1. Introduction
Mangroves are unique wetland forests that thrive in the coastal intertidal zone. Mangroves have long served as natural barriers, defending coastal areas against storms and huge waves. For example, a six-year-old mangrove forest about 100 meters wide can withstand the impact of a super typhoon[
1]. Mangroves have an ecological service value of including preventing coastal erosion, raw material supply, regulating water resources, climate regulation, and air quality maintenance[
2], with an average value of USD 4,185 per hectare per year[
3]. However, with accelerated anthropogenic processes and climate fluctuations, the extent of mangrove range degenerated at a visible rate[
4,
5]. At the same time, mangroves are increasingly recognized for their role in providing food, coastal conservation, biodiversity reserves[
6], and serving as a large carbon bank[
7]. As a result, the number of individuals, organizations and countries involved in the conservation of mangrove forests are increasing, and the efforts in research in the field of mangrove forests are increasing. Therefore, in order to cope with the risk of mangrove degradation and disappearance, artificial intervention is urgent. Meanwhile, in order to meet the afforestation and restoration needs of mangroves, it is necessary to develop the cultivation technology and breeding improvement. Cultivating mangrove seedling is crucial to support mangrove ecosystem protection, management, and recovery activities, as well as for scientifically and judiciously planting mangrove plants to reverse the gradual degradation of ecological function.
The production of mangrove plant germplasm and seedlings is the basis of all mangrove ecological restoration projects[
8]. Mangrove germplasm is the repository of mangrove genetic resources and acts as the guarantor and source of mangrove genetic diversity[
9]. Under natural conditions, the diversity of plant germplasm is generally screened by environmental heterogeneity, resulting in the distribution and abundance of different genotypes[
10,
11]. Mangroves are limited by climatic conditions, possessing thin species resources. Therefore, the conservation of genetic diversity of mangrove species needs to be considered from different regional environments, including provenance, genealogy, or even individual.
Cultivating seedlings is one of the ways of large-scale plant production, playing an important role in the yield and quality of seedlings, germplasm resource preservation, and cost savings. Due to the particularity of mangroves, cultivating mangrove seedlings is a challenging task. The germination, growth, and maturation process of mangrove seeds need to take into account many aspects, including growth media, water salinity, and seed quality[
12,
13]. The growth media is a crucial factor in seedling cultivation, which can provide the basic conditions for seedling growth and development, and address the issue of damage or death due to poor site conditions in the young age. Many studies have shown that different soil substrates have different effects on seedling growth, and overall growth quality[
14,
15,
16,
17]. However, the majority of these studies are found in terrestrial species, but there are few reports on true mangrove species.
Additionally, salinity is also one of the important factors to be considered in mangrove seedling cultivation. Mangroves are renowned plant communities growing in "drinking seawater". Although seawater can produce salt stress on plants, mangrove plants have evolved unique salt management strategies, such as exclude, secete, and accumulate, that are well adapted to the hypersaline environment[
18,
19,
20]. In fact, access to fresh water can also boost mangrove productivity[
21,
22]. Many believe that salt water is an ecological advantage for mangroves, rather than a physiological need[
23,
24,
25]. Contrarily, some people argue that mangroves cannot grow in freshwater for a long time, and that mangrove plants store enough salt in their bodies for growth[
26,
27,
28]. Therefore, the cultivation of mangrove seedlings needs to take into account the salinity of the water.
Kandelia obovata is the most widely distributed typical true mangrove species and is also one of the most commonly used mangrove species in afforestation and restoration[
29,
30]. Therefore, we chose the
K. obovata species, and used nursery experiments to find suitable breeding conditions. In addition, we examined phenotypic differences in the growth of various genealogies and conducted interactive experiments to test whether there is available genetic diversity among different genealogies of the same provenance. Based on the purpose of this study, the results will offer a theoretical and practical foundation for the nursery cultivation technology and germplasm breeding of
K. obovata.
2. Materials and Methods
2.1. Materials and Designs
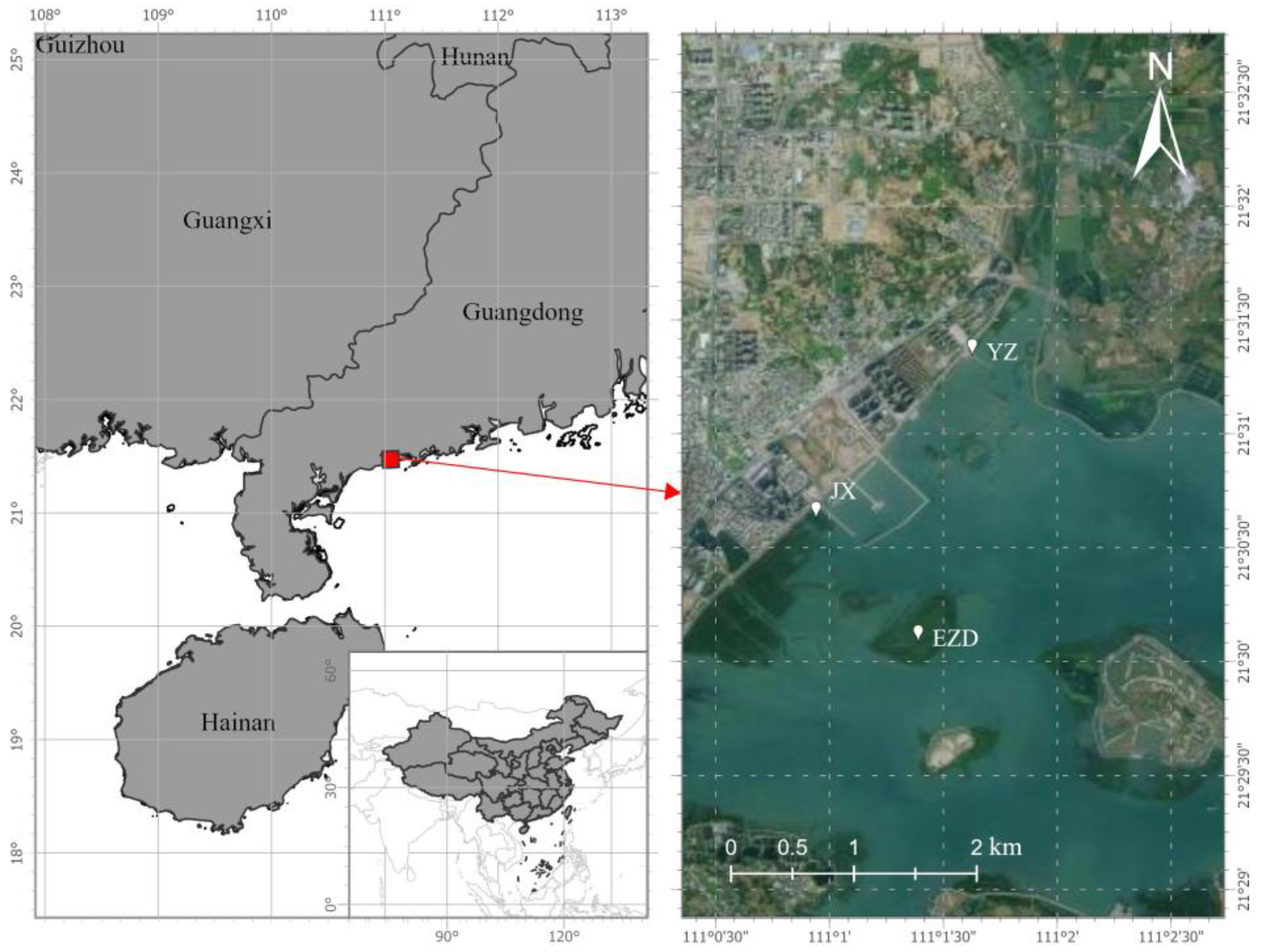
The plant materials originate from Dianbai District, Maoming City, Guangdong Province (
Figure 1, 110°1′37″E, 21°30′11″N), where the salinity ranges around 10.3 ppt, with irregular semidiurnal tides. The climate type falls under the tropical monsoon climate category, featuring an average annual temperature of 23.5°C, a temperature range of 19.6°C annually, and an average annual precipitation of 1909 mm. In March 2022, three representative maternal trees(i.e. three genealogies) from the local
K. obovata populations, named JX, YZ, and EZD, were selected (
Figure 1). Distance over 30 m between these genealogies for ensure genetic independence.
Salt water was prepared by mixing solarized sea salt and fresh water, creating three salinity levels: S1 (0 ppt), S2 (10 ppt), and S3 (20 ppt). Salinity was measured using a pen-type salinometer (Model 5250, China). The salt water was stored in containers, and monitoring occurs weekly. Given that the soil in mangrove growth environments is predominantly sandy or sticky[
31], this experiment focused on five growth media: M1 (100% loess), M2 (100% sandy), M3 (50% loess + 50% sandy), M4 (40% loess + 40% sandy + 20% peat), and M5 (40% loess + 40% sandy + 20% coir).
The study was conducted at the nursery base of Guangxi Forestry Research Institute (108°21′24″E, 22°55′1″N), designated as a dry land facility nursery[
8]. The area experiences a subtropical monsoon climate with abundant light, heat, and year-round rainfall. Throughout the experiment, the average monthly temperature was 22.9°C, the highest monthly temperature reached 36°C, and the lowest monthly temperature was 5°C. Each treatment includes twelve plants, with one propagule inserted into each seedling bag (size 30 × 20 cm), resulting in a total of 540 plants.
2.3. Measurements
2.3.1. Properties of the Media
In May 2022, cutting rings with volume of 100 cm
3 and the weight of W0 (including bottom net and top cover) were used for sampling randomly, and the process was repeated 3 times. Then weights were recorded in the laboratory after soaking for 6 hours (W1), standing for 12 hours (W2), and drying in a 105°C oven (DHG, China) until a constant weight (W3). Soil Bulk Density (BD), total porosity (TP), capillary porosity (CP), and non-capillary porosity (NCP) were calculated using formulas (1-4). The media pH was determined by the potentiometric method, and total N, P, and K were measured using the elemental analyzer (Elementar EL, Germany), colorimetry, and flame photometer (WGH6431, China), respectively. The results are shown in
Table 1.
2.3.2. Seedling Height, Diameter and Mortality Rate
After sowing in April 2022, the seedling height (H) was measured every 30 days to determine the length from the upper end to the top end of the stem. A death plant was recorded if the whole shoot was withered or necrotic. From June, the seedling basal diameter (D) was measured every 30 days from the stem at the upper end of the propagule. The plant was recorded as dead if the stem was total necrotic. The calculation formula for mortality is as follows: mortality (%) = number of dead plants / total number of plants × 100. All the measurements were finished in April 2023. Considering the monthly average temperature changes during various seasons and throughout the experiment, our attention is directed to specific period: T1 (from April 2022 to July 2022), T2 (from July 2022 to October 2022), T3 (from October 2022 to January 2023), and T4 (from January 2023 to April 2023)
2.3.3. Biomass
In April 2023, three plants were randomly selected for each treatment. The attached soil was carefully washed off, and the plants were separated into roots, stems and leaves. These plant parts were placed into envelopes and dried in a 105°C oven for 30 minutes, followed by further drying at 80°C until a constant weight was achieved. After cooling in the oven to room temperature, the dry biomass of plant parts were weigh by 1 / 100 electronic balance.
2.4. Data Processing and Analysis
The fuzzy comprehensive evaluation method of membership index (MI) (4) and Dickson quality index (DQI) (5) were used to evaluate the seedlings comprehensively. The formula for calculating values was as follows:
where X
i represents the determined value of a treatment index; X
min is the minimum value of a specific processing index; and X
max is the maximum value of that processing index (5). Shoot biomass includes the sum of leaf biomass and stem biomass (6). Both values for each treatment index were calculated within groups, and the mean for different treatment groups was then sorted. A higher mean value indicates better performance.
Both the mean and standard error (SE) were calculated. The one-way analysis of variance (ANOVA) was employed to assess statistical significance. In the case of statistically significant main effects, the Tukey test was applied for multiple comparisons. All reported differences were deemed statistically significant at a threshold of p < 0.05. Data collation, statistical analysis, and figure production were conducted using Excel 2016 and SPSS version 26.
3. Results
3.1. Difference of the Media Properties
TP, CP, total N and total P showed no significant difference between M1 and M2. The aeration (NCP) and pH of the medium were significantly increased by M2, while the total K was decreased (p < 0.05) in comparison to M1. Compared with M3, the peat (M4) or coir (M5) additions obviously decreased BD, and M5 had considerably higher porosity levels than M4 and M3.
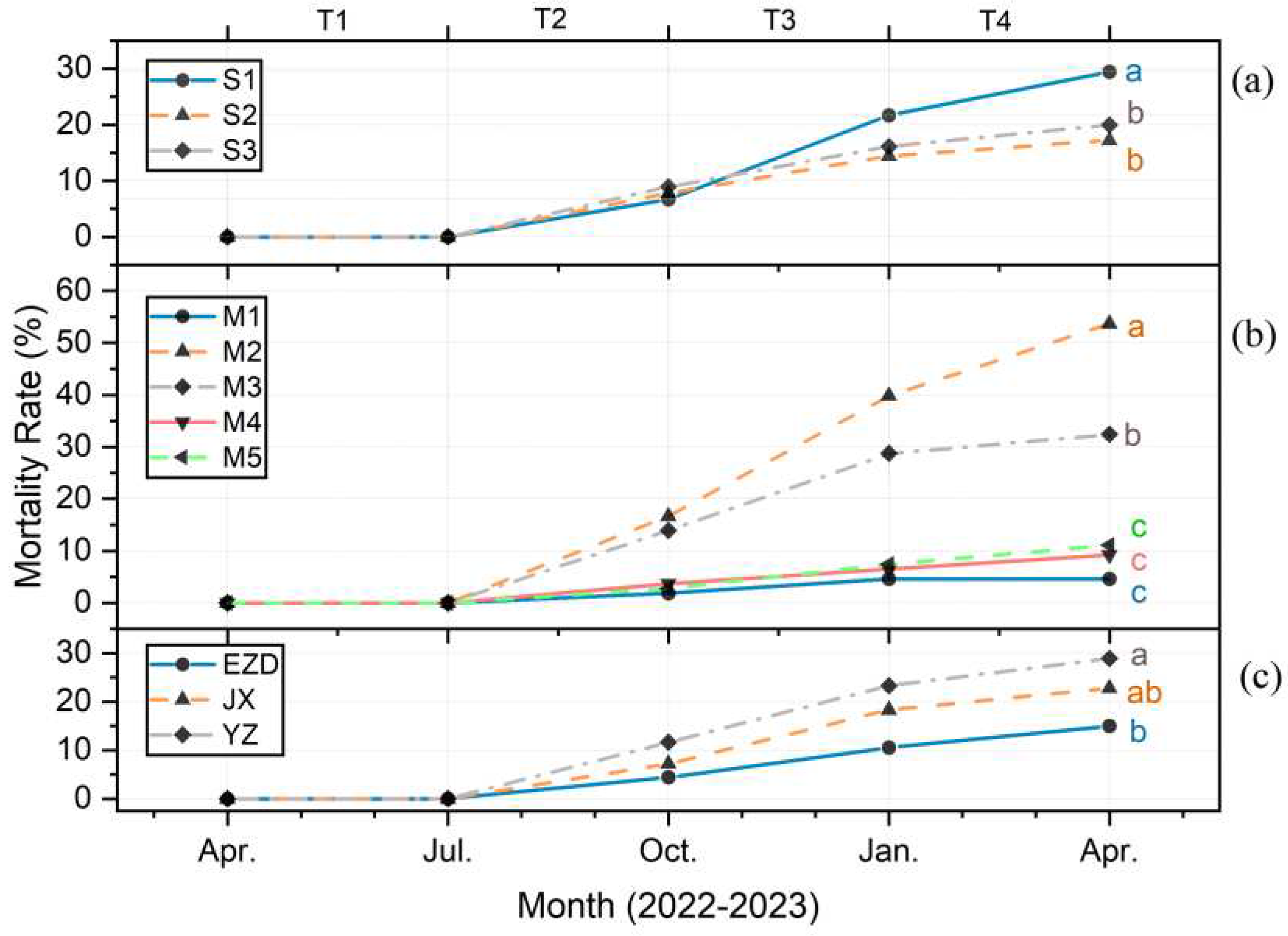
3.2. Changes of Seedling Mortality Rate
Salinity(
Figure 1a), growth media(
Figure 1b), and genealogy(
Figure 1c) were all had significant effect on the mortality rate (
p < 0.05). It can be observed that the death rate increased obviously from July 2022 . After one year of observation, the lowest mortality rate was recorded at on S2 (17.22%± 6.38%), M1 (4.63% ± 2.02% ), and EZD (15.00% ± 5.22%). Conversely, the rate in death increased the most on S1 (29.44% ± 5.07%) , M2 (53.71% ± 3.43%), and YZ (28.89% ± 6.80%). Moreover, salinity between S2 and S3 was a little difference and both were significantly lower than S1. The medium with a high sand concentration(M2, M3) displayed a higher death rate significantly, where adding peat (M4) or coir (M5) obviously reduced the mortality rate. The various in mortality rate between genealogies was relatively stable and followed a specific order (EZD > JX > YZ).
Figure 2.
Variation in salinity (a), growth media (b), and genealogy (c) on the mortality rate of K. obovata seedlings. Different letters indicate significant differences among levels in April, p < 0.05.
Figure 2.
Variation in salinity (a), growth media (b), and genealogy (c) on the mortality rate of K. obovata seedlings. Different letters indicate significant differences among levels in April, p < 0.05.
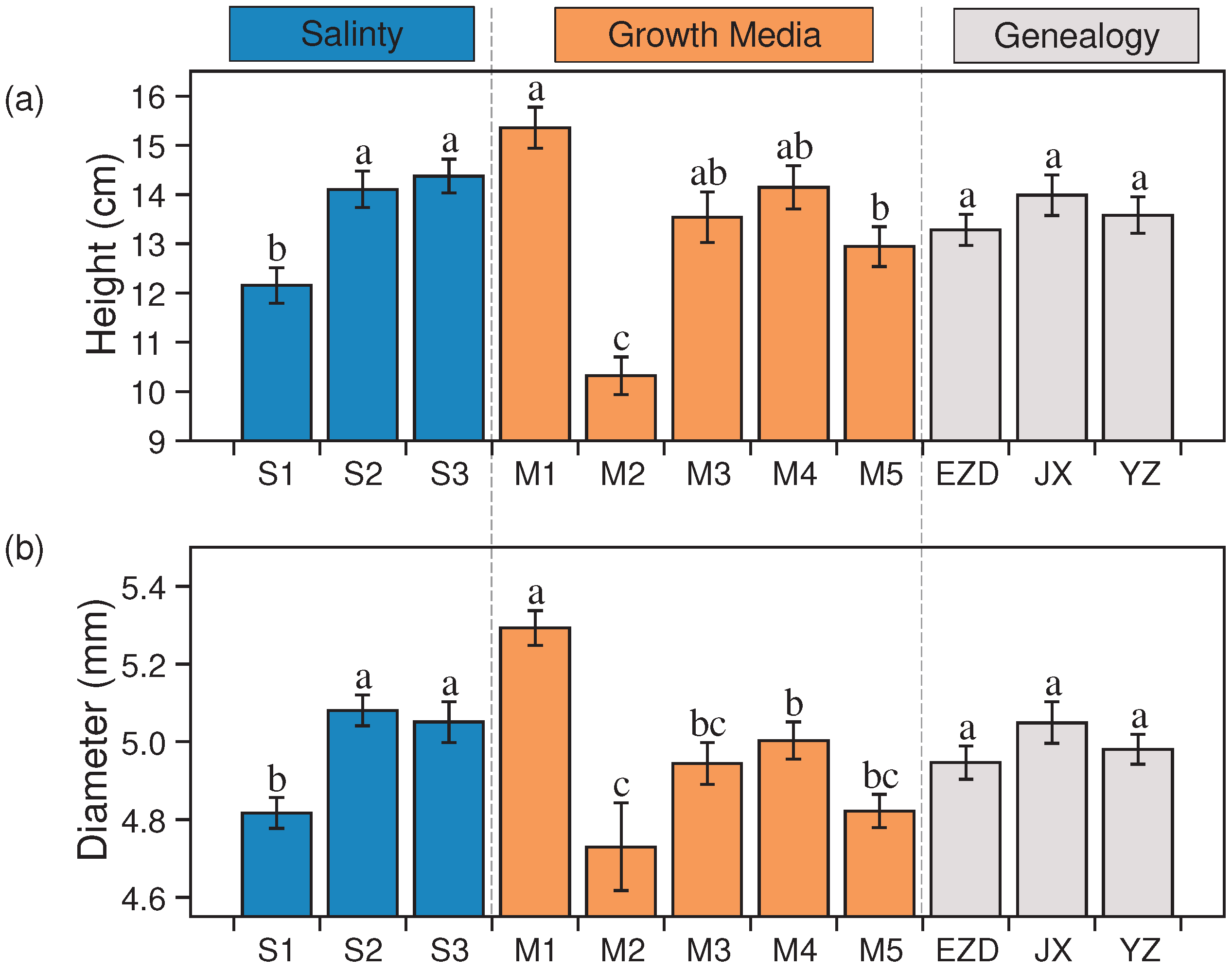
3.3. Seedling Height and Diameter
Variations in growing medium and salinities had significant influence on the height (
Figure 3a) and basal diameter (
Figure 3b) of seedlings. 20 ppt (S3) was the salinity condition that promoted the height, which reached the highest value at 14.37 cm ± 0.34 cm, and basal diameter in 10 ppt (S2) reached the highest value at 5.08 mm ± 0.04 mm. Salt water had more advantages than fresh water (S2>S3>S1). Media had a greater effect on seedling growth (
Table 4). Loess (M1) was the medium that increased seedling height the most (15.35 cm ± 0.42 cm), ordered by M1>M4>M3>M5>M2. Similar variations existed for basal diameter (5.29 mm ± 0.04 mm; M1>M4>M3>M5>M2). JX was the genealogy that grew the best, and the height and diameter were 13.98 cm ± 0.41 cm and 5.05 mm ± 0.63 mm, respectively. However, the genealogy effect on seedling height and diameter was not obvious (
p > 0.05).
In the univariate analysis, both salinity and growth media demonstrated highly significant effects on the seedling height and basal diameter of
K. obovata (
Table 2), and the influence of growth media was higher than sailnity and genealogy, which consistently observed across different seasons. While genealogy only had a significant effect during the early stages of growth. In the context of multi-factor interaction, the interaction between salinity and growth media exerted the most significant effect on seedling height, while the interaction between salinity and genealogy had the greatest impact on basal diameter. This pattern remained consistent across seasonal observations. Seedling height showed significant differences in the interaction of salinity, media and genealogy at T2, T3 and T4, but not at T1, which was the opposite of basal diameter. Besides, interactions with growth media primarily influenced seedling height, while interactions with genealogy predominantly affected basal diameter.
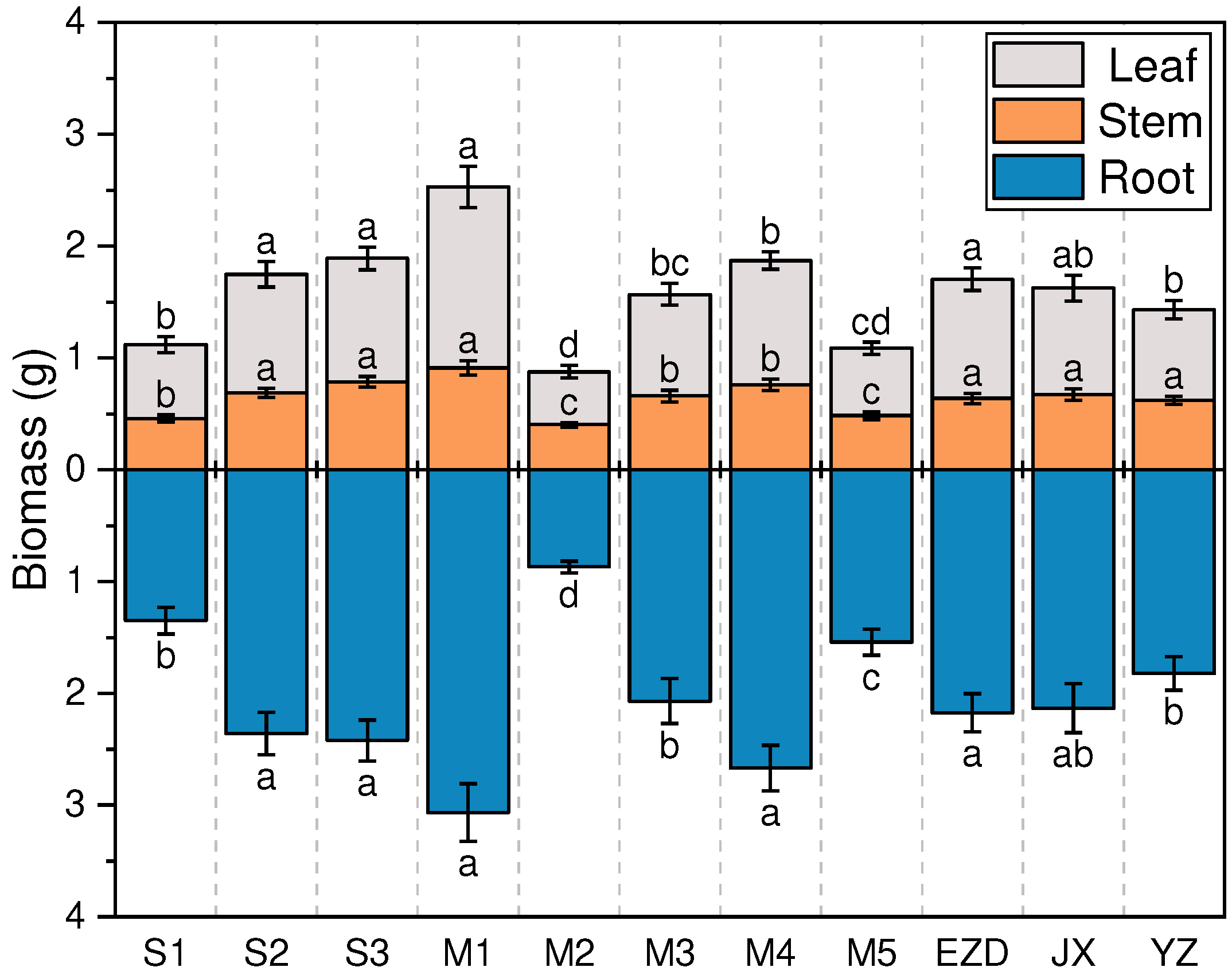
3.4. Biomass Accumulation
The total biomass was affect by salinity, growth media, and genealogy, and reached the highest value at S3 (4.31 ± 0.30 g), M1 (5.59 ± 0.47 g), and EZD (3.87 ± 0.28 g), respectively (
Figure 4). The biomass of roots, stems, and leaves exhibited increased with rising salinity. Biomass in S2 and S3 were significant higher compared with S1. Under the influenced of various media, the biomass of
K. obovata seedlings followed the order M2, M5, M3, M4, and M1 in ascending order, consistently observed across different plant parts. Genealogies between EZD and YZ exhibited significant differences in the biomass of roots and leaves (order: EZD > JX > YZ), while stem biomass did not differ significantly.
In the analysis of one-way ANOVA, salinity exhibited the most significant impact on stem biomass accumulation (
F = 38.60), while growth media exerted the greatest influence on root(
F = 48.93), leaf(
F = 27.18), and total biomass(
F = 54.75) accumulation (
Table 4). The interaction between salinity and growth media attained the highest value of
F, and this consistency was observed across all parts of the biomass. Salinity and growth media emerged as the primary factors influencing the biomass accumulation of
K. obovata in multifactor interaction. There was no significant effect of three-factor interaction on the biomass accumulation of
K. obovata.
3.5. Evaluate the Influence of Growth Media, Salinity, Genealogy, and Their Combination
The MI and DQI were employed to assess the seedling effect of each treatment combination, and a similar pattern was observed for the evaluation results (
Table 5). In terms of fuzzy evaluation (MI), S3, M1, and EZD obtained the highest values of 0.516, 0.688, and 0.459, respectively The combinations of S2-M1 and S3-M1-JX recorded the highest values of 0.862 and 0.932. Similarly, in quality evaluation (DQI), S3, M1, and EZD also displayed the highest values of 1.179, 1.478, and 1.089, respectively. And the combinations of S2-M1 (1.893) and S2-M1-JX (2.123) achieved the highest values. These results suggest that S3, M1, and EZD have consistently excelled in both relative and absolute evaluation measures. Furthermore, the combinations involving these treatments also yielded highly favorable results.
4. Discussion
Seawater is a special component of the mangrove habitat, with fluctuating salinity levels that influence the growth and development of mangrove plants. While the debate continues regarding whether mangrove plants require or tolerate salt, salinity has undoubtedly emerged as a key factor in cultivating mangrove plants. Earlier investigations have indicated that the optimal salinity for mangrove plants, including
K. obovata and
Avicennia marina, is 50% or less of the standard seawater [
32,
33,
34]. In this study, saltwater proved beneficial in reducing the death of seedlings, promoting diameter growth, and enhancing biomass accumulation. This suggests that the growth of
K. obovata seedlings in a freshwater environment was inferior to that in a saltwater environment. Studies by Lv et al. [
35], and Liao[
36] examining the salinity adaptability of
K. obovata suggested that saltwater with a salinity of 10 ppt was more conducive to the growth of
K. obovata, aligning with the findings of this study. Concurrently, ANOVA analysis suggested that salinity had a high influence on seedling growth and biomass accumulation. Additionally, we found the death of seedlings was increasing rapidly during T3 (October to January). It may be caused by drop in temperature. Mangrove plants are sensitive to temperature, especially at low temperatures[
37]. The survival rate of
K. obovata seedlings, originating from a similar latitude, gradually decreased at 5°C[
38]. Throughout this study, the average monthly low temperature experienced a rapid decrease after October. Consequently, the mortality rate of
K. obovata seedlings under freshwater irrigation increased swiftly with the temperature decrease, significantly surpassing that under saltwater treatment. Hence, saltwater irrigation proved beneficial in enhancing the survival rate of
K. obovata seedlings.
Growth media plays a pivotal role in plant seedling growth, and an appropriate media can furnish container seedlings with an optimal growth environment characterized [
39]. In this study, significant differences among growth media were observed in growing of
K.
obovata seedlings. Seedlings exhibited a low death rate and positive growth in loess but the opposite in sandy. However, previous studies have suggested that soil with a high sandy content enhances the growth and development of
K.
obovata [
40,
41,
42]. This difference may be attributed to various water levels. We infer that sandy soil is more suitable for cultivation in a soaking environment, because soil media with high concents of sand exhibit high non-capillary porosity. Furthermore, the incorporation of a organic media decreased soil bulk density and reduced the death of
K.
obovata seedlings. Regarding growth and biomass, the growth performance of seedlings in peat-containing growth media outperforms that of seedlings in coir.. Peat has higher mineral nutrient content [
43], and peat can be recommended as the preferred choice for cultivating
K. obovata with an organic media. Moreover, being grown in a freshwater environment, M4 seedlings possess the highest quality available. It may be attributed to the beneficial effects of nutrient additions, such as soil fertilizer, on the growth of mangrove plants in freshwater[
44]. Consequently, in the process of raising
K. obovata seedlings, an appropriate growth media can be selected based on diverse conditions and requirements.
Varied populations, encompassing provenances and genealogies, contribute to differences in the growth and development of seeds and seedlings. These variations are typically influenced by genotype, environmental conditions, and other factors. Previous studies have revealed substantial differences in phenotypes among various provenances of
K. obovata [
13,
45]. In this study, the mortality rate of various genealogies rises, displaying gradually distinctions among genealogies. Distinct genealogies exhibit varied advantages in basal diameter, seedling height, and biomass, indicating the potential for germplasm mining within
K. obovata genealogies. Based on the evaluation results, EZD and JX are superior in growing, possibly because they have stronger parent trees. Additionally, plants may exhibit varying salt tolerance across different provenances [
46,
47]. In this study, the membership value of genealogy JX is highest at 20 ppt saltwater, differing from that of genealogies EZD and YZ at 10 ppt salinity. This suggests potential genetic differences in salinity adaptability among different genealogies. Three genealogies of
K. obovata seedlings, on the other hand, were thought to come from the same provenance and have similar initial environmental conditions; all plants flourished in a uniform garden setting. Therefore, the differences in their growth primarily stem from genetic variations among the original genealogy and the interactions between these genetic factors and their growth environment [
48]. In summary, when selecting suitable germplasm resources for cultivation, it is essential to consider not only the impact of environmental heterogeneity but also the diversity of genetic factors among individuals.
5. Conclusion
Our study evaluated the strategy of cultivating K. obovata seedlings from the aspects of water salinity, growth medium, and genealogies in order to obtain high-growth and high-quality seedlings. All growth indexes and evaluation results showed that (1) seedlings with 10 ppt or 20 ppt salt water were significantly better than fresh water (0 ppt); (2) The medium of loess produced the best seedlings, though peat added was also a good option; (3) EZD was the genealogy growing the best, followed by JX. Therefore, we recommend using loess as a growth medium (or added peat) in cultivating K. obovata, while fresh water and pure sand should be avoided. In additions, it is imperative that we also emphasize the collection and development of genealogy resources. Generally speaking, these results can be used for mangrove nursery cultivation, providing a theoretical reference for the artificial cultivation of K. obovata and aiding in the identification of potentially valuable germplasm resources.
Author Contributions
Conceptualization, J.Z., J.Y., X.L. and P.W.; methodology, J.Z., J.Y., J.Q., J.H., X.L. and P.W.; software, J.Y. and J.Y.; validation, J.Z., J.Y., X.L. and P.W.; investigation, J.Z., J.Y., J.Q., and J.H.; resources, P.W.and X.L.; data curation, J.Z., J.Y. and J.Q.; writing—original draft preparation, J.Z.; writing—review and editing, J.Z., J.Y., X.L. and P.W.; visualization, J.Z.; supervision, J.Y. X.L. and P.W.; project administration, J.Z., J.Y., J.Q., J.H., X.L.and P.W.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangxi Forestry Science and Technology Project (GLKY [2021]1).
Data Availability Statement
Not applicable.
Acknowledgments
The authors truly thankful the members of Mangrove and Wetland Research Team, Guangxi Forestry Research Institute for measuring and collecting experimental data.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Zhang, X.X.; Lin, P.Z.; Chen, X.P. Coastal Protection by Planted Mangrove Forest during Typhoon Mangkhut. J Mar Sci Eng 2022, 10. [CrossRef]
- Lin, C.Y.; Fu, C.Y.; Liu, Y.; Zhang, M.Q.; Liu, Y.; Wu, W.Y.; Wang, L.X.; Lin, X.H.; Fu, X.M. Assessing the changes of the monetary value of mangrove ecosystem services in China and its application. Front Env Sci-Switz 2022, 10. [CrossRef]
- Brander, L.M.; Wagtendonk, A.J.; Hussain, S.S.; McVittie, A.; Verburg, P.H.; de Groot, R.S.; van der Ploeg, S. Ecosystem service values for mangroves in Southeast Asia: A meta-analysis and value transfer application. Ecosyst Serv 2012, 1, 62-69. [CrossRef]
- Fan, H.Q.; Wang, W.Q. Some Thematic Issues for Mangrove Conservation in China. Journal of Xiamen University. Natural Science 2017, 56, 323-330.
- Wong, W.Y.; Al-Ani, A.K.I.; Hasikin, K.; Khairuddin, A.S.M.; Razak, S.A.; Hizaddin, H.F.; Mokhtar, M.I.; Azizan, M.M. Water, Soil and Air Pollutants’ Interaction on Mangrove Ecosystem and Corresponding Artificial Intelligence Techniques Used in Decision Support Systems - A Review. Ieee Access 2021, 9, 105532-105563. [CrossRef]
- Malik, A.; Fensholt, R.; Mertz, O. Mangrove exploitation effects on biodiversity and ecosystem services. Biodivers Conserv 2015, 24, 3543-3557. [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 2011, 4. [CrossRef]
- Hu, H.Y.; Chen, S.Y.; Wang, W.Q.; Dong, K.Z.; Lin, G.H. Current status of mangrove germplasm resources and key techniques for mangrove seedling propagation in China. Chin J Appl Ecol 2012, 23, 939-946.
- Gu, W.C.; Li, W.Y. Analysis and Suggestions of Benefit Sharing Policies Relating to Forest Tree Germplasm Resources in China. World Forestry Research 2007, 20, 66-69.
- Kordrostami, M.; Rahimi, M. Molecular markers in plants: Concepts and applications. Genetics in the Third Millennium 2015, 13, 4024-4031.
- Rossetto, M.; Rymer, P.D. Applications of Molecular Markers in Plant Conservation. In Molecular Markers in Plants, John Wiley & Sons, Ltd: New York, USA, 2012; pp 81-98.
- Budiadi, B.; Widiyatno, W.; Nurjanto, H.H.; Hasani, H.; Jihad, A.N. Seedling Growth and Quality of Avicennia marina (Forssk.) Vierh. under Growth Media Composition and Controlled Salinity in an Ex Situ Nursery. Forests 2022, 13, 684. [CrossRef]
- Yang, S.; Liu, X.; Deng, R.; Chen, Q.; Wang, J.; Lu, X. Geographic variations of hypocotyl and seedling growth traits for Kandelia obovata with different provenances. Chinese Journal of Ecology 2020, 39, 1769-1777.
- Hong, Z.; Liu, X.; Zhang, N.; Yang, Z.; Cui, Z.; Xu, D. Effects of different mediums and container sizes on the growth performance of Dalbergia odorifera at seedling and early afforestation stages. Journal of Central South University of Forestry & Technology 2022, 42, 22-29, 38. [CrossRef]
- Li, X.J.; Long, Z.W.; She, Z.L.; Shi, F.H.; Shen, Y.B. Effects of Substrate Composition and Container Size on Growth of Container Seedlings of Tilia miquelianal. Journal of Northeast Forestry University 2023, 51, 46-52.
- Liu, B.E.; Liao, B.W.; Han, J. Effect of Different Substrates on Seedlings Growth and Physiological and Biochemical Indices of P.pinnata and H.littoralis. Journal of Anhui Agricultural Sciences 2011, 39, 3449-3453. [CrossRef]
- Liu, Z.X.; Li, R.S.; Zou, W.T.; Qiu, Z.F.; Yu, N.; Yang, J.C. Transplantation Effects of Different Nursery Substrates on the Growth of Tissue Culture Seedlings of Mytilaria laosensis. Chinese Journal of Tropical Crops 2020, 41, 655-660. [CrossRef]
- Hanagata, N.; Takemura, T.; Karube, I.; Dubinsky, Z. Salt water relationships in mangroves. Isr J Plant Sci 1999, 47, 63-76. [CrossRef]
- Parida, A.K.; Jha, B. Salt tolerance mechanisms in mangroves: a review. Trees (Berlin, West) 2010, 24, 199-217. [CrossRef]
- Reef, R.; Lovelock, C.E. Regulation of water balance in mangroves. Ann Bot-London 2015, 115, 385-395. [CrossRef]
- Hayes, M.A.; Jesse, A.; Welti, N.; Tabet, B.; Lockington, D.; Lovelock, C.E. Groundwater enhances above-ground growth in mangroves. The Journal of Ecology 2019, 107, 1120-1128. [CrossRef]
- Santini, N.S.; Reef, R.; Lockington, D.A.; Lovelock, C.E. use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiologia 2015, 745, 59-68. [CrossRef]
- Mitsch, W.; Gosselink, J. Wetlands, 3rd edition. John Wiley & Sons Inc: New York, USA, 2000; p 335-373.
- Saenger, P. Mangrove Ecology, Silviculture and Conservation. Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; p 11-18.
- Suárez, N.; Medina, E. Influence of salinity on Na+ and K+ accumulation, and gas exchange in Avicennia germinans. Photosynthetica 2006, 44, 268-274. [CrossRef]
- Ball, M.C. Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees (Berlin, West) 2002, 16, 126-139. [CrossRef]
- Xin, C. Mangroves Distribution Pattern along the River and the Adapted Mechanism to Salt Water and Fresh Water Rotation. Master Type, Xiamen University, 2008.
- Werner, A.; Stelzer, R. Physiological responses of the mangrove Rhizophora mangle grown in the absence and presence of NaCl. Plant, Cell and Environment 1990, 13, 243-255. [CrossRef]
- Wu, W.Z.; Zhao, Z.X.; Yang, S.; Liang, L.C.; Chen, Q.X.; Lu, X.; Liu, X.; Zhang, X.W. The mangrove forest distribution and analysis of afforestation effect in Zhejiang Province. Journal of Tropical Oceanography 2022, 41, 67-74.
- Zhao, Y.Z.; Zhong, Y.F.; Ye, C.T.; Liang, P.P.; Pan, X.B.; Zhang, Y.Y.; Zhang, Y.H.; Shen, Y.J. Multi-omics analyses on Kandelia obovata reveal its response to transplanting and genetic differentiation among populations. Bmc Plant Biol 2021, 21. [CrossRef]
- Li, M.; Guan, W.; Jiang, Z.; Liao, B.; Chen, Y. Dynamics of Plant Community Structures and Soil Properties in Artificial Mangrove Area of Nansha Wetlands in Guangzhou. Wetland Science 2023, 21, 716-722. [CrossRef]
- Hutahaean, E.E.; Kusmana, C.; Dewi, H.R. Studi Kemampuan Tumbuh Anakan Mangrove Jenis Rhizophora mucronata, Bruguiera gimnorrhiza, dan Avicennia Marina Pada Berbagai Tingkat Salinitas. J Manaj Hutan Tropik 1999, 5, 77-85.
- Clough, B.F. Growth and Salt Balance of the Mangroves Avicennia marina (Forsk.) Vierh. And Rhizophora stylosa Griff. In Relation to Salinity. Funct Plant Biol 1984, 11, 419-430. [CrossRef]
- Chang, Y.S.; Peng, L.; Tsuneo, N. Influence of Soil Particle on Salt Effect of Kandelia candel Seedling. Journal of Natural Science Xiamen 2002, 41, 559-564. [CrossRef]
- Lv, X.; Li, D.; Yang, X.; Zhang, M.; Deng, Q. Leaf Enzyme and Plant Productivity Responses to Environmental Stress Associated with Sea Level Rise in Two Asian Mangrove Species. Forests 2019, 10, 250. [CrossRef]
- Liao, B. The Adaptability of Seedlings of Three Mangrove Species to Tide-flooding and Water Salinity. Doctor Type, Chinese Academy of Forestry, 2010.
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr Clim Change Rep 2015, 1, 30-39. [CrossRef]
- Lu, W. Experimental Study on Cultivation of Viviparous Seedlings of Mangrove Plant Kandelia obovata. Master Type, Guangdong Ocean University, 2019.
- Zhou, X.H.; Li, Y.Q.; Xiao, Z.Y.; Sun, J.J. Influences of Growth Substrate Ratio,Container Size and SRF on Cunninghamia lanceolata(Lamb.)Hook Container Seedling. Acta Agriculturae Universitatis Jiangxiensis 2017, 39, 72-81.
- Chou, J.B.; Chen, S.B.; Huang, L.; Zheng, C.F.; Chi, W.; Xie, Q.L.; Li, S.L.; Zeng, G.Q. Effects of Different Soils on Hypocotyles Grow th of Kandelia candel (L.)Druce. Journal of Anhui Agricultural Sciences 2010, 38, 17514-17517.
- Ye, Y.; Tan, F.Y.; Lu, C.Y. Effects of soil texture and light on growth and physiology parameters in Kandelia candel. Chinese Journal of Plant Ecology 2001, 42-49.
- He, Q.F.; Peng, Y.H.; Liu, X.; Jiang, Y.; Lu, G.D. Experiment on hypocotyle seedling growth of Kandelia candel in controlled habitats. China Forestry Science and Technology 2014, 28, 108-110.
- Liu, Q.C.; Ling, W.K.; Xiang, Z.Q.; Hua, L.Q. Test on The Physical and Chemical Characteristics of Several Organic Materials And Compare to The Traditional Peat Moss Cultural Media. Northern Horticulture 2007, 7, 37-39.
- Auni, A.H.; Bachtiar, B.; Paembonan, S.A.; Larekeng, S.H. Growth analysis of mangrove (Rhizophora apiculata bl) propagule toward differences in types of water and planting media at Makassar mangrove center. Iop Conference Series. Earth and Environmental Science 2020, 575, 12137. [CrossRef]
- Deng, R. Studies on Physiological Response of Kandelia obovata for Natural and Artificial Cooling. Master Type, Zhejiang Agriculture & Forest University, 2018.
- Ouahzizi, B.; Elbouny, H.; Sellam, K.; Alem, C.; Bakali, A.H. Effects of temperature, provenance, drought stress and salinity on seed germination response and early seedling stage of Thymus atlanticus (Ball) Roussine. J Appl Res Med Aroma 2023, 34, 100482. [CrossRef]
- Qi, W.W.; Ma, H.Y.; Li, S.Y.; Wu, H.T.; Zhao, D.D. Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances. Biology 2023, 12, 1343. [CrossRef]
- Saenger, P.; West, P.W. Phenotypic variation of the mangrove species Avicennia marina (Forssk.) Vierh. from seven provenances around Australia. Aquat Bot 2018, 149, 28-32. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).