4. Conclusion:
Cleft lip and/or palate are complex congenital malformations influenced by a combination of genetic, environmental, and teratogenic factors. Understanding the characteristics of these factors is essential for both prevention and treatment strategies. This comprehensive analysis of cases and literature contributes to our knowledge of the etiology of CL/P, facilitating better clinical management and genetic counseling for affected individuals and their families. Further research is warranted to uncover additional insights into the multifaceted nature of CL/P.
Clefts in the oral and facial region can be classified into two main categories: syndromic clefts and isolated clefts. Syndromic clefts, which encompass over 400 different types, are primarily attributed to genetic, chromosomal, and teratogenic abnormalities. They are often associated with additional congenital defects and physical anomalies. Common genetic causes of syndromic clefts include Van der Woude syndrome, Velocardiofacial syndrome, 22q11.2 Deletion Syndrome, Pierre Robin Syndrome, and related syndromes.[
1]
Isolated clefts, constituting approximately 70% of cleft lip and palate (CL/P) cases and around 50% of cleft palate (CP) cases, exhibit distinct morphological features. Their development is influenced by a combination of endogenous and exogenous factors [
2,
3].
The prevalence of clefts varies across different races and geographic regions, with occurrences ranging from 1 in 500 among Asians and Native Americans to 1 in 1,000 in Caucasian, Spanish, and Latino populations. The lowest incidence is observed among African populations at 1 in 2,500.[
4] Worldwide, CL/P is the most common congenital facial anomaly, with a frequency of 7.94 per 10,000 live births [
5] ]. In Poland, it is among the most frequent congenital defects, affecting approximately 10 in 10,000 births. [
6]
Clefts show a gender-based distribution, being more prevalent in males. Specifically, cleft lip occurs in 59.5% of male and 40.5% of female cases. For cleft palate, the prevalence is 57.62% in males and 38.58% in females. When considering cleft lip and palate combined, the occurrence is 42.38% in males and 29.52% in females. Moreover, isolated cleft lip (without cleft palate) occurs in 61.12% of males, with a significantly lower prevalence of 2.52% in females. It's also noted that cleft lip more commonly occurs on the left side, with a prevalence of 61.2% in males and 38.8% in females. Furthermore, approximately 50% of patients with cleft lip also have a cleft palate, attributed to issues in facial element fusion before palate formation. [
7].
Furthermore, in 50% of patients with cleft lip, a cleft palate is also observed due to the lack of fusion of facial elements before palate formation. Etiological factors for cleft lip and palate can be divided into the following groups: non-genetic factors, which include various environmental factors, e.g. smoking, drinking alcohol and genetic factors when the cleft is associated with other malformations and when the cleft occurs as an isolated feature. [
8] Pathogenic variants causing clefts to have been identified in many different genes. A correlation between phenotype and type of mutation has been found. Treatment of patients is adapted to age and must be provided at the right time. Usually, patients require support from many different specialists: otolaryngologist, geneticist, speech pathologist, orthodontist. Most researchers consider that clefts are caused by a combination of genetic and environmental factors. Several genes have been identified whose mutations can lead to the development of non-syndromic cleft lip palate (
Table 1). A few syndromes in which cleft lip and/or palate occur have been described (Table2).The latest research suggests that three types of clefts—cleft lip, cleft palate, and cleft lip and palate—may have different etiological sources, although the molecular dynamics behind these variations remain unclear. [
9] Scientists confirm that different DNA methylation patterns during embryonic development can influence the occurrence of clefts. [
10] The analysis of blood samples from children with non-syndromic cleft lip, cleft palate, and cleft lip and palate has revealed gene sequences previously associated with facial clefts, as well as 250 new sequences with various methylation patterns occurring during embryonic development.[
11]
These findings support the hypothesis that the three subtypes may have different etiological sources. [
12] In addition to evidence supporting the role of genetics in the etiology of clefts, scientific research and epidemiological data indicate that environmental risk factors (such as smoking, alcohol consumption, malnutrition during pregnancy, viral infections, teratogenic drugs, folate deficiency, and body mass) significantly influence the complex embryonic development that determines congenital anomalies.[
12,
2,
13,
14]
Infants with cleft lip and palate experience difficulties in speech, feeding, hearing, and cosmetic and psychological issues. They require multidisciplinary healthcare both in the short and long term. Moreover, these infants exhibit higher mortality rates in the early years of life, up to the age of 55, and an increased risk of developing cancer. Embryologically, the face derives from five processes, namely the frontonasal process, and the two maxillary and mandibular processes. The frontonasal process originates from the medial and lateral nasal processes, which surround the bilateral nasal pits. Between the 4th and 12th weeks of fetal life, the lip and palate develop through the migration of cells originating from the neural crest to the first pharyngeal arch. As a result of complex cell differentiation processes, the fusion of the nasal placodes with the maxillary process forms the upper lip and primary palate. Subsequent elongation and thickening of the palatal processes lead to their fusion and differentiation into bone and muscle tissue, forming the hard and soft palate. Failure of fusion between the maxillary processes and nasal processes leads to cleft lip (CL), while failure of fusion of the palatal processes results in cleft palate (CP).[
15]
Depending on the location of the cleft, two main categories are distinguished: cleft lip with or without cleft palate (CL/P) and cleft palate (CP), unilateral or bilateral. Additionally, various subtypes of clefts exist. Various forms of pictographic notation have been developed to depict the precise anatomical involvement and its severity. The most popular of these diagrams was the "Y" diagram, developed by Kernahan. It was widely used during the era of paper-based medical records, but its use has significantly decreased with the advent of electronic health records. Examples of various forms of pictographic notation are available.
The most widely used system for classifying cleft phenotypes is the LAHSHAL Cleft Classification. Thank you for providing context for "LAHSHAL." It seems to represent anatomical structures progressing from the right side of the patient to the left side. Here is the layout:
- -
L: Left lip
- -
A: Left alveolus (left tooth ridge)
- -
H: Left hard palate
- -
S: Soft palate midline
- -
H: Right hard palate
- -
A: Right alveolus (right tooth ridge)
- -
L: Right lip
This notation is commonly used to describe and classify the specific involvement of cleft structures in individuals with cleft lip and palate conditions.
"LAHSHAL" is indeed an abbreviation or notation used to represent the sequence of anatomical structures in the oral cavity and palate from the right side to the left side of the patient. Further modifications to the LAHSHAL system have been made to allow for the marking of the skin bridge (sometimes called the Simonart's band) that occurs in a complete cleft lip using a plus sign (+) in the L column if the skin bridge exists.
The LAHSHAL system can describe over 12,000 combinations (pertaining to anatomy and severity) of cleft lip and palate, making it extremely detailed. Despite its conciseness, which makes it useful in computing, its steep learning curve limits its practical utility.. [
16]
4.1. Aim of Study
Identifying and understanding associated risk factors will guide prevention and/or prenatal
detection for early intervention and better health outcomes; and identifying and understanding genetic mechanisms will lead to the development of clinical protocols for surgery and rehabilitation of children with cleft palate. To contribute to personalized therapies, postoperative rehabilitation thus improving the overall health of children with cleft palate and their families. Expand the scope of research to include a thorough evaluation of the origins of patients' families, considering factors like ethnicity, genetic history, and socio-economic backgrounds. This comprehensive analysis will help in identifying specific risk factors associated with different groups, enabling more targeted prevention strategies.
Incorporate an assessment of the educational background and age of patients and their families. Understanding the educational level can assist in developing more effective communication and educational materials for families, tailored to their comprehension levels. Age-specific strategies can also be devised for early intervention and education about the condition. Conduct gender-specific studies to understand if there are any differences in the incidence, severity, or outcomes of cleft palate between male and female patients. This can lead to more personalized treatment and intervention plans. Intensify research into the genetic mechanisms of cleft palate to develop personalized clinical protocols. This would involve not just surgery, but also comprehensive rehabilitation programs tailored to the individual genetic makeup of the child. Focus on developing advanced postoperative rehabilitation methods. This includes physical therapies, speech and language therapy, and psychological support, customized to the needs of each child. Recognize the impact of a child's condition on their family. Develop support systems and resources for families, addressing their mental, emotional, and financial needs, thereby improving the overall well-being of both the child and their family. Aim to not only treat the cleft palate but also to ensure the overall long-term health and quality of life of the child. This includes regular follow-ups, addressing any associated health issues, and ensuring their integration into society.
Build collaborative networks with healthcare providers, educators, and community organizations to ensure a holistic approach to the treatment and support of children with cleft palate and their families. By broadening the scope of these objectives, the initiative can provide a more holistic, personalized, and effective approach to the treatment and care of children with cleft palate, considering their diverse backgrounds and specific needs.
4.2. Data and Data Preparation
Participants were recruited during their hospital visit at the Department of Head and Neck Surgery Clinic for Children and Young Adults, Department of Clinical Pediatrics, University of Warmia and Mazury, Olsztyn. The appropriate consent/assent forms were collected. The potential participants, patient’s representatives, were also contacted remotely by telephone to introduce the retrospective study. Once eligible participants provide verbal consent to participate, the researcher either collects data face-to-face or remotely. The study included children whose parents or legal caregivers, after reading the aim, scope, and course of the study, provided written informed consent to conduct study procedures and to process the personal data of the child in accordance with the Ordinance of the European Parliament and of the Council of EU of April 27, 2016, on the protection of individuals. The study was approved by the Ethics Committee of the Academy of Physical Education, Katowice, Poland; (ID KBE- 1/2021/28/10/21; date of approval: 28.10.2021) and was conducted in accordance with the Declaration of Helsinki of the World Medical Association concerning ethical procedures for medical studies involving human participants.
The data collected in case report forms were entered in a secure electronic database. Descriptive statistics were used to summarize relevant data regarding frequency, risk factors, interventions, and status of children with cleft palate.
4.3. Material
The initial sample of 103 children were reduced by 18 individuals due to missing data, which included: 7 birth weights, 5 orders of a live child born, 2 histories of cleft in any of parents, 11 education levels of mother, and 1 cleft record. The remaining data includes 36 girls and 46 boys, 14 children being born third or higher in order, 13 children having small or very small body mass (<2500g).
4.4. Statistical Methods
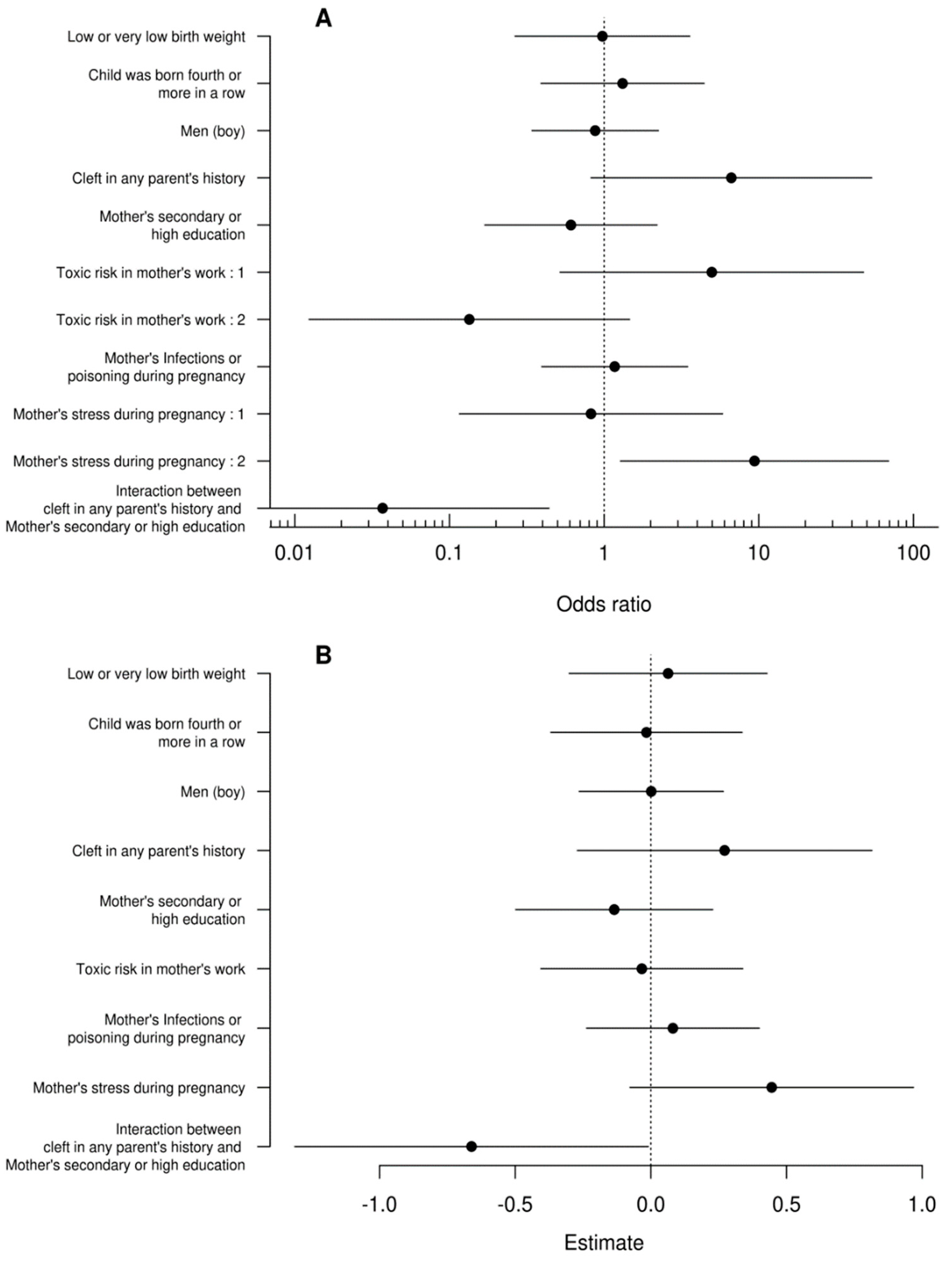
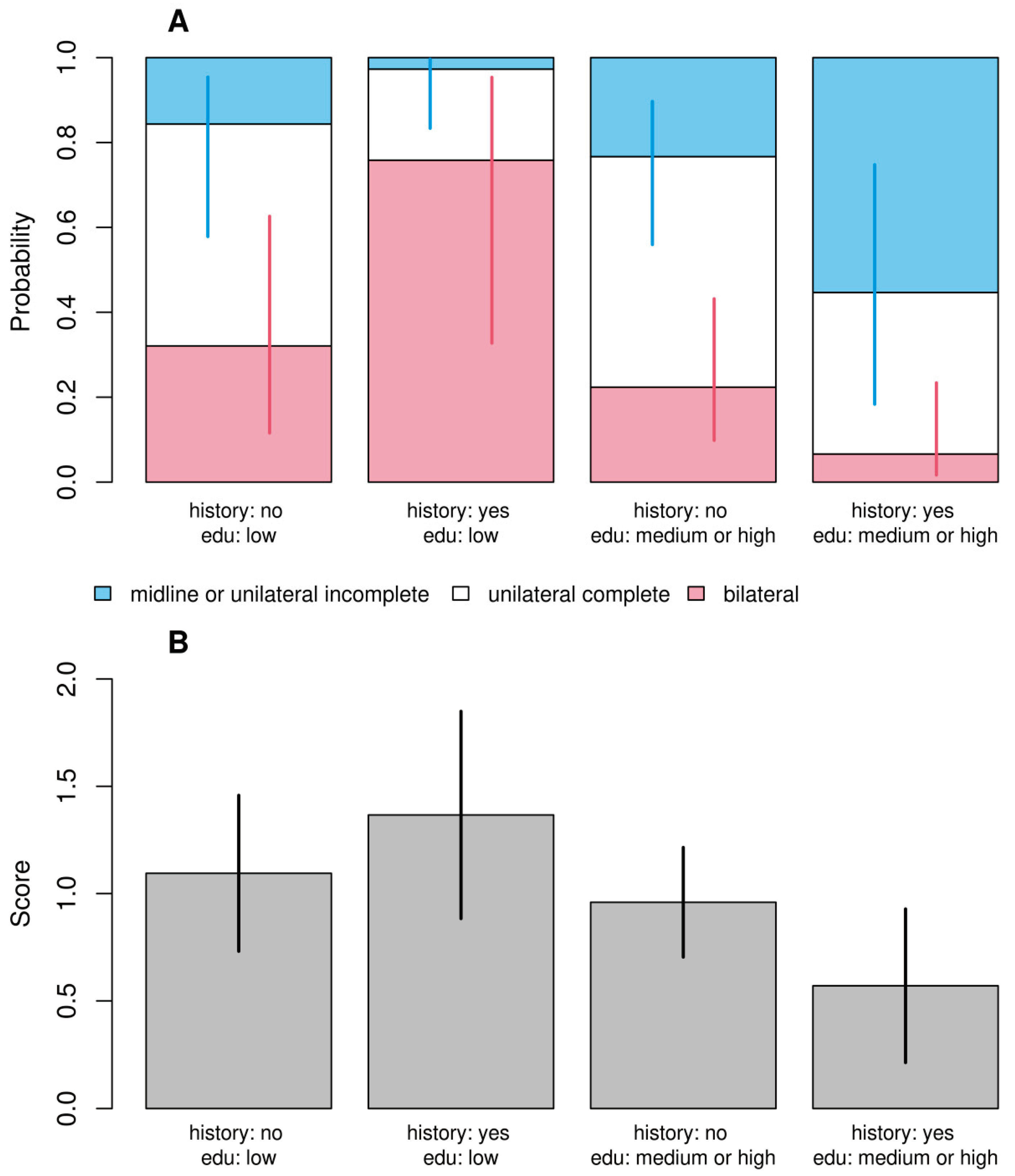
The data were analyzed using R (R core team 2021). In this article, we focus on modeling a cleft whose level can be coded in many ways. Since arbitrarily different coding methods for this variable can theoretically lead to qualitatively different outcomes, we performed a type of sensitivity analysis using two models in which the dependent variable was either (A) an ordered tertiary classification or (B) a score. The first type of coding (A) includes 3 classes: midline or unilateral incomplete, unilateral complete, and bilateral. Table 3 shows the classification of cleft cases in correspondence to LASHSAL coding. The second model (B) is based on score, which is computed by counting the presence of cleft in any lip (L or l), alveolus (A or a), hard palate (H or h), soft palate (S or s), and uvula (U) as well as presence of bilateral cleft. The score is standardized for better comparability with model (A), so that the midline or unilateral incomplete clefts fall in range from 0 to 1, unilateral complete clefts have a value of 1, and bilateral clefts fall in range from 1 to 2.
Table 3.
Tertiary classification of cleft cases and corresponding LASHSAL coding, where L (or l) denotes lip, A (or a) denotes alveolus, H (or h) denotes hard palate, S (or s) denotes soft palate, and U denotes uvula. Capital letters describe the cases where anatomic feature was completely clefted, while lowercase letters describe cases with partial clefting. Values denote number of cases (children). The last column score represents.
Table 3.
Tertiary classification of cleft cases and corresponding LASHSAL coding, where L (or l) denotes lip, A (or a) denotes alveolus, H (or h) denotes hard palate, S (or s) denotes soft palate, and U denotes uvula. Capital letters describe the cases where anatomic feature was completely clefted, while lowercase letters describe cases with partial clefting. Values denote number of cases (children). The last column score represents.
| |
Midline or unilateral incomplete |
Unilateral complete |
Bilateral |
Score |
| l |
1 |
. |
. |
0 |
| L |
3 |
. |
. |
0 |
| S |
6 |
. |
. |
0 |
| al |
1 |
. |
. |
0.333 |
| LA |
1 |
. |
. |
0.333 |
| sh |
1 |
. |
. |
0.333 |
| SL |
1 |
. |
. |
0.333 |
| SU |
1 |
. |
. |
0.333 |
| HSH |
4 |
. |
. |
0.333 |
| Lal |
1 |
. |
. |
0.333 |
| laHS |
. |
2 |
. |
1 |
| LAHS |
. |
15 |
. |
1 |
| Shal |
. |
1 |
. |
1 |
| SHAL |
. |
24 |
. |
1 |
| HSHAl |
. |
. |
1 |
1.333 |
| HSHAL |
. |
. |
2 |
1.333 |
| LAHSH |
. |
. |
2 |
1.333 |
| LAHSHL |
. |
. |
1 |
1.667 |
| lHSHAL |
. |
. |
1 |
1.667 |
| lahSHAL |
. |
. |
1 |
2 |
| LAHSHal |
. |
. |
1 |
2 |
| LAHSHAL |
. |
. |
11 |
2 |
Since the first model (A) has an ordered categorical dependent variable, we use generalized ordinal logistic regression (VGAM R package, Yee 2010 (5)). This type of regression assumes that at least some of the variables violate the proportional odds assumption. The set of variables inconsistent with this assumption was determined using the AICc selection [
17] from among all possible subsets of the set of all independent variables. The selection of the AICc model revealed that only intercept, toxic risk at mother’s work, and maternal stress violates the proportional odds assumption. The second model (B), which has points as the dependent variable, was fitted using the standard linear model.
Table 4.
Estimates of the generalized ordinal logit model.
Table 4.
Estimates of the generalized ordinal logit model.
| |
Odds ratio |
97.5 % |
2.5 % |
Estimate |
Std. Error |
z value |
Pr(>|z|) |
| (Intercept):1 |
5.3849 |
0.7187 |
0.0480 |
-1.6836 |
0.6905 |
-2.4383 |
0.0148 |
| (Intercept):2 |
0.4718 |
7.7150 |
0.5822 |
0.7511 |
0.6592 |
1.1395 |
0.2545 |
| Low or very low birth weight |
0.9747 |
3.7668 |
0.2794 |
0.0256 |
0.6636 |
0.0385 |
0.9693 |
| Child was born fourth or more in a row |
1.3162 |
2.5587 |
0.2256 |
-0.2748 |
0.6195 |
-0.4435 |
0.6574 |
| Men (boy) |
0.8755 |
2.9256 |
0.4459 |
0.1329 |
0.4799 |
0.2770 |
0.7818 |
| Cleft in any parent’s history |
6.6475 |
1.2151 |
0.0186 |
-1.8942 |
1.0659 |
-1.7772 |
0.0755 |
| Mother’s secondary or high education |
0.6102 |
5.9077 |
0.4546 |
0.4940 |
0.6542 |
0.7551 |
0.4502 |
| Toxic risk in mother’s work : 1 |
4.9688 |
1.9260 |
0.0210 |
-1.6032 |
1.1524 |
-1.3912 |
0.1642 |
| Toxic risk in mother’s work : 2 |
0.1344 |
80.8591 |
0.6845 |
2.0068 |
1.2173 |
1.6486 |
0.0992 |
| Mother’s Infections or drugs toxicity during pregnancy |
1.1702 |
2.5310 |
0.2885 |
-0.1572 |
0.5540 |
-0.2838 |
0.7766 |
| Mother’s stress during pregnancy : 1 |
0.8226 |
8.6182 |
0.1715 |
0.1953 |
0.9993 |
0.1955 |
0.8450 |
| Mother’s stress during pregnancy : 2 |
9.3879 |
0.7837 |
0.0145 |
-2.2394 |
1.0182 |
-2.1994 |
0.0278 |
| Interaction between cleft in any parent’s history and Mother’s secondary or high education |
0.0370 |
322.1006 |
2.2732 |
3.2980 |
1.2637 |
2.6098 |
0.0091 |
Table 5.
Estimates of the standard linear model.
Table 5.
Estimates of the standard linear model.
| |
Estimate |
2.5 % |
97.5 % |
Std. Error |
t value |
Pr(>|t|) |
| (Intercept) |
1.0946 |
0.7251 |
1.4641 |
0.1854 |
5.9050 |
0.0000 |
| Low or very low birth weight |
0.0635 |
-0.3011 |
0.4280 |
0.1829 |
0.3470 |
0.7296 |
| Child was born fourth or more in a row |
-0.0163 |
-0.3688 |
0.3362 |
0.1768 |
-0.0921 |
0.9269 |
| Men (boy) |
0.0013 |
-0.2641 |
0.2668 |
0.1332 |
0.0100 |
0.9921 |
| Cleft in any parent’s history |
0.2718 |
-0.2709 |
0.8146 |
0.2723 |
0.9984 |
0.3214 |
| Mother’s secondary or high education |
-0.1350 |
-0.4982 |
0.2281 |
0.1822 |
-0.7413 |
0.4609 |
| Toxic risk in mother’s work |
-0.0333 |
-0.4055 |
0.3389 |
0.1867 |
-0.1784 |
0.8589 |
| Mother’s Infections or poisoning during pregnancy |
0.0812 |
-0.2370 |
0.3993 |
0.1596 |
0.5088 |
0.6125 |
| Mother’s stress during pregnancy |
0.4454 |
-0.0770 |
0.9678 |
0.2620 |
1.6996 |
0.0935 |
| Interaction between cleft in any parent’s history and Mother’s secondary or high education |
-0.6610 |
-1.3124 |
-0.0095 |
0.3268 |
-2.0227 |
0.0468 |
6. Discussion
In a recent groundbreaking study, researchers have delved deep into the complex interplay of genetic, socio-economic, and environmental factors contributing to the development of bilateral cleft palates in children. This comprehensive analysis, leveraging two distinct modeling approaches, has yielded consistent and thought-provoking results.
A striking aspect of the study is the emphasis on the impact of maternal stress during pregnancy. Children born to stressed mothers were found to have 9.4 times higher odds of developing bilateral clefts. This significant correlation underscores the potential influence of prenatal environmental factors on craniofacial development. Equally compelling is the discovery of a nuanced interaction between a family's cleft history and the mother's educational level. The data reveal that children with a parental history of clefts and mothers with lower educational attainment face a heightened risk of more severe cleft forms. This finding suggests that socioeconomic factors, possibly influencing access to healthcare or environmental exposures, play a critical role in the manifestation of this congenital condition.
The study also brings attention to the concerning lack of comprehensive literature on rehabilitation strategies for children with cleft palates, especially in the context of various surgical methods. The existing protocols, largely limited to physiotherapy and speech therapy, indicate a significant gap in the holistic care required for these children, particularly in rare disease scenarios.
The demographic composition of the study's participants further enriches the analysis. Researchers included children from diverse backgrounds, including those with different levels of maternal education and exposure to potential occupational toxins. Intriguingly, the study categorizes professions like hairdressing and chemistry as higher-risk occupations for toxicity, in contrast to lower-risk fields such as teaching and economics. This classification sheds light on the potential environmental risk factors that may influence prenatal development.
The impact of risk factors of cleft palate is still not well known. In the presented study we observed that children of stressed mothers during pregnancy had 9.4 times higher odds of having bilateral cleft. This agrees with our study and confirms in the randomized studies of Mahapure KS, et al. [
18] They found a significant association between increased maternal age and increased stress levels in the cleft group, but the sample size could be not efficient. In the last decade, most publications on cleft palate assess the morphological changes as in our study, on the example of over a hundred cases [
19]
Non-modifiable risk factors for non-syndromic cleft lip and palate include gender, which can serve as both a protective and risk factor. In some cases, studies have shown correlations between gender and specific types of clefts. Specifically, several authors have found that males are a risk factor for CL/P (cleft lip with or without cleft palate) and CL (cleft lip) while CP (cleft palate) occurs more frequently in females. [
20,
21] Ethnic differences may potentially influence the occurrence of clefts. However, estimates of these associations significantly vary in studies. This can be explained by differences in the measurement and categorization of ethnic groups and variability in the classification of clefts within the oral cavity. [
22]. International literature indicates that Asians, white populations, and indigenous peoples have higher rates of cleft occurrences. In the American and Asian continents, 1 in 500 births is affected by this condition, compared to 1 in 2500 births in African populations. Belonging to a non-white group may serve as a protective factor against the occurrence of clefts. [
23]
This study has shown that the incidence rate of this anomaly is higher among newborns of non-white ethnicity.
The nutritional status of the mother plays a crucial role in significantly reducing the occurrence of congenital anomalies in newborns. For example, a raw vegetarian diet of the mother increases the risk of clefts in newborns 15-fold compared to omnivorous individuals. [
24]
Low levels of vitamin B12 and folate are often found in vegetarian women, and a diet deficient in folate and vitamin B12, especially in the early stages of pregnancy, is associated with the occurrence of clefts.
Caffeine belongs to the class of methylxanthines and increases the level of homocysteine by interfering with the metabolic pathways of vitamin B6. [
25]
On the other hand, for some individuals, prevention of clefts may not be as straightforward. Potatoes are generally considered a reliable source of nutrients. However, high levels of the glycoalkaloid solanine, which is considered toxic and teratogenic to the human body, can be found in certain foods. Consuming such foods during pregnancy may increase the risk of clefts and neural tube defects. [
26]
Kolejnym istotnym czynnikiem środowiskowym we współczesnych czasach jest stres.
Stresujące wydarzenie może spowodować wzrost poziomu kortyzolu we krwi matki, a co za tym idzie u płodu. Teratogenny efekt kortykosteroidów (został dawno wykazany. [
27,
28]. Enzym 11-beta-hydroksysteroid dehydrogenazy typu 2 (11beta HSD2), odpowiedzialny za regulację przepuszczalności bariery łożyskowej, jest regulowany w dół w przypadku stresu [
29]. Ponadto w wczesnych etapach ciąży - krytycznym momencie dla formowania masy twarzowej - ten enzym jest mniej reprezentowany. Stres Skutkujący wzrostem poziomu krążących kortykosteroidów może również powodować hiperinsulinemię i oporność na insulinę, negatywnie wpływając na rozwój płodu [
30]. Indeed, it has been shown that taking vitamin B6 supplements can help reduce the adverse effects of corticosteroids because this vitamin acts as a tissue receptor suppressor for these hormones[
31]. Deficiency of folate (vitamin B9) and cobalamin (vitamin B12) can indeed result from inadequate dietary intake and/or mutations in genes involved in the folate cycle. These vitamins are essential for various metabolic processes in the body, including DNA synthesis and repair, and their deficiency can lead to various health problems. It's important to maintain a balanced diet to ensure an adequate intake of these vitamins and to address any genetic factors that may affect their absorption or utilization. [
32] Although genetic polymorphisms are considered among non-modifiable risk factors, proper supplementation with folate and vitamin B12 during pregnancy compensates for the deficiency of enzymes and the accumulation of teratogenic metabolites. The enzyme MTHFR converts 5,10-MTHF into 5-MTHF, which is an active metabolite of folic acid and acts as a cofactor in many biochemical reactions, such as the conversion of homocysteine into methionine, leading to DNA, RNA, and histone protein methylation, one of the main mechanisms of gene expression regulation. Reduced levels of the active form of folic acid and the accumulation of homocysteine negatively affect cell differentiation and tissue growth during embryogenesis, leading to neural tube defects and cleft lip and palate. The common source of these defects is that the cells of the neural crest of the craniofacial tissues and teeth are located in the lateral-dorsal regions of the neural tube. Moreover, hyperhomocysteinemia is an independent risk factor for brain vascular diseases associated with atherosclerosis, hypertension, inflammation, neurodegenerative diseases, pregnancy complications, and congenital defects. Even though it is clear that supplementation with folic acid and cobalamin during pregnancy significantly reduces the occurrence of clefts and neural tube defects in both mice and humans, the correlation between clefts and the most common folate pathway polymorphisms remains controversial.
The most commonly prescribed medications during pregnancy are vitamins, followed by antibiotics. Current evidence is somewhat inconclusive when it comes to supplementation with folic acid and multivitamins to prevent clefts. According to most studies, taking folic acid or multivitamins containing folate at a dose of nearly 400 mcg per day is mandatory to reduce the risk of neural tube defects and clefts, not only during the first 3 months of pregnancy but also from the time of conception to 12 weeks after conception. [
33]. In other words, the lack of vitamin supplementation increases the risk of cleft lip and palate, but not specifically palate alone. These data may support the hypothesis that these are genetically and embryologically distinct entities.
Stress also increases the levels of catecholamines, which reduce blood flow to the uterus and increase the risk of fetal oxygen deprivation and damage. [
34] There is an association between cleft lip and palate and maternal psychological stress during the 15th week of pregnancy.[
35]
When it comes to maternal physical activity, its role has also been examined in the workplace environment. More intense movements such as twisting, lifting weights, running, and kneeling have shown a weak association with clefts; however, longer periods of sitting have been found to be a protective factor. Nevertheless, further research is necessary to more precisely investigate and confirm the relationship between maternal physical activity and clefts.[
36]
In the study presented, we found that children of mothers experiencing stress during pregnancy had a 9.4 times higher likelihood of developing bilateral clefts. This is consistent with our research and has been confirmed in randomized studies conducted by Mahapure KS et all.. [
37]. They demonstrated a significant association between advanced maternal age and increased stress levels in the cleft group, but the sample size may have been insufficient. In the last decade, most publications on cleft palate have assessed morphological changes, as in our study, using examples of over a hundred cases. [
6]
Diabetes is a metabolic disorder characterized by hyperglycemia, an elevated level of blood glucose. The regulation of blood glucose levels is achieved through the production of insulin by the pancreatic beta cells, a hormone responsible for controlling the absorption and utilization of glucose by cells. Depending on the mechanisms responsible for hyperglycemia, various forms of this condition can be distinguished: (a) Type 1 (or insulin-dependent diabetes), in which the pancreas does not produce insulin; (b) Type 2 (or non-insulin-dependent diabetes), in which the amount of insulin produced is insufficient to meet the body's needs, or it does not work properly in the tissues (insulin resistance); (c) Gestational diabetes, which occurs during pregnancy and leads to an increase in blood glucose levels.
Globally, the prevalence of different forms of diabetes is 8.3%. It has been observed that the association between gestational diabetes and obesity triples the risk of fetal congenital defects, including clefts.[
38,
39]
Hyperglycemia during pregnancy increases the risk of newborns developing clefts. Therefore, rigorous and systematic monitoring of maternal blood glucose levels is necessary during pregnancy to prevent the risk of clefts and other congenital defects.. [
40] Similarly, the use of antihypertensive medications in the early stages of pregnancy has been shown to have teratogenic effects on the fetus. Therefore, careful consideration and monitoring of medication use in pregnancy are crucial to minimize potential risks to the developing fetus, including the risk of congenital defects like clefts.[
41]
The development of the lip and palate occurs in the early stages of pregnancy. Exposure to infections during this period can indeed increase the risk of clefts. It's essential for expectant mothers to take precautions to reduce the risk of infections during pregnancy to help protect the developing fetus from congenital defects like cleft lip and palate. [
42] Hyperthermia, often associated with viral infections, plays an important role in the occurrence of clefts. Elevated body temperature, especially during critical periods of fetal development, can increase the risk of congenital defects like cleft lip and palate. It's crucial for pregnant individuals to avoid situations that may lead to hyperthermia, such as high fever due to viral infections, to reduce the risk of such birth defects.. [
43]
The most common conditions associated with clefts typically occur in early pregnancy and include colds, acute respiratory infections, the flu, appendicitis, urinary tract infections, and genital infections. It's important for pregnant individuals to seek prompt medical attention and treatment for these conditions to minimize potential risks to the developing fetus. [
44]
These data suggest the importance of vaccinations for pregnant women, such as influenza vaccination. There is no evidence of a potential correlation between SARS-CoV-2 infection during pregnancy and the risk of clefts. The association between infants born with cleft palate and any acute or chronic maternal illnesses during pregnancy suggests that maternal anemia may be related to this condition. This would align with the theory that embryonic hypoxia occurring during the first three months of pregnancy may hinder proper facial formation.
Furthermore, an increase in inflammatory cytokines may also be responsible for this issue.[
45] The risk of cleft palate may be higher in cases of maternal thyroid hyperactivity during pregnancy. Painkillers and antiepileptic medications are often used during pregnancy. However, in these cases, one must also consider the potential teratogenic effect of antiepileptic drugs that the mother is taking. Even muscle relaxants and painkillers used for conditions such as gallstones, urinary tract stones, and neuro-musculoskeletal pain syndromes can be considered potential contributors to the problem. It's important for pregnant individuals and healthcare providers to carefully weigh the benefits and risks of medications during pregnancy to make informed decisions that minimize potential harm to the developing fetus. [
46]
Specifically, the most used antibiotics are beta-lactams, followed by sulfonamides and macrolides. Antibiotics are prescribed more frequently in the second trimester of pregnancy, especially for women under 25 years of age. It's important for healthcare providers to carefully consider the choice of antibiotics and their potential effects on the developing fetus when prescribing them during pregnancy. The goal is to provide effective treatment while minimizing any potential risks to the unborn child. [
47] While antibiotic prescribing during pregnancy has increased somewhat in the last decade, the association between antibiotics and clefts remains a controversial issue in the literature. This controversy exists even for molecules traditionally considered safe during pregnancy. For example, trimethoprim is associated with an increased risk of cleft palate in the first trimester, while doxycycline/tetracyclines and sulfamethoxazole are strongly associated with cleft lip and palate in the second month of pregnancy. It's essential for healthcare providers to carefully assess the potential risks and benefits of antibiotic use during pregnancy and choose antibiotics that are considered the safest option for both the mother and the developing fetus.[
48]
In the third trimester, amoxicillin is associated with an increased risk of both cleft palate and cleft lip and palate. It's crucial for healthcare providers to consider these potential risks and benefits when prescribing antibiotics during pregnancy and to weigh the necessity of antibiotic treatment against the potential risks to the developing fetus. The choice of antibiotics and the timing of their use should be carefully evaluated in each individual case. [
49]
Corticosteroids are widely used as anti-inflammatory and immunomodulatory drugs during pregnancy and are used to induce fetal lung maturation. They are primarily used in pregnancy in dermatological preparations to treat rashes, psoriasis, skin inflammation, and eczema, as well as in inhalation preparations to treat nasal congestion.
High-dose corticosteroids in early pregnancy are considered a risk factor for clefts due to their potential teratogenic effects. It's essential for healthcare providers to carefully consider the dosage and timing of corticosteroid use during pregnancy to minimize potential risks while ensuring that the treatment is necessary for the health and well-being of both the mother and the developing fetus. The benefits and risks should be carefully weighed on a case-by-case basis. [
50]
An increase in the levels of endogenous corticosteroids can affect the fusion of palatal folds in mice. Considering that the fetus has lower levels of endogenous corticosteroids, even low doses of corticosteroids and dermatological preparations like betamethasone can cross the placenta, enter the fetal circulation, and induce teratogenic effects during embryogenesis. As mentioned earlier, excessive production of endogenous cortisol due to maternal stress can also contribute to the risk of cleft formation.
Among oral corticosteroids, prednisone has shown a slight association with cleft lip and palate. Regarding nasal spray/inhalation forms, triamcinolone has the strongest association with cleft palate.[
51]
When it comes to the timing of exposure, the most critical periods of exposure to corticosteroids are from 1 to 4 weeks and from 5 to 8 weeks after fertilization. Subsequently, exposure only during the 4 weeks before fertilization and exposure during weeks 5 to 8 and 9 to 12 after fertilization are also significant in terms of potential risk. These specific windows of exposure during pregnancy are important to consider when assessing the potential impact of corticosteroid use on fetal development and the risk of congenital defects like clefts.[
52]
In the latest literature, various medications have been associated with an increased risk of clefts. These medications include antiepileptic drugs, retinoic acid, painkillers, benzodiazepines, antidepressants, stimulants, antihypertensive drugs, as well as medications containing iron and folic acid. It's essential for healthcare providers to be aware of these potential risks and carefully consider the use of these medications during pregnancy, weighing the benefits against the potential risks to the developing fetus. Each case should be evaluated individually, and appropriate precautions should be taken to minimize any potential harm to the unborn child.[
53]
The misuse of opioids during pregnancy is a highly risky behavior that can lead to various congenital defects, including cleft lip and palate. The risk of clefts associated with opioid misuse is nearly three times higher than in individuals who do not misuse opioids. Newborns exposed to opioids before birth are also at risk of developing Neonatal Abstinence Syndrome (NAS), which affects approximately half of all exposed newborns. It's crucial for individuals who are pregnant or planning to become pregnant to seek help and support to address opioid misuse, as it poses significant health risks to both the mother and the developing fetus. [
54]
Smoking cigarettes during early pregnancy is a well-documented risk factor for cleft lip and palate. It can significantly increase the risk, especially for cleft lip and palate. Maternal exposure to chemicals released during tobacco combustion, such as polycyclic aromatic hydrocarbons, carbon monoxide, and heavy metals, has been linked to smoking and causes embryonic tissue hypoxia during palate development, which can lead to congenital defects. It's essential for pregnant individuals to avoid smoking and exposure to secondhand smoke to reduce the risk of these birth defects and other adverse pregnancy outcomes.[
55] [
56]
Consuming alcohol during pregnancy, especially during the first trimester, is associated with an increased risk of cleft lip and palate. This risk is dose-dependent, and excessive alcohol consumption can have serious detrimental effects on fetal development, affecting the nervous system and neurodevelopment. The period between the fifth and tenth week of pregnancy, when the structures of the palate and lip fuse, is particularly vulnerable to the effects of alcohol. The quantity and frequency of alcohol consumption during pregnancy, as well as the genetic susceptibility linked to the mother's alcohol-metabolizing genes, can influence the risk of cleft lip and palate malformations. It is strongly recommended for pregnant individuals to avoid alcohol completely to minimize the risk of these birth defects and other potential complications.[
57] [
58,
59]
Summarizing, maternal substance abuse, including opioids and tobacco, as well as alcohol consumption and exposure to pollutants and environmental substances, may contribute to the risk of cleft lip and palate in offspring. The specific mechanisms and interactions involved in these associations are complex and may vary depending on the substance or pollutant involved.
In this study, there was no evidence that the child's gender, birth order, body weight, maternal occupational toxicity risk, or reporting of infections or chemical toxicity had a significant impact on the dependent variable. We demonstrated statistically significant interaction between parental cleft history and maternal education in both models. Children of parents with a history of cleft and low-educated mothers had a higher probability of bilateral cleft, a lower probability of simple cleft, and a higher score than children of parents with a cleft history and mothers with medium to high education. Genetic etiology is emphasized in research, but cleft lip and palate can occur in isolation in 61.6% of cases [
9]. This study calls for further prospective research with randomization, but at the same time, it is not possible to eliminate risk factors without identifying them in cleft lip and palate prevention.[
60]
This study not only provides critical insights into the factors influencing the development of cleft palates in children but also opens several avenues for future research. There is a clear need for a deeper understanding of the underlying mechanisms of these associations and the development of more effective, comprehensive rehabilitation approaches. Additionally, this research highlights the importance of considering a wide range of genetic, socio-economic, and environmental factors in the study and treatment of congenital conditions like cleft palates. As we move forward, a multidisciplinary approach will be essential in addressing the complexities of these developmental challenges.
To incorporate the aspect of education and awareness about vitamin intake among mothers. In conclusion, navigating the complexities of cleft palate from its origins to management reveals numerous layers that necessitate scientific investigation. This includes exploring the intertwining of genetic, biochemical, environmental, socio-economic, and clinical factors.
Notably, our findings underscore the significant correlation between maternal education level and awareness of prenatal care, particularly regarding vitamin intake and its impact on cleft palate risk. Enhanced education and awareness among expectant mothers, especially those under stress, could potentially reduce the incidence of cleft palate. Furthermore, through extensive epidemiological studies examining various risk factors across different populations, alongside the development of comprehensive and diverse rehabilitation protocols in areas like physiotherapy, the scientific community can make strides towards a more holistic understanding and management of cleft palate. This approach aims to provide individuals affected with a complete and optimized pathway from prevention to rehabilitation, ultimately improving their quality of life and facilitating better societal integration."
This revised conclusion ties in the role of maternal education and awareness in relation to cleft palate, emphasizing the importance of preventive measures and comprehensive management strategies.