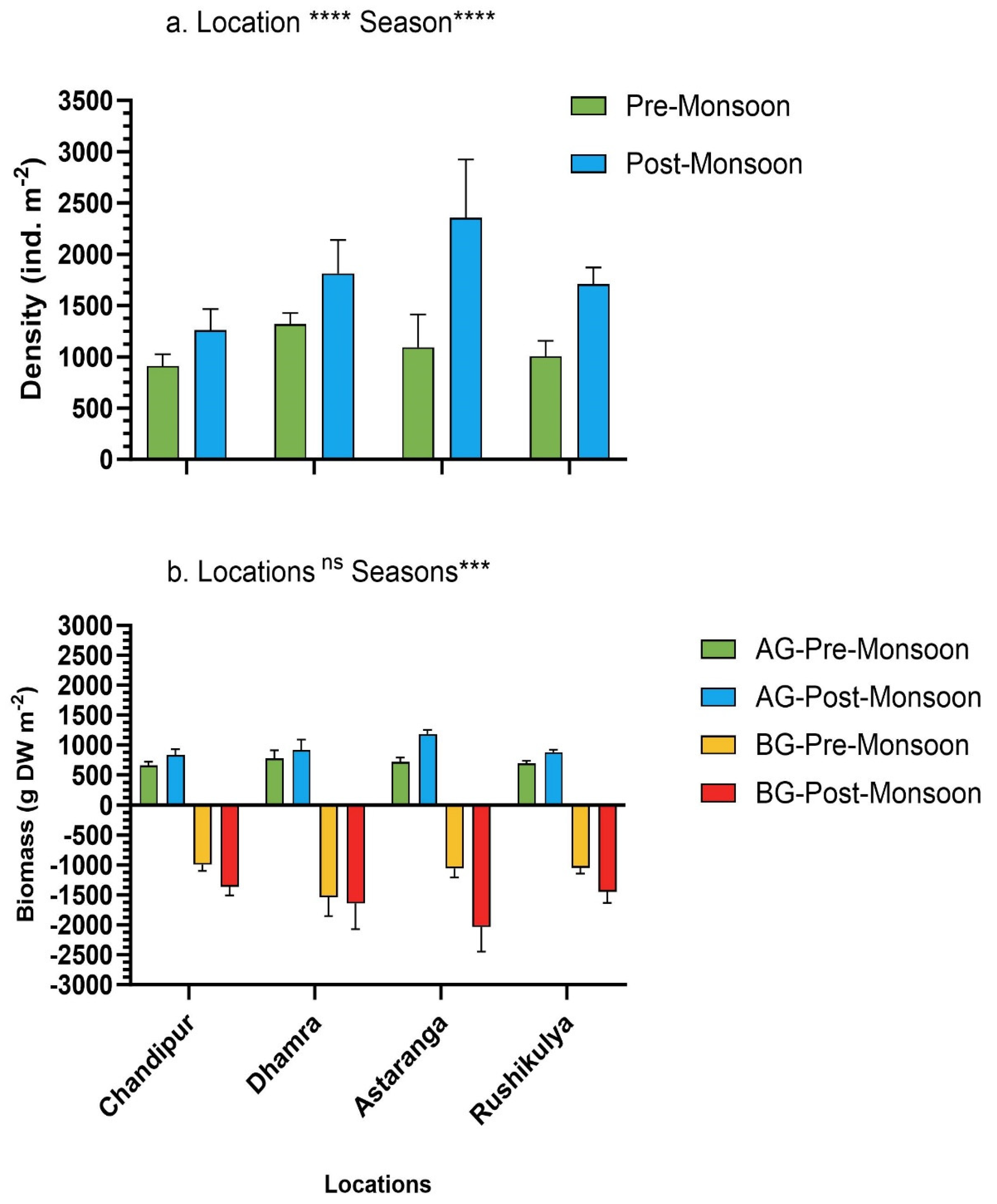
3.3. Seasonal Variation in P. coarctata Density, Biomass and Carbon Stocks
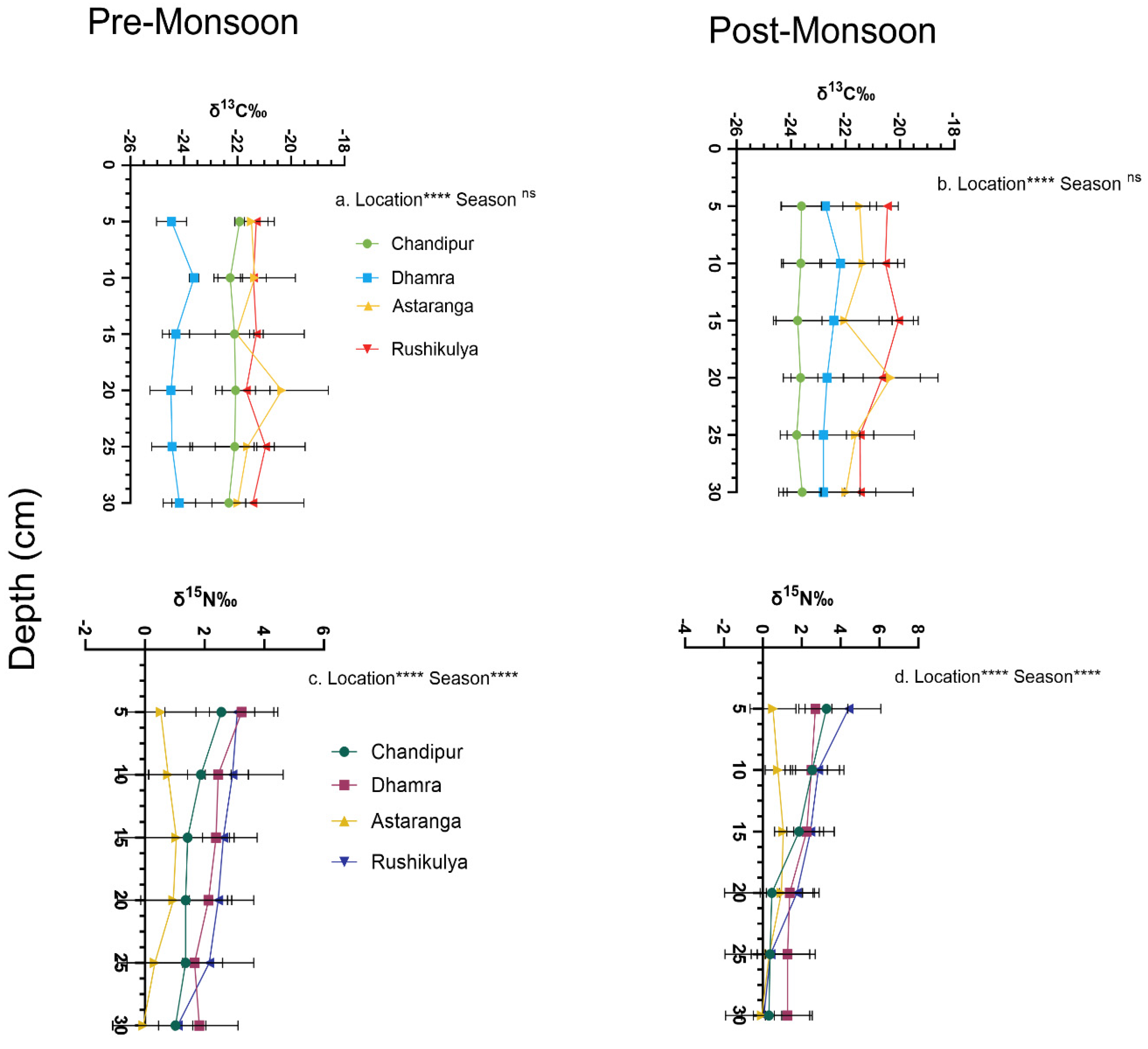
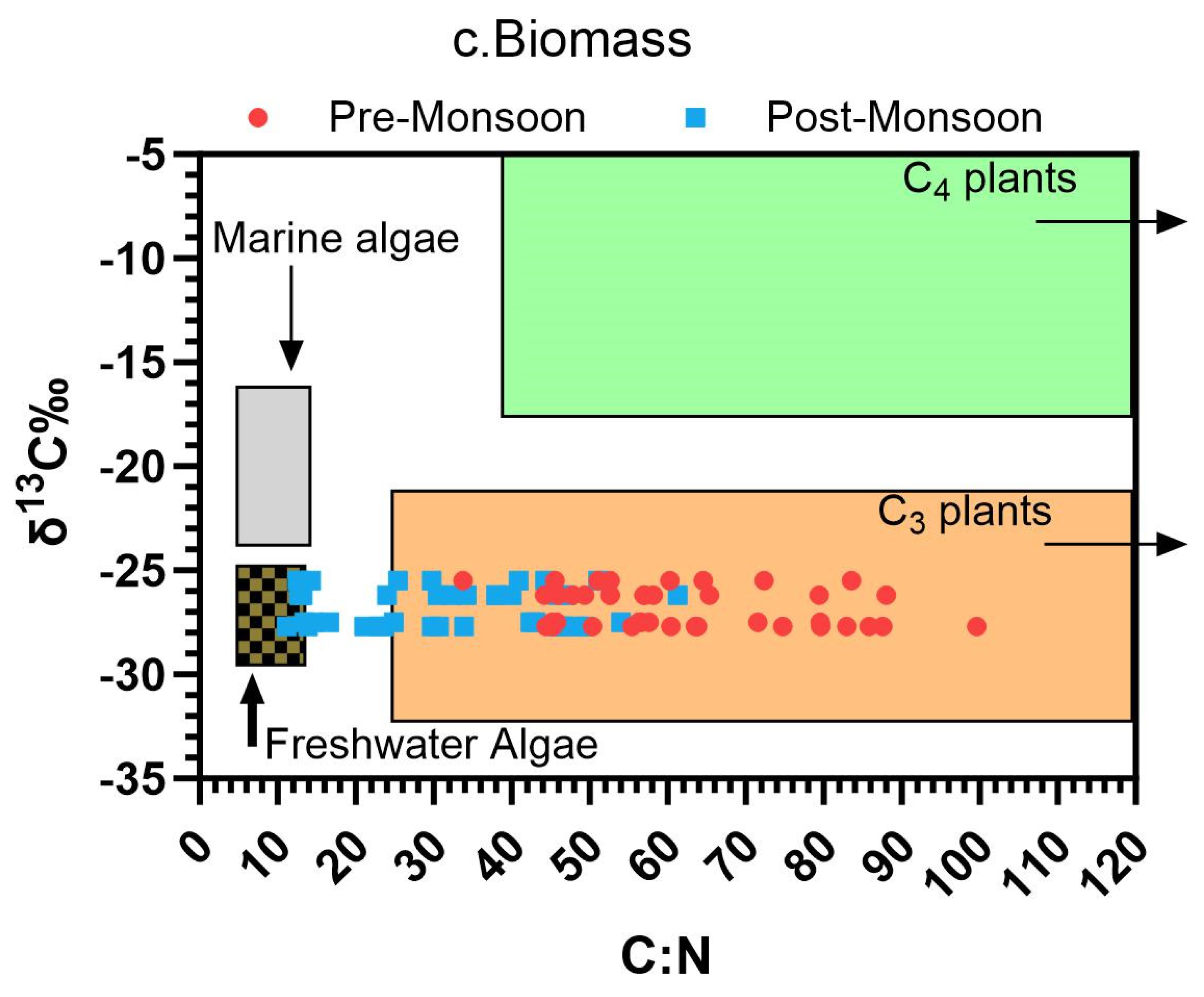
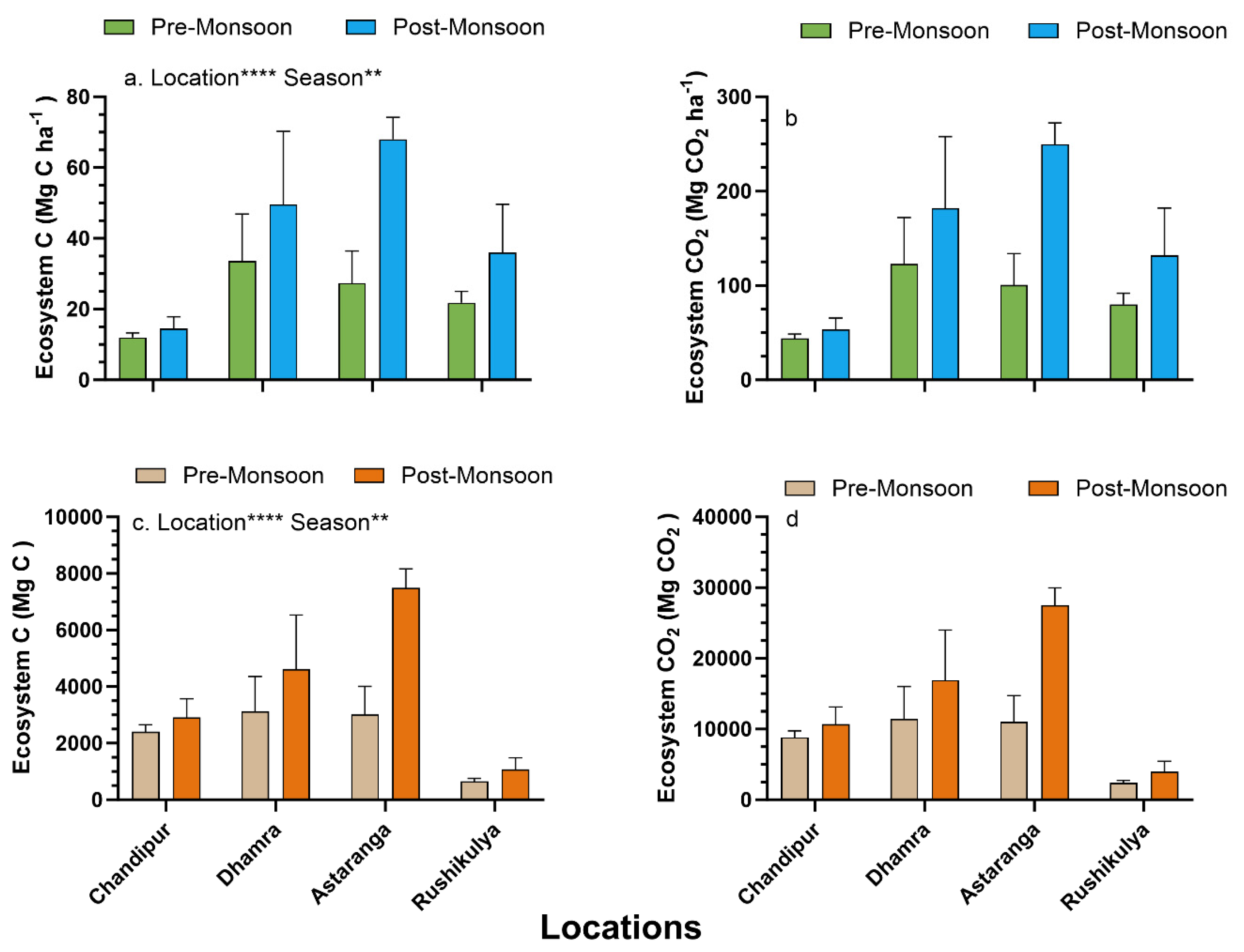
3.4. Ecosystem Carbon Stocks and Different Source Contribution to Sediment Organic Carbon Pool
4. Discussion
4.1. Influence of Physical Parameters on the Sediment Variables (DBD, OM, Corg%) and Carbon Stocks of P. coarctata Meadows
4.2. Influence of Seasonality on P. coarctata Traits and Carbon Stocks
4.3. Total Carbon Stocks of P. coarctata Ecosystems
4.4. P. coarctata Ecosystem Carbon Stocks and Their Role in India’s NDC
5. Conclusion
Funding
Acknowledgement
References
- Akhtar, S., Equeenuddin, Sk.Md., Bastia, F., 2021. Distribution of pCO2 and air-sea CO2 flux in Devi estuary, eastern India. Applied Geochemistry 131, 105003. [CrossRef]
- Alongi, D.M., 2020. Carbon balance in salt marsh and mangrove ecosystems: A global synthesis. J Mar Sci Eng 8, 1–21. [CrossRef]
- Ambade, B., Sethi, S.S., Kurwadkar, S., Mishra, P., Tripathee, L., 2022. Accumulation of polycyclic aromatic hydrocarbons (PAHs) in surface sediment residues of Mahanadi River Estuary: Abundance, source, and risk assessment. Mar Pollut Bull 183, 114073. [CrossRef]
- Bal, G., Banerjee, K., 2019. Carbon storage potential of tropical wetland forests of South Asia: a case study from Bhitarkanika Wildlife Sanctuary, India. Environ Monit Assess 191. [CrossRef]
- Banerjee, K., Mallik, K., Sahoo, C.K., Paul, R., 2022. Aquatic and edaphic determinants influencing carbon storage in a salt marsh grass, Porteresia coarctata, of the Bhitarkanika wildlife sanctuary and the Mahanadi estuary. Environmental Quality Management 31, 11–28. [CrossRef]
- Banerjee, K. Banerjee, K., Sappal, S.M., Ramachandran, P., Ramesh, R., 2017. Salt Marsh: Ecologically Important, Yet Least Studied Blue Carbon Ecosystems in India. Journal of Climate Change 3, 59–72. [CrossRef]
- Begam, M.M., Sutradhar, T., Chowdhury, R., Mukherjee, C., Basak, S.K., Ray, K., 2017. Native salt-tolerant grass species for habitat restoration, their acclimation and contribution to improving edaphic conditions: a study from a degraded mangrove in the Indian Sundarbans. Hydrobiologia 803, 373–387. [CrossRef]
- Bhadury, P., Sen, A., 2020. Understanding Impact of Seasonal Nutrient Influx on Sedimentary Organic Carbon and Its Relationship With Ammonia spp. in a Coastal Lagoon. Front Mar Sci 7. [CrossRef]
- Bhomia, R.K., Mackenzie, R.A., Murdiyarso, D., Sasmito, S.D., Purbopuspito, J., 2016. Impacts of land use on Indian mangrove forest carbon stocks: Implications for conservation and management. Ecological Applications 26, 1396–1408. [CrossRef]
- Billah, M.M., Zamal, H., Mustafa Kamal, A.H., Hoque, A.T.M.R., Rahman, M.M., Hoque, M.M., Akhtar, A., Hoque, M.N., 2016. Saltmarsh and seagrass beds on the south-eastern coast of Bangladesh: vegetation characteristics and adjacent fisheries diversity. Zoology and Ecology 26, 313–322. [CrossRef]
- Campbell, A.D., Fatoyinbo, L., Goldberg, L., Lagomasino, D., 2022a. Global hotspots of salt marsh change and carbon emissions. Nature 612, 701–706. [CrossRef]
- Campbell, A.D., Fatoyinbo, L., Goldberg, L., Lagomasino, D., 2022b. Global hotspots of salt marsh change and carbon emissions. Nature 612, 701–706. [CrossRef]
- Chatterji, A., Kotnala, S., Mathew, R., 2003. Effect of salinity on larval growth of horseshoe crab, Tachypleus gigas (Müller) 2002–2004.
- Chen, S., Torres, R., Goñi, M.A., 2016. The Role of Salt Marsh Structure in the Distribution of Surface Sedimentary Organic Matter. Estuaries and Coasts 39, 108–122. [CrossRef]
- Chowdhury, A., Naz, A., Bhattacharyya, S., Sanyal, P., 2018. Cost–benefit analysis of ‘Blue Carbon’ sequestration by plantation of few key mangrove species at Sundarban Biosphere Reserve, India. Carbon Manag 9, 575–586. [CrossRef]
- Chowdhury, A., Naz, A., Dasgupta, R., Maiti, S.K., 2023. Blue Carbon: Comparison of Chronosequences from Avicennia marina Plantation and Proteresia coarctata Dominated Mudflat, at the World’s Largest Mangrove Wetland. Sustainability (Switzerland) 15. [CrossRef]
- Cruz de Carvalho, R., Feijão, E., Kletschkus, E., Marques, J.C., Reis-Santos, P., Fonseca, V.F., Papenbrock, J., Caçador, I., Duarte, B., 2020. Halophyte bio-optical phenotyping: A multivariate photochemical pressure index (Multi-PPI) to classify salt marsh anthropogenic pressures levels. Ecol Indic 119, 106816. [CrossRef]
- Dencer-Brown, A.M., Shilland, R., Friess, D., Herr, D., Benson, L., Berry, N.J., Cifuentes-Jara, M., Colas, P., Damayanti, E., García, E.L., Gavaldão, M., Grimsditch, G., Hejnowicz, A.P., Howard, J., Islam, S.T., Kennedy, H., Kivugo, R.R., Lang’at, J.K.S., Lovelock, C., Malleson, R., Macreadie, P.I., Andrade-Medina, R., Mohamed, A., Pidgeon, E., Ramos, J., Rosette, M., Salim, M.M., Schoof, E., Talukder, B., Thomas, T., Vanderklift, M.A., Huxham, M., 2022. Integrating blue: How do we make nationally determined contributions work for both blue carbon and local coastal communities? Ambio. [CrossRef]
- Di Bella, C.E., Rodríguez, A.M., Jacobo, E., Golluscio, R.A., Taboada, M.A., 2015. Impact of cattle grazing on temperate coastal salt marsh soils. Soil Use Manag 31, 299–307. [CrossRef]
- Du, J., Chen, Z., Xie, M., Chen, M., Zheng, X., Liao, J., Chen, B., 2019. Analysis of organic carbon sources in tropical seagrass fish: a case study of the east coast of Hainan Province. Marine Biology Research 15, 513–522. [CrossRef]
- Ermgassen, P.S.E., Baker, R., Beck, M.W., Dodds, K., Ermgassen, S.O.S.E., Mallick, D., Taylor, M.D., Turner, R.E., 2021. Ecosystem Services : Delivering Decision-Making for Salt Marshes Why Quantify the Value of Ecosystem. Estuaries and Coasts 44, 1691–1698.
- Gilby, B.L., Weinstein, M.P., Baker, R., Cebrian, J., Alford, S.B., Chelsky, A., Colombano, D., Connolly, R.M., Currin, C.A., Feller, I.C., Frank, A., Goeke, J.A., Goodridge Gaines, L.A., Hardcastle, F.E., Henderson, C.J., Martin, C.W., McDonald, A.E., Morrison, B.H., Olds, A.D., Rehage, J.S., Waltham, N.J., Ziegler, S.L., 2021. Human Actions Alter Tidal Marsh Seascapes and the Provision of Ecosystem Services. Estuaries and Coasts 44, 1628–1636. [CrossRef]
- Gopi, M., Pravin Kumar, M., Joyson Joe Jeevamani, J., Raja, S., Muruganandam, R., Deepak Samuel, V., Simon, N.T., Viswanathan, C., Abhilash, K.R., Krishnan, P., Purvaja, R., Ramesh, R., 2019. Distribution and biodiversity of tropical saltmarshes: Tamil nadu and Puducherry, southeast coast of India. Estuar Coast Shelf Sci 106393. [CrossRef]
- Gorham, C. Gorham, C., Lavery, P., Kelleway, J.J., Salinas, C., Serrano, O., 2020a. Soil Carbon Stocks Vary Across Geomorphic Settings in Australian Temperate Tidal Marsh Ecosystems. Ecosystems. [CrossRef]
- Gorham, C., Lavery, P., Kelleway, J.J., Salinas, C., Serrano, O., 2020b. Soil carbon stocks vary across geomorphic settings in Australian temperate tidal marsh ecosystems. Ecosystems In review. [CrossRef]
- Hena, M.K.A., Short, F.T., Sharifuzzaman, S.M., Hasan, M., Rezowan, M., Ali, M., 2007. Salt marsh and seagrass communities of Bakkhali Estuary, Cox’s Bazar, Bangladesh. Estuar Coast Shelf Sci 75, 72–78. [CrossRef]
- Hossain, M., Saha, C., Rubaiot Abdullah, S.M., Saha, S., Siddique, M.R.H., 2016. Allometric biomass, nutrient and carbon stock models for Kandelia candel of the Sundarbans, Bangladesh. Trees - Structure and Function 30, 709–717. [CrossRef]
- Howard, J., Hoyt, S., Isensee, K., Pidgeon, E., Telszewski, M., 2014. Coastal Blue Carbon. National Wetlands Newsletter 36, 5–7.
- Hughes, A.R., Cebrian, J., Heck, K., Goff, J., Hanley, T.C., Scheffel, W., Zerebecki, R.A., 2018. Effects of oil exposure, plant species composition, and plant genotypic diversity on salt marsh and mangrove assemblages. Ecosphere 9. [CrossRef]
- Human, L.R.D., Els, J., Wasserman, J., Adams, J.B., 2022. Blue carbon and nutrient stocks in salt marsh and seagrass from an urban African estuary. Science of the Total Environment 842, 156955. [CrossRef]
- Islam, M.S., Pervez, A., Asseri, A.H., Al-Mutair, M., Sumon, M.A.A., Taleb, M.A., Ashik, A.A., Rahman, M.A., Molla, M.H.R., 2022. Diversity and seasonal succession of resident and migratory macrobenthic fauna of saltmarsh restoration site at Sonadia Island, Cox’s Bazar, Bangladesh. Reg Stud Mar Sci 53, 102460. [CrossRef]
- Jiang, Z., Zhao, C., Yu, S., Liu, S., Cui, L., Wu, Y., Fang, Y., Huang, X., 2019. Contrasting root length, nutrient content and carbon sequestration of seagrass growing in offshore carbonate and onshore terrigenous sediments in the South China Sea. Science of The Total Environment 662, 151–159. [CrossRef]
- Jinks, K.I., Rasheed, M.A., Brown, C.J., Olds, A.D., Schlacher, T.A., Sheaves, M., York, P.H., Connolly, R.M., 2020. Saltmarsh grass supports fishery food webs in subtropical Australian estuaries. Estuar Coast Shelf Sci 238, 106719. [CrossRef]
- Karstensen, J., Roy, J., Deb Pal, B., Peters, G., Andrew, R., 2020. Key drivers of Indian greenhouse gas emissions. Econ Polit Wkly 55, 46–53.
- Kaviarasan, T., Dahms, H.U., Gokul, M.S., Henciya, S., Muthukumar, K., Shankar, S., Arthur James, R., 2019. Seasonal Species Variation of Sediment Organic Carbon Stocks in Salt Marshes of Tuticorin Area, Southern India. Wetlands 39, 483–494. [CrossRef]
- Koshy, N.E., Bhatt, J.R., Vakily, J.M., 2018. Synthesis of the Conference on Management and Conservation of Seagrass Ecosystems in India. Ocean Coast Manag 159, 3–6. [CrossRef]
- Lamb, A.L., Wilson, G.P., Leng, M.J., 2006. A review of coastal palaeoclimate and relative sea-level reconstructions using d 13 C and C / N ratios in organic material 75, 29–57. [CrossRef]
- Lin, J., Liu, X., Lai, T., He, B., Du, J., Zheng, X., 2021. Trophic importance of the seagrass Halophila ovalis in the food web of a Hepu seagrass bed and adjacent waters, Beihai, China. Ecol Indic 125, 107607. [CrossRef]
- Mason, V.G., Burden, A., Epstein, G., Jupe, L.L., Wood, K.A., Skov, M.W., 2023a. Blue carbon benefits from global saltmarsh restoration. Glob Chang Biol 1–29. [CrossRef]
- Mason, V.G., Burden, A., Epstein, G., Jupe, L.L., Wood, K.A., Skov, M.W., 2023b. Blue carbon benefits from global saltmarsh restoration. Glob Chang Biol 1–29. [CrossRef]
- Mcleod, E., Chmura, G.L., Bouillon, S., Salm, R., Björk, M., Duarte, C.M., Lovelock, C.E., Schlesinger, W.H., Silliman, B.R., 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO 2. Front Ecol Environ 9, 552–560. [CrossRef]
- McMahon, L., Ladd, C.J.T., Burden, A., Garrett, E., Redeker, K.R., Lawrence, P., Gehrels, R., 2023. Maximizing blue carbon stocks through saltmarsh restoration. Front Mar Sci 10, 1–16. [CrossRef]
- Mcowen, C.J., Weatherdon, L. V., Van Bochove, J.W., Sullivan, E., Blyth, S., Zockler, C., Stanwell-Smith, D., Kingston, N., Martin, C.S., Spalding, M., Fletcher, S., 2017. A global map of saltmarshes. Biodivers Data J 5. [CrossRef]
- Miller, C.B., Rodriguez, A.B., Mctigue, N.D., Bost, M.C., Mckee, B.A., n.d. Carbon accumulation rates are highest at young and expanding salt marsh edges. [CrossRef]
- Miller, L.C., Smeaton, C., Yang, H., Austin, W.E.N., 2023. Carbon accumulation and storage across contrasting saltmarshes of Scotland. Estuar Coast Shelf Sci 282, 108223. [CrossRef]
- Mishra, A., Apte, D., 2020. Ecological connectivity with mangroves influences tropical seagrass population longevity and meadow traits within an island ecosystem. Mar Ecol Prog Ser 644, 47–63. [CrossRef]
- Mishra, A.K., Acharya, P., Apte, D., Farooq, S.H., 2023. Seagrass ecosystem adjacent to mangroves store higher amount of organic carbon of Andaman and Nicobar Islands, Andaman Sea. Mar Pollut Bull 193, 115135. [CrossRef]
- Mishra, A.K., Farooq, S.H., 2022a. Lack of ecological data hinders management of ecologically important saltmarsh ecosystems: A case study of saltmarsh plant Porterasia coarctata (Roxb.). J Environ Manage 321, 115957. [CrossRef]
- Mishra, A.K., Farooq, S.H., 2022b. Trace metal accumulation in seagrass and saltmarsh ecosystems of India: comparative assessment and bioindicator potential. Mar Pollut Bull 174, 113251. [CrossRef]
- Mishra, A.K., Narayana, S., Apte, D., 2021. Loss of Dugong Grass [Halophila Ovalis (R. Brown)] Population Structure Due to Habitat Disturbance in an Island Ecosystem. Indian Journal of Geo-Marine Sciences 50, 115–121.
- Mishra, R.K., Naik, S., Mishra, S., Mahapatro, D., Khadanga, M.K., 2022. Mass nesting of sea turtles along the east coast of India: A sustainable environmental management approach. Ecol Inform 69, 101648. [CrossRef]
- Misra, S., Choudhury, A., Chattopadhyay, S., Ghosh, A., 1988. Lipid composition of Porteresia coarctata from two different mangrove habitats in India. Phytochemistry 27, 361–364. [CrossRef]
- Mohanty, B., Nayak, A., Dash, B., Rout, S.S., Charan Kumar, B., Patnaik, L., Dev Roy, M.K., Raman, A., Raut, D., 2019. Biodiversity and ecological considerations of brachyuran crabs (Crustacea: Decapoda) from Devi estuary–mangrove region on the east coast of India. Reg Stud Mar Sci 32, 100865. [CrossRef]
- Naik, S., Mishra, R.K., Sahu, K.C., Lotliker, A.A., Panda, U.S., Mishra, P., 2020. Monsoonal Influence and Variability of Water Quality, Phytoplankton Biomass in the Tropical Coastal Waters – A Multivariate Statistical Approach. Front Mar Sci 7, 1–14. [CrossRef]
- Nazneen, S., Kumar, A., Raju, N.J., Mehmood, G., 2022. Coastal macrophytes as bioindicators of trace metals in the Asia ’ s largest lagoon ecosystem. Mar Pollut Bull 178, 113576. [CrossRef]
- Nordhaus, W.D., 2017. Revisiting the social cost of carbon. Proc Natl Acad Sci U S A 114, 1518–1523. [CrossRef]
- Pattnayak, S., Kumar, M., Sahu, S.C., Dhal, N.K., Behera, R.K., 2019. Comparison of soil characteristics and carbon content of contrastingly different moist-mixed deciduous and evergreen mangrove forest in Odisha, India. Geology, Ecology, and Landscapes 3, 239–246. [CrossRef]
- Perera, N., Lokupitiya, E., Halwatura, D., Udagedara, S., 2022. Quantification of blue carbon in tropical salt marshes and their role in climate change mitigation. Science of the Total Environment 820, 153313. [CrossRef]
- Phillips, D.L., Gregg, J.W., 2001. Uncertainty in source partitioning using stable isotopes. Oecologia 127, 171–179. [CrossRef]
- Phillips, D.L., Newsome, S.D., Gregg, J.W., 2005. Combining sources in stable isotope mixing models: alternative methods. Oecologia 144, 520–527. [CrossRef]
- Phillips, J.,& Yang, L., 2017. Oryza coarctata. The IUCN Red List of Threatended Species 2017.
- Pradhan, U.K., Shirodkar, P. V., Sahu, B.K., 2009. Physico-chemical characteristics of the coastal water off Devi estuary, Orissa and evaluation of its seasonal changes using chemometric techniques. Curr Sci 96, 1203–1209.
- Pramanik, D.S., 2019. Fish species diversity and their assemblages of Devi estuary in north east coast of India. Int J Fish Aquat Stud 7, 265–273.
- Prasad, M.B.K., Kumar, A., Datta, D.K., Ramanathan, L., 2014. Spectrofluorometric analysis of organic matter in the Sundarban mangrove, Bangladesh. Indian Journal of Geo-Marine Sciences 43, 999–1006.
- Qu, W., Li, J., Han, G., Wu, H., Song, W., Zhang, X., 2019. Effect of salinity on the decomposition of soil organic carbon in a tidal wetland 609–617.
- Quevedo, J.M.D., Uchiyama, Y., Kohsaka, R., 2023. Progress of blue carbon research: 12 years of global trends based on content analysis of peer-reviewed and ‘gray literature’ documents. Ocean Coast Manag 236, 106495. [CrossRef]
- Radabaugh, K.R., Powell, C.E., Bociu, I., Clark, B.C., Moyer, R.P., 2017. Plant size metrics and organic carbon content of Florida salt marsh vegetation. Wetl Ecol Manag 25, 443–455. [CrossRef]
- Rahaman, S.M.B., Sarder, L., Rahaman, M.S., Ghosh, A.K., Biswas, S.K., Siraj, S.M.S., Huq, K.A., Hasanuzzaman, A.F.M., Islam, S.S., 2013. Nutrient dynamics in the Sundarbans mangrove estuarine system of Bangladesh under different weather and tidal cycles. Ecol Process 2, 1–13. [CrossRef]
- Ramesh, R., Banerjee, K., Paneer Selvam, A., Lakshmi, A., Krishnan, P., Purvaja, R., 2018. Legislation and policy options for conservation and management of seagrass ecosystems in India. Ocean Coast Manag 159, 46–50. [CrossRef]
- Rasquinha, D.N., Mishra, D.R., 2021. Impact of wood harvesting on mangrove forest structure, composition and biomass dynamics in India. Estuar Coast Shelf Sci 248, 106974. [CrossRef]
- Rendón, O.R., Garbutt, A., Skov, M., Möller, I., Alexander, M., Ballinger, R., Wyles, K., Smith, G., McKinley, E., Griffin, J., Thomas, M., Davidson, K., Pagès, J.F., Read, S., Beaumont, N., 2019. A framework linking ecosystem services and human well-being: Saltmarsh as a case study. People and Nature 1, 486–496. [CrossRef]
- Ricke, K., Drouet, L., Caldeira, K., Tavoni, M., 2018. Country-level social cost of carbon. Nat Clim Chang 8, 895–900. [CrossRef]
- Saha, K., Sanyal, P., Saha, S., 2022. Source assessment of tropical-marshland sediment for evaluating seawater intrusion in Chandipur, India: An integrated granulometric and stable isotope approach. Estuar Coast Shelf Sci 278, 108096. [CrossRef]
- Sahu, S.C., Kumar, M., Ravindranath, N.H., 2016. Carbon stocks in natural and planted mangrove forests of Mahanadi Mangrove Wetland, East Coast of India. Curr Sci 110, 2253–2260. [CrossRef]
- Samantaray, S., Sanyal, P., 2022. Sources and fate of organic matter in a hypersaline lagoon: A study based on stable isotopes from the Pulicat lagoon, India. Science of the Total Environment 807, 150617. [CrossRef]
- Serrano, O., Lovelock, C.E., B. Atwood, T., Macreadie, P.I., Canto, R., Phinn, S., Arias-Ortiz, A., Bai, L., Baldock, J., Bedulli, C., Carnell, P., Connolly, R.M., Donaldson, P., Esteban, A., Ewers Lewis, C.J., Eyre, B.D., Hayes, M.A., Horwitz, P., Hutley, L.B., Kavazos, C.R.J., Kelleway, J.J., Kendrick, G.A., Kilminster, K., Lafratta, A., Lee, S., Lavery, P.S., Maher, D.T., Marbà, N., Masque, P., Mateo, M.A., Mount, R., Ralph, P.J., Roelfsema, C., Rozaimi, M., Ruhon, R., Salinas, C., Samper-Villarreal, J., Sanderman, J., J. Sanders, C., Santos, I., Sharples, C., Steven, A.D.L., Cannard, T., Trevathan-Tackett, S.M., Duarte, C.M., 2019. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat Commun 10, 1–10. [CrossRef]
- Shaik, A. ur R., Biswas, H., Reddy, N.P.C., Srinivasa Rao, V., Bharathi, M.D., Subbaiah, C. V., 2015. Time series monitoring of water quality and microalgal diversity in a tropical bay under intense anthropogenic interference (SW coast of the Bay of Bengal, India). Environ Impact Assess Rev 55, 169–181. [CrossRef]
- Shrinivas, S., Sumitra, P., Sangeeta, N., Bhabani, M., Panda, S., Mahala, S.S., 2023. A comprehensive study of the estuary sea environment in the Bay of Bengal, near the Mahanadi River confluence. Discover Water. [CrossRef]
- Srinivasan, V., Natesan, U., Parthasarathy, A., 2013. Seasonal Variability of Coastal Water Quality in Bay of Bengal and Palk Strait, Tamilnadu, Southeast Coast of India 56, 875–884.
- Stankovic, M., Mishra, A.K., Rahayu, Y.P., Lefcheck, J., Murdiyarso, D., Friess, D.A., Corkalo, M., Vukovic, T., Vanderklift, M.A., Farooq, S.H., Gaitan-Espitia, J.D., Prathep, A., 2023. Blue carbon assessments of seagrass and mangrove ecosystems in South and Southeast Asia: Current progress and knowledge gaps. Science of The Total Environment 904, 166618. [CrossRef]
- Sundaray, S.K., Panda, U.C., Nayak, B.B., Bhatta, D., 2006. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river-estuarine system (India) - A case study. Environ Geochem Health 28, 317–330. [CrossRef]
- Swain, S., Sahu, B.K., Pattanaik, S., Sahoo, R.K., Majhi, A., Satapathy, D.R., Panda, C.R., Roy, R., Choudhury, S.B., 2021. Anthropogenic influence on the physico-chemical parameters of Dhamra estuary and adjoining coastal water of the Bay of Bengal. Mar Pollut Bull 162, 111826. [CrossRef]
- Tang, H., Xin, P., Ge, Z., Gong, Z., Yang, Y., Zhang, Y., 2020. Estuarine, Coastal and Shelf Science Response of a salt marsh plant to sediment deposition disturbance. Estuar Coast Shelf Sci 237, 106695. [CrossRef]
- Tessier, M., Vivier, J.P., Ouin, A., Gloaguen, J.C., Lefeuvre, J.C., 2003. Vegetation dynamics and plant species interactions under grazed and ungrazed conditions in a western European salt marsh. Acta Oecologica 24, 103–111. [CrossRef]
- Viswanathan, C., Purvaja, R., Joyson Joe Jeevamani, J., Deepak Samuel, V., Sankar, R., Abhilash, K.R., Gejo Anna Geevarghese, Muruganandam, R., Gopi, M., Raja, S., Rocktim Ramen Das, Shesdev Patro, Krishnan, P., Ramesh, R., 2020. Salt marsh vegetation in India: Species composition, distribution, zonation pattern and conservation implications. Estuar Coast Shelf Sci 242, 106792. [CrossRef]
- Mariotti, G., Carr, J., 2014. Dual role of salt marsh retreat: Long-term loss and short-term resilience. Water Resour Res 50, 2963–2974. [CrossRef]
- Whitfield, A.K., 2017. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev Fish Biol Fish 27, 75–110. [CrossRef]
- Wu, F., Pennings, S.C., Ortals, C., Ruiz, J., Farrell, W.R., Mcnichol, S.M., Angelini, C., Spivak, A.C., Alber, M., Tong, C., 2021. Disturbance is complicated : Headward-eroding saltmarsh creeks produce multiple responses and recovery trajectories 1–15. [CrossRef]
- Yando, E.S., Jones, S.F., James, W.R., Colombano, D.D., Montemayor, D.I., Nolte, S., Raw, J.L., Ziegler, S.L., Chen, L., Daffonchio, D., Fusi, M., Rogers, K., Sergienko, L., 2023. An integrative salt marsh conceptual framework for global comparisons. Limnol Oceanogr Lett. [CrossRef]
- Yang, D., Miao, X.Y., Wang, B., Jiang, R.P., Wen, T., Liu, M.S., Huang, C., Xu, C., 2020. System-specific complex interactions shape soil organic carbon distribution in coastal salt marshes. Int J Environ Res Public Health 17, 1–11. [CrossRef]
- Yuan, Y., Li, X., Xie, Z., Xue, L., Yang, B., Zhao, W., Craft, C.B., 2022. Annual Lateral Organic Carbon Exchange Between Salt Marsh and Adjacent Water: A Case Study of East Headland Marshes at the Yangtze Estuary. Front Mar Sci 8, 1–15. [CrossRef]
- Zhang, Z., Zhang, H., Yu, D., Song, J., Zhou, J., Liu, H., Zhao, X., Jiang, Y., Wang, M., 2020. Influence of spatial heterogeneity of artificial reefs on food sources and trophic levels of marine animals based on stable isotope ratios. Ecol Indic 118, 106779. [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




