Submitted:
13 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
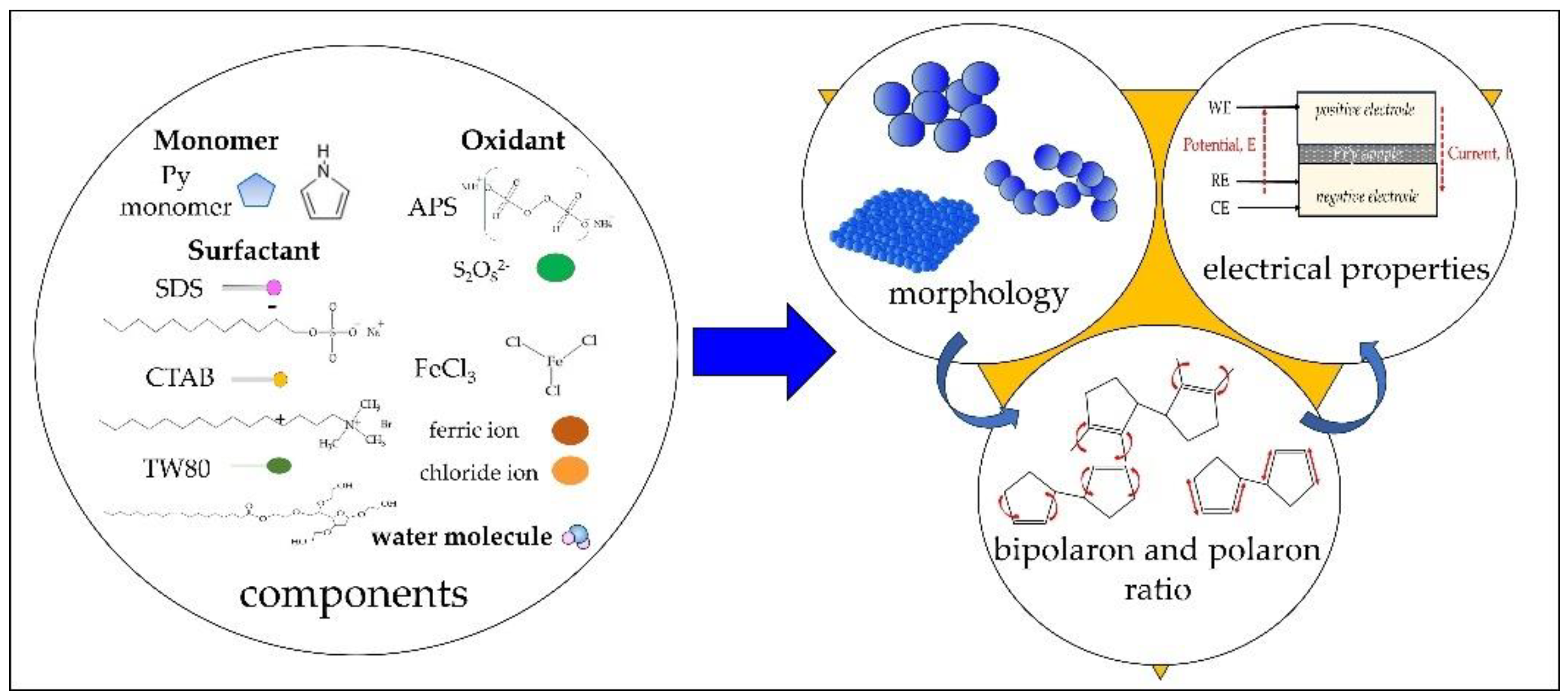
1. Introduction
2. Results
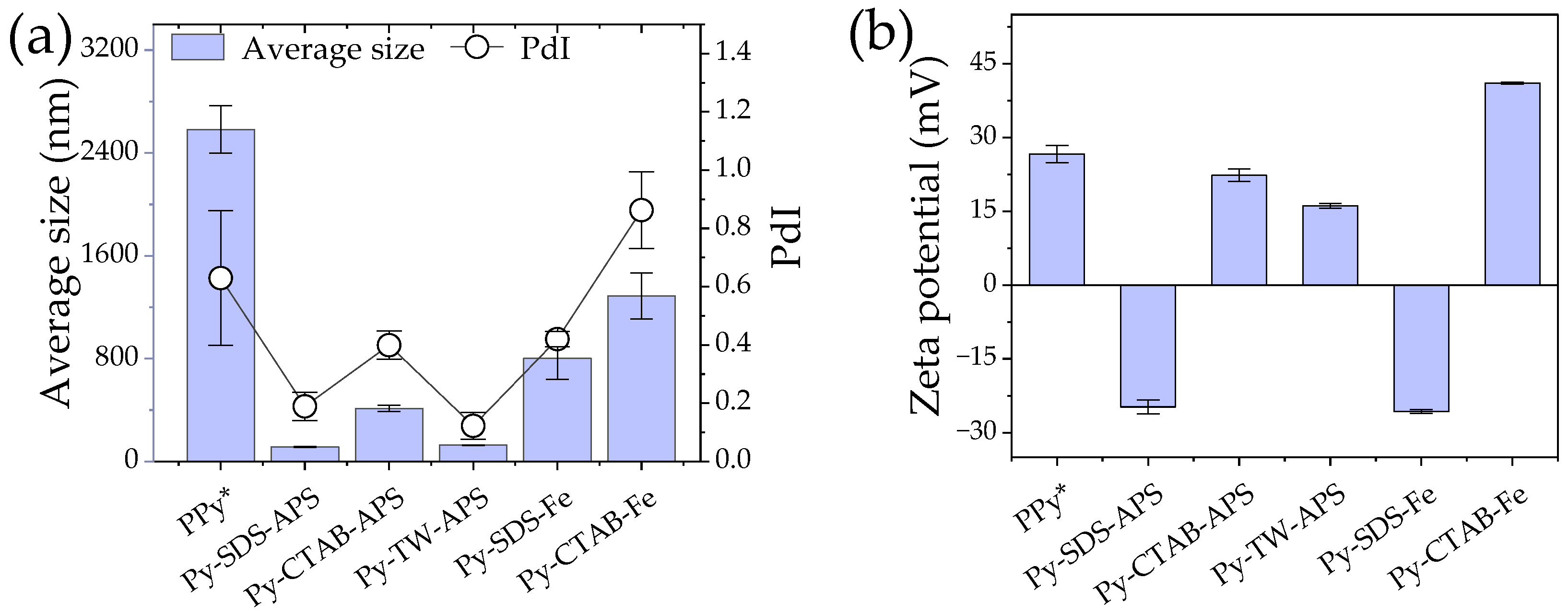
2.1. Average Size and Zeta Potential
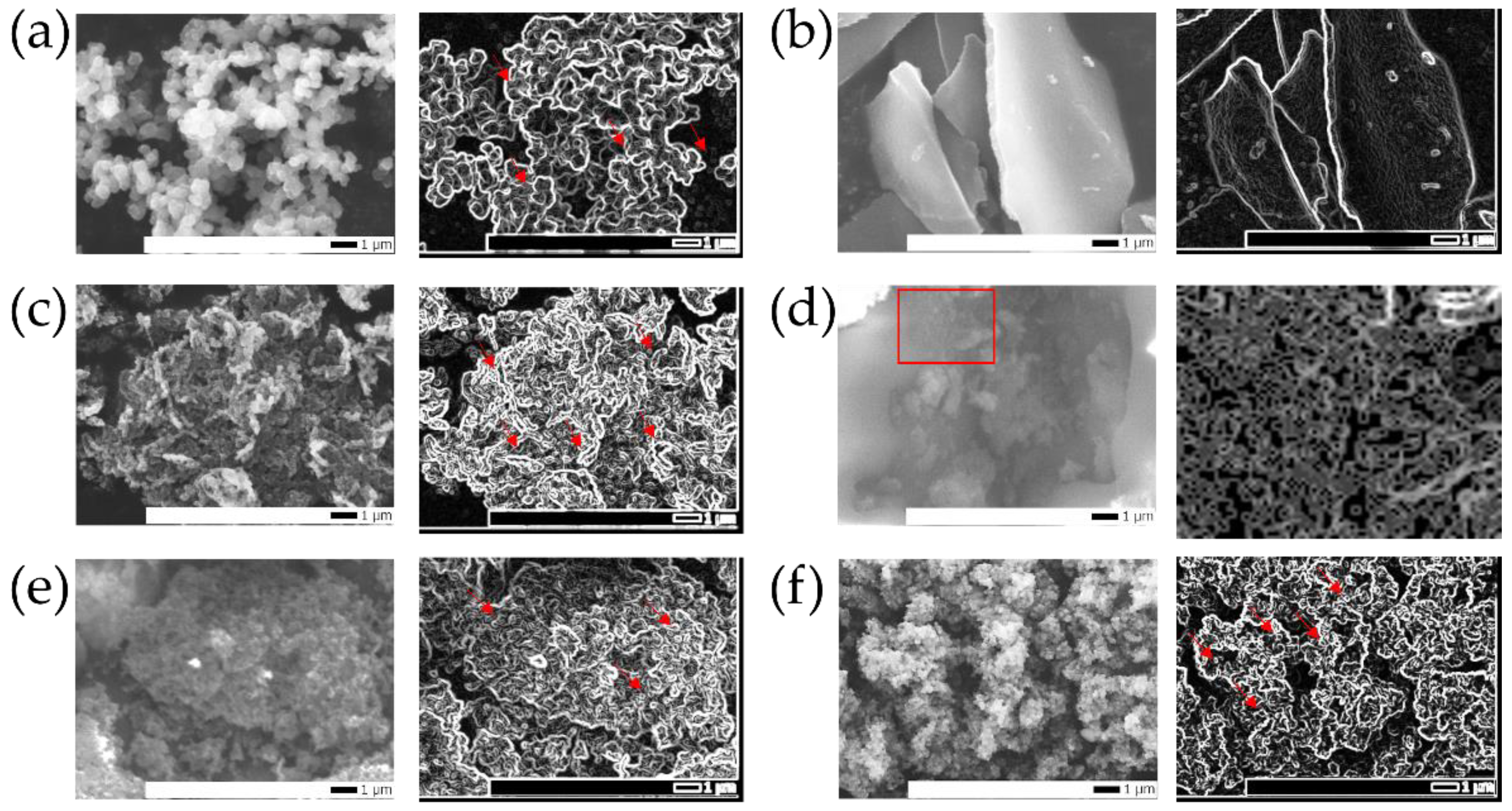
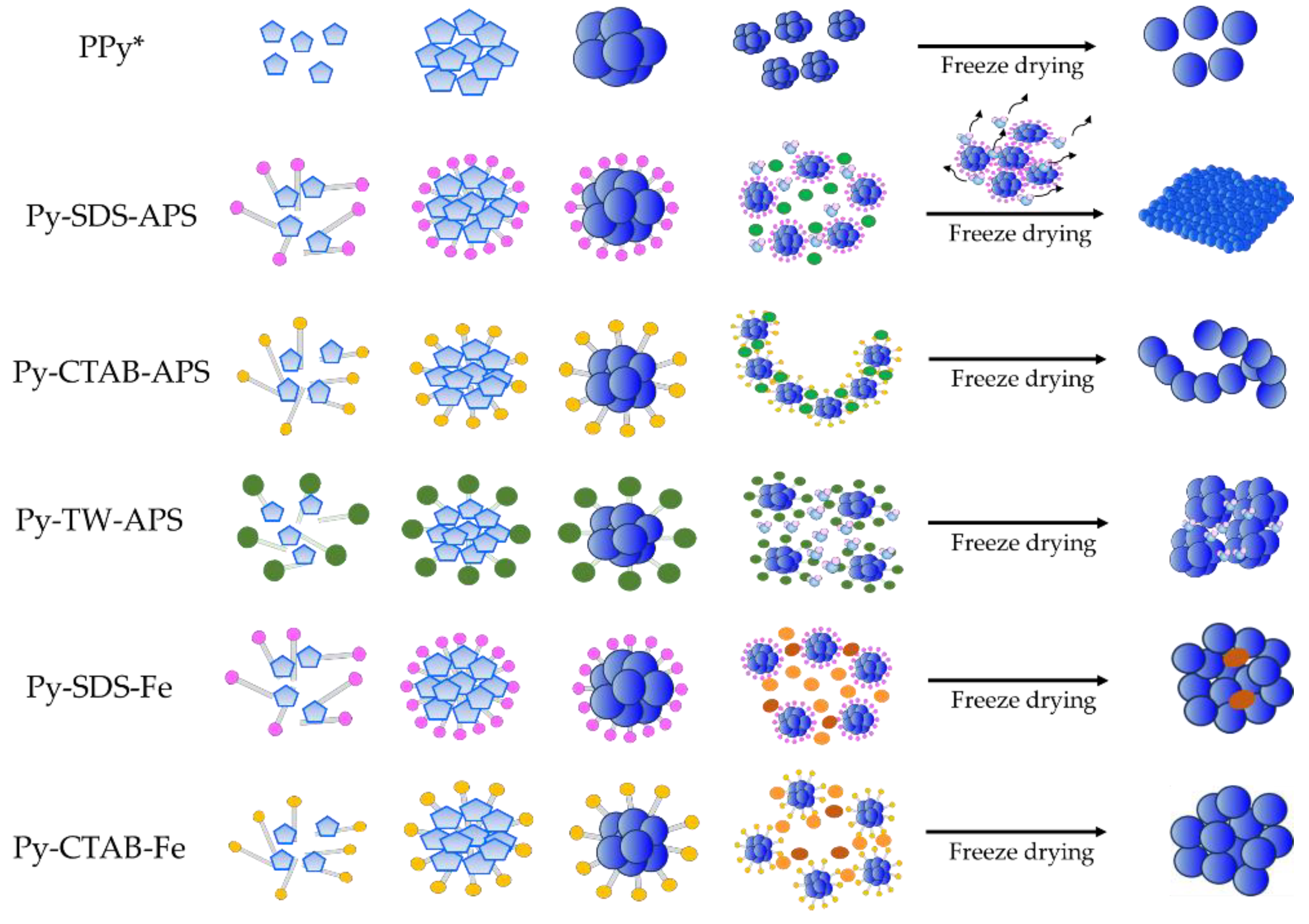
2.2. Morphology and Structure Characteristics
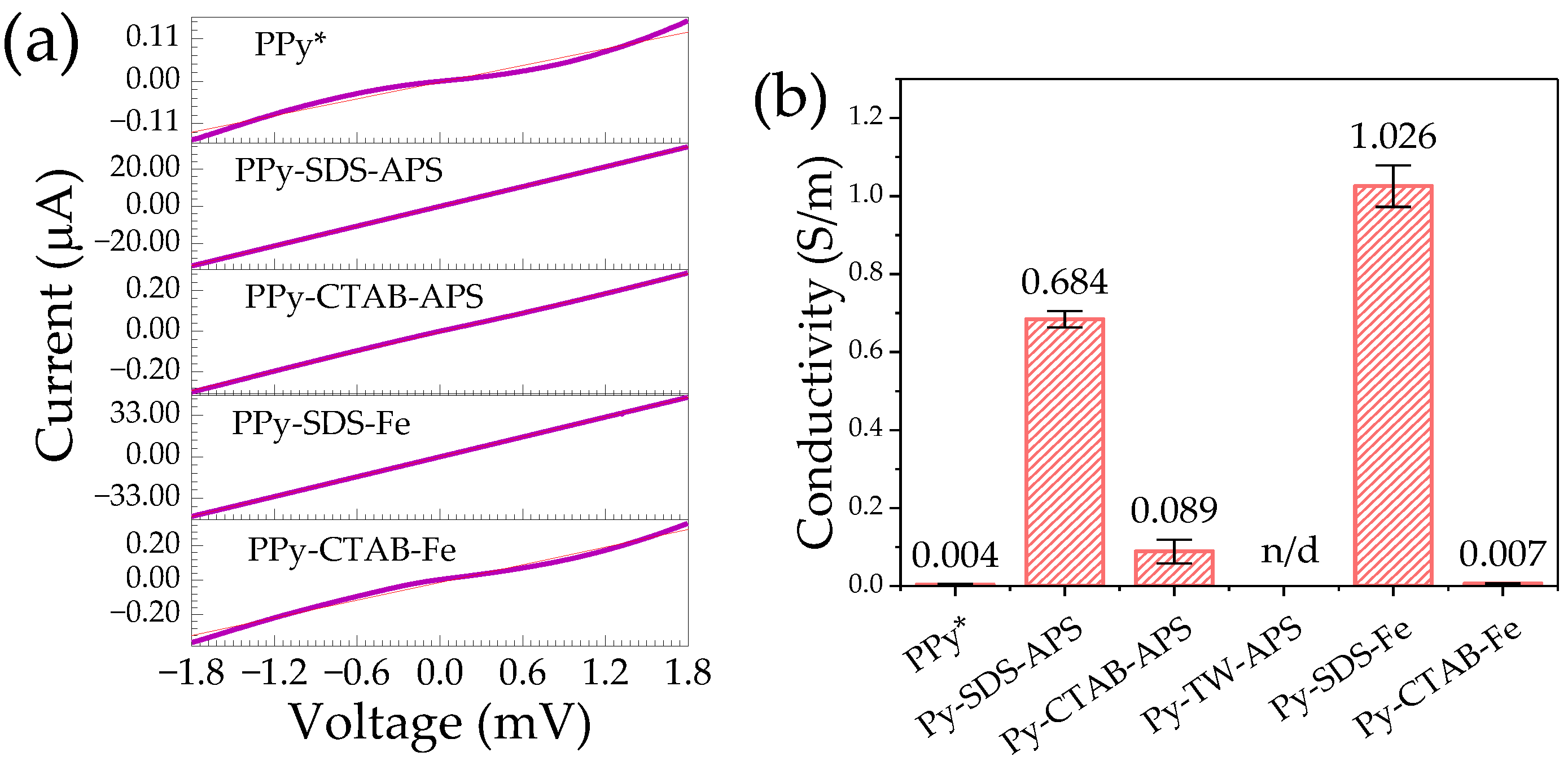
2.3. Electrical Conductivity
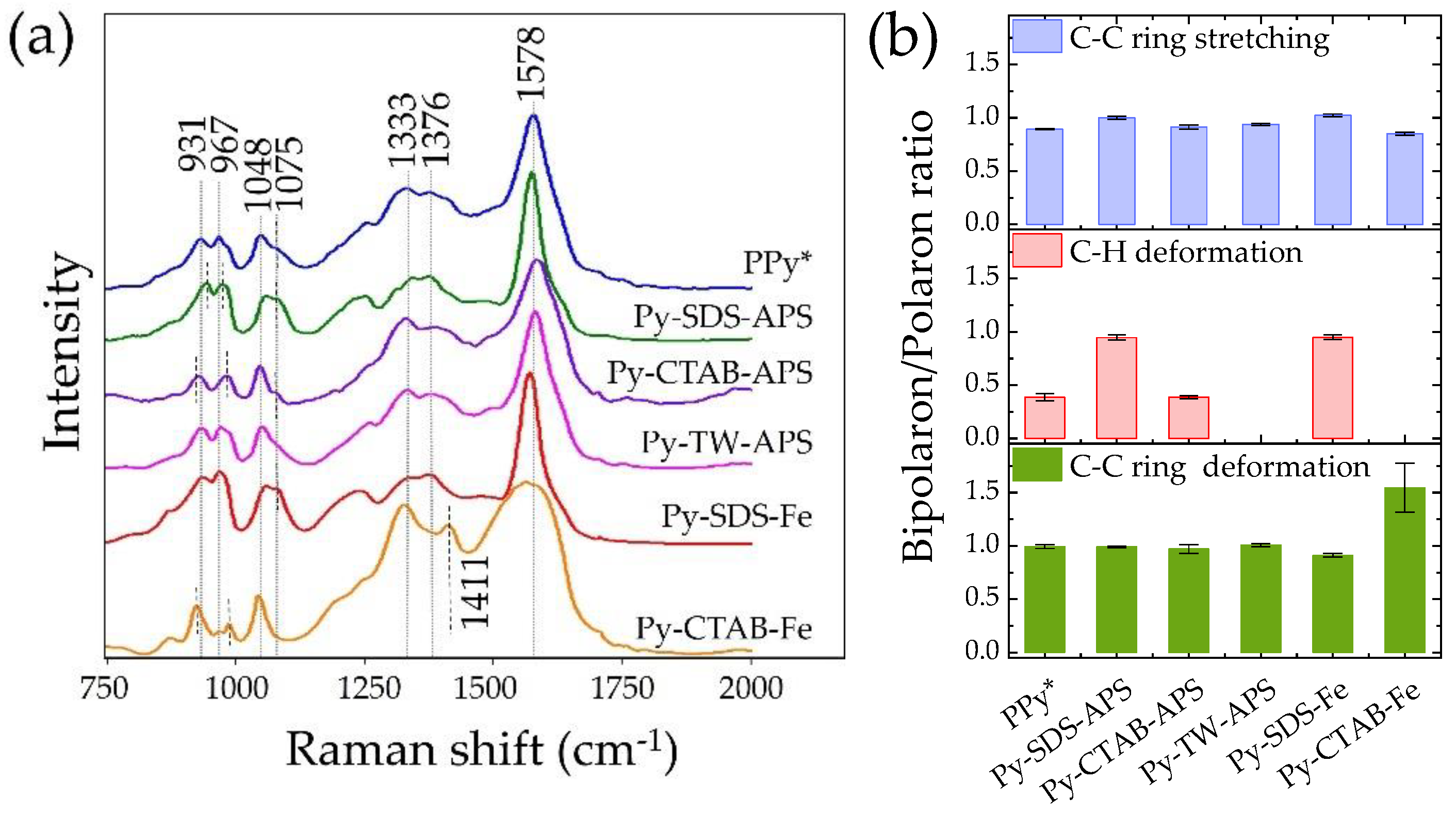
2.4. Raman Spectra and Bipolarons/Polarons Ratio
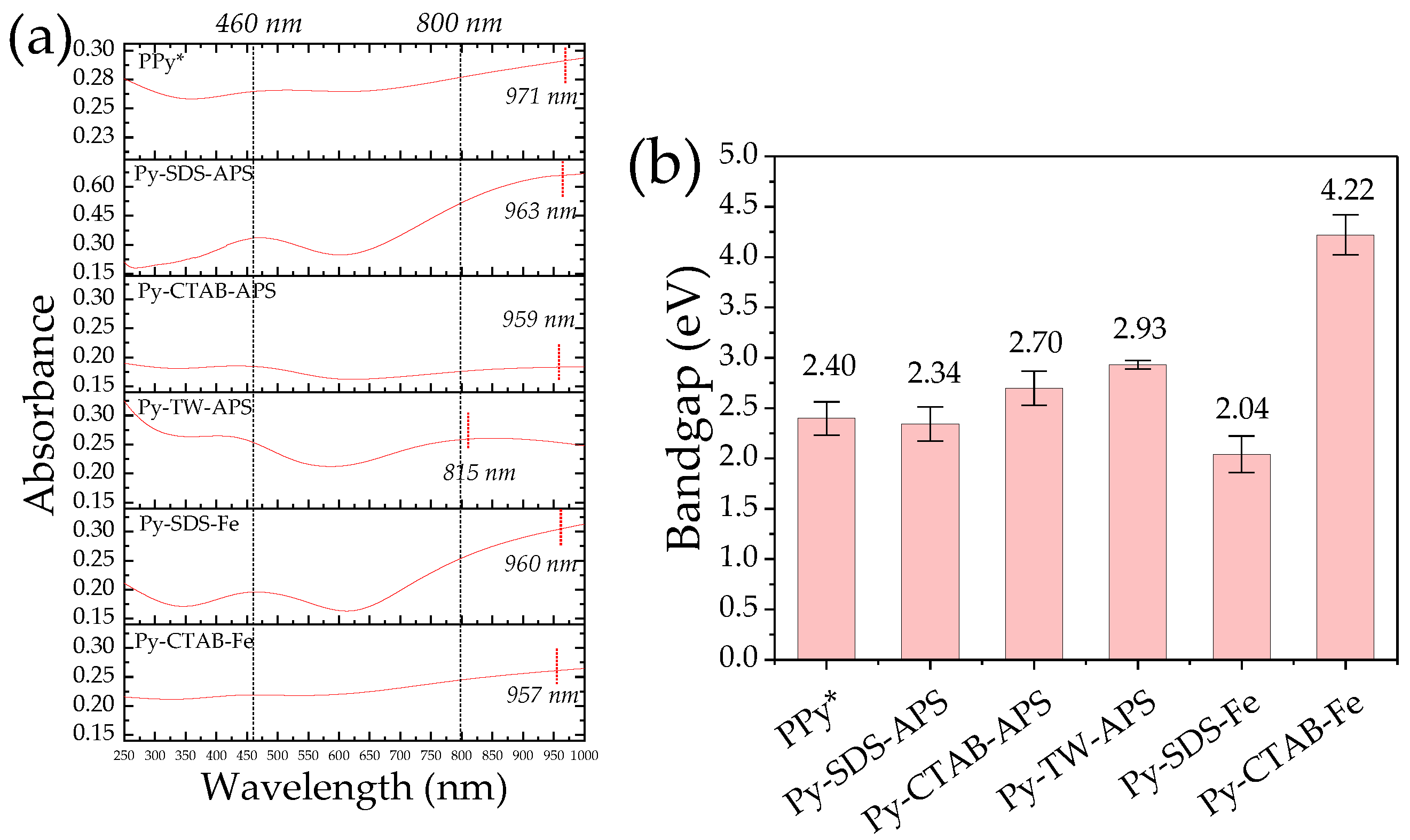
2.5. UV-Vis-NIR Absorption
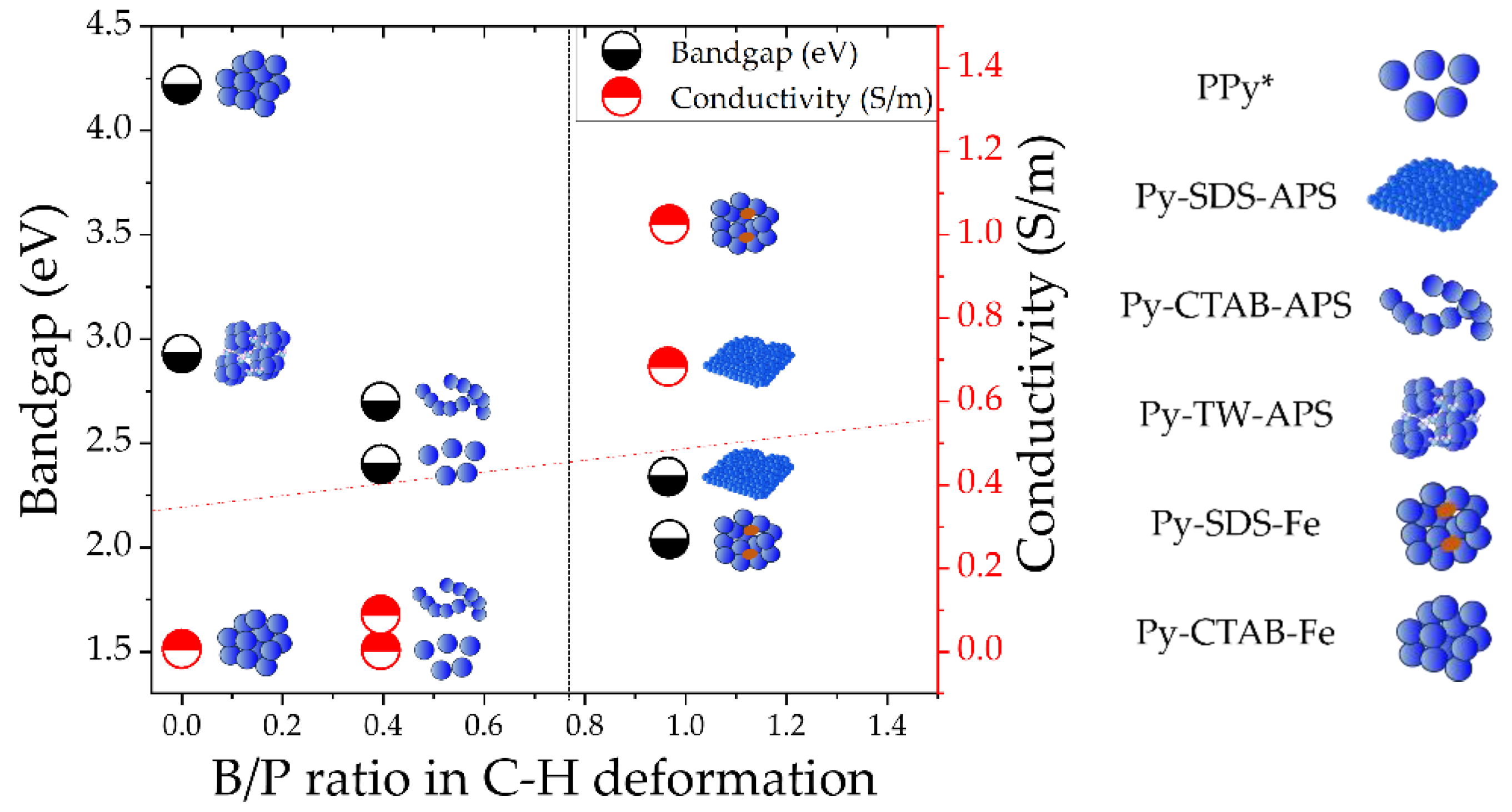
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. PPy Synthesis
4.3. Particle Size Distribution and Zeta Potential Measurement
4.4. Morphology Observation by Scanning Electron Microscopy (SEM)
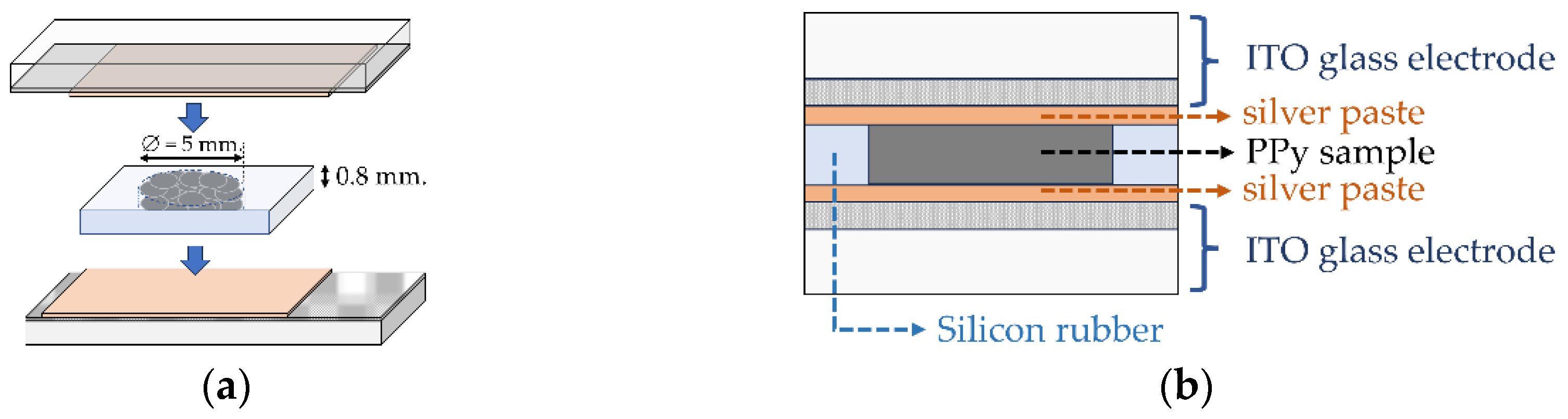
4.5. Electrical Conductivity Analysis
4.6. Raman Spectroscopy
4.7. Ultraviolet/Visible/Near Infrared (UV-Vis-NIR) Absorbance and Bandgap Calculations
5. Conclusions
- Comparing Py-SDS-APS, Py-CTAB-APS, and Py-TW-APS, the morphology shifts towards a sheet-like configuration when using SDS as a surfactant and showed higher bipolaron and polaron ratio in the C-H deformation and higher electronic properties. In contrast, Py-SDS-Fe showed a cluster of particle-like form, and the conductivity and the bipolaron and polaron ratio in the C-H deformation of Py-SDS-Fe surpasses that of Py-CTAB-Fe. The results showed that if different oxidant were used, the shape morphology of PPy might not have an effect on the bipolaron and polaron ratio in C-H deformation. However, the ratio of bipolaron and polaron has a strong relation with conductivity.
- This work highlights that SDS contributes to elevating the bipolaron content within the C-H deformation region of PPy, which may occur through the electrostatic interaction between PPy and the head group of SDS, resulting in the enhancement of its conductivity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Q.; Yang, Y.; Ma, Y.; Zhang, R.; Wang, J.; Chen, X.; Shao, Z. Bandgap Engineered Polypyrrole–Polydopamine Hybrid with Intrinsic Raman and Photoacoustic Imaging Contrasts. Nano Lett. 2018, 18, 7485–7493. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef]
- Veisi, H.; Varshosaz, J.; Rostami, M.; Mirian, M. Thermosensitive TMPO-Oxidized Lignocellulose/Cationic Agarose Hydrogel Loaded with Deferasirox Nanoparticles for Photothermal Therapy in Melanoma. Int. J. Biol. Macromol. 2023, 238, 124126. [Google Scholar] [CrossRef] [PubMed]
- Xiaoqiang, L.; Qian, S.; Yan, K.; Yanan, Z.; Zengyuan, P.; Yang, J.; Mengjuan, L.; Chronakis, I.S. Self-Powered Humidity Sensor Based on Polypyrrole Modified Melamine Aerogel. Mater. Lett. 2020, 277, 128281. [Google Scholar] [CrossRef]
- Miao, J.; Chen, Y.; Li, Y.; Cheng, J.; Wu, Q.; Ng, K.W.; Cheng, X.; Chen, R.; Cheng, C.; Tang, Z. Proton Conducting Polyoxometalate/Polypyrrole Films and Their Humidity Sensing Performance. ACS Appl. Nano Mater. 2018, 1, 564–571. [Google Scholar] [CrossRef]
- De Aguiar, M.F.; Leal, A.N.R.; De Melo, C.P.; Alves, K.G.B. Polypyrrole-Coated Electrospun Polystyrene Films as Humidity Sensors. Talanta 2021, 234, 122636. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.R.; Yang, M.; Khandaker, S.; Bashar, M.M.; Alsukaibi, A.K.D.; Hassan, H.M.A.; Znad, H.; Awual, Md. R. Polypyrrole-Based Sensors for Volatile Organic Compounds (VOCs) Sensing and Capturing: A Comprehensive Review. Sens. Actuators Phys. 2022, 347, 113933. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and Fabrication of Conductive Polymer Hydrogels and Their Applications in Flexible Supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Shabeeba, A.; Rajan, L.; Sidheekha, M.P.; Thayyil, M.S.; Ismail, Y.A. Polypyrrole/Hydrogel Hybrid Films as Multi Sensing Supercapacitor Electrodes. J. Energy Storage 2022, 55, 105724. [Google Scholar] [CrossRef]
- Brusamarello, C.Z.; Santos, L.M.; Romio, A.P.; Domenico, M.D.; Santos, A.F.; de Araújo, P.H.H.; Sayer, C. Polypyrrole Production through Chemical Polymerization Using Anionic and Cationic Dopants: The Influence of Synthesis Conditions and Reaction Kinetics. Mater. Today Commun. 2021, 26, 101740. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Abdel-Aziz, M.H.; Bassyouni, M.; Abusorrah, A.M.; Attar, A.; Baghdadi, N.; Salah, N. Polypyrrole Sheets Composed of Nanoparticles as a Promising Room Temperature Thermo-Electric Material. Phys. E Low-Dimens. Syst. Nanostructures 2021, 134, 114889. [Google Scholar] [CrossRef]
- Alekseeva, E.; Bober, P.; Trchová, M.; Šeděnková, I.; Prokeš, J.; Stejskal, J. The Composites of Silver with Globular or Nanotubular Polypyrrole: The Control of Silver Content. Synth. Met. 2015, 209, 105–111. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, L.; Chen, L.Y.; Liu, P.; Hirata, A.; Chen, M.W. Raman Characterization of Pseudocapacitive Behavior of Polypyrrole on Nanoporous Gold. Phys. Chem. Chem. Phys. 2014, 16, 3523. [Google Scholar] [CrossRef]
- Deljoo Kojabad, Z.; Shojaosadati, S.A. Chemical Synthesis of Polypyrrole Nanostructures: Optimization and Applications for Neural Microelectrodes. Mater. Des. 2016, 96, 378–384. [Google Scholar] [CrossRef]
- Shen, X.; Wei, X.; Wang, T.; Li, S.; Li, H. Polypyrrole Embedded in Nickel-Cobalt Sulfide Nanosheets Grown on Nickel Particles Passivated Silicon Nanowire Arrays for High-Performance Supercapacitors. Chem. Eng. J. 2023, 461, 141745. [Google Scholar] [CrossRef]
- Parayangattil Jyothibasu, J.; Lee, R.-H. Green Synthesis of Polypyrrole Tubes Using Curcumin Template for Excellent Electrochemical Performance in Supercapacitors. J. Mater. Chem. A 2020, 8, 3186–3202. [Google Scholar] [CrossRef]
- Zhou, Z.; Shao, Y.; Gao, X.; Liu, Z.; Zhang, Q. Structural Regulation of Polypyrrole Nanospheres Guided by Hydrophobic Chain Length of Surfactants. J. Mater. Sci. 2019, 54, 14309–14319. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Du, H.; Zhang, Z.; Lv, J.; Sun, Y.; Wang, L. Mechanism Research of SDBS-Functionalized Polypyrrole to Improve Electrochemical Performance of Screen-Printed Graphene Electrode. Electrochimica Acta 2023, 454, 142408. [Google Scholar] [CrossRef]
- Jamdegni, M.; Kaur, A. Role of Polarity of Surfactants on the Morphology of Electrochemically Synthesized Polyaniline Nanostructures: Towards Faster and Efficient Electrochromic Response. Thin Solid Films 2020, 714, 138373. [Google Scholar] [CrossRef]
- Samwang, T.; Watanabe, N.M.; Okamoto, Y.; Srinives, S.; Umakoshi, H. Study of Chemical Polymerization of Polypyrrole with SDS Soft Template: Physical, Chemical, and Electrical Properties. ACS Omega 2023, 8, 48946–48957. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.J.; Jayakannan, M. Amphiphilic Azobenzenesulfonic Acid Anionic Surfactant for Water-Soluble, Ordered, and Luminescent Polypyrrole Nanospheres. J. Phys. Chem. B 2007, 111, 12772–12780. [Google Scholar] [CrossRef]
- Molina, B.G.; Cianga, L.; Bendrea, A.-D.; Cianga, I.; Del Valle, L.J.; Estrany, F.; Alemán, C.; Armelin, E. Amphiphilic Polypyrrole-Poly(Schiff Base) Copolymers with Poly(Ethylene Glycol) Side Chains: Synthesis, Properties and Applications. Polym. Chem. 2018, 9, 4218–4232. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Song, W.; Liu, Z. Controllable Synthesis of Conducting Polypyrrole Nanostructures. J. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef]
- Dubey, N. A Study on Surfactant Modified Polypyrrole Nanostructures and Its Applications in Supercapacitors. Int. J. Polym. Anal. Charact. 2023, 28, 625–646. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and Structural Study of Polypyrroles Prepared in the Presence of Surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, G. Morphology, Structure, and Conductivity of Polypyrrole Prepared in the Presence of Mixed Surfactants in Aqueous Solutions. J. Appl. Polym. Sci. 2007, 104, 1987–1996. [Google Scholar] [CrossRef]
- Ren, H.; Sun, R.; Jin, Y.; Xu, M.; Pei, Y.; Wang, Q.; Yan, M. Electrochemical Properties of Multi-Morphology Polypyrrole Electrode Materials Depended on Template Agents. ChemistrySelect 2023, 8, e202302155. [Google Scholar] [CrossRef]
- Khadem, F.; Pishvaei, M.; Salami-Kalajahi, M.; Najafi, F. Morphology Control of Conducting Polypyrrole Nanostructures via Operational Conditions in the Emulsion Polymerization. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Zhou, M.; Pagels, M.; Geschke, B.; Heinze, J. Electropolymerization of Pyrrole and Electrochemical Study of Polypyrrole. 5. Controlled Electrochemical Synthesis and Solid-State Transition of Well-Defined Polypyrrole Variants. J. Phys. Chem. B 2002, 106, 10065–10073. [Google Scholar] [CrossRef]
- Gupta, S. Hydrogen Bubble-Assisted Syntheses of Polypyrrole Micro/Nanostructures Using Electrochemistry: Structural and Physical Property Characterization. J. Raman Spectrosc. 2008, 39, 1343–1355. [Google Scholar] [CrossRef]
- Appel, G.; Böhme, O.; Mikalo, R.; Schmeißer, D. The Polaron and Bipolaron Contributions to the Electronic Structure of Polypyrrole Films. Chem. Phys. Lett. 1999, 313, 411–415. [Google Scholar] [CrossRef]
- Brédas, J.L.; Scott, J.C.; Yakushi, K.; Street, G.B. Polarons and Bipolarons in Polypyrrole: Evolution of the Band Structure and Optical Spectrum upon Doing. Phys. Rev. B 1984, 30, 1023–1025. [Google Scholar] [CrossRef]
- Santos, M.J.L.; Brolo, A.G.; Girotto, E.M. Study of Polaron and Bipolaron States in Polypyrrole by in Situ Raman Spectroelectrochemistry. Electrochimica Acta 2007, 52, 6141–6145. [Google Scholar] [CrossRef]
- Pang, S.-K. Comprehensive Study of Polymerization of Pyrrole: A Theoretical Approach. J. Electroanal. Chem. 2020, 859, 113886. [Google Scholar] [CrossRef]
- Ishpal; Kaur, A. Spectroscopic and Electrical Sensing Mechanism in Oxidant-Mediated Polypyrrole Nanofibers/Nanoparticles for Ammonia Gas. J. Nanoparticle Res. 2013, 15, 1637. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Tumacder, D.V.; Morávková, Z.; Bober, P. Enhanced Electrochemical Performance of Electrosynthesized Fibrillar Polypyrrole Film. Mater. Lett. 2022, 308, 131295. [Google Scholar] [CrossRef]
- Paúrová, M.; Šeděnková, I.; Hromádková, J.; Babič, M. Polypyrrole Nanoparticles: Control of the Size and Morphology. J. Polym. Res. 2020, 27, 366. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Morávková, Z.; Taboubi, O.; Minisy, I.M.; Bober, P. The Evolution of the Molecular Structure of Polypyrrole during Chemical Polymerization. Synth. Met. 2021, 271, 116608. [Google Scholar] [CrossRef]
- Pandit, V.K.; Soudagar, N.M.; Lokhande, C.D.; Joshi, S.S. Galvanostatically Deposited Polypyrrole Thin Films for Supercapacitor Application: Effect of Surfactant. Mater. Today Proc. 2020, 23, 246–259. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Hwang, B.-J. Identification of Oxidized Polypyrrole on Raman Spectrum. Synth. Met. 2000, 113, 203–207. [Google Scholar] [CrossRef]
- Vigmond, S.J.; Ghaemmaghami, V.; Thompson, M. Raman and Resonance-Raman Spectra of Polypyrrole with Application to Sensor – Gas Probe Interactions. Can. J. Chem. 1995, 73, 1711–1718. [Google Scholar] [CrossRef]
- Bayat, M.; Izadan, H.; Santiago, S.; Estrany, F.; Dinari, M.; Semnani, D.; Alemán, C.; Guirado, G. Study on the Electrochromic Properties of Polypyrrole Layers Doped with Different Dye Molecules. J. Electroanal. Chem. 2021, 886, 115113. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Mazeiko, V.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of Chemical Synthesis of Polypyrrole Particles. Colloids Surf. Physicochem. Eng. Asp. 2015, 483, 224–231. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, Bipolarons, and Solitons in Conducting Polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Iurchenkova, A.A.; Kallio, T.; Fedorovskaya, E.O. Relationships between Polypyrrole Synthesis Conditions, Its Morphology and Electronic Structure with Supercapacitor Properties Measured in Electrolytes with Different Ions and pH Values. Electrochimica Acta 2021, 391, 138892. [Google Scholar] [CrossRef]
- Hazarika, J.; Kumar, A. Controllable Synthesis and Characterization of Polypyrrole Nanoparticles in Sodium Dodecylsulphate (SDS) Micellar Solutions. Synth. Met. 2013, 175, 155–162. [Google Scholar] [CrossRef]
- Hoshina, Y.; Zaragoza-Contreras, E.A.; Farnood, R.; Kobayashi, T. Nanosized Polypyrrole Affected by Surfactant Agitation for Emulsion Polymerization. Polym. Bull. 2012, 68, 1689–1705. [Google Scholar] [CrossRef]
- Bo, J.; Luo, X.; Huang, H.; Li, L.; Lai, W.; Yu, X. Morphology-Controlled Fabrication of Polypyrrole Hydrogel for Solid-State Supercapacitor. J. Power Sources 2018, 407, 105–111. [Google Scholar] [CrossRef]
- Talens-Alesson, F.I.; Hall, S.T.; Hankins, N.P.; Azzopardi, B.J. Flocculation of SDS Micelles with Fe3+. Colloids Surf. Physicochem. Eng. Asp. 2002, 204, 85–91. [Google Scholar] [CrossRef]
- Dai, T.; Yang, X.; Lu, Y. Controlled Growth of Polypyrrole Nanotubule/Wire in the Presence of a Cationic Surfactant. Nanotechnology 2006, 17, 3028–3034. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic Diameter and Zeta Potential of Nanostructured Lipid Carriers: Emphasizing Some Parameters for Correct Measurements. Colloids Surf. Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Wen, J.; Tian, Y.; Mei, Z.; Wu, W.; Tian, Y. Synthesis of Polypyrrole Nanoparticles and Their Applications in Electrically Conductive Adhesives for Improving Conductivity. RSC Adv. 2017, 7, 53219–53225. [Google Scholar] [CrossRef]
- Ravichandran, S.; Nagarajan, S.; Kokil, A.; Ponrathnam, T.; Bouldin, R.M.; Bruno, F.F.; Samuelson, L.; Kumar, J.; Nagarajan, R. Micellar Nanoreactors for Hematin Catalyzed Synthesis of Electrically Conducting Polypyrrole. Langmuir 2012, 28, 13380–13386. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Samuelson, L.A. Enzymatic Synthesis of Conducting Polyaniline in Micelle Solutions. Langmuir 2002, 18, 9696–9704. [Google Scholar] [CrossRef]
- Izza, N.; Suga, K.; Okamoto, Y.; Watanabe, N.; Bui, T.T.; Wibisono, Y.; Fadila, C.R.; Umakoshi, H. Systematic Characterization of Nanostructured Lipid Carriers from Cetyl Palmitate/Caprylic Triglyceride/Tween 80 Mixtures in an Aqueous Environment. Langmuir 2021, 37, 4284–4293. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, P.; Chen, H.; Yao, Y.; Zhao, L.; Zhao, M.; Zhu, L.; Sun, H. Porous Polypyrrole with a Vesicle-like Structure for Efficient Removal of per- and Polyfluoroalkyl Substances from Water: Crucial Role of Porosity and Morphology. J. Hazard. Mater. 2024, 462, 132748. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, W.; Fei, G.; Shu, K.; Sun, L.; Tian, S.; Niu, H.; Wang, M.; Hu, G.; Duan, Y. A Simple Route to Fabricate Ultralong and Uniform Polypyrrole Nanowires with High Electrochemical Capacitance for Supercapacitor Electrodes. ACS Appl. Polym. Mater. 2023, 5, 1254–1263. [Google Scholar] [CrossRef]
- Tang, F.; Jiang, W.; Xie, J.; Zhao, D.; Meng, Y.; Yang, Z.; Lv, Z.; Xu, Y.; Sun, W.; Jiang, Z. PPy-Coated Mo3S4/CoMo2S4 Nanotube-like Heterostructure for High-Performance Lithium Storage. Molecules 2024, 29, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bober, P.; Trchová, M.; Stejskal, J. Polypyrrole Prepared in the Presence of Methyl Orange and Ethyl Orange: Nanotubes versus Globules in Conductivity Enhancement. J. Mater. Chem. C 2017, 5, 4236–4245. [Google Scholar] [CrossRef]
- Bober, P.; Li, Y.; Acharya, U.; Panthi, Y.; Pfleger, J.; Humpolíček, P.; Trchová, M.; Stejskal, J. Acid Blue Dyes in Polypyrrole Synthesis: The Control of Polymer Morphology at Nanoscale in the Promotion of High Conductivity and the Reduction of Cytotoxicity. Synth. Met. 2018, 237, 40–49. [Google Scholar] [CrossRef]
| Sample ID | Py concentration (M) | surfactant | surfactant concentration (M) | Oxidizing agent |
|---|---|---|---|---|
| PPy* | 0.10 | - | - | APS |
| Py-SDS-APS | 0.10 | SDS | 0.10 | APS |
| Py-CTAB-APS | 0.10 | CTAB | 0.10 | APS |
| Py-TW-APS | 0.10 | TW | 5 wt% | APS |
| Py-SDS-Fe | 0.10 | SDS | 0.10 | FeCl3 |
| Py-CTAB-Fe | 0.10 | CTAB | 0.10 | FeCl3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





