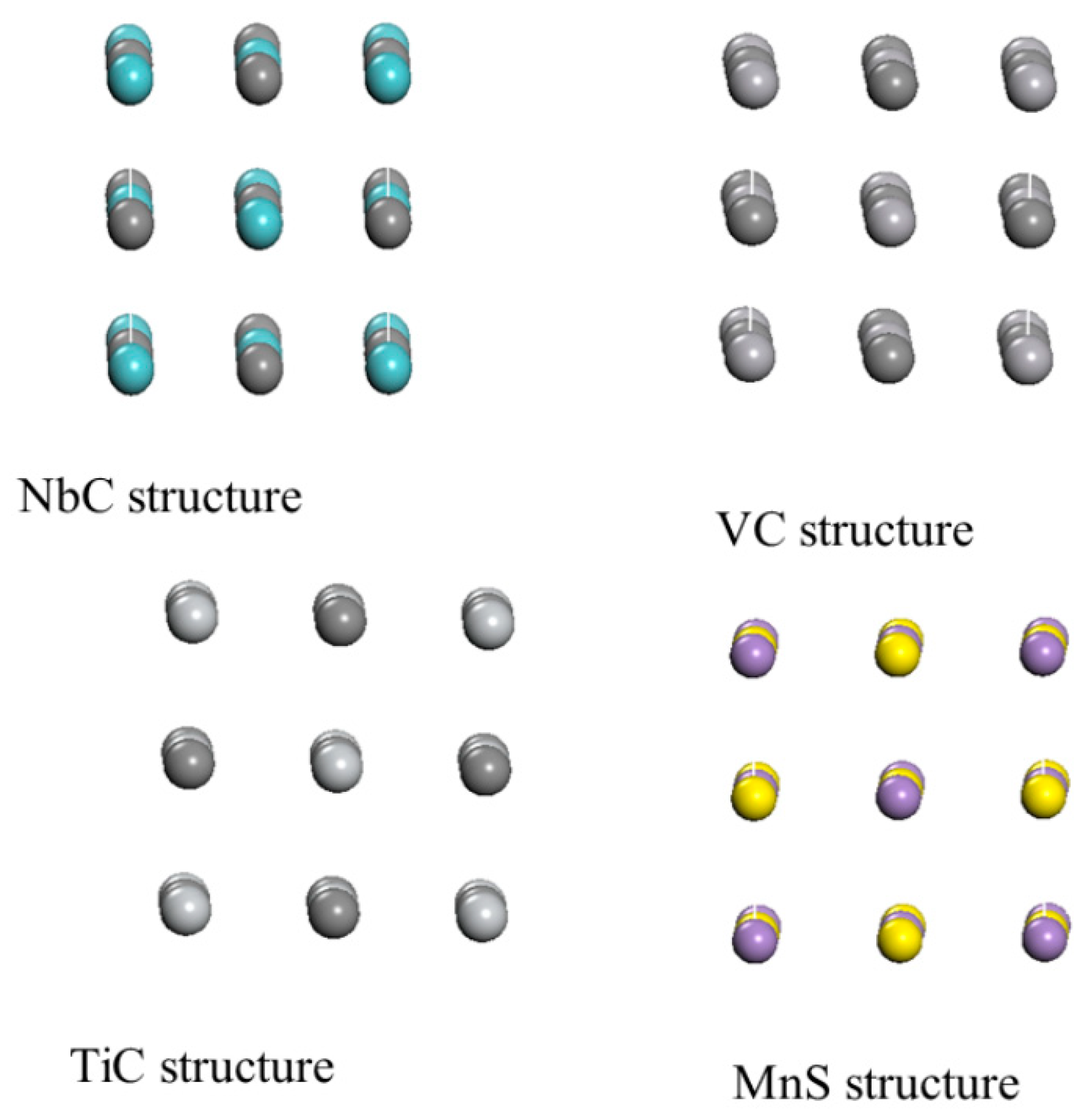
3.1. Hydrogen Interaction with NbC,TiC, VC and MnS Structure
Hydrogen embrittlement is a serious issue in the pipeline industry. Therefore, to mitigate this issue many researchers are using transition metal carbides as an alternative. In this study, we use niobium carbide (NbC), Titanium carbide (TiC), Vanadium carbide (VC) and Manganese sulfide (MnS) to investigate their effect in the hydrogen embrittlement. To begin with, we display the bulk structure of the under investigated metals carbide in
Figure 1.
The calculated bulk properties such as: the lattice parameter, the band gap energy are listed in
Table 1.
Table1 showed that the calculated lattice parameter of niobium carbide (NbC) is 4.40 Å compared to 4.51 Å [
19], and 4.43 [
20,
21] as reported in the literature. Also, as indicated in
Table 1, the calculated lattice parameter of vanadium carbide is 4.180 Å compared the experimental value of 4.16Å [
22,
23], and the calculated lattice parameter of titanium carbide is 4.36Å compared to its experimental value of 4.32 Å [
24,
25,
26]. Finally, the calculated lattice parameter and manganese sulfide is 5.07 Å compared to its experimental value of 5.60 Å [
27]. The good agreement between the theoretical and experimental value, and also, the small deviation between the experimental and theoretical values showed that our approximation to model these structures is reliable. Furthermore, we study the chemical stability of these metal carbides by determining the energy band gap using equation 2.
where
is the energy of the lowest unoccupied molecular orbital (LUMO), and
is the energy of the highest occupied molecular orbital (HOMO).
Vacuum space of 15 Å was created in order to avoid the images interaction and to model accurate the adsorption of the metal carbides with hydrogen.
The calculated energy band gap of niobium carbide crystal is 0.04 eV, which is slightly deviated from the zero gap as reported by Delgado et al. [
28]. In the same trend, Lin et al. [
29] stated that the vanadium carbide has good metal properties, which is in good agreement with our energy band gap calculation (
) of 0.007eV. Also, Mohsen et.al [
24] predicted similar characteristic for titanium carbide for a zero band gap energy compared to our computed value of 0.09eV. In contrast with manganese sulfide (MnS) where the experimental value the energy band gap is 3 eV [
27,
30] compared to our calculated value of 3.2 eV.
Moreover, knowing their lattice parameter and their chemical stability, we further study the most two common ways that molecular hydrogen interacts with those metal carbides (physisorption and chemisorptions).
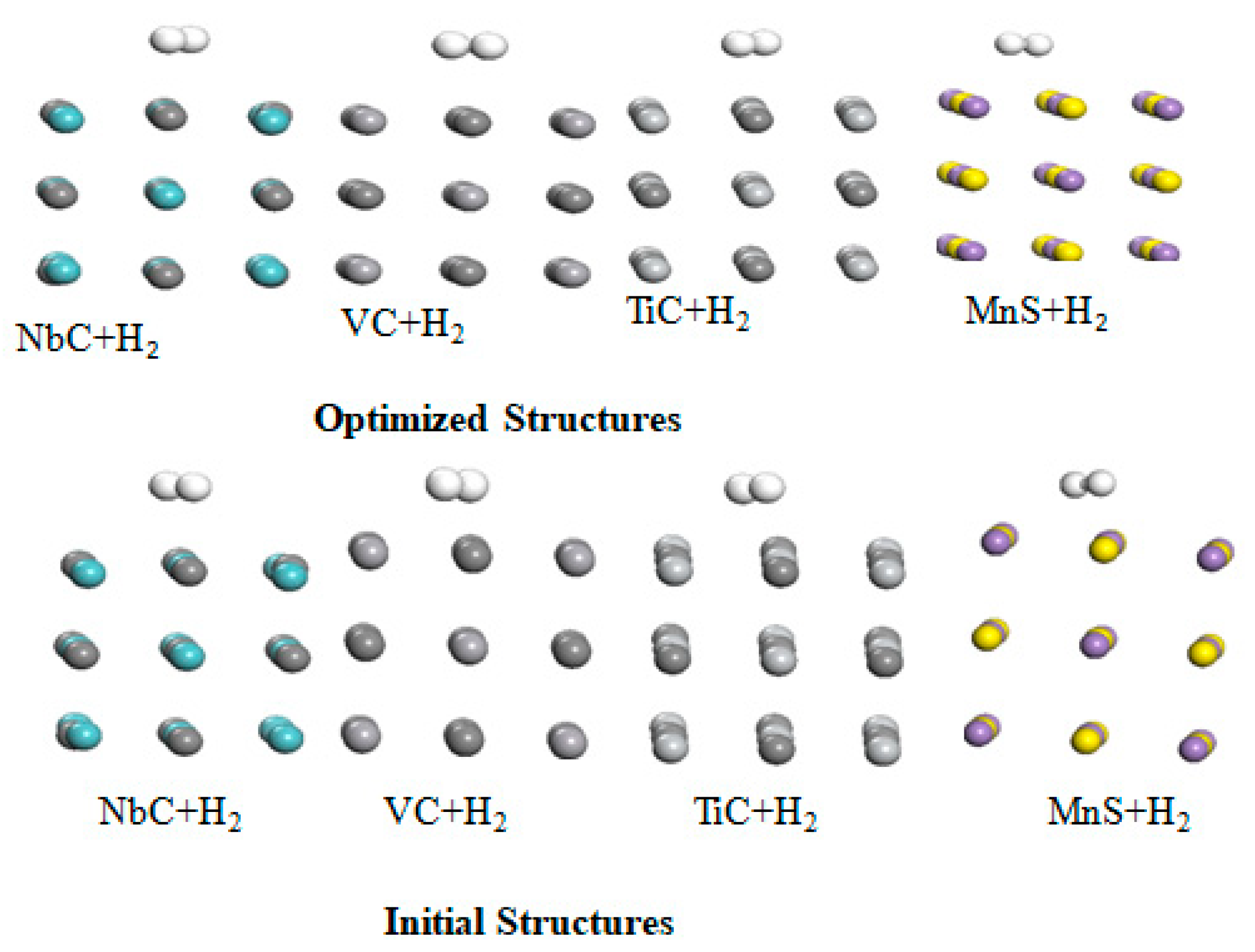
Firstly, we study the interaction of hydrogen molecule in the physisorption case with niobium carbide (NbC), titanium carbide (TiC), vanadium carbide (VC) and manganese sulfide (MnS). The initial and optimized structures in the physisorption interaction of hydrogen with these metal carbides are shown in
Figure 2.
To do so, we evaluate the strength of the interaction of H
2 molecule with those metal carbides we use the equation (2):
where
stands for the total energy of the metal carbide in it primitive unit cell,
the ground energy of hydrogen molecule in a cubic cell of lattice parameter (a=20 Å) or, and
the total energy of the metal carbide plus the H
2 molecule.
The calculated binding energy along with their equilibrium distance of these optimized structures are summarized in
Table 2.
Table 2 revealed that the binding energy between hydrogen molecule and these metal carbides in the physisorption case are the wan der Waal interaction, where the adsorption energy ranging from 0.043 eV to 0.70 eV. In addition, their equilibrium distance falls in the range 1.204 Å to 1.810 Å, which are determined from their optimize structure as displayed in
Figure 2. These calculated results align with the earlier results reported in the literature [
1,
31,
32].
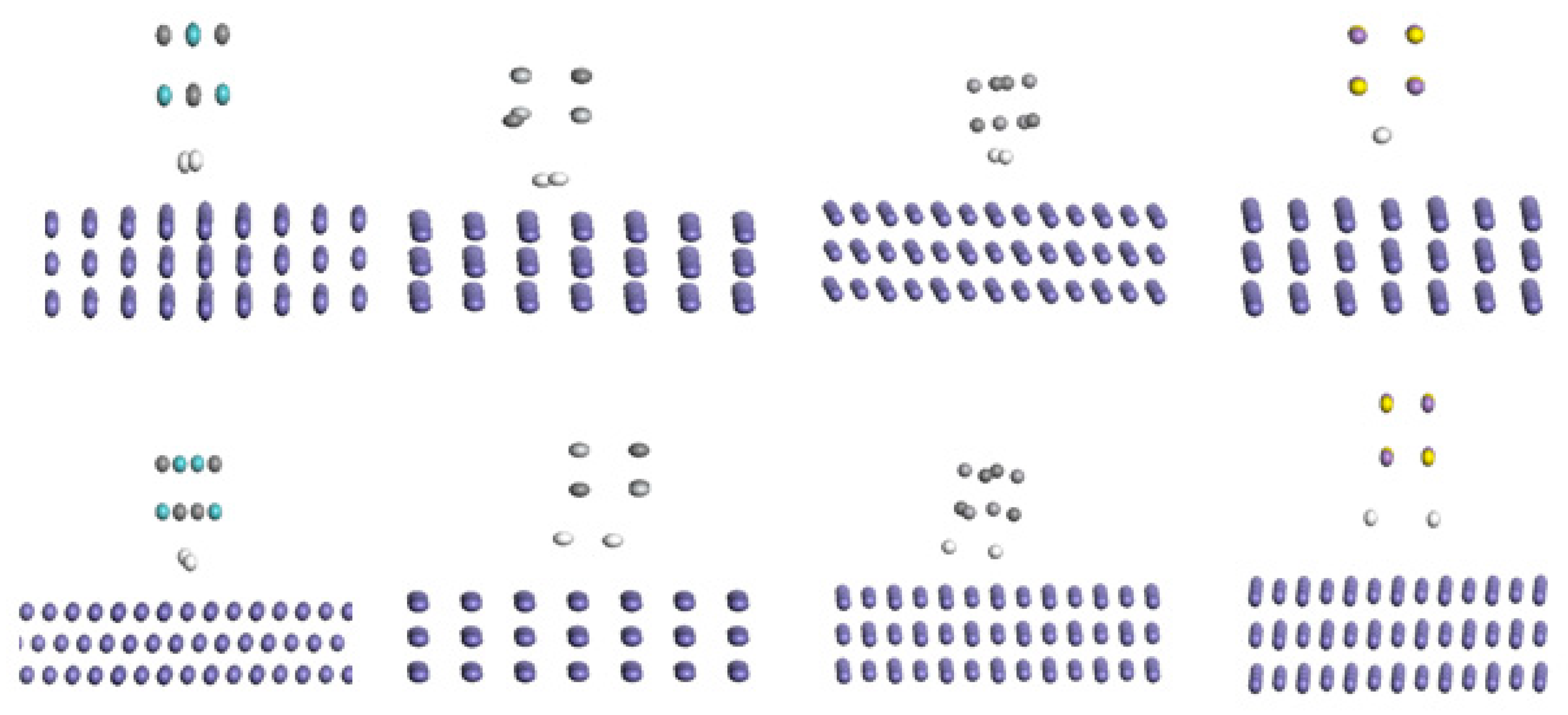
Furthermore, we investigate the interaction of hydrogen with the precipitate in the presence steel. To do so, Fe crystal was cleaved along {111}, {110}, and {100} surfaces. After calculation, Fe {110} surface was identified to have the most stabilization compared to other Fe surfaces. Therefore, Fe{110} surface was selected to study the interaction of hydrogen molecules sandwiched between the Fe{110} surface and the four precipitates (NbC, TiC, VC, and MnS). The initial and optimized structures of this interaction are displayed on
Figure 3.
Figure 3 reveals total dissociation of H
2 molecule in all cases. The binding energy, and the equilibrium distance of H
2 between Fe {110}, and the precipitates are summarized in
Table 3. Initially we placed H
2 molecule at a distance of 2.6 Å with respect the Fe{110} and 2.8Å with respect the precipitate atom.
The equilibrium distance between the hydrogen atom and the closest top atoms of Fe{110} surface fall in the interval [2.6 to 3.21Å], while [2.38 to 2.65Å] in the cases of the precipitates as shown
Table 3. From our results, we can state that the presence of Fe surface favors the dissociation of H
2 molecule. Also, the dissociated hydrogen atoms are closer to the precipitate atoms than the Fe{110} surface.
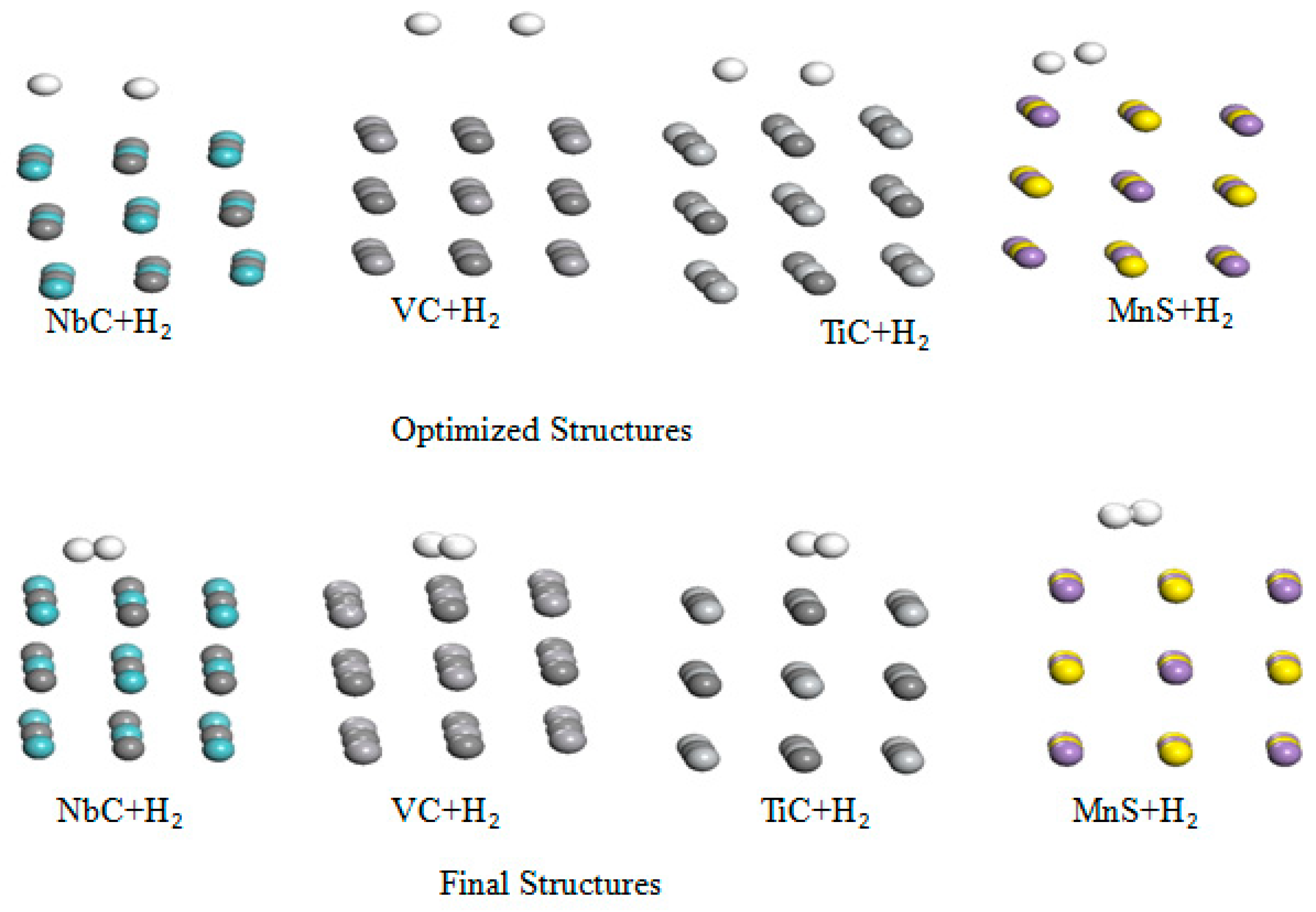
Furthermore, the interaction of H
2 in the chemisorptions case with the aforementioned metal carbides was investigated. Their initial and optimized structures of these complexes are portrayed in
Figure 4 to better visualize this interaction.
Our calculations reveal spontaneous dissociation of H
2 molecule occurs in all cases in the chemisorptions of hydrogen with these metals carbides as shown in
Figure 3. This result aligns with the earlier result of Silveri et al. [
31], where, they showed a spontaneous dissociative chemisorption was observed to happen when the molecule was placed less than 2.0 Å above the topmost surface atomic plane. To better visualize these results, we summarized the equilibrium parameters such as binding energy (
), and the optimized distance of hydrogen with the matrix (
are summarized in
Table 4.
From
Table 4, the strength of the hydrogen trapping can be arranged in the following order: TiC+H
2 >VC+H
2>MnS+H
2> NbC+H
2 and the equilibrium distance varies in this interval [1.67 Å to 2.04 Å]. According to the literature, the trapping of hydrogen can be generally classified as strong or weak. Weak trapping occurs when the binding energy is less than 0.31eV (30 KJ mol) while a strong trapping happens when the trapping energy is greater than 0.63eV (60 KJ/mol) [
1]. These results predict that all these metal carbides with their binding energy with hydrogen molecule are greater than 0.63 eV is irreversible. Therefore, NbC, TiC and VC are potential precipitates to enhance the strength of the steel due to their strong hydrogen trapping energy. Since, the hydrogen embrittlement occurs essential in the atom state of hydrogen. Therefore, in the next section, we investigate the interaction of hydrogen atom with those metals carbides.
3.2. Interaction of the Metal Carbide (NbC, TiC, VC, and MnS) with Hydrogen Atom
Since, the hydrogen can interact with metal from two types of sources: hydrogen generate at the metal surface and the gaseous hydrogen. At gaseous state, it is in the molecular form at standard conditions, therefore it does not produce embrittlement. However, the atomic hydrogen creates embrittlement due to the fact that atomic hydrogen dissolves rapidly into the metal at room temperature [
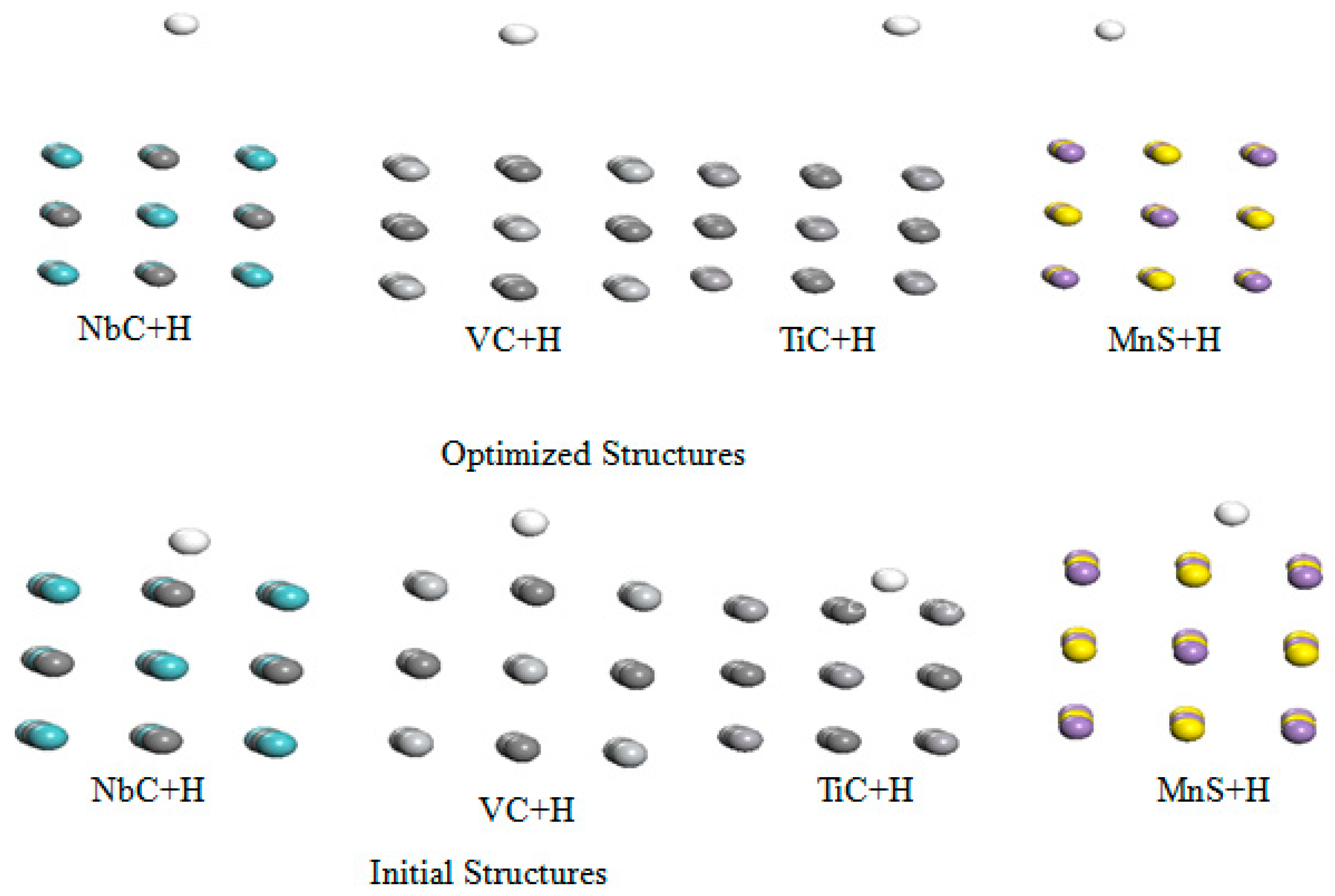
33]. Therefore, to have more insight in the hydrogen embrittlement on these carbides, we propose to investigate the interaction of hydrogen atom with these metal carbides. To do so we created two layers of bulk crystal separate by a vacuum of 15 Å and 15 Å vacuum or the top layer in order to avoid the strong interaction between these layers.
We display the initial and optimized structures of this interaction in
Figure 5.
From these optimized structures, we summarize their optimized parameters such as binding energy (
and final distance between hydrogen atom and these metal carbides (
on
Table 5.
Table 5 reveals that the most favorable trap site for hydrogen atom among these metal carbides are the TiC with
=0.840 eV, VC with
=0.480 eV, NbC with
=0.240 eV, and MnS with
=0.127 eV. In addition, their corresponding equilibrium distance (
) ranges in the interval [1.13 Å, 2.08 Å]. Moreover, we study the most favorable adsorption site between the metal and the carbon atom in these metal carbides. To do so, we chemisorbed the hydrogen atom on the carbon and metal site successively, and determine the binding energy on these sites using equation 1.
The adsorption energy (
), and their corresponding final distance (
of hydrogen atom with these metal carbide are summarized in
Table 6.
From
Table 6, we learnt that the most favorable adsorption site is the carbon site in NbC, VC and TiC with a binding energy lying in the energy window [1.22 eV, 1.55 eV]. While the binding energy of the metal site (Nb, V, and Ti) varying in this interval [0.96 eV, 1.04 eV]. However, for manganese sulfide (MnS) the most favorable trapping site is the sulfur atom with
= 1.28 eV compared to
= 0.88 eV for the manganese atom (Mn). The trend of this results follows the earlier results reported in the literature [
31]. The last section, will discuss the effect of layer on the trapping capacity of the carbides.
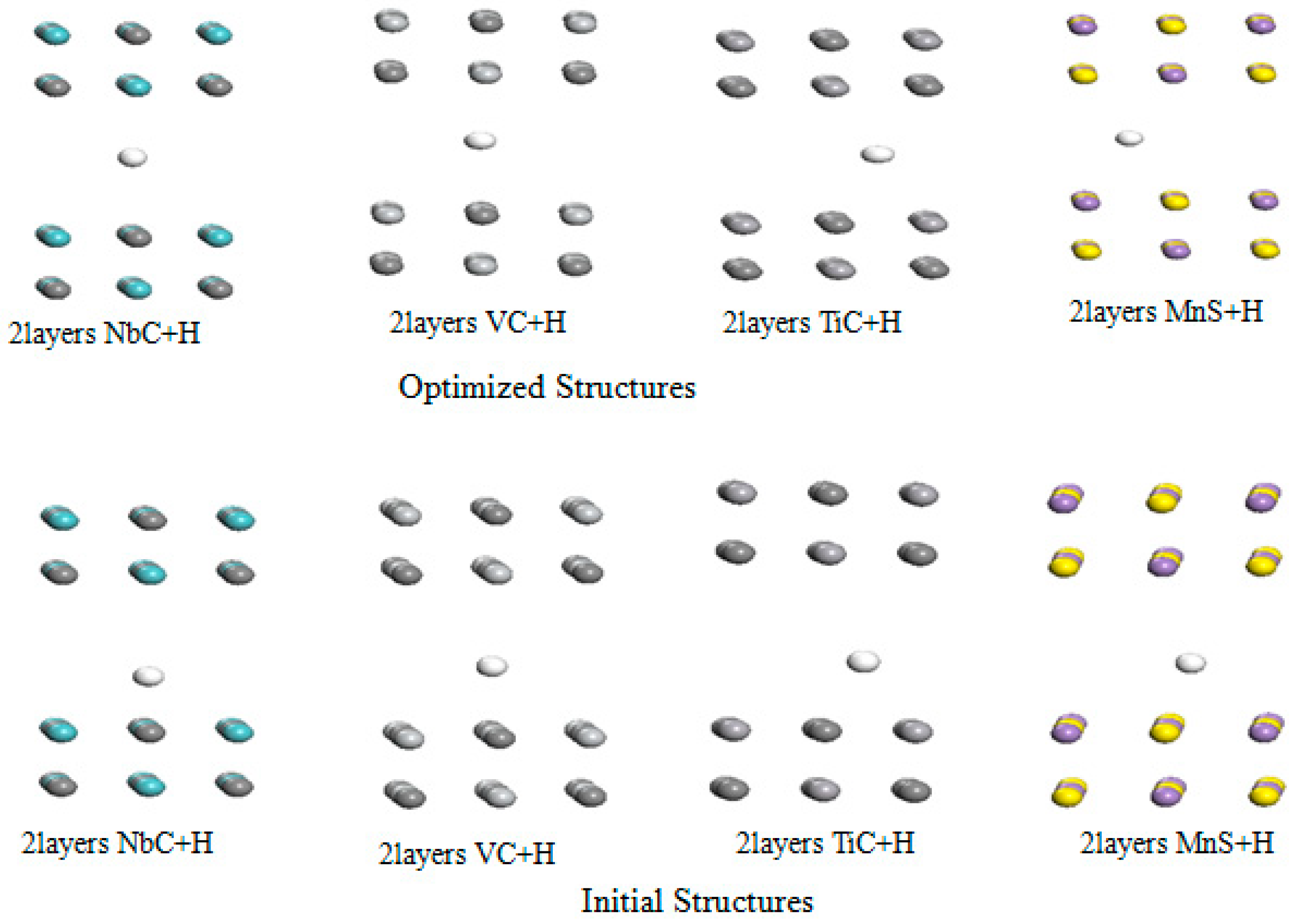
3.3. The Effect Layer on Hydrogen Interaction with NbC, TiC, and VC
Understanding the interaction of H
2 molecule and hydrogen atom help us to find new direction of research. In this section we investigate the effect layer on the hydrogen embrittlement on niobium carbide, Titanium carbide, and Vanadium carbide and manganese sulfide. Firstly, we use two layers with a vacuum spacing of 15 Å. The initial and optimize structures of these metals carbides are showed in
Figure 6 below.
Furthermore, we determine the strength of the interaction of hydrogen atom with the two layers of NbC, TiC, VC, and MnS using equation (2).
The optimized parameters such as: binding energy of hydrogen atom with those metals carbides are summarized in
Table 7.
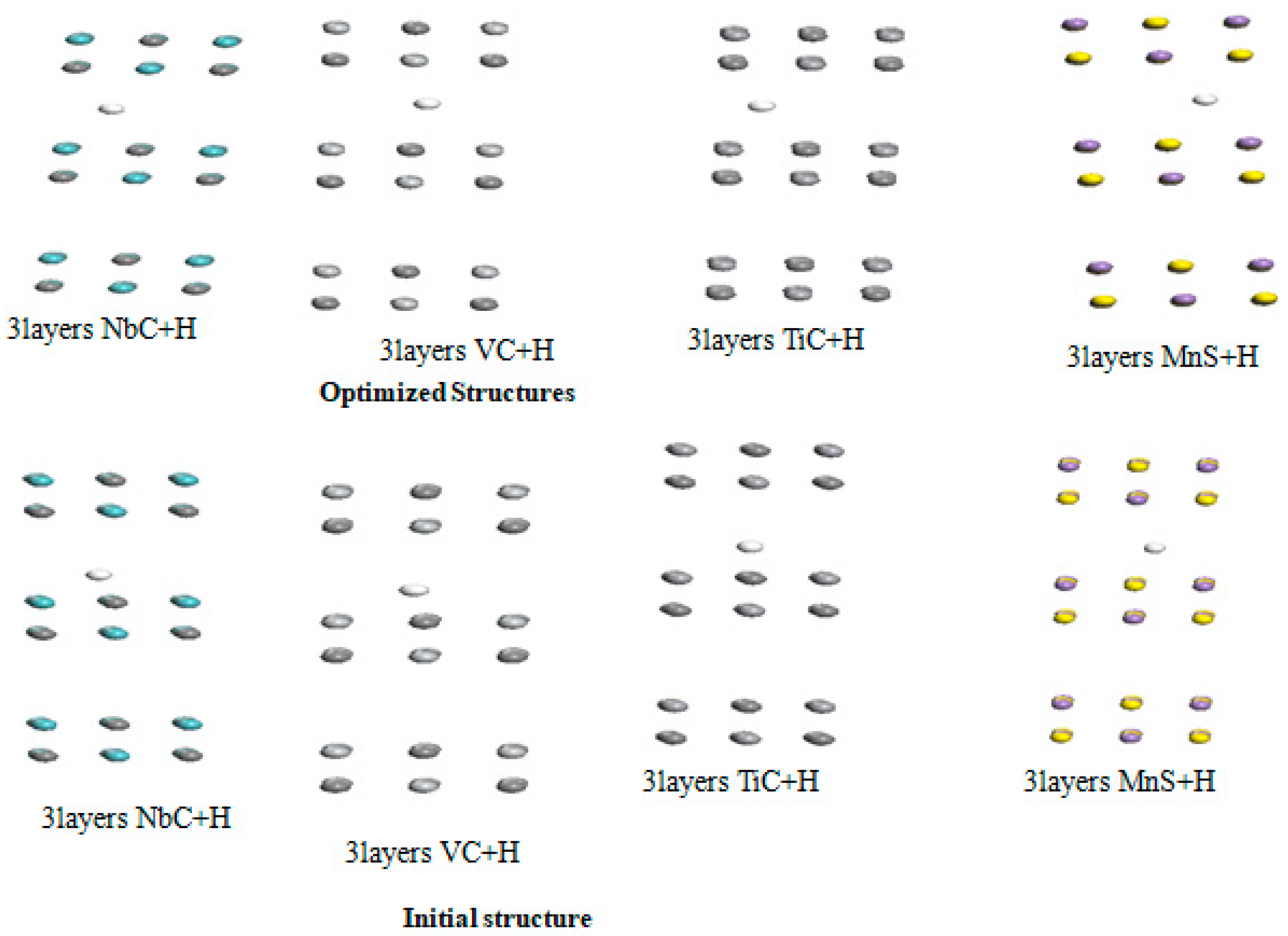
Table 7 reveals that the adsorption energy is in the energy window 0.21 to 0.88 eV. The binding of H atom is more favorable in the case of NbC layer. In addition, their optimized distance is between 1.24 Å to 1.76 Å. We notice a slight increase in the binding energy of hydrogen atom in these carbides. Furthermore, we increase the number of layer from two to three for all carbides. The initial and optimized structures in this interaction are shown
Figure 7.
Moreover, we summarized in
Table 7 the resulting parameters like adsorption energy and equilibrium distance for the interaction between hydrogen atom and the carbides under study.
Table 7.
Summarized the adsorption energy of NbC, VC, TiC and MnS along with their equilibrium distance.
Table 7.
Summarized the adsorption energy of NbC, VC, TiC and MnS along with their equilibrium distance.
| Metal carbide |
(eV) |
|
| 2NbC +H |
0.34 |
1.78 |
| 2VC+H |
0.56 |
1.45 |
| 2TiC+H |
0.93 |
1.32 |
| 2MnS+H |
0.28 |
1.87 |
From
Table 7, we notice that the adsorption energy increases by increasing the number of layers. These results prove that increasing the layer of these carbides can also be an efficient way to enhance the trapping of hydrogen on these carbides.