1. Introduction
Myelodysplastic neoplasms (MDS) were first reported in the 1920s by Di Guglielmo, describing patients with cytopenias and abnormal appearing erythrocytes. Over the last 100 years, our understanding of this clinical entity has improved drastically, as reflected in the progression from the primary symptomatic terminology used during the early 20th century to the morphologic definitions used in the French American British (FAB) system, and most recently to the more complex classification systems incorporating blast count and molecular alterations. Currently, two competing MDS classification schemes are accepted: the 5th edition of the World Health Organization Classification of Hematolymphoid Tumors (WHO5) and International Consensus Classification 2022 (ICC 2022), which will hopefully be unified as our understanding of the biology of MDS continues to improve. Evolution of treatment from supportive care to molecularly-targeted disease modifying therapy has reflected our increasing understanding of the pathogenesis of this heterogenous disease.
2. Molecular Evolution of MDS from Genotype to Phenotype to Prognostication
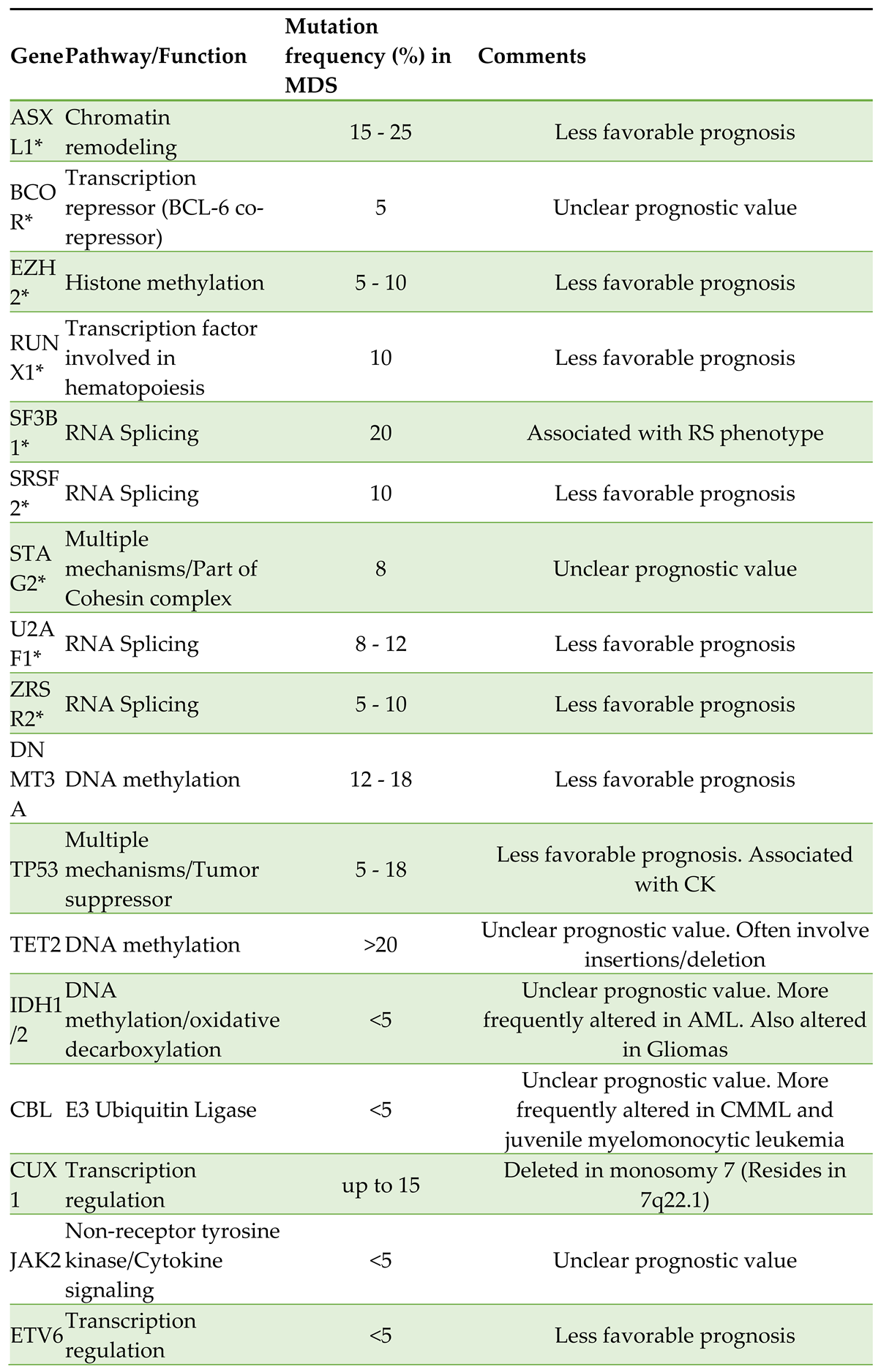
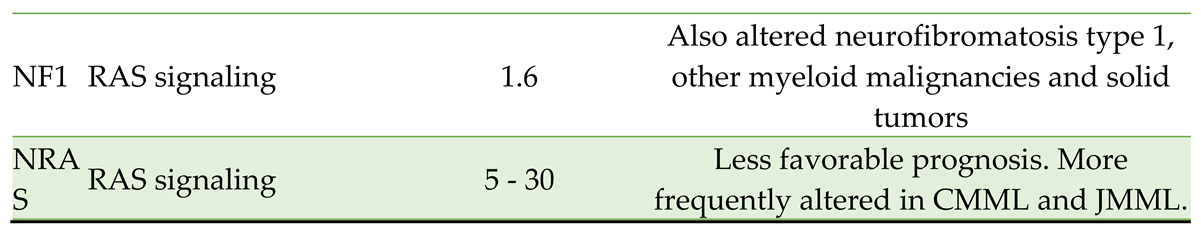
The biology of MDS remains incompletely understood, and is constantly refined based on research correlating genetic alterations with clinical phenotypes, as reflected in the current WHO5 and the ICC 2022 classifications. It is well-established that the development of MDS involves a multistep acquisition of genomic alterations arising in hematopoietic stem cells. The most common mutations occur in genes involved in RNA splicing (SF3B1, SRSF2, U2AF1, and ZRSR2), epigenetic modification (TET2, ASXL1, and DNMT3A), signal transduction regulation (NRAS and JAK2), and transcription (RUNX1 and TP53) [
1]. Some of the more common mutations seen in MDS are listed in
Table 1.
Although recognizable MDS mutations have been described in more than 40 genes, reliable genotype-to-phenotype relationships have been identified in less than 5 percent of cases. For example, mutations in SF3B1 and MDS with ringed sideroblasts (MDS-RS), and mutations in SRSF2 and CMML are both established associations. As another example, Seethy et al. in 2023 demonstrated that there was a reduction in TET2 expression among patients with higher risk MDS per the Revised International Prognostic Scoring System (IPSS-R) and myelodysplasia related AML (AML-MR in WHO5), as compared to patients with lower risk disease. The authors also noted that levels of
5-hydroxymethyl cytosine were concordantly lower as well [
8]. The ICC 2022 recognizes MDS founding lesions and mutations in the following genes: ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, and ZRSR2 [
9].
Karyotypic abnormalities correlate in some cases with specific phenotypic features that influence management decisions and modify outcomes. It is well known that MDS with isolated 5q deletion with or without additional cytogenetic aberrancy (except monosomy 7) responds well to lenalidomide, portending a superior prognosis [
10]. Conversely, certain chromosomal abnormalities, such as the deletion of 17p, associated with loss of TP53, independently predict poorer prognosis and resistance to treatment. Other common chromosomal aberrations in MDS include del(7q), trisomy (+8), del(20q) and complex karyotype (CK). CK, which is also frequently associated with TP53 pathogenic alterations, has yet to be uniformly defined. The definition of CK can vary geographically and nosologically, although in most studies CK is generally defined as the presence of ≥3 independent cytogenetic abnormalities. However, the Medical Research Council Acute Myeloid Leukemia 10 trial (MRC AML10), required ≥5 independent cytogenetic abnormalities. It is known that CK is associated with a poor prognosis in hematological malignancies [
11].
The molecular pathology of MDS has exploded recently, spearheaded in part by advances in massively parallel sequencing techniques. In 2011, a seminal study by Bejar et al. examined DNA from 439 cases and identified point mutations in 18 genes. In addition, they discovered that TP53 mutations were observed mainly in patients with intermediate-2 or high-risk disease according to the International Prognostic Scoring System (IPSS), and described a correlation with thrombocytopenia, increased blasts, and CK. Furthermore, severe thrombocytopenia and increased blasts were more prevalent when mutations in RUNX1, TP53, and NRAS were present. Combined alterations in five or more genes were associated with an increased risk of death from any cause, and the specific hazard ratio for specific genetic alterations was reported as follows: TP53 (2.48), EZH2 (2.13), ETV6 (2.04), RUNX1 (1.41), and ASXL1 (1.38) [
12].
More recently, Bersanelli et al. identified distinct MDS clusters linked to specific genomic features, defining distinct genomic groups with unique outcomes: No specific genomic abnormalities, SF3B1 related, TP53/complex karyotype, SRSF2 related, U2AF1 associated 20q deletion or chromosome 7 abnormalities, and finally AML-like mutation patterns. Isolated SF3B1-related MDS portends superior survival [
13]. The authors also noted that specific co-mutation patterns accounted for clinical heterogeneity within SF3B1- and SRSF2-related MDS. Unsurprisingly, MDS with CK and/or TP53 gene abnormalities and MDS with AML–like mutations showed the poorest prognosis. Interestingly MDS with 5q deletion clustered into two distinct subgroups according to the number of mutated genes and/or presence of TP53 mutations. The study concluded that individuals with 5q deletion who have either no additional mutations or only a single additional mutation tend to have a more favorable prognosis compared to those with coexisting mutated TP53 [
13].
3. Prognostication
MDS prognostication based on genomic information is evolving. The IPSS-R is widely used and describes five categories ranging from very low to very high risk utilizing cytogenetic as well as, hematologic parameters including bone marrow blast count, hemoglobin levels, platelet count, absolute neutrophil count, and transfusion dependency [
14].
In 2022, the Molecular IPSS for Myelodysplastic Syndromes (IPSS-M) was proposed as a risk stratification tool based on combined profiling of 152 genes along with clinical data representing a major advancement in the field. We consider the IPSS-M to be the heir to the widely used IPSS-R. The IPSS-M utilizes six prognostic categories, and re-stratified nearly half (46 percent) of 1281 cases of MDS, including not only primary but also secondary and therapy-related cases, demonstrating improved risk stratification and prognostic accuracy based on a refined list of 31 mutated genes [
15,
16]. The IPSS-M has also been noted to be a more accurate predictor of outcomes (leukemia free survival and overall survival) compared to the IPSS-R [
17]. Notably, the IPSS-M allows for missing data facilitating practical application in settings where routine genomic testing is still unavailable.
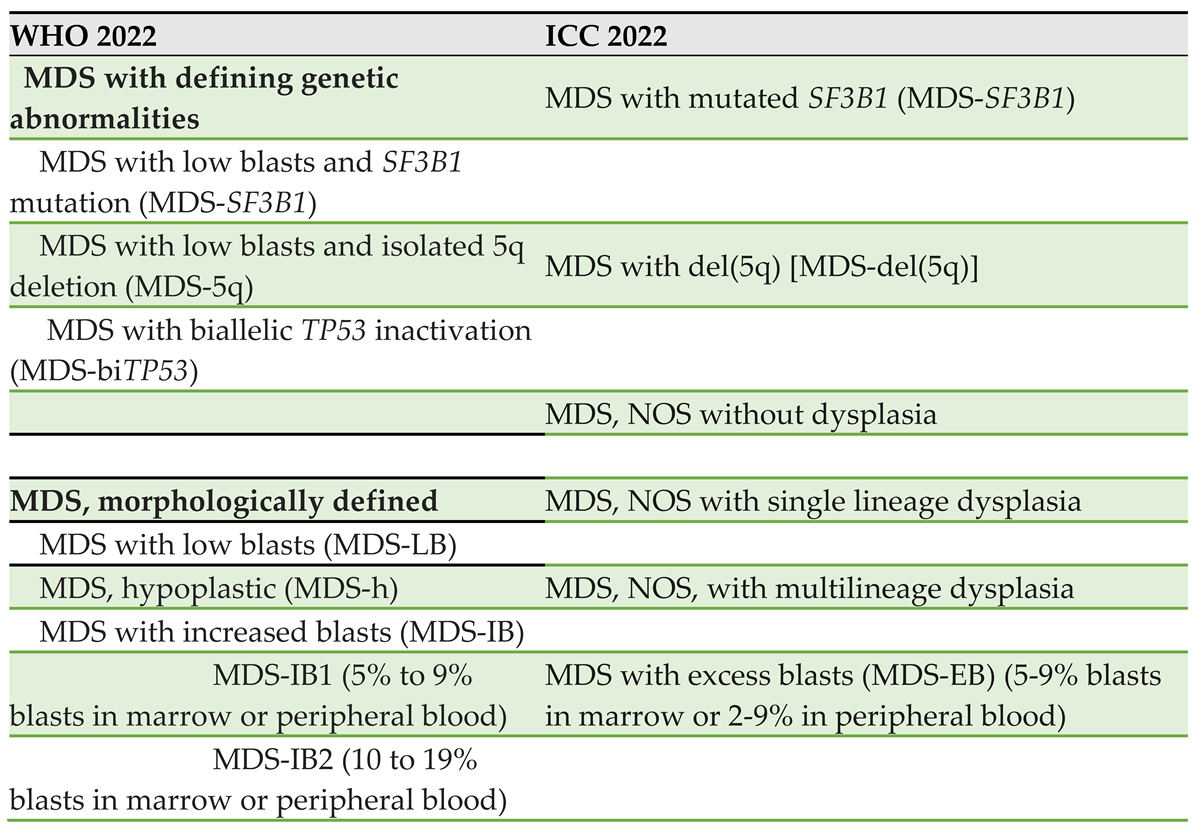
4. Classification Schemes
The revised WHO5 and ICC 2022 both reflect the increasing understanding of the impact of genomics on the clinical presentation of MDS. For example, in the ICC 2022 classification,
MDS-RS has been replaced by an entity called MDS with mutated SF3B1. Likewise, the new schema differentiates MDS with mutated TP53 (0-9% blasts) from MDS/AML (with mutated p53 and
10-19% blasts), because the presence of multi-hit TP53 mutations in cytopenic myeloid neoplasms corresponds to a highly aggressive disease with short survival, regardless of morphologic features such as blast count [
12]
. Interestingly, unlike other MDS variants, the prognosis of MDS with multi-hit TP53 does not appear to depend on the blast percentage [
9]
. Table 2 lists the recognized MDS subgroups according to both WHO5 and ICC2022.
5. Treatment of Lower Risk Disease
MDS is a clinically diverse disease entity. It can present as a mild, asymptomatic hematologic abnormality (most commonly anemia) found incidentally in some patients, or as symptomatic disease in the setting of bone marrow failure in other patients. Symptoms classically include fatigue, infections, and bleeding. Goals of therapy in lower risk MDS are generally aimed at reducing symptoms, improving quality of life, decreasing transfusion burden, and preventing other complications such as iron overload, infections, or bleeding. Observation is universally accepted as the management modality of choice in asymptomatic patient with low-risk disease. The following discussion will review the evidence supporting various treatment modalities for “lower-risk” MDS, which encompass patients classified as very low risk, low risk and intermediate risk according to the IPSS-R.
Blood Product Transfusion
An international survey of patients conducted by the MDS Foundation revealed that approximately three-fourths of all patients with MDS in Europe and the U.S. receive packed red blood cell (pRBC) transfusions [
19]. Patients are generally transfused in order to minimize symptoms and keep hemoglobin (Hb) in a safe, near physiologic range. Often a Hb level of 7.0 or 8.0gm/dL is used as trigger for pRBC transfusion, based on expert opinion, although prospective clinical data are lacking. Absence of standardization for RBC transfusion impedes a thorough cost-benefit analysis of this approach, which represents a high financial expense to the healthcare system [
20].
Patients with MDS who are transfusion dependent have lower overall survival, increased rates of progression to AML and a subjective lower quality of life [
20,
21]. Transfusion dependent patients also have a higher incidence of acute complications, most notably transfusion associated circulatory overload (TACO) and transfusion related acute lung injury (TRALI). Furthermore, there is a significant risk of developing iron overload, a notable chronic complication of pRBC transfusions, although this may be reduced with chelation therapy. The TELESTO Trial, a phase II study published by Angelucci et al. in 2020, included lower risk MDS patients who have received between 15 and 75 pRBC transfusions with elevated ferritin. Iron chelation with oral deferasirox resulted in improved event free survival (EFS) although the study was not powered to show a survival benefit [
22]. Given the high proportion of patients who receive transfusions, determining those who will benefit most from chelation therapy is an important goal.
Although up to 65 percent of patients with MDS have thrombocytopenia, routine platelet transfusions outside of certain clinical situations (such as bleeding or peri procedural) are not currently recommended in the treatment guidelines. There are no clinical trials that we are aware of exploring outcomes of platelet transfusion in patients with low risk MDS, and clinical triggers can vary widely.
Erythropoiesis Stimulating Agents (ESA)
Erythropoietin (EPO), an ESA, is an established treatment modality for anemia in lower risk MDS and is included in the NCCN guidelines [
23]. EPO alone is expected to improve anemia in 15 to 20 percent of allcomers with MDS, as shown by Hellstrom-Lindberg et al. in a 1995 meta-analysis including 205 participants [
24]. However, the authors noted that certain patients seemed to derive more benefit from EPO therapy than others. For example, patients without a previous transfusion requirement or with lower endogenous EPO levels (below 200mU/mL) were more likely to respond to EPO treatment. The authors noted a lack of response to EPO therapy in patients with refractory anemia with ringed sideroblasts (RAS) and EPO level above 200 mU/mL. Patients with RAS and EPO level below 200 mU/mL who did not previously require transfusion showed response rates of about 33 percent. In contrast, patients without RAS not previously requiring transfusions showed response rates that were above 50 percent. These findings nicely demonstrate the utility of using readily available clinical data in order to determine the potential efficacy of a treatment modality.
Furthermore, in 1998, Hellstrom et al. demonstrated that the combination of EPO and granulocyte colony-stimulating factor (G-CSF) improved hemoglobin levels in approximately 38 percent of patients with MDS, indicating a synergistic effect between these therapies [
25]. Notably, those with RAS had the highest response rate (46 percent) to combination therapy, and those with refractory anemia (RA) had the lowest (20 precent). When using a validated decision model including pretreatment pRBC transfusion burden as well as EPO level, a subgroup of patients (EPO level below 500 mU/mL and receiving less than 2 units pRBC per month) responded to the combination particularly well, with a 61 percent response rate [
26].
Investigators more recently have continued to explore ways to improve anemia in MDS patients using ESA therapy. Darbepoetin is a longer acting ESA, with a half-life approximately three times that of EPO, that has also been studied in the context of MDS. Platzbecker et al. published a randomized-placebo controlled trial in 2017 evaluating the use of darbepoetin in 147 patients with anemia and lower risk MDS, which found that the need of
pRBC transfusion was significantly lower in the darbepoetin alfa treated group as compared to the placebo group (36.1 percent vs. 59.2 percent) [
27].
No new safety concerns were raised and the study included a 48 week open label extension in which most of the patients received darbepoetin every two weeks (as opposed to every three weeks) and demonstrated improved hematologic response. In 2018, Fenaux et al. published a randomized, controlled trial assessing the efficacy of ESA therapy in 130 patients with lower risk MDS.
Participants were randomized in a 2:1 fashion to receive ESA (epoetin-alpha, 450 IU/kg/week) or placebo for 24 weeks, followed by a treatment extension period in responders. The primary endpoint was erythroid response (using a modified IWG-2006 criteria) at week 24, and a response rate of 45.9 percent in the treatment group vs. 4.4 percent in the placebo group was observed [
28]
. The ESA treated group had reduced RBC transfusion requirement and increased the time-to-first-transfusion as compared to the placebo group. There is currently no data comparing different ESA formulations.
Thrombopoietin Receptor Agonists
Thrombocytopenia poses a significant clinical problem in patients with lower-risk MDS. It has been estimated that the prevalence of thrombocytopenia is between 40 to 65 percent in patients with MDS. Thrombocytopenia is an adverse risk factor associated with increased risk of bleeding, and patients with thrombocytopenia are more likely to have higher risk MDS [
29]. Platelet transfusion, a commonly utilized treatment, has limited therapeutic utility, and is associated with transfusion-related adverse effects.
Thrombopoietin receptor agonists (TPO-RA), such as Romiplostim and Eltrombopag have been shown to facilitate megakaryocyte differentiation and increase platelet count in diseases such as immune thrombocytopenia (ITP), aplastic anemia and certain hematological malignancies such as CML. At the present time, the use of TPO-RA for MDS specifically remains under investigation although there are studies that suggest benefit.
The efficacy and safety of eltrombopag was explored in a phase II trial published in 2017 by Oliva et al., where platelet responses (median follow up time of 11 weeks) occurred in 47 percent of patients in the eltrombopag group versus only 3 percent in the placebo group. The authors concluded that this drug was well tolerated and clinically effective [
30]. During the initial follow up period, 21 trial participants experienced a severe bleeding event (as defined by WHO bleeding score of 2 or more), 13 of which were enrolled in the placebo arm, representing a statistically significant difference between the groups.
A follow up to this trial published in 2023 confirmed the longer term efficacy and safety of eltrombopag. Results at week 11 or week 25 were indistinguishable, and the updated data showed that 25 percent of patients who responded to eltrombopag at week 25 lost response by 60 months [
31]. However, a statistically significant decrease in bleeding events was maintained in the treatment group.
A phase II dose-escalation study of eltrombopag (starting at 50 mg daily and increasing to a maximum dose of 150 over 16 weeks) in patients with lower risk MDS showed hematologic response between 16 and 20 weeks in 11 of 25 patients, as per the International Working Group definition. The predictors of response were the presence of a paroxysmal nocturnal hemoglobinuria clone, marrow hypocellularity, thrombocytopenia, and elevated plasma thrombopoietin levels at study entry [
32]. Reassuringly, eltrombopag was well-tolerated and there were no discontinuation events.
Similarly, romiplostim can improve platelet counts by approximately 40 to 50 percent, reduce platelet transfusions, and decrease bleeding risk in MDS patients with lower risk disease [
33]. However, the long term safety and efficacy of this drug are not fully elucidated. An open label extension study by Fenaux et al. published in 2017, included 60 participants from previous TPO-RA trials [
34,
35,
36] with lower risk MDS and a platelet count of 50,000 or less using Romiplostim for a mean duration of 57 weeks. Treatment-related adverse effects (AE) were found in 30 percent of participants, and progression to AML occurred in 2 patients after 44 and 46 weeks. The median response duration was 33 weeks, and 82 percent of participants had a continuous response without new safety concerns [
33].
The role of TPO-RA therapy in MDS has yet to be fully elucidated, with trials showing variable benefit. In 2000, a systematic review and meta-analysis from Meng et al. analyzed 8 studies with over 1000 patients collectively, including lower risk and higher risk MDS (and AML), treated either with Romiplostim or Eltrombopag. The authors found that TPO receptor agonists decreased all bleeding events (including grade 3 or 4) without increasing risk for AML transformation [
37]. However, a decrease in overall response rate (ORR) in patients receiving TPO-RA therapy was detected, suggesting that TPO-agonists may have a detrimental effect on hematologic indices, seemingly more pronounced with Eltrombopag and in higher risk patients. Different treatment regimens used in dissimilar studies (e.g. azacitidine vs. decitabine) may have contributed to this finding. More trials are clearly needed to determine which subset of MDS patients are most likely to benefit from TPO-RA therapy.
Luspatercept
Luspatercept is a recombinant fusion protein that binds to certain transforming growth factor β (TGF- β) superfamily ligands, decreasing SMAD protein signaling and ultimately accelerating erythroblast maturation. Although luspatercept was approved by the FDA in 2019 for beta-thalassemia, its use has been extended to include anemia in MDS-RS or in lower risk MDS, respectively in 2020 and 2023.
The 2017 PACE-MDS open-label, phase II trial by Platzbecker et al. included patients with lower risk MDS with and without increased RS. High rates of hematologic response and decreased transfusion requirements were observed in Luspatercept treated patients with low risk MDS, which was notably more striking in MDS-RS and MDS with SF3B1 mutation (hematologic response rates of 69 percent and 77 percent respectively) [
38].
In November of 2022, an update to the PACE-MDS trial confirmed the benefit of luspatercept across lower risk MDS, with erythroid hematologic response seen in approximately 54 percent of patients per the International Working Group criteria, without new safety concerns. Specifically, responses were noted in approximately 68, 36 and 71 percent of MDS-RS, MDS without RS and non-transfusion dependent MDS, respectively [
39].
The phase III, placebo controlled MEDALIST trial included participants with lower risk MDS who had been receiving frequent transfusions and who were less likely to respond to ESA therapy. Thirty eight percent of patients who received luspatercept were transfusion independent for eight weeks or longer [
40].
The interim results of the highly anticipated open label, phase III randomized trial COMMANDS trial was recently published in July 2023, and included transfusion dependent, ESA naïve participants with lower risk MDS (regardless of RS subtype). Patients received either epoetin alfa or luspatercept and at 24 weeks of treatment, 59 percent of participants in the luspatercept arm had reached the primary end point (increase in Hb of 1.5g/dL and were transfusion independent for at least 12 weeks), in comparison to only 31 percent of the participants who received epoetin [
41]. Although longer term studies are warranted, this does provide further compelling evidence for the role of luspatercept in the low risk setting.
Immunomodulation/Immune Suppression
Immunosuppression therapy is a mainstay of treatment in lower risk MDS, represented mainly by lenalidomide and anti-thymocyte globulin (ATG) paired with various agents, including cyclosporine, prednisone, tacrolimus.
Lenalidomide is an immunomodulatory agent with multiple mechanisms of action widely accepted to treat del(5q) [
23]. Fenaux et al. conducted the first randomized, placebo controlled trial in 2011, which demonstrated the efficacy of lenalidomide in 205 patients with del(5q) and lower risk MDS. Lenalidomide was shown to significantly increase transfusion independence (TI) at 26 weeks and beyond in a dose-dependent manner (56%, 42.6% and only 5.9% TI after10mg, 5mg or 0mg, respectively) [
42].
A study by List et al. examined long term outcomes from the single arm MDS-003 [
43], and demonstrated that the rate of TI in lower risk participants with del(5q) 8 weeks after receiving lenalidomide was 65.5 percent, with 63 of 88 evaluable patients achieving a partial or complete cytogenetic response [
44]. Importantly, the duration of response was sustained, with a median duration of response 2.2 years. TI was associated with an overall survival benefit, and increased time to development of AML.
In 2006, Santini et al. demonstrated that lower risk patients without del(5q) may benefit from the use of lenalidomide as well. The authors noted a 26.9 percent response rate in achieving RBC-TI in this population, as well as improved quality of life [
45]. Of note, this study included patients who were transfusion dependent and refractory or otherwise not eligible for ESA therapy.
Immune suppression, including ATG and cyclosporine, are currently incorporated in the NCCN guidelines in lower risk patients with elevated endogenous EPO whose cytopenia would be expected to improve with immune suppressive therapy [
23]. Although it has long since been proposed that T-cell driven immunity may play a role in MDS associated cytopenias, large trials to prove these therapies beneficial are still lacking.
A retrospective analysis published by Stahl et al. in 2018 explored outcomes of immune suppressive therapy from multiple centers in the U.S. and Europe. The authors found that the most commonly utilized regimen was ATG with prednisone (43 percent), followed by ATG plus cyclosporine (21 percent). ATG was also paired with tacrolimus and etanercept among others. In general, overall response rate to immunosuppressive therapy (three-quarters of participants were treated with ATG) was approximately 49 percent, and approximately 11 percent achieved complete response, while 30 percent achieved transfusion independence [
46]. The presence of hypocellular marrow was associated with transfusion independence.
A 2014 non-randomized, phase 2 trial by Komrokji et al. demonstrated that rabbit ATG resulted in one-third of the 27 participants achieving durable hematologic response following 4 daily doses of therapy. Interestingly, participants with a higher percentage of CD8 memory T cells, as well as CD4 T-cells with a higher proliferative index were more likely to benefit from treatment with ATG [
47].
Hypomethylating Agents (HMA)
Hypomethylating agents (HMA) are currently used in AML for transplant-ineligible patients who are not candidates for intensive chemotherapy. Furthermore, HMA therapy is well established as frontline treatment in patients with higher risk MDS [
23]. Two commonly prescribed agents are azacitidine, which was initially FDA approved for MDS in 2004, and decitabine, approved two years later.
The use of HMA therapy is currently included in the NCCN guidelines for lower risk MDS without del(5q) for symptomatic anemia with elevated endogenous EPO levels (>500) and a low likelihood of hematologic response to immunosuppressive therapy [
23]. Several trials over recent decades have demonstrated the efficacy and safety of using lower doses of HMA in lower risk MDS patients. In 2017, Jabbour et al. compared decitabine 20mg/m2 compared to azacitidine 75mg/m2, with both drugs given daily for 3 consecutive days. Although the authors concluded that using lower doses of HMA was safe and effective in general, there was a significantly increased ORR and a significantly higher cytogenetic response rate with decitabine compared to azacitidine, especially among patients with higher risk features such as mutations in TP53
or ZRSR2. The authors also noted a higher transfusion independence rate with decitabine [
48]. A 2020 phase-III, placebo controlled study by Garcia-Manero et al. evaluated the use of an oral hypomethylating agent (CC 486) in patients with lower risk, transfusion dependent MDS. This study included 216 participants and demonstrated an improvement in the rate of transfusion independence, however this benefit came at a cost of increased deaths related to infection [
49]. Choosing the optimal HMA based on patient specific disease characteristics remains to be determined.
Telomerase Inhibition
Telomere length appears to be shorter in mononuclear cells in MDS patients, and perhaps somewhat paradoxically, telomerase activity and expression of human telomerase reverse transcriptase are often increased in MDS cells [
50]. It has been hypothesized that this activity may contribute to the growth of the malignant clone, suggesting that blocking telomerase may be an effective therapeutic target.
Efficacy of telomerase inhibition was demonstrated by a single arm, phase II study of 57 lower risk and transfusion-dependent MDS patients who relapsed or were refractory to ESA therapy. Thirty seven percent of those treated with the telomerase inhibitor imetelstat achieved transfusion independence at 8 weeks, and 23 percent were transfusion independent at 24 weeks [
51]. Median duration of transfusion independence was 65 weeks. Results appeared to be more robust for patients without del(5q), as well as those who were HMA or lenalidomide naïve. Cytopenias, especially thrombocytopenia and neutropenia, were common AEs and occurred in 61 percent and 67 percent of study participants respectively.
Of note, we await the result of the iMERGE trial (NCT02598661), a phase III, placebo controlled study which will shed further light on the efficacy of imetelstat in low risk MDS.
6. Higher Risk Disease
Higher risk MDS encompasses patients with International Prognostic Scoring System (IPSS) categories of Intermediate-2 and High risk groups, or IPSS-R intermediate-, high-, or very-high-risk disease. This category also corresponds to the 2016 World Health Organization histologic subtypes of refractory anemia with excess blasts (RAEB-1 and RAEB-2). Treatment is typically indicated at the time of diagnosis of higher risk MDS, as outlined in 2013 by Sekeres and Cutler [
52].
TPO Mimetics in Higher Risk Disease
TPO mimetics have a role in the treatment of patients with higher risk MDS. Platzbecker et al. in a 2015 international, multicenter study demonstrated that eltrombopag (dosed up to 300 mg daily) had an acceptable safety profile in patients with MDS and AML. Adverse events occurred in 53 percent of participants in the eltrombopag group and 32 percent of those in the placebo group, and included fever, nausea, diarrhea, fatigue, decreased appetite and pneumonia [
53]. Importantly, no significant increase in circulating peripheral blasts or marrow blasts were seen within three months of treatment initiation.
The phase II ASPIRE trial explored the benefit of eltrombopag therapy in higher risk MDS and AML with grade 4 thrombocytopenia. In the non-randomized, dose escalation phase of the study, 10 of the 11 participants who completed treatment with eltrombopag had decreased transfusion needs, and 4 of these participants had a significant increase in platelet count. The randomized phase of the study demonstrated that average weekly thrombocytopenic events (defined as platelet transfusion need, grade 3 or higher bleeding event, or platelet count less than 10,000) were significantly lower in those treated with eltrombopag (54 percent), as compared to the control arm (69 percent), during weeks 5 through 12. Serious adverse events were reported in 58 percent of eltrombopag-treated participants and 68 percent of those in the placebo group. Two fatalities attributable due to the drug were reported [
54].
Conversely, the phase III, placebo controlled SUPPORT Trial published by Dickinson et al. in 2018 showed that the addition of eltrombopag to azacitidine did not improve overall or progression free survival in higher risk MDS. Furthermore, participants who received eltrombopag had inferior outcomes, both in terms of survival and platelet transfusion independence (55). There was also a trend toward progression to AML with eltrombopag therapy.
Ultimately more data is needed to determine the characteristics of higher risk MDS patients most likely to benefit from TPO-RA therapy.
HMA
Aberrant DNA methylation is a well described molecular characteristic of higher risk MDS. In the current guidelines, HMA therapy, including the DNA methyltransferase inhibitors (DNMTi) azacitidine and decitabine, are first-line therapy for patients with higher risk MDS who are unfit for hematopoietic stem cell transplantation [
23].
A randomized, phase 3, multicenter, open label trial by Fenaux et al. in 2009 assessed the efficacy of azacitidine compared to conventional treatment. The conventional treatment arm in the study included either supportive care, cytarabine, or conventional chemotherapy, which was decided prior to randomization based on what was deemed appropriate for each individual. There was an improvement in overall survival in participants who received azacitidine when compared to the “conventional care” arm with a median survival of 24.5 months vs. 15 months respectively [
56]. At two year follow up approximately 51 percent of those randomized to the azacitidine group were still alive, compared to 26 percent of those randomized to the “conventional treatment” group.
In 2011, a randomized, phase III trial compared decitabine to best supportive care in higher risk, elderly patients (median age of 70) who were deemed ineligible for chemotherapy. Progression free survival was significantly longer in those that received decitabine (approximately 6.5 months) versus supportive care (3 months). AML transformation at one year was also significantly reduced with decitabine treatment. Importantly, decitabine treatment was also associated with improved patient reported quality of life metrics. There was no statistical difference in overall survival or acute myeloid leukemia (AML) –free survival (AMLFS) with decitabine treatment [
57].
Oral HMAs decrease the need for transfusions, thereby possibly alleviating the burden of extra-domiciliary treatment. However, developing agents with adequate oral bioavailability has been difficult. A phase 2 study including 80 participants assessing bioavailability of oral cedazuridine with decitabine found that oral cedazuridine with decitabine allowed similar systemic decitabine area under the curve (AUC) exposure compared with standard dose IV decitabine. It is important to note that this study only assessed for AUC in the blood, not specific outcomes with this regimen [
58]. More data is clearly needed to assess viability of oral HMA therapy in higher risk MDS, and to determine those most likely to benefit.
Venetoclax
Although HMA are considered first line medications for high-risk MDS, only about 50 percent of patients respond to these agents. Furthermore, patients who do respond can develop drug resistance, after which prognosis is poor. Venetoclax, an oral selective inhibitor of the anti-apoptotic protein BCL-2, which is utilized in the treatment of AML, has been explored as an additional treatment option for high risk MDS.
A retrospective study of 44 patients assessed the impact of venetoclax added to HMA therapy. Overall response to venetoclax was seen in 59 percent of participants. Encouragingly, a response rate of 44 percent was observed even among patients with a previous HMA failure [
59].
Venetoclax in combination with chemotherapy may also be a therapeutic option in higher risk MDS patients. Kadia et al. published a phase II study in 2021 exploring treatment with cladribine, idarubicin and cytarabine in combination with venetoclax. This study primarily included AML patients, although of the 50 study participants, only 5 had high risk MDS. Fourty seven of the 50 patients achieved either complete response (CR) or CR with incomplete count recovery (CRi), as defined by the International Working Group criteria. Thirty seven patients achieved minimal residual disease negativity. Notably, 29 of the 47 responders were eventually taken to transplant [
60], and neutropenic fever was a common grade 3 AE affecting 42 participants. More research is needed to effectively ascertain the cost/benefit of venetoclax.
Chemotherapy
Chemotherapy is a treatment option in higher risk MDS. In the NCCN guidelines chemotherapy regimens similar to those used in AML (such as an anthracycline in combination with cytarabine) are considered, especially before pursuing allogeneic transplant [
23]. However, there is currently no consensus on the role of chemotherapy in higher risk MDS.
Allogenic Hematopoietic Stem Cell Transplant (Allo HSCT)
Allo HSCT remains the only curative treatment option for MDS, and is the recommended standard of care for any suitable candidate. The literature on stem cell transplantation for MDS has recently been reviewed elsewhere [
61]. It is noteworthy that several challenges remain regarding standard pre-transplant risk stratification using disease specific data (i.e. cytogenetic lesions), selection of optimal disease-directed therapy, selection of optimal conditioning regimens, and of course selection of optimal post-transplant therapy.
IDH Inhibition
Approximately 5 percent of patients with MDS harbor mutations in isocitrate dehydrogenase (IDH)-1 or -2, with IDH-2 alterations occurring more commonly (the converse is true in AML). In addition, mutational stability over time has been demonstrated, although new IDH mutations have been reported during leukemic transformation [
62]. IDH mutations are associated with older age, higher platelet counts, and concurrent alterations in DNMT3A, ASXL1 and SRSF2 [
63]. Interestingly, mutated IDH-2 is a marker of poorer prognosis with overall worse survival in lower-risk MDS, but not in higher-risk groups [
63].
The IDH-1 inhibitor ivosidenib is currently FDA approved for patients with relapsed/refractory AML or chemotherapy-ineligible AML with sensitizing IDH-1 mutations.
The IDIOME trial is an ongoing phase II trial exploring the safety and efficacy of ivosidenib in patients with higher risk MDS. The trial encompasses three arms: (A) high risk MDS after azacitidine failure following 6 cycles, (B) high risk treatment-naïve MDS without life threatening cytopenia, and (C) lower risk MDS with anemia that did not respond to ESA therapy. The interim results, presented at the American Society of Hematology (ASH) annual meeting in 2021, showed an ORR of 54 percent in arm A, 91 percent in arm B, and 50 percent in arm C [
64]
.
This suggests that IDH-1 inhibition may be an effective modality in various clinical scenarios, although unsurprisingly treatment naïve patients had superior response. Although the data is encouraging, more mature data is needed for firm conclusions.
Enasidenib, an inhibitor of IDH-2, is approved for treatment of relapsed/refractory AML patients who harbor an IDH-2 mutations, which is associated with a response rate of nearly 40 percent in patients with AML in this setting [
65]. In 2022, DiNardo et al. conducted a trial investigating the efficacy of enasidenib in combination with azacitidine as upfront therapy, or as monotherapy in those previously treated with HMA. Encouragingly, the overall response rate was 74 percent in the upfront setting, and 35 percent in the previously treated group [
66]. Approximately 16 percent of patients experienced differentiation syndrome associated with IDH inhibitor treatment in this study.
Experimental Targeted Agents
Due to the poor prognosis of higher risk MDS failing treatment with HMA other targeted therapies for MDS are continuously being investigated.
Glasdegib
Glasdegib is an oral inhibitor of the transmembrane G protein-coupled receptor Smoothened, a component of the Hedgehog signaling pathway that functions as an oncogene ultimately promoting leukemic cell survival. A phase 2, single center open label trial assessing the impact of glasdegib in patients with MDS or CMML found an overall 6 percent response rate with an acceptable safety profile [
67]. A 2019 study by Cortes et al. randomized 132 patients with AML or higher risk MDS to receive low dose cytarabine with or without glasdegib. Median survival was 8.8 months for patients receiving both glasdegib and low dose cytarabine compared to 4.9 months for those receiving low dose cytarabine alone. Within the subset of higher risk MDS patients, the combination therapy showed an overall response rate of 20 percent, whereas cytarabine alone has a response rate of 0 percent [
68]. It should be noted that 35 percent of participants discontinued the study due to adverse effects. Further clinical studies are needed to fully characterize the role of glasdegib in high-risk MDS.
Rigosertib
Rigosertib acts on the RAS signaling pathway by binding to the RAS-binding domain of multiple kinases including RAF, PI3K and PLK. Preclinical trials demonstrated in vitro mitotic arrest and apoptosis in MDS cells. A 2016 randomized, controlled, phase 3 trial was conducted in patients who failed to respond to, relapsed after, or were ineligible for bone marrow transplant. This study compared overall survival in patients who received rigosertib versus those receiving best supportive care. There was no significant difference in terms of overall survival between the study arms. Subgroup analysis did demonstrate a possible benefit of rigosertib in patients with very high-risk disease, patients with monosomy 7 or trisomy 8 or HMA failure [
69]. Consequently, further investigation into rigosertib is warranted.
Pevonedistat
Pharmacologic targeting of proteasome activity is considered standard of care in the treatment of multiple myeloma. In 2023, De Carvalho et al. published data suggesting that certain genes involved in the ubiquitination pathway are differentially expressed in patients with MDS. For example, UBE2O, UBE2T, and USP7 expression was increased while USP15 expression was decreased in marrow mononuclear cells [
70], raising the possibility of targeting the ubiquitin-proteasome pathway in MDS. NEDD8 is a ubiquitin-like protein that targets the cullin subunits of E3 ubiquitin ligases, resulting activation of ubiquitination [
71]. Pevonedistat is a NEDD8 activating enzyme that has been evaluated in combination with HMA therapy in patients with higher risk MDS [
72]. This study also included participants with AML with lower blast count as well as CMML. Interestingly, treatment with pevonedistat and azacitidine resulted in a median OS of 23.9 months compared to 19.1 months for those treated with azacitidine alone in participants with higher risk MDS. Pevonedistat plus azacitidine led to longer event free survival (EFS) compared with azacitidine alone and duration of response (DOR) was also markedly improved in the combination arm. Impressively, the CR rate in the combination arm was nearly double that of azacitidine alone, 51.7% versus 26.7% [
72]. Although this was a small trial with only 120 participants, it certainly suggests that further studies are warranted in high risk MDS.
Magrolimab
Magrolimab is a first-in-class monoclonal antibody directed against CD47, intended to promote macrophage mediated phagocytosis of cancerous cells. A phase 1b study by Sallman et al. explored the addition of Magrolimab to HMA in IPSS-R intermediate to very high risk MDS. Magrolimab plus azacitidine resulted in an ORR of 75%, with a 33% CR rate. Notable, there was a CR of 40% among patients with TP53 mutation. The authors note that over one third of study participants would go on to have an allo HSCT with 77 percent OS at two years [
73]. The drug had a reasonable safety profile with constipation, thrombocytopenia and anemia being the most commonly reported AEs.
The phase III ENHANCE study was a randomized, double blinded, placebo controlled trial that included 539 IPSS-R intermediate, high or very high risk treatment naïve participants, randomized to receive azacitidine with or without magrolimab. Disappointingly, in July of 2023 the trial was discontinued due to futility [
74].
Sabatolimab
T-cell immunoglobulin domain and mucin domain-3 (TIM-3) is a membrane receptor expressed by myeloid cells and most AML blasts but not by normal hematopoietic stem cells, and is believed to play a role in leukemic stem cell self-renewal. TIM-3 is also expressed on T-cells and dendritic cells and is involved in the downregulation of cell-mediated immunity. As such, targeting of this protein represents a rational therapeutic target [
75]. Sabatolimab, a monoclonal antibody that targets TIM-3, in preclinical studies showed efficacy by mediating immune activation and phagocytosis of myeloblasts in vitro. Two clinical trials evaluating the efficacy and safety of front line treatment with Sabatolimab in combination with HMA in patients with IPSS-R intermediate to very high risk MDS are ongoing: the phase II STIMULUS-MDS1 trial, and the phase III STIMULUS-MDS2 trial, which also include participants with CMML [
76]. The primary outcomes of STIMULUS-MDS1 are complete remission rate and PFS, and the primary outcome of STIMULUS-MDS-2 is OS. We eagerly await the results of these studies.
Eprenetapopt (APR-246)
TP53 has been described as the “guardian of the genome” due to its multitude of effects, including its role in DNA damage repair. It is well known that patients with MDS and AML with TP53 mutations have a notably worse prognosis, often with a less robust response to therapy. Therefore, TP53 directed treatment represents an unmet need in the current treatment paradigm.
Eprenetapopt (APR-246) is a small organic molecule thought to bind to mutant p53 protein and induce a conformational change that results in restored functionality. Treatment with APR-246 alone results in in-vitro tumor cell death, and is believed to synergize with HMA in human myeloid cell lines [
77], raising hopes for the use of p53 activators in MDS.
A phase Ib/II clinical trial published by Sallman et al. in 2021 included 55 participants (44 with MDS or MDS/MPN and 11 with AML) and evaluated the safety and efficacy of adding APR-246 to HMA therapy. This therapeutic combination showed a 73 percent response rate in MDS, with 50 percent of participants achieving CR and 58 percent achieving a cytogenetic response [
78]. Importantly, 35 percent of participants underwent allo HSCT, with a median overall survival of 14.7 months. It is notable that approximately one third of the patients in this trial experienced febrile neutropenia, underscoring the challenges of P53 reactivation therapy
.
Conclusions
Despite significant progress in the understanding and treatment of MDS over recent decades, allo HSCT remains the only available curative therapy, which is explained in part by the heterogeneity of this disease with variable risk of progression to AML. We have summarized here the state-of-the-art management strategies for MDS in the context specific clinical features, as well as, the molecular genetic landscape of patients. We hope that continuous research on the molecular underpinnings and the pathogenesis of this complex entity produces a better understanding and allows further opportunities for more effective and less toxic targeted intervention, with the ultimate goal to continue improving morbidity and survival.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia-Manero, G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023 Aug;98, 1307-1325. [CrossRef] [PubMed]
- Ogawa, S. Genetics of MDS. Blood. 2019 Mar 7;133, 1049-1059. PMCID: PMC6587668. [CrossRef] [PubMed]
- Haider, M.; Duncavage, E.J.; Afaneh, K.F.; Bejar, R.; List, A.F. New Insight into the Biology, Risk Stratification, and Targeted Treatment of Myelodysplastic Syndromes. Am Soc Clin Oncol Educ Book. 2017, 37:480-494. [CrossRef] [PubMed]
- Abuhadra, N.; Mukherjee, S.; Al-Issa, K.; Adema, V.; Hirsch, C.M.; Advani, A.; Przychodzen, B.; Makhoul, A.; Awada, H.; Maciejewski, J.P.; Sekeres, M.A.; Nazha, A. BCOR and BCORL1 mutations in myelodysplastic syndromes (MDS): clonal architecture and impact on outcomes. Leuk Lymphoma. 2019 Jun;60, 1587-1590. PMCID: PMC8694070. [CrossRef] [PubMed]
- Katamesh, B.; Nanaa, A.; He, R.; Viswanatha, D.; Nguyen, P.; Greipp, P.; Bessonen, K.; Gangat, N.; Begna, K.; Mangaonkar, A.; Patnaik, M.; Hogan, W.J.; Tefferi, A.; Litzow, M.; Shah, M.V.; Yi, C.A.; Foran, J.; Badar, T.; Alkhateeb, H.B.; Al-Kali, A. Clinical and prognostic impact of STAG2 mutations in myeloid neoplasms: the Mayo Clinic experience. Blood Adv. 2023 Apr 25;7, 1351-1355. PMCID: PMC10139934. [CrossRef] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017 Aug;7, 818-831. PMCID: PMC5611790. [CrossRef] [PubMed]
- Aly, M.; Ramdzan, Z.M.; Nagata, Y.; Balasubramanian, S.K.; Hosono, N.; Makishima, H.; Visconte, V.; Kuzmanovic, T.; Adema, V.; Nazha, A.; Przychodzen, B.P.; Kerr, C.M.; Sekeres, M.A.; Abazeed, M.E.; Nepveu, A.; Maciejewski, J.P. Distinct clinical and biological implications of CUX1 in myeloid neoplasms. Blood Adv. 2019 Jul 23;3, 2164-2178. PMCID: PMC6650742. [CrossRef] [PubMed]
- Seethy, A.A.; Pethusamy, K.; Kushwaha, T.; Kumar, G.; Talukdar, J.; Chaubey, R.; Sundaram, U.D.; Mahapatra, M.; Saxena, R.; Dhar, R.; Inampudi, K.K.; Karmakar, S. Alterations of the expression of TET2 and DNA 5-hmC predict poor prognosis in Myelodysplastic Neoplasms. BMC Cancer. 2023 Oct 26;23, 1035. PMCID: PMC10601240. [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; Bueso-Ramos, C.E.; Cortes, J.E.; Dal Cin, P.; DiNardo, C.D.; Dombret, H.; Duncavage, E.J.; Ebert, B.L.; Estey, E.H.; Facchetti, F.; Foucar, K.; Gangat, N.; Gianelli, U.; Godley, L.A.; Gökbuget, N.; Gotlib, J.; Hellström-Lindberg, E.; Hobbs, G.S.; Hoffman, R.; Jabbour, E.J.; Kiladjian, J.J.; Larson, R.A.; Le Beau, M.M.; Loh, M.L.; Löwenberg, B.; Macintyre, E.; Malcovati, L.; Mullighan, C.G.; Niemeyer, C.; Odenike, O.M.; Ogawa, S.; Orfao, A.; Papaemmanuil, E.; Passamonti, F.; Porkka, K.; Pui, C.H.; Radich, J.P.; Reiter, A.; Rozman, M.; Rudelius, M.; Savona, M.R.; Schiffer, C.A.; Schmitt-Graeff, A.; Shimamura, A.; Sierra, J.; Stock, W.A.; Stone, R.M.; Tallman, M.S.; Thiele, J.; Tien, H.F.; Tzankov, A.; Vannucchi, A.M.; Vyas, P.; Wei, A.H.; Weinberg, O.K.; Wierzbowska, A.; Cazzola, M.; Döhner, H.; Tefferi, A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15;140, 1200-1228. PMCID: PMC9479031. [CrossRef] [PubMed]
- Fenaux, P.; Adès, L. How we treat lower-risk myelodysplastic syndromes. Blood. 2013 May 23;121, 4280-6. [CrossRef] [PubMed]
- Shahjahani, M.; Hadad, E.H.; Azizidoost, S.; Nezhad, K.C.; Shahrabi, S. Complex karyotype in myelodysplastic syndromes: Diagnostic procedure and prognostic susceptibility. Oncol Rev. 2019 Feb 4;13, 389. PMCID: PMC6379782. [CrossRef] [PubMed]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; Ebert, B.L. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011 Jun 30;364, 2496-506. PMCID: PMC3159042. [CrossRef] [PubMed]
- Bersanelli, M.; Travaglino, E.; Meggendorfer, M.; Matteuzzi, T.; Sala, C.; Mosca, E.; Chiereghin, C.; Di Nanni, N.; Gnocchi, M.; Zampini, M.; Rossi, M.; Maggioni, G.; Termanini, A.; Angelucci, E.; Bernardi, M.; Borin, L.; Bruno, B.; Bonifazi, F.; Santini, V.; Bacigalupo, A.; Voso, M.T.; Oliva, E.; Riva, M.; Ubezio, M.; Morabito, L.; Campagna, A.; Saitta, C.; Savevski, V.; Giampieri, E.; Remondini, D.; Passamonti, F.; Ciceri, F.; Bolli, N.; Rambaldi, A.; Kern, W.; Kordasti, S.; Sole, F.; Palomo, L.; Sanz, G.; Santoro, A.; Platzbecker, U.; Fenaux, P.; Milanesi, L.; Haferlach, T.; Castellani, G.; Della Porta, M.G. Classification and Personalized Prognostic Assessment on the Basis of Clinical and Genomic Features in Myelodysplastic Syndromes. J Clin Oncol. 2021 Apr 10;39, 1223-1233. PMCID: PMC8078359. [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; Kantarjian, H.; Kuendgen, A.; Levis, A.; Malcovati, L.; Cazzola, M.; Cermak, J.; Fonatsch, C.; Le Beau, M.M.; Slovak, M.L.; Krieger, O.; Luebbert, M.; Maciejewski, J.; Magalhaes, S.M.; Miyazaki, Y.; Pfeilstöcker, M.; Sekeres, M.; Sperr, W.R.; Stauder, R.; Tauro, S.; Valent, P.; Vallespi, T.; van de Loosdrecht, A.A.; Germing, U.; Haase, D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012 Sep 20;120, 2454-65. PMCID: PMC4425443. [CrossRef] [PubMed]
- Kewan, T.; Bahaj, W.; Durmaz, A.; Aly, M.M.; Ogbue, O.D.; Carraway, H.E.; Sekeres, M.A.; Visconte, V.; Gurnari, C.; Maciejewski, J.P. Validation of the Molecular International Prognostic Scoring System in patients with myelodysplastic syndromes. Blood. 2023 Jan 30:blood.2022018896. [CrossRef] [PubMed]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Delvin, S.M.; Papaemmanuil, E. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. June 12, 2022. NEJM Evid 2022;1(7). [CrossRef]
- Sabile, J.M.G.; Kaempf, A.; Tomic, K.; Manu, G.P.; Swords, R.; Migdady, Y. A retrospective validation of the IPSS-M molecular score in primary and therapy-related myelodysplastic syndromes (MDS). Leuk Lymphoma. 2023 Jul 13:1-6. [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; Chen, W.; Chen, X.; Chng, W.J.; Choi, J.K.; Colmenero, I.; Coupland, S.E.; Cross, N.C.P.; De Jong, D.; Elghetany, M.T.; Takahashi, E.; Emile, J.F.; Ferry, J.; Fogelstrand, L.; Fontenay, M.; Germing, U.; Gujral, S.; Haferlach, T.; Harrison, C.; Hodge, J.C.; Hu, S.; Jansen, J.H.; Kanagal-Shamanna, R.; Kantarjian, H.M.; Kratz, C.P.; Li, X.Q.; Lim, M.S.; Loeb, K.; Loghavi, S.; Marcogliese, A.; Meshinchi, S.; Michaels, P.; Naresh, K.N.; Natkunam, Y.; Nejati, R.; Ott, G.; Padron, E.; Patel, K.P.; Patkar, N.; Picarsic, J.; Platzbecker, U.; Roberts, I.; Schuh, A.; Sewell, W.; Siebert, R.; Tembhare, P.; Tyner, J.; Verstovsek, S.; Wang, W.; Wood, B.; Xiao, W.; Yeung, C.; Hochhaus, A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul;36, 1703-1719. [CrossRef]
- Heptinstall, K.; Myelodysplastic Syndromes Foundation, Inc. Quality of life in myelodysplastic syndromes. A special report from the Myelodysplastic Syndromes Foundation, Inc. Oncology (Williston Park). 2008 Feb;22(2 Suppl Nurse Ed):13-8; discussion 19. [PubMed]
- Platzbecker, U.; Hofbauer, L.C.; Ehninger, G.; Hölig, K. The clinical, quality of life, and economic consequences of chronic anemia and transfusion support in patients with myelodysplastic syndromes. Leuk Res. 2012 May;36, 525-36. [CrossRef] [PubMed]
- Cermak, J.; Kacirkova, P.; Mikulenkova, D.; Michalova, K. Impact of transfusion dependency on survival in patients with early myelodysplastic syndrome without excess of blasts. Leuk Res. 2009 Nov;33, 1469-74. [CrossRef] [PubMed]
- Angelucci, E.; Li, J.; Greenberg, P.; Wu, D.; Hou, M.; Montano Figueroa, E.H. Iron chelation in transfusion-dependent patients with low- to intermediate-1-risk myelodysplastic syndromes: a randomized trial. Ann Intern Med. 2020;172:513–22. [CrossRef]
- National Comprehensive Cancer Network. Myelodysplastic Syndromes (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf (accessed on 24 July 2023).
- Hellström-Lindberg, E. Efficacy of erythropoietin in the myelodysplastic syndromes: a meta-analysis of 205 patients from 17 studies. Br J Haematol. 1995 Jan;89, 67-71. [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Ahlgren, T.; Beguin, Y.; Carlsson, M.; Carneskog, J.; Dahl, I.M.; Dybedal, I.; Grimfors, G.; Kanter-Lewensohn, L.; Linder, O.; Luthman, M.; Löfvenberg, E.; Nilsson-Ehle, H.; Samuelsson, J.; Tangen, J.M.; Winqvist, I.; Oberg, G.; Osterborg, A.; Ost, A. Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: results from a randomized phase II study and long-term follow-up of 71 patients. Blood. 1998 Jul 1;92, 68-75. [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Gulbrandsen, N.; Lindberg, G.; Ahlgren, T.; Dahl, I.M.; Dybedal, I.; Grimfors, G.; Hesse-Sundin, E.; Hjorth, M.; Kanter-Lewensohn, L.; Linder, O.; Luthman, M.; Löfvenberg, E.; Oberg, G.; Porwit-MacDonald, A.; Rådlund, A.; Samuelsson, J.; Tangen, J.M.; Winquist, I.; Wisloff, F. ; Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003 Mar;120, 1037-46. [CrossRef] [PubMed]
- Platzbecker, U.; Symeonidis, A.; Oliva, E.N.; Goede, J.S.; Delforge, M.; Mayer, J.; Slama, B.; Badre, S.; Gasal, E.; Mehta, B.; Franklin, J. A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes. Leukemia. 2017 Sep;31, 1944-1950. PMCID: PMC5596208. [CrossRef] [PubMed]
- Fenaux, P.; Santini, V.; Spiriti, M.A.A.; Giagounidis, A.; Schlag, R.; Radinoff, A.; Gercheva-Kyuchukova, L.; Anagnostopoulos, A.; Oliva, E.N.; Symeonidis, A.; Berger, M.H.; Götze, K.S.; Potamianou, A.; Haralampiev, H.; Wapenaar, R.; Milionis, I.; Platzbecker, U. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia. 2018 Dec;32, 2648-2658. PMCID: PMC6286328. [CrossRef] [PubMed]
- Kantarjian, H.; Giles, F.; List, A.; Lyons, R.; Sekeres, M.A.; Pierce, S.; Deuson, R.; Leveque, J. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007 May 1;109, 1705-14. [CrossRef] [PubMed]
- Oliva, E.N.; Alati, C.; Santini, V.; Poloni, A.; Molteni, A.; Niscola, P.; Salvi, F.; Sanpaolo, G.; Balleari, E.; Germing, U.; Fenaux, P.; Stamatoullas, A.; Palumbo, G.A.; Salutari, P.; Impera, S.; Avanzini, P.; Cortelezzi, A.; Liberati, A.M.; Carluccio, P.; Buccisano, F.; Voso, M.T.; Mancini, S.; Kulasekararaj, A.; Morabito, F.; Bocchia, M.; Cufari, P.; Spiriti, M.A.; Santacaterina, I.; D'Errigo, M.G.; Bova, I.; Zini, G.; Latagliata, R. Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial. Lancet Haematol. 2017 Mar;4, e127-e136. [CrossRef] [PubMed]
- Oliva, E.N.; Riva, M.; Niscola, P.; Santini, V.; Breccia, M.; Giai, V.; Poloni, A.; Patriarca, A.; Crisà, E.; Capodanno, I.; Salutari, P.; Reda, G.; Cascavilla, N.; Ferrero, D.; Guarini, A.; Tripepi, G.; Iannì, G.; Russo, E.; Castelli, A.; Fattizzo, B.; Beltrami, G.; Bocchia, M.; Molteni, A.; Fenaux, P.; Germing, U.; Ricco, A.; Palumbo, G.A.; Impera, S.; Di Renzo, N.; Rivellini, F.; Buccisano, F.; Stamatoullas-Bastard, A.; Liberati, A.M.; Candoni, A.; Delfino, I.M.; Arcadi, M.T.; Cufari, P.; Rizzo, L.; Bova, I.; D'Errigo, M.G.; Zini, G.; Latagliata, R. Eltrombopag for Low-Risk Myelodysplastic Syndromes With Thrombocytopenia: Interim Results of a Phase-II, Randomized, Placebo-Controlled Clinical Trial (EQOL-MDS). J Clin Oncol. 2023 Jun 9:JCO2202699. [CrossRef] [PubMed]
- Vicente, A.; Patel, B.A.; Gutierrez-Rodrigues, F.; Groarke, E.; Giudice, V.; Lotter, J.; Feng, X.; Kajigaya, S.; Weinstein, B.; Barranta, E.; Olnes, M.J.; Parikh, A.R.; Albitar, M.; Wu, C.O.; Shalhoub, R.; Calvo, K.R.; Townsley, D.M.; Scheinberg, P.; Dunbar, C.E.; Young, N.S.; Winkler, T. Eltrombopag monotherapy can improve hematopoiesis in patients with low to intermediate risk-1 myelodysplastic syndrome. Haematologica. 2020 Dec 1;105, 2785-2794. PMCID: PMC7716353. [CrossRef] [PubMed]
- Fenaux, P.; Muus, P.; Kantarjian, H.; Lyons, R.M.; Larson, R.A.; Sekeres, M.A.; Becker, P.S.; Orejudos, A.; Franklin, J. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: long-term safety and efficacy. Br J Haematol. 2017 Sep;178, 906-913. PMCID: PMC5600084. [CrossRef] [PubMed]
- Giagounidis, A.; Mufti, G.J.; Fenaux, P.; Sekeres, M.A.; Szer, J.; Platzbecker, U.; Kuendgen, A.; Gaidano, G.; Wiktor-Jedrzejczak, W.; Hu, K.; Woodard, P.; Yang, A.S.; Kantarjian, H.M. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014 Jun 15;120, 1838-46. PMCID: PMC4298760. [CrossRef] [PubMed]
- Kantarjian, H.; Fenaux, P.; Sekeres, M.A.; Becker, P.S.; Boruchov, A.; Bowen, D.; Hellstrom-Lindberg, E.; Larson, R.A.; Lyons, R.M.; Muus, P.; Shammo, J.; Siegel, R.; Hu, K.; Franklin, J.; Berger, D.P. Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J Clin Oncol. 2010 Jan 20;28, 437-44. [CrossRef] [PubMed]
- Sekeres, M.A.; Kantarjian, H.; Fenaux, P.; Becker, P.; Boruchov, A.; Guerci-Bresler, A.; Hu, K.; Franklin, J.; Wang, Y.M.; Berger, D. Subcutaneous or intravenous administration of romiplostim in thrombocytopenic patients with lower risk myelodysplastic syndromes. Cancer. 2011 Mar 1;117, 992-1000. [CrossRef] [PubMed]
- Meng, F.; Chen, X.; Yu, S.; Ren, X.; Liu, Z.; Fu, R.; Li, L. Safety and Efficacy of Eltrombopag and Romiplostim in Myelodysplastic Syndromes: A Systematic Review and Meta-Analysis. Front Oncol. 2020 Nov 26;10:582686. PMCID: PMC7727449. [CrossRef] [PubMed]
- Platzbecker, U.; Germing, U.; Götze, K.S.; Kiewe, P.; Mayer, K.; Chromik, J.; Radsak, M.; Wolff, T.; Zhang, X.; Laadem, A.; Sherman, M.L.; Attie, K.M.; Giagounidis, A. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017 Oct;18, 1338-1347. Erratum in: Lancet Oncol. 2017 Oct;18, e562. [CrossRef] [PubMed]
- Platzbecker, U.; Götze, K.S.; Kiewe, P.; Germing, U.; Mayer, K.; Radsak, M.; Wolff, T.; Chromik, J.; Sockel, K.; Oelschlägel, U.; Haase, D.; Illmer, T.; Al-Ali, H.K.; Silling, G.; Reynolds, J.G.; Zhang, X.; Attie, K.M.; Shetty, J.K.; Giagounidis, A. Long-Term Efficacy and Safety of Luspatercept for Anemia Treatment in Patients With Lower-Risk Myelodysplastic Syndromes: The Phase II PACE-MDS Study. J Clin Oncol. 2022 Nov 20;40, 3800-3807. PMCID:PMC9671752. [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; Sekeres, M.A.; Falantes, J.F.; Arrizabalaga, B.; Salvi, F.; Giai, V.; Vyas, P.; Bowen, D.; Selleslag, D.; DeZern, A.E.; Jurcic, J.G.; Germing, U.; Götze, K.S.; Quesnel, B.; Beyne-Rauzy, O.; Cluzeau, T.; Voso, M.T.; Mazure, D.; Vellenga, E.; Greenberg, P.L.; Hellström-Lindberg, E.; Zeidan, A.M.; Adès, L.; Verma, A.; Savona, M.R.; Laadem, A.; Benzohra, A.; Zhang, J.; Rampersad, A.; Dunshee, D.R.; Linde, P.G.; Sherman, M.L.; Komrokji, R.S.; List, A.F. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N Engl J Med. 2020 Jan 9;382, 140-151. [CrossRef] [PubMed]
- Platzbecker, U.; Della Porta, M.G.; Santini, V.; Zeidan, A.M.; Komrokji, R.S.; Shortt, J.; Valcarcel, D.; Jonasova, A.; Dimicoli-Salazar, S.; Tiong, I.S.; Lin, C.C.; Li, J.; Zhang, J.; Giuseppi, A.C.; Kreitz, S.; Pozharskaya, V.; Keeperman, K.L.; Rose, S.; Shetty, J.K.; Hayati, S.; Vodala, S.; Prebet, T.; Degulys, A.; Paolini, S.; Cluzeau, T.; Fenaux, P.; Garcia-Manero, G. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial. Lancet. 2023 Jul 29;402, 373-385. [CrossRef] [PubMed]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Te Boekhorst, P.; Sanz, G.; Del Cañizo, C.; Guerci-Bresler, A.; Nilsson, L.; Platzbecker, U.; Lübbert, M.; Quesnel, B.; Cazzola, M.; Ganser, A.; Bowen, D.; Schlegelberger, B.; Aul, C.; Knight, R.; Francis, J.; Fu, T.; Hellström-Lindberg, E. ; MDS-004 Lenalidomide del5q Study Group. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011 Oct 6;118, 3765-76. [CrossRef] [PubMed]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; Reeder, C.; Wride, K.; Patin, J.; Schmidt, M.; Zeldis, J.; Knight, R. ; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006 Oct 5;355, 1456-65. [CrossRef] [PubMed]
- List, A.F.; Bennett, J.M.; Sekeres, M.A.; Skikne, B.; Fu, T.; Shammo, J.M.; Nimer, S.D.; Knight, R.D.; Giagounidis, A. ; MDS-003 Study Investigators. Extended survival and reduced risk of AML progression in erythroid-responsive lenalidomide-treated patients with lower-risk del(5q) MDS. Leukemia. 2014 May;28, 1033-40. Erratum in: Leukemia. 2015 Dec;29, 2452.. PMCID: PMC4017258. [CrossRef] [PubMed]
- Santini, V.; Almeida, A.; Giagounidis, A.; Gröpper, S.; Jonasova, A.; Vey, N.; Mufti, G.J.; Buckstein, R.; Mittelman, M.; Platzbecker, U.; Shpilberg, O.; Ram, R.; Del Cañizo, C.; Gattermann, N.; Ozawa, K.; Risueño, A.; MacBeth, K.J.; Zhong, J.; Séguy, F.; Hoenekopp, A.; Beach, C.L.; Fenaux, P. Randomized Phase III Study of Lenalidomide Versus Placebo in RBC Transfusion-Dependent Patients With Lower-Risk Non-del(5q) Myelodysplastic Syndromes and Ineligible for or Refractory to Erythropoiesis-Stimulating Agents. J Clin Oncol. 2016 Sep 1;34, 2988-96. [CrossRef] [PubMed]
- Stahl, M.; DeVeaux, M.; de Witte, T.; Neukirchen, J.; Sekeres, M.A.; Brunner, A.M.; Roboz, G.J.; Steensma, D.P.; Bhatt, V.R.; Platzbecker, U.; Cluzeau, T.; Prata, P.H.; Itzykson, R.; Fenaux, P.; Fathi, A.T.; Smith, A.; Germing, U.; Ritchie, E.K.; Verma, V.; Nazha, A.; Maciejewski, J.P.; Podoltsev, N.A.; Prebet, T.; Santini, V.; Gore, S.D.; Komrokji, R.S.; Zeidan, A.M. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018 Jul 24;2, 1765-1772. [CrossRef]
- Komrokji, R.S.; Mailloux, A.W.; Chen, D.T.; Sekeres, M.A.; Paquette, R.; Fulp, W.J.; Sugimori, C.; Paleveda-Pena, J.; Maciejewski, J.P.; List, A.F.; Epling-Burnette, P.K. A phase II multicenter rabbit anti-thymocyte globulin trial in patients with myelodysplastic syndromes identifying a novel model for response prediction. Haematologica. 2014 Jul;99, 1176-83. [CrossRef]
- Jabbour, E.; Short, N.J.; Montalban-Bravo, G.; Huang, X.; Bueso-Ramos, C.; Qiao, W.; Yang, H.; Zhao, C.; Kadia, T.; Borthakur, G.; Pemmaraju, N.; Sasaki, K.; Estrov, Z.; Cortes, J.; Ravandi, F.; Alvarado, Y.; Komrokji, R.; Sekeres, M.A.; Steensma, D.P.; DeZern, A.; Roboz, G.; Kantarjian, H.; Garcia-Manero, G. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood. 2017 Sep 28;130, 1514-1522. [CrossRef]
- Garcia-Manero, G.; Santini, V.; Almeida, A.; Platzbecker, U.; Jonasova, A.; Silverman, L.R.; Falantes, J.; Reda, G.; Buccisano, F.; Fenaux, P.; Buckstein, R.; Diez Campelo, M.; Larsen, S.; Valcarcel, D.; Vyas, P.; Giai, V.; Olíva, E.N.; Shortt, J.; Niederwieser, D.; Mittelman, M.; Fianchi, L.; La Torre, I.; Zhong, J.; Laille, E.; Lopes de Menezes, D.; Skikne, B.; Beach, C.L.; Giagounidis, A. Phase III, Randomized, Placebo-Controlled Trial of CC-486 (Oral Azacitidine) in Patients With Lower-Risk Myelodysplastic Syndromes. J Clin Oncol. 2021 May 1;39, 1426-1436. [CrossRef]
- Asai, A.; Oshima, Y.; Yamamoto, Y.; Uochi, T.A.; Kusaka, H.; Akinaga, S.; Yamashita, Y.; Pongracz, K.; Pruzan, R.; Wunder, E.; Piatyszek, M.; Li, S.; Chin, A.C.; Harley, C.B.; Gryaznov, S. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003 Jul 15;63, 3931-9. [PubMed]
- Steensma, D.P.; Fenaux, P.; Van Eygen, K.; Raza, A.; Santini, V.; Germing, U.; Font, P.; Diez-Campelo, M.; Thepot, S.; Vellenga, E.; Patnaik, M.M.; Jang, J.H.; Varsos, H.; Bussolari, J.; Rose, E.; Sherman, L.; Sun, L.; Wan, Y.; Dougherty, S.; Huang, F.; Feller, F.; Rizo, A.; Platzbecker, U. Imetelstat Achieves Meaningful and Durable Transfusion Independence in High Transfusion-Burden Patients With Lower-Risk Myelodysplastic Syndromes in a Phase II Study. J Clin Oncol. 2021 Jan 1;39, 48-56. [CrossRef] [PubMed]
- Sekeres, M.A.; Cutler, C. How we treat higher-risk myelodysplastic syndromes. Blood. 2014 Feb 6;123, 829-36. [CrossRef] [PubMed]
- Platzbecker, U.; Wong, R.S.; Verma, A.; Abboud, C.; Araujo, S.; Chiou, T.J.; Feigert, J.; Yeh, S.P.; Götze, K.; Gorin, N.C.; Greenberg, P.; Kambhampati, S.; Kim, Y.J.; Lee, J.H.; Lyons, R.; Ruggeri, M.; Santini, V.; Cheng, G.; Jang, J.H.; Chen, C.Y.; Johnson, B.; Bennett, J.; Mannino, F.; Kamel, Y.M.; Stone, N.; Dougherty, S.; Chan, G.; Giagounidis, A. Safety and tolerability of eltrombopag versus placebo for treatment of thrombocytopenia in patients with advanced myelodysplastic syndromes or acute myeloid leukaemia: a multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial. Lancet Haematol. 2015 Oct;2, e417-26. [CrossRef] [PubMed]
- Mittelman, M.; Platzbecker, U.; Afanasyev, B.; Grosicki, S.; Wong, R.S.M.; Anagnostopoulos, A.; Brenner, B.; Denzlinger, C.; Rossi, G.; Nagler, A.; Garcia-Delgado, R.; Portella, M.S.O.; Zhu, Z.; Selleslag, D. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018 Jan;5, e34-e43. [CrossRef] [PubMed]
- Dickinson, M.; Cherif, H.; Fenaux, P.; Mittelman, M.; Verma, A.; Portella, M.S.O.; Burgess, P.; Ramos, P.M.; Choi, J.; Platzbecker, U. ; SUPPORT study investigators. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood. 2018 Dec 20;132, 2629-2638. PMCID: PMC6337824. [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; Gore, S.D.; Seymour, J.F.; Bennett, J.M.; Byrd, J.; Backstrom, J.; Zimmerman, L.; McKenzie, D.; Beach, C.; Silverman, L.R. ; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009 Mar;10, 223-32. PMCID: PMC4086808. [CrossRef] [PubMed]
- Lübbert, M.; Suciu, S.; Baila, L.; Rüter, B.H.; Platzbecker, U.; Giagounidis, A.; Selleslag, D.; Labar, B.; Germing, U.; Salih, H.R.; Beeldens, F.; Muus, P.; Pflüger, K.H.; Coens, C.; Hagemeijer, A.; Eckart Schaefer, H.; Ganser, A.; Aul, C.; de Witte, T.; Wijermans, P.W. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011 May 20;29, 1987-96. [CrossRef] [PubMed]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; O'Connell, C.; Michaelis, L.C.; Brandwein, J.; Kantarjian, H.; Oganesian, A.; Azab, M.; Savona, M.R. Oral cedazuridine/decitabine for MDS and CMML: a phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood. 2020 Aug 6;136, 674-683. PMCID: PMC7414597. [CrossRef] [PubMed]
- Ball, B.J.; Famulare, C.A.; Stein, E.M.; Tallman, M.S.; Derkach, A.; Roshal, M.; Gill, S.I.; Manning, B.M.; Koprivnikar, J.; McCloskey, J.; Testi, R.; Prebet, T.; Al Ali, N.H.; Padron, E.; Sallman, D.A.; Komrokji, R.S.; Goldberg, A.D. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020 Jul 14;4, 2866-2870. PMCID: PMC7362378. [CrossRef] [PubMed]
- Kadia, T.M.; Reville, P.K.; Borthakur, G.; Yilmaz, M.; Kornblau, S.; Alvarado, Y.; Dinardo, C.D.; Daver, N.; Jain, N.; Pemmaraju, N.; Short, N.; Wang, S.A.; Tidwell, R.S.S.; Islam, R.; Konopleva, M.; Garcia-Manero, G.; Ravandi, F.; Kantarjian, H.M. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021 Aug;8, e552-e561. [CrossRef]
- DeFilipp, Z.; Ciurea, S.O.; Cutler, C.; Robin, M.; Warlick, E.D.; Nakamura, R.; Brunner, A.M.; Dholaria, B.; Walker, A.R.; Kröger, N.; Bejanyan, N.; Atallah, E.; Tamari, R.; Solh, M.M.; Percival, M.E.; de Lima, M.; Scott, B.; Oran, B.; Garcia-Manero, G.; Hamadani, M.; Carpenter, P.; DeZern, A.E. Hematopoietic Cell Transplantation in the Management of Myelodysplastic Syndrome: An Evidence-Based Review from the American Society for Transplantation and Cellular Therapy Committee on Practice Guidelines. Transplant Cell Ther. 2023 Feb;29, 71-81. [CrossRef] [PubMed]
- DiNardo, C.D.; Jabbour, E.; Ravandi, F.; Takahashi, K.; Daver, N.; Routbort, M.; Patel, K.P.; Brandt, M.; Pierce, S.; Kantarjian, H.; Garcia-Manero, G. IDH1 and IDH2 mutations in myelodysplastic syndromes and role in disease progression. Leukemia. 2016 Apr;30, 980-4. PMCID: PMC4733599. [CrossRef] [PubMed]
- Lin, C.C.; Hou, H.A.; Chou, W.C.; Kuo, Y.Y.; Liu, C.Y.; Chen, C.Y.; Lai, Y.J.; Tseng, M.H.; Huang, C.F.; Chiang, Y.C.; Lee, F.Y.; Liu, M.C.; Liu, C.W.; Tang, J.L.; Yao, M.; Huang, S.Y.; Ko, B.S.; Wu, S.J.; Tsay, W.; Chen, Y.C.; Tien, H.F. IDH mutations are closely associated with mutations of DNMT3A, ASXL1 and SRSF2 in patients with myelodysplastic syndromes and are stable during disease evolution. Am J Hematol. 2014 Feb;89, 137-44. [CrossRef] [PubMed]
- Sebert, M.; Cluzeau, T.; Beyne Rauzy, O. Ivosidenib monotherapy is effective in patients with IDH1 mutated myelodysplastic syndrome (MDS): the IDIOME phase 2 study by the GFM group. Blood. 2021;138:62. [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Pollyea, D.A.; Stone, R.M.; Altman, J.K.; Roboz, G.J.; Patel, M.R.; Collins, R.; Flinn, I.W.; Sekeres, M.A.; Stein, A.S.; Kantarjian, H.M.; Levine, R.L.; Vyas, P.; MacBeth, K.J.; Tosolini, A.; VanOostendorp, J.; Xu, Q.; Gupta, I.; Lila, T.; Risueno, A.; Yen, K.E.; Wu, B.; Attar, E.C.; Tallman, M.S.; de Botton, S. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019 Feb 14;133, 676-687. [CrossRef]
- DiNardo, C.D.; Venugopal, S.; Lachowiez, C.; Takahashi, K.; Loghavi, S.; Montalban-Bravo, G.; Wang, X.; Carraway, H.; Sekeres, M.; Sukkur, A.; Hammond, D.; Chien, K.; Maiti, A.; Masarova, L.; Sasaki, K.; Alvarado, Y.; Kadia, T.; Short, N.J.; Daver, N.; Borthakur, G.; Ravandi, F.; Kantarjian, H.M.; Patel, B.; Dezern, A.; Roboz, G.; Garcia-Manero, G. Targeted therapy with the mutant IDH2 inhibitor enasidenib for high-risk IDH2-mutant myelodysplastic syndrome. Blood Adv. 2023 Jun 13;7, 2378-2387. PMCID: PMC10220255. [CrossRef] [PubMed]
- Sallman, D.A.; Komrokji, R.S.; Sweet, K.L.; Mo, Q.; McGraw, K.L.; Duong, V.H.; Zhang, L.; Nardelli, L.A.; Padron, E.; List, A.F.; Lancet, J.E. A phase 2 trial of the oral smoothened inhibitor glasdegib in refractory myelodysplastic syndromes (MDS). Leuk Res. 2019 Jun;81:56-61. PMCID: PMC7787349. [CrossRef] [PubMed]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; Ma, W.W.; Shaik, M.N.; Laird, A.D.; Zeremski, M.; O'Connell, A.; Chan, G.; Heuser, M. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019 Feb;33, 379-389. PMCID: PMC6365492. [CrossRef] [PubMed]
- Garcia-Manero, G.; Fenaux, P.; Al-Kali, A.; Baer, M.R.; Sekeres, M.A.; Roboz, G.J.; Gaidano, G.; Scott, B.L.; Greenberg, P.; Platzbecker, U.; Steensma, D.P.; Kambhampati, S.; Kreuzer, K.A.; Godley, L.A.; Atallah, E.; Collins RJr Kantarjian, H.; Jabbour, E.; Wilhelm, F.E.; Azarnia, N.; Silverman, L.R. ; ONTIME study investigators. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016 Apr;17, 496-508. [CrossRef] [PubMed]
- de Carvalho, L.G.A.; Komoto, T.T.; Moreno, D.A.; Goes, J.V.C.; de Oliveira, R.T.G.; de Lima Melo, M.M.; Roa, M.E.G.V.; Gonçalves, P.G.; Montefusco-Pereira, C.V.; Pinheiro, R.F.; Ribeiro Junior, H.L. USP15-USP7 Axis and UBE2T Differential Expression May Predict Pathogenesis and Poor Prognosis in De Novo Myelodysplastic Neoplasm. Int J Mol Sci. 2023 Jun 13;24, 10058. PMCID: PMC10298103. [CrossRef] [PubMed]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; Cullis, C.A.; Doucette, A.; Garnsey, J.J.; Gaulin, J.L.; Gershman, R.E.; Lublinsky, A.R.; McDonald, A.; Mizutani, H.; Narayanan, U.; Olhava, E.J.; Peluso, S.; Rezaei, M.; Sintchak, M.D.; Talreja, T.; Thomas, M.P.; Traore, T.; Vyskocil, S.; Weatherhead, G.S.; Yu, J.; Zhang, J.; Dick, L.R.; Claiborne, C.F.; Rolfe, M.; Bolen, J.B.; Langston, S.P. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009 Apr 9;458, 732-6. [CrossRef] [PubMed]
- Sekeres, M.A.; Watts, J.; Radinoff, A.; Sangerman, M.A.; Cerrano, M.; Lopez, P.F.; Zeidner, J.F.; Campelo, M.D.; Graux, C.; Liesveld, J.; Selleslag, D.; Tzvetkov, N.; Fram, R.J.; Zhao, D.; Bell, J.; Friedlander, S.; Faller, D.V.; Adès, L. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia. 2021 Jul;35, 2119-2124. Erratum in: Leukemia. 2021 Dec;35, 3637. PMCID: PMC8257476. [CrossRef] [PubMed]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N.; Zeidner, J.F.; Garcia-Manero, G.; Jeyakumar, D.; Komrokji, R.; Lancet, J.; Kantarjian, H.M.; Gu, L.; Zhang, Y.; Tan, A.; Chao, M.; O'Hear, C.; Ramsingh, G.; Lal, I.; Vyas, P.; Daver, N.G. Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study. J Clin Oncol. 2023 May 20;41, 2815-2826. [CrossRef] [PubMed]
- Magrolimab + azacitidine versus azacitidine + placebo in untreated participants with myelodysplastic syndrome (MDS) (ENHANCE). ClinicalTrials.gov identifier: NCT04313881. Updated September 25, 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04313881 (accessed on 1 December 2023).
- Zeidan, A.M.; Giagounidis, A.; Sekeres, M.A.; Xiao, Z.; Sanz, G.F.; Hoef, M.V.; Ma, F.; Hertle, S.; Santini, V. STIMULUS-MDS2 design and rationale: a phase III trial with the anti-TIM-3 sabatolimab (MBG453) + azacitidine in higher risk MDS and CMML-2. Future Oncol. 2023 Mar;19, 631-642. [CrossRef] [PubMed]
- Zeidan, A.M.; Esteve, J.; Giagounidis, A. The STIMULUS program: clinical trials evaluating sabatolimab (MBG453) combination therapy in patients (pts) with higher-risk myelodysplastic syndromes (HR-MDS) or acute myeloid leukemia (AML). Blood. 2020;136(suppl 1)45-46. [CrossRef]
- Maslah, N.; Salomao, N.; Drevon, L.; Verger, E.; Partouche, N.; Ly, P.; Aubin, P.; Naoui, N.; Schlageter, M.H.; Bally, C.; Miekoutima, E.; Rahmé, R.; Lehmann-Che, J.; Ades, L.; Fenaux, P.; Cassinat, B.; Giraudier, S. Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica. 2020 Jun;105, 1539-1551. PMCID: PMC7271596. [CrossRef] [PubMed]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; Puskas, J.; Zhang, L.; Yao, J.; Mo, Q.; Nardelli, L.; Al Ali, N.H.; Padron, E.; Korbel, G.; Attar, E.C.; Kantarjian, H.M.; Lancet, J.E.; Fenaux, P.; List, A.F.; Komrokji, R.S. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J Clin Oncol. 2021 May 10;39, 1584-1594. [CrossRef] [PubMed]
Table 1.
List of common genetic mutations seen in MDS with frequency and selected characteristics [
2,
3,
4,
5,
6,
7].* indicates founding lesion per ICC2022. RS, Ring Sideroblast. CK, complex karytype. AML, acute myeloid leukemia. CMML, Chronic myelomonocytic leukemia. JMML, Juvenile myelomonocytic leukemia.
Table 1.
List of common genetic mutations seen in MDS with frequency and selected characteristics [
2,
3,
4,
5,
6,
7].* indicates founding lesion per ICC2022. RS, Ring Sideroblast. CK, complex karytype. AML, acute myeloid leukemia. CMML, Chronic myelomonocytic leukemia. JMML, Juvenile myelomonocytic leukemia.
Table 2.
MDS subtypes within the WHO5 and ICC 2022 classification systems. Both groups recognize presence of del(5q) and SF3B1 mutation, however WHO5 also recognizes the presence of bilallelic inactivation of TP53 as a distinct subgroup. Both groups take into account the presence of increased blasts. The MDS/AML category within the ICC 2022 schema includes presence of 10% or more blasts without an AML defining genetic lesion such as bZIP CEBPA (which notably is not considered AML defining in WHO5), NPM1 or TP53 [
9,
18].
Table 2.
MDS subtypes within the WHO5 and ICC 2022 classification systems. Both groups recognize presence of del(5q) and SF3B1 mutation, however WHO5 also recognizes the presence of bilallelic inactivation of TP53 as a distinct subgroup. Both groups take into account the presence of increased blasts. The MDS/AML category within the ICC 2022 schema includes presence of 10% or more blasts without an AML defining genetic lesion such as bZIP CEBPA (which notably is not considered AML defining in WHO5), NPM1 or TP53 [
9,
18].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).