Abstract : Docosahexaenoic acid (DHA) is an essential fatty acid (FA) with proven health effects, whom bioavailability improvement is becoming a public health issue. Unlike fish oils, the bioavailability of DHA from microalgal (A) has not been fully assessed, particularly with regard to the molecular structuring capabilities offered by A-oil. We explored the impact of 5 formulas rich in DHA, different by i) the molecular structure: ethyl ester (EE) or monoglyceride (MG) or Triglyceride (TG) and ii) the supramolecular form: emulsified TG or TG+phospholipids (blend PL), on the lymphatic kinetics of DHA absorption and the lipid characteristics of resulting lipoproteins. We demonstrated that in rat, the DHA absorption of the conventional A-DHA TG structure was more effective than the EE structure (+ 23%). More, the A-DHA MG and A-DHA Emulsion were the most favourable DHA-vectors (AUC: 89% and +42%, respectively), thanks to an improved lipolysis. The A-DHA MG and -Emulsion presented the richest DHA content in TG (+40%) and PL (+50%) of lymphatic chylomicrons, which could impact the metabolic fate of DHA. We concluded that structuring A-DHA in TG or EE would be more prone for tissue and hepatic metabolism whereas in A-DHA MG and A-DHA Emulsion could target nerve tissues.
1. Introduction
Long chain omega-3 fatty acids (n-3 LC PUFAs), especially eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA) are recognized for their beneficial health benefits. In particular, DHA is recognized to reduce the risk to develop several chronic pathologies such as, cardiovascular diseases, stroke, neurodegenerative diseases and inflammation [
1,
2,
3,
4,
5,
6]. In humans and animals, the synthesis of DHA is very limited [
7,
8,
9] and therefore it must be provided by the diet, whom main dietary sources are seafood and fatty fish derivatives. However, based on data consumption, the intake of EPA and DHA is twice lower than the current recommendations [
10,
11,
12]. In a context where halieutic resources are declining and to overcome the challenge of the insufficient n-3 LC PUFAs intake, the industry needs to innovate and expand its scientific research.
Microalgal oils as primary source of n-3 LC-PUFA oils are promising and sustainable alternative to fish oils [
13]. They are allergen-free and contaminants free unlike fish oils[
14,
15]. For some years now, in addition to increase the n-3 LC PUFA intakes, current studies are focusing on the benefits of improving their bioavailability by modifying the physical and chemical parameters of fatty acid ( FA) presentation, in order to upgrade the nutritional status of the population. The FA bioavailability is conditioned both, by the molecular form in which the FAs are embedded but also by the supramolecular structure in which they are solubilized [
16,
17,
18,
19,
20,
21].
In the diet, fatty acids (FA), including DHA, are mainly triglyceride (TG) supply (95% of dietary lipids). However other structures such as diglycerides (DG), monoglycerides (MG), ethyl esters (EE), phospholipids (PL) or free fatty acids (FFA) bring FA. Several studies highlighted the impact of the molecular structure on the FA absorption and bioavailability, but with regards to DHA data are heterogeneous and no consensus emerged. Most of studies agree that the EE form appeared to be the least bioavailable form compared to the conventional TG structure [
22,
23]. Conversely studies assessed that the hydrolysate forms, such as free fatty acids (FFA), MG or diglyceride (DG), seemed to be good candidates to increase the amount of DHA in plasma but also its accretion in target tissues [
22,
24,
25,
26,
27,
28,
29] . Overall, the various short- and long-term studies based on human and animal models point out a better bioavailability of DHA when it was incorporated into FFA or MG structures compared with TG structure, and even more compared with EE form. The greater bioaccessibility observed in these studies is mostly explained by the digestion step, which is crucial for FA absorption. Indeed dietary lipids, mainly TG and to a lesser extend PL, need to be hydrolyzed under the enzymatic action of the digestive lipases to be absorbed [
30,
31]. The degree and velocity of lipolysis depends on the chemical form in which FAs are integrated: TG, PL, FFA or EE and also, according to the FA profile of the lipid structures. Thus, it has been described that the hydrolysis level of EE was slower and lower than a DHA-TG structure [
32,
33,
34]. Conversely, the hydrolysis of FFA and MG would be faster and more efficient than TG, as they are lipolysis products and mostly bypass the digestion step [
34,
35]. The lipolysis level directly conditions the FA micellisation by which FA are structured to allow their absorption by the intestinal barrier [
36].
Interestingly by comparing the TG vs. PL as molecular form of n-3 vectorization, the overall intestinal absorption of FA was not influenced but rather their incorporation into lymph [
16,
37] and plasma lipids, altering the size and composition of lipoproteins [
18,
23,
38]. As lipoproteins are the carriers of fatty acids in organism, any change in their composition, in terms of both quantity and quality, would have an impact on the metabolic fate of FA. Thus, by structuring essential FA, we can hope to drive their distribution to specific target tissues. In particular, the PL form appeared to improve PUFAs status in brain and retina, whereas the TG form promoted the incorporation of n-3 LC PUFAs into hepatic lipids [
26,
39,
40].
In addition to the molecular structure, the supramolecular form has been also reported to impact the FA bioavailability, particularly with regards to n-3 PUFA [
20,
36,
39,
41,
42,
43,
44,
45,
46,
47]. Lipid structuring has been widely promoted on FA bioavailability, by structuring physically dietary lipids in emulsions with vegetable PLs, used either as lipid carriers for essential FA or as surfactants in lipid emulsions. The supramolecular form in which lipids are solubilized, plays a role in the FA absorption which ultimately determines the bioavailability of n-3 PUFAs in lymph [
19,
20,
36,
45] or in plasma compartments[
37,
46]. The explanation lies by the improvement of the gastrointestinal lipolysis. Indeed, by creating lipid droplets in suspension, the emulsification process provides a large lipid-water interface available for the adsorption of pancreatic lipase and promotes a faster and more efficient lipolysis compared to the bulk phase oil [
36,
46,
48,
49]. Vegetable PLs (lecithins) are good candidates for improving the interface and hence the digestibility of lipid matrices [
37,
46,
50,
51,
52,
53]. The rate of lipolysis is thus strongly influenced by the supramolecular structures of the lipid matrix [
18,
54,
55] making the gastrointestinal lipolysis a crucial step for FA bioavailability.
The bioavailability of a FA takes into account the various upstream steps: since the digestion and the micellization before the absorption at the intestinal level. Studies highlighted that the digestion step is crucial to FA absorption and conditions its bioavailability. Lipid formulation via their emulsification with lecithin or by structuring FA in different chemical forms are ways for improving their bioavailability. However, many disparities exist between studies, and the link is not to date well established. This is mainly due to the lack of a global study covering all these formulas. Moreover, current data has been focused on fish oil but to date, few studies monitored the bioavailability of DHA from microalgal oils. In this context of lacking both in omega 3 intake and in halieutic resources, microalgal oils could be useful to vectorize DHA by different carrying structures whose bioavailability needs to be still explored. It would be innovative form derived from microalgal oils to enhance in fine, the DHA with the possibility of targeting specific tissues. Lymph is a compartment of choice to study the FA absorption and bioavailability, since after digestion, lipids are directly absorbed through the enterocytes to enter the lymphatic pathway before being metabolized by the liver.
In this context, we assessed the intestinal absorption of DHA-microalgal oil through two aspects: the molecular structure and the supramolecular form of DHA delivery. The potential nutritional effect of algal oil has been assessed in rat lymph fed with a microalgal DHA rich oil (A-DHA), whom DHA was provided either in TG form alone (A-DHA TG), blended with vegetable PL (A-DHA PL blend) or TG in emulsion (A-DHA emulsion), or supplied either in monoacylglycerol structure (A-DHA MG) or ethyl esters (A-DHA EE). Lymph was collected sequentially over a 6-hour post-feeding kinetic and lymph samples were characterized according to their fatty acid composition and quantification. In order to follow the DHA incorporation into lymph lipoproteins as the structure vectorization in the organism, the incorporation of DHA was assessed in the main lipid fractions of lymphatic chylomicrons (CM), TG and PL fractions.
2. Materials and Methods
Material
Omegavie® DHA 800 Algae oil was provided from Polaris (Quimper, France) and the oil emulsion (A-DHA Emulsion) was realized by Polaris under pattern WO 2021224940A1. Polaris synthetized the A-DHA MG, A-DHA EE, A-DHA blend PL formulas. The lipid characterization as, glyceridic composition, fatty acid profile and oil structure, was determined according to the standardization methods of IUPAC 6.002 and NF EN 14105 and NF EN ISO 12966, respectively (Table1).
Acetyl chloride, acetic acid, potassium chloride (KCl), and sodium carbonate (Na2CO3), sodium chloride (NaCl), sodium methoxide (CH3ONa), sulfuric acid (H2SO4) and all organic solvents used; di-ethyl ether, heptane, hexane, iso-octane and methanol (analytical or HPLC grades) were provided by Thermo Fisher Scientific (Strasbourg, France). Internal standard, 1,2-diheptadecanoyl-sn-glycero-3-phosphatidylcholine (PC 17:0), 1,2,3-triheptadecanoyl-sn-glycerol (TG 17:0) were obtained from Avanti Polar Lipids INC (Alabaster, Alabama, USA).
Ketamine, xylazine, buprenorphine and sodium pentobarbital were provided by Axience (Pantin, France).
Experimental design - Animal and surgical procedures
Male Wistar rats (8 weeks-old, body weight 300-350 g) were obtained from Elevage Janvier (Saint-Berthevin, France) and were randomly assigned to one of the 5 dietary groups (A-DHA TG, A-DHA EE, A-DHA PL blend, A-DHA Emulsion, A-DHA MG). Animals were treated in accordance with the European Communities Council Guidelines for the Care and Use of Laboratory Animals (2010/63/ EU). All experiments were conformed to the Guidelines for the Handling and Training of Laboratory Animals. The experiments and procedures were approved by the French ministry, recorded under the APAFIS n° DAP38317-V5-2022083015242376, and were carried out in compliance with the local ethics committee in Bordeaux, France (CEEA50). Rats were housed for at least 3 days before the experiment in a controlled environment, with constant temperature and humidity, and with free access to food and water. The day before surgery, rats were fed a fat-free diet (Epinay, France) and had free access to water. The day of surgery, each rat was placed under anesthesia by an injection of ketamine/xylazine (100/10 mg/kg, respectively). After visualization of the main mesenteric lymph duct, a polyethylene catheter (0.95 mm x 15 cm Biotrol, Paris, France) was inserted, as described by [
46,
56]. After surgery, rats were placed in individual restraining cages, in a warm environment with water freely available. To prevent pain, rats received an intra-peritoneal injection of buprenorphine (0.02 mg/kg) 1h before and 2h after surgery.
In each experiment, an equivalent amount of 300 mg of DHA, were administered to rats (n=6 rats per group), whatever the DHA-rich microalgal oil formulation used. Lymph was collected hourly for 6 h post feeding. At the end of the experiment, rats were euthanized by an intra-peritoneal injection of sodium pentobarbital.
The sequential collection of lymph allows to define the kinetics of intestinal absorption of n-3, according to their DHA-rich microalgal oil formulation administered, as well as the maximum lymphatic concentration (Cmax) of and the time (Tmax) for which, this maximal concentration of n-3 PUFA has been reached.
Fatty acid profile and n-3 LC PUFAs composition in lymph fatty acids
Total fatty acid composition from each batch time of lymph was directly obtained by the method described by Lepage &Roy[
57].
The resulting FA methyl esters (FAME) were analyzed by GC (TRACE GC, Thermo Scientific, Waltham, Massachusetts, USA) equipped with a flame ionization detector (FID) and a split injector. A fused-silica capillary column (BPX 70, 60 m×0.25 mm i.d., 0.25 μm film; SGE, France) was used with hydrogen as a carrier gas (inlet pressure: 120 kPa). The split ratio was 1:33. The column temperature program was as follow: from 150°C, the temperature increased to 200°C at 1.5°C/min, and maintained for 30 minutes before increasing at 20 °C/ min until 225°C for 15 minutes. The injector and detector were maintained at 250°C and 250°C, respectively. GC peaks were integrated using Chromeleon software (Thermofinnigan, Courtaboeuf, France). FA were quantified using tri-heptadecaenoic acid as an internal standard, and added at 10% of the lipid weight before the (trans) methylation procedure.
DHA incorporation in TG and PL fractions of lymph
The collection of lymph samples obtained during the kinetics of lipid uptake (6 points per animal) were pooled for each animal. The resulting single sample per animal represents the DHA absorption over the 6 hours post-feeding. Triglycerides (TG) and phospholipids (PL) are the major lipid fractions in lymph and therefore the major constituents of lymph chylomicrons. They represent around 85% and 10% of lymph lipids respectively.
Total lipids were first extracted using the method of Folch[
58].
PL, FFA, TG and cholesterol ester (CE) fractions from lymph were separated by TLC (glass plates 20 × 20 cm pre-coated with silica gel 60H) using a solvent mixture composed of hexane/ diethyl ether/acetic acid (80/20/1, v/v/v). After vaporisation of 2,7-dichlorofluorescein and visualisation under UV-light, spots corresponding to PL, FFA, TAG and CE were identified by external standards spotted on the plate and extracted from silica gel by addition of 2·5 ml of chloroform/methanol (2/1, v/v). After homogenisation and centrifugation of the scrapped fractions (1050 g, 5 min, 20°C), the organic phase was collected. A 100 μl distilled water volume and chloroform/methanol (2/1, v/v, 2 ml) were added to the silica gel phase. The extraction step was repeated and the organic phase was collected. Lipid extraction from silica gel ended by the addition of 2 ml of methanol to the silica gel phase, homogenisation and centrifugation. The organic phases were collected and dried under N. Finally, 2,7- dichlorofluorescein was removed using 0·4 ml of a KCl solution (0·8 % in distilled water w/v) and 2 ml of chloroform/methanol (2/1, v/v). The organic phase was washed twice by addition of 0·8 ml of a mixture composed of chloroform/methanol/KCL 0·8 % in distilled water (15/240/235, v/v/v). Samples were stored at −20°C until analysis.
The fatty acid composition of the triglyceride (TG) and phospholipid (PL) fractions was determined by gas chromatography (GC-FID) after trans-methylation using the Castro-Gomez method [
59], and quantified by using an internal standard: TG C17:0 or PL 17:0 for TG fraction and PL fraction determinations respectively.
Data are reported as the DHA concentration (µg/µL lymph) and proportion (%) in total fatty acids in the lipid fraction for the 5 formulas studied: A-DHA TG, A-DHA EE, A-DHA PL blend, A-DHA Emulsion, A-DHA MG).
Statistical analysis
Data were expressed as mean values with their standard deviation (SD) and were analyzed by XLStat software, to evaluate the kinetics of absorption of DHA, over the 6 hours following lipid administration. More, it has been determined through the kinetics curve, (i) the area under the curve (AUC; expressed in mg x h/ml) to assess the amount of DHA absorbed and (ii) the maximum lymphatic concentration of DHA (Cmax) and the maximum time (Tmax) to reach it. p-values lower than 0.05 were considered to be statistically significant.
3. Results
3.1. Influence of the lipid carrier on the intestinal absorption of DHA
3.1.1. Total fatty acid absorption
The amount of total fatty acids was determined in animals, over the time-kinetics from 1 to 6 hours after DHA administration from lipid formulations. DHA was supplied either by crude TG, or in emulsion, or as EE, MG or associated with vegetable PL (
Figure 1).
In all groups, lipid administration induced an increase in total FA concentration as early as 3 hours post prandial.
For most formulations, the maximal concentration in total FA in lymph raised 20mg/mL of lymph. The time to reach the Cmax depended on the lipid structure and formulation. More precisely, when DHA was vectorized by TG structure (A-DHA TG), total lipid concentration reached a plateau 5 hours post feeding, whereas provided in emulsion or in MG structure, the plateau was reached 1 hour earlier (Tmax=4hours). In contrast, when lipids were provided as EE structure the Tmax was 1 hour delayed (Tmax=6 hours).
It should be noticed that when lipids (mostly TG structure) were combined to vegetable PLs, the Cmax was doubled (Cmax= 40mg/mL) compared to the other groups, and never reached a steady state until the end of lipid absorption (Tmax=6hours).
More, by considering the AUC data (Insert,
Figure 1), the data showed that AUCs varied by a factor of two, depending on the lipid formulation. In particular, the lowest AUC was found when lipids were provided as TG structure (50mg/mL.h) whereas it was significantly higher when lipids were provided in emulsion or in EE forms (70 mg/mL.h). The AUCs relative to the overall lipid absorption was most favorable in the MG-vectorized lipids group (87mg/mL.h) or when microalgal TGs was combined with plant PL (100mg/mL.h).
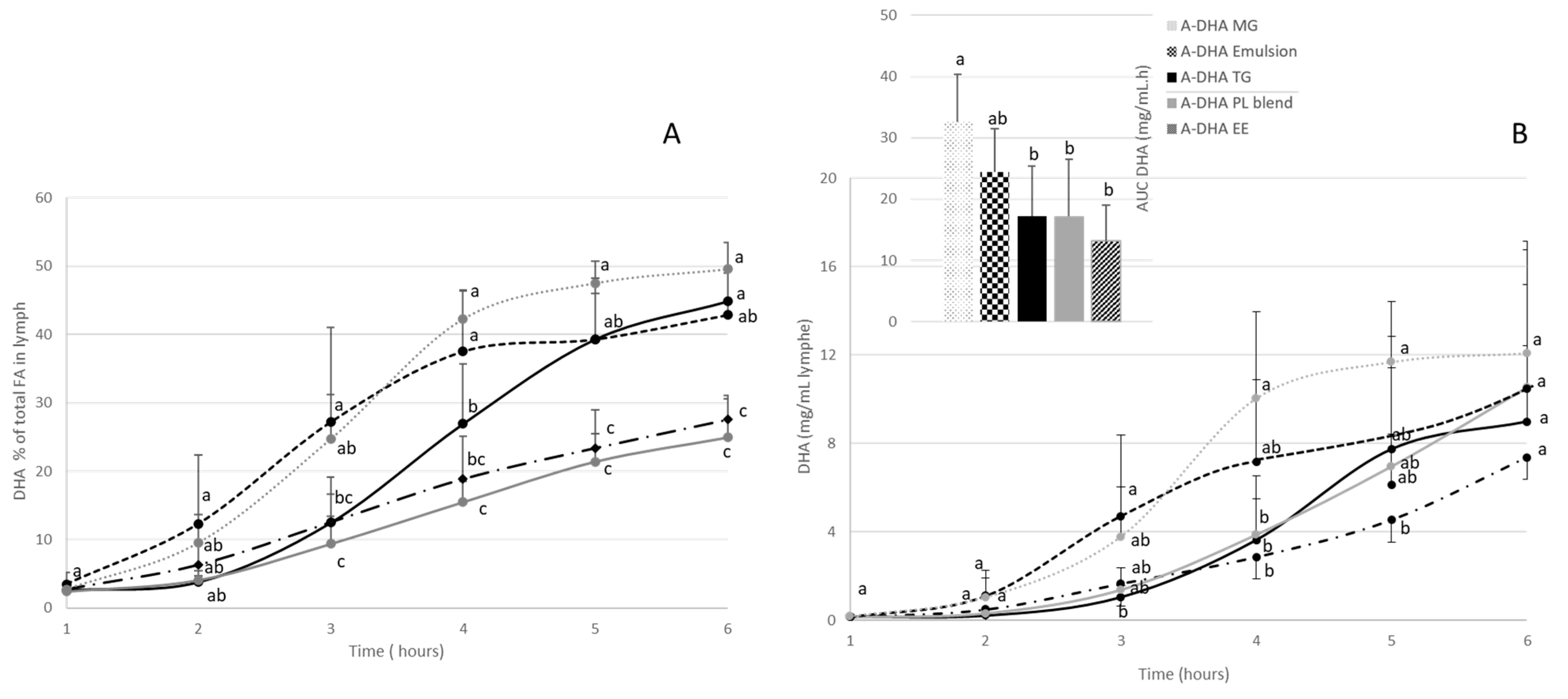
3.1.2. Lymphatic recovery of DHA
With the aim of comparing the DHA bioavailability according to the lipid structure, the DHA was monitored in lymph compartment.
Figure 2 shows the absorption kinetics of DHA in lymph compartment over the 6 hours following lipid administration, both qualitatively (
Figure 2A: % of total fatty acids) and quantitatively (
Figure 2B: mg/mL/g lipid intake).
The kinetics showed that over the 6 hours following lipid administration, the lymph is progressively enriched in DHA, reaching a plateau at the end of the kinetics of lipid absorption (between 4 and 6 hours).
Qualitatively (
Figure 2A), DHA was gradually incorporated into lymph lipids, regardless of the formula used. At the end of the kinetics, DHA represented between 27 and 50% of total fatty acids, depending on the formula used. More precisely, A-DHA PL Blend and A-DHA EE groups had the lowest DHA proportion (around 27% at T 6hours), while the, A-DHA emulsion, A-DHA TG and A-DHA MG groups presented the highest DHA proportion (43%, 45% and 49% of total fatty acids, respectively).
From a quantitative point of view (
Figure 2B and insert), when the DHA levels plateaued, the Cmax values depended on the formula used. More precisely, the A-DHA EE group had the lowest kinetics of DHA incorporation characterized by a Cmax=7mg/mL at T6h, whereas A-DHA TG (9mg DHA/mL) and A-DHA PL Blend and of A-DHA Emulsion (both 10mg DHA/mL) presented intermediate Cmax values. The greater Cmax value (12mg DHA/mL) was obtained for the A-DHA MG formula.
It is to notice that the kinetics of DHA absorption for A-DHA MG and A-DHA Emulsion groups differed from the other groups, particularly in terms of curve shape. In these two groups, the Cmax were close (Cmax of 10 and 12 mg/mL; respectively) and tended to be higher than the Cmax observed in A-DHA EE and A-DHA TG groups (7 and 9mg/mL, respectively). For these two groups their Tmax were 1-2 hour earlier compared to the other groups (4 hours versus 5-6 hours for the other groups).
The data related to the area under the curve (AUC;
Figure 2B) confirmed that the "overall" intestinal absorption of DHA was the lowest for A-DHA EE group (13mg/mL*h). The A-DHA MG group presented significant higher AUC (32mg/mL*h) compared to the AUCs of A-DHA Emulsion (24mg/mL*h), A-DHA TG (17 mg/mL*h) and A-DHA PL Blend (17 mg/mL*h). We noted a trend towards higher DHA bioaccessibility values for the A-DHA Emulsion and A-DHA MG groups.
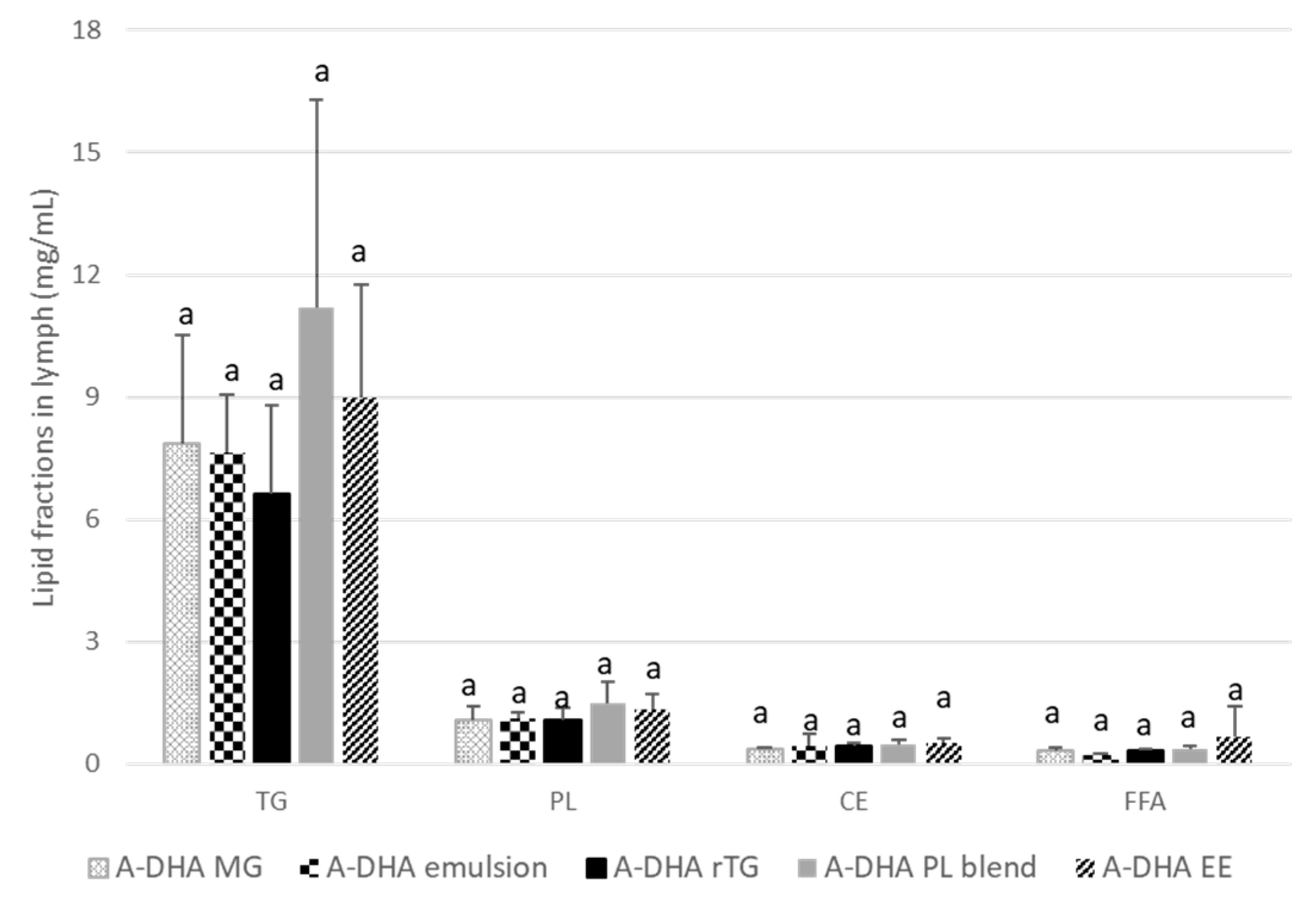
3.1.3. DHA incorporation in the main lipid fractions of lymph
From a constitutive point of view, lymph is composed of TG, PL and to a lesser extent cholesterol ester (CE) and free fatty acids (FFA). The incorporation of DHA was monitored in, TG and PL, which are the major lipid fractions constitutive of the lymphatic lipoproteins, the chylomicrons (
Figure 3).
Regardless of the formula administered, the main lipid fractions in lymph were TGs (78-82% of lymph lipids) and to a lesser extent PLs, which constitute the second lipid fraction of lymph (between 11-13% of lymph lipids). FFA and CE represented less than 5% of lymph lipids.
These proportions slightly fluctuated between groups; particularly with regard to the TG fractions. Indeed, the TG concentrations of the A-DHA PL blend and A-DHA EE groups tended to be higher (11 and 9 µg/µL lymph, respectively) compared to the other groups (on average 7.4µg/µL).
Qualitatively, DHA accounted for 23% to 45% of total FA in lymph TG fraction (
Table 2). DHA was less represented in the lymph TGs of the A-DHA EE and A-DHA PL blend groups, whereas it was significantly higher when DHA was vectorized in A-DHA MG, A-DHA TG and A-DHA Emulsion. On the other hand, from a quantitative point of view, the DHA concentrations were similar and ranging from 2.3 to 3.5 µg/µL lymph.
PLs are the second major fraction of lymph lipids; they are the structural component of chylomicrons and are located at the lipoprotein interface. The DHA was therefore monitored in PL fraction, which constitute with TG, the "membrane" of chylomicrons.
Data
Table 2 showed that, qualitatively, DHA accounted for 3.4% to 10.4% of total fatty acids in lymph PL. It was less represented in the A-DHA EE and A-DHA PL blend formula groups (on average 3.45% of total fatty acids), while it was significantly better incorporated in PLs from the A-DHA MG (10.4%) and A-DHA Emulsion (9.9%) groups. The PL incorporation of DHA was intermediate in A-DHA TG group and represented 6.2%.
Quantitatively, DHA concentrations ranged between 45 to 124 ng/µL lymph. More precisely, DHA concentrations in PLs were significantly higher in A-DHA MG (124 ng/µL lymph) and A-DHA Emulsion (109 ng/µL lymph) groups compared to the A-DHA EE (45 ng/µL lymph), A-DHA PL blend (55 ng/µL lymph and A-DHA TG groups (68 ng/µL lymph).
4. Discussion
To date, microalgal oils are showing a strong interest as new sustainable sources and are promising alternative to fish oils to meet the nutritional requirements of the population in n-3-LC PUFAs [
13,
14]. Studies on the bioavailability of DHA have focused almost exclusively on fish oils, but data on microalgae oils are still scattered. In this context, we determined in rat model, the lymphatic absorption of DHA from microalgal oils according to its molecular and supramolecular forms of vectorization, during a 6-hour post-prandial kinetics. Omegavie® algae (A) has been used in the study for its complete range of microalgae oils, from natural to concentrates up to 800 mg of TG-DHA per gram. DHA from microalgal was designed in different lipid structures: ethyl ester (A-DHA EE), monoglyceride (A-DHA MG) or triglyceride (A-DHA -TG) based on internal processes and patents. In addition to the impact of the molecular structure to vectorize DHA, we also monitored the impact of the supramolecular structure of DHA supply, through the emulsification of TG-DHA (A-DHA emulsion) and by the association of TG-DHA with vegetable PLs (A-DHA blend PL). Rats with lymphatic duct fistulation were submitted to a single bolus of each DHA-formula, so that the DHA intake was strictly the same and represented 300mg/rat. As the endogenous proportion of DHA lymph is negligible (less than 0.3% of total fatty acids), any DHA increase in this compartment can only be associated with its dietary intake.
We clearly showed that the administration of lipids in any form of intake induced an increase in postprandial lipemia in lymph over the 6 hours following lipid formula administration. By considering DHA as the “target molecule” for its nutritional interest and health effect, the addition of DHA-rich formulas significantly enriched lymph in DHA. As previously reported in the literature, the fatty acid composition of lymph reflects that of dietary lipids [
60,
61] and the DHA inter-group variability observed in rat lymph (27-50%) was representative of the FA composition of formulas. On the one hand, we found a direct impact of the molecular structure on lipid absorption. More precisely, although the AUC values were similar between groups, the kinetics of lipid appearance in lymph differed according to their form of vectorization. The Cmax value for total FA was twice higher when TG were associated with PL (A-DHA with PL; 40mg/mL) compared to the other formulas (DHA-TG, DHA-emulsion, DHA-MG and DHA-EE groups (20mg/mL)). By comparing the overall intestinal absorption of DHA, the TG structure blended or not with PL (A-DHA TG and A-DHA Blend PL) was intermediate. The Tmax values for reaching the Cmax was faster (2hours earlier) by vectoring DHA in Emulsion and by MG structure (Tmax=4hours) compared with the EE structure or the TG blended with PL (Tmax =6hours). Our results indicate that the emulsification of lipids and the MG structure were the best formulas for improving the intestinal absorption of DHA. Our data are in line with other works in which the impact of the supramolecular structure impacted the intestinal absorption and bioavailability of FA. In particular, the emulsification process of fish oil enhanced the absorption of n-3 LC PUFA [
39,
41,
42,
62] and advanced the peak of FA absorption (Tmax) with a greater amplitude compared to the oil in bulk phase. Some authors established the link with the digestion step to explain the increased bioavailability. They highlighted the efficiency of gastric emptying via the preformed lipid droplets[
63,
64]. More, the presence of a lipid/water interface favors the anchoring of pancreatic lipase necessary for TG hydrolysis and favors the FA micellization [
30,
65]. In contrast, it should be pointed out that the conformation of DHA generates a steric hindrance limiting the access to lipases during the digestion process [
30,
60,
66]. As a result, by creating a larger interface, the emulsification facilitates the access to lipases and thus the lipolysis of structures containing DHA. In the present study, the emulsification process makes it easier to generate lipid droplets during the digestion step and therefore a broader interface, which one is essential for the anchoring and activity of the lipases involved in lipolysis [
65,
66,
67]. By promoting the action of lipase and lipolysis, the hydrolysis products (MG and FFA) will be faster generated and micellized into mixed lipid/bile salt micelles ; a primordial stage that promotes the intestinal FA absorption.
On the other hand, the absorption of FA and DHA was also improved by modifying the molecular structure of lipid. Thus, we observed that the MG structure seemed to better vectorize FAs compared to the conventional TG structure and to a greater extent than the EE structure. When DHA was vectorized by MG (formula A-DHA MG), the better absorption observed in our study is not due to a better digestibility but rather to an optimal FA solubilization and a faster FA absorption by the intestinal cells. Indeed, MGs are lipid hydrolysates and as a result they can mostly bypass the lipolysis step to enter directly in the micellization phase and being faster absorbed at the gut level [
26,
27,
28]. Lipases are stereospecific for the external positions of the glycerol backbone. However, when grafted at the external position of glyceride structures, DHA generate a the stereochemical hindrance which limits the hydrolysis of the ester bond. Thus, due to the presence of DHA on the external position of our formula, only a small part of the sn-1(3)DHA-MGs formula would be hydrolyzed. In this instance, by these 2 specifications (faster micellization and DHA hindrance), the resulting profile of our mixed micelles would be mainly composed of non-hydrolyzed sn-1(3)DHA-MG and by a small proportion of DHA-FFA.
At cellular level, the acute and faster FA uptake occurred with the MG and emulsion formulas can induce some metabolic changes during the synthesis of lymphatic lipoproteins (Chylomicrons; CM) and modify the morphological characteristics of lymphatic CM [
18,
36]. As, CM are vectors of FA from lymph to target tissues via the bloodstream, their analysis allows to predict the lipid form of FA vectorization. After being absorbed by the intestinal mucosa, FFA and MG are re-used in the enterocytes to synthetize, mostly TGs (85% of total lipids) which form the core of CM, and to a lesser extent, some PLs (approx.10% of total lipids) to form the CM membrane [
68,
69]. By monitoring the incorporation of DHA into lipid fractions of lymph, we get an overview of the lipid characteristics of CM in which DHA will be vectorized to tissues.
The TG fraction tended to be higher in A-DHA PL blend (+57%) and in A-DHA EE groups (+28%) compared to the FA structuration in TG, MG and in emulsion. However, the A-DHA TG, A-DHA MG and A-DHA Emulsion structuration allowed to improve the DHA content by 40% in lymphatic TG. Only the MG and Emulsion structuration increased the DHA content in lymphatic PL by 50%. The enrichment in DHA occurred in both the TG and PL lymphatic fractions follow the initial observation of an improved FA uptake by the enterocytes and could induce some metabolic changes related to the lipid synthesis pathways in cells. After their intestinal absorption, FA are predominantly incorporated into the “re-synthesized” TG via the main metabolic pathway of "2-MG". The second and minor metabolic pathway (G3P pathway) is also involved in the synthesis of TG but also of PL and occurs during the interprandial period or at the end of the lipid absorption [
70]. Thus, the presence of DHA-PL in CM in A-DHA MG and A-DHA Emulsion groups would be the result of the activation and the earlier stimulation of this 2nd pathway. The G3P activation enable a threefold increase of DHA incorporation into the lymph PL of CM. PLs are the constituents of CM membrane and any change of FA composition in PL fraction modifies therefore the characteristics of the CM membrane and the FA metabolic fate. These data are in agreement with previous works in which authors observed the activation of both 2-MG and G3P pathways, to rapidly compensate the "influx" of FA in the enterocyte following the improved lipolysis[
18,
37].
The kinetics of CM synthesis has been described as a two-step process, starting by an increased diameter as lipids were absorbed, particularly at the beginning of the kinetic and second, and then beyond a certain diameter by a stimulated synthesis of the number of particle. Based on the literature, we conclude that the CMs of both TG blend PL and EE groups were either, larger in diameter with a denser core of TG, or ii) more numerous compared to the CM in A-DHA MG and A-DHA Emulsion groups. The CMs produced by the TG-DHA formula appear to be intermediates with medium-sized CMs rich in TG-DHA. In contrast, CM rich in PL-DHA and TG-DHA produced with A-DHA emulsion and A-DHA MG formulas could differently vectorize DHA in the organism. For instance, it has been reported that PL-DHA is more prone for incorporating DHA in nervous tissue, such as the retina or brain, whereas TG-DHA is more likely to be used by the liver [
26,
71].
5. Conclusion
In our study, we clearly demonstrated that the molecular structure obtained with microalgal oil (MG, EE or TG) to carry DHA, but also the supramolecular structure (TG emulsified or blended with vegetable PL) modulated the digestibility of DHA-containing structures and directly impacted its absorption and metabolic fate.
On the one hand, the DHA-TG form enables to vectorized DHA similarly when carried alone or blended with vegetable PLs and was found to be an intermediate compared to the other structures assessed (EE or MG, or emulsified). On the other hand, the vectorization of DHA by EE structure (A-DHA EE formula) was the least conducive to DHA bioavailability, and presented, as for A-DHA PL blend, voluminous CM with a core of TGs and a membrane poor in DHA. Conversely the A-DHA MG and A-DHA Emulsion formulas were the most favorable systems to carry DHA as the A-DHA MG formula mostly bypassed the lipolysis step whereas the A-DHA Emulsion formula would accelerate the lipid digestion by creating a larger interface. Both formulations contributed, in different ways, to improve the digestion and absorption steps to enhance the intestinal FA absorption process. The resulting lymphatic lipoproteins presented a core dense in TG-DHA and a membrane 3 times more concentrated in PL-DHA compared to A-DHA EE and A-DHA PL blend. By modifying the characteristics of CMs, Omegavie DHA-TG blended or not with PLs would be more prone for tissues and hepatic metabolism whereas the formulas DHA-MG or DHA-TG in emulsion would be more specifically directed to nervous tissues (retina or brain tissue).
6. Patents
The emulsion of Omegavie® oil has been developed by Polaris under pattern n° WO 2021224940A1.
Author Contributions
Conceptualization, Leslie Couëdelo, Stéphanie Lennon, Carole Vaysse and Gildas Breton; Data curation, Leslie Couëdelo; Formal analysis, Hélène Abrous, Ikram Chamekh, Corentin Bouju, Hugues Griffon and Lionel Larvol; Investigation, Leslie Couëdelo, Hélène Abrous, Ikram Chamekh and Carole Vaysse; Methodology, Leslie Couëdelo, Stéphanie Lennon and Carole Vaysse; Project administration, Leslie Couëdelo, Stéphanie Lennon, Carole Vaysse and Gildas Breton; Resources, Lionel Larvol and Gildas Breton; Software, Leslie Couëdelo and Carole Vaysse; Supervision, Leslie Couëdelo and Stéphanie Lennon; Validation, Leslie Couëdelo, Hélène Abrous, Ikram Chamekh and Carole Vaysse; Writing – original draft, Leslie Couëdelo, Stéphanie Lennon, Lionel Larvol and Gildas Breton; Writing – review & editing, Leslie Couëdelo, Stéphanie Lennon, Carole Vaysse, Lionel Larvol and Gildas Breton.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of University of Bordeaux, France (CE050), and approved by the French ministry, recorded under the n° DAP38317-V5-2022083015242376 (accepted on March 20th 2023). Animals were treated in accordance with the European Communities Council Guidelines for the Care and Use of Laboratory Animals (2010/63/ EU). All experiments were conformed to the Guidelines for the Handling and Training of Laboratory Animals.
Acknowledgments
Authors are members of the “Unité mixte technologique” ACTIA (UMT Profeel)
Conflicts of Interest
The authors declare no conflicts of interest. Polaris funded and contracted ITERG for the experiments.
References
- Dyall, S.C.; Michael-Titus, A.T. Neurological Benefits of Omega-3 Fatty Acids. Neuromolecular Med. 2008, 10, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A Systemic Review of the Roles of N-3 Fatty Acids in Health and Disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Tressou, J.; Buaud, B.; Simon, N.; Pasteau, S. Inadequate Daily Intakes of N-3 Polyunsaturated Fatty Acids (PUFA) in the General French Population of Children (3-10 Years) and Adolescents (11-17 Years): The INCA2 Survey. Eur. J. Nutr. 2019, 58, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H.; Eskin, N.A.M. Functional Foods and Cardiovascular Disease; CRC Press, 2012; ISBN 978-1-4200-7111-5.
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and Conversion of Plant Based Sources of Omega-3 Fatty Acids – a Scoping Review to Update Supplementation Options for Vegetarians and Vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of Alpha-Linolenic Acid to Eicosapentaenoic, Docosapentaenoic and Docosahexaenoic Acids in Young Women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, Interconversion, and Dose Response of n-3 Fatty Acids in Humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Actualisation Des Apports Nutritionnels Conseillés Pour Les Acides Gras - Version Intégrant Les Modifications Apportées Par l’erratum Du 28 Juillet 2011 | Anses - Agence Nationale de Sécurité Sanitaire de l’alimentation, de l’environnement et Du Travail. Available online: https://www.anses.fr/fr/content/actualisation-des-apports-nutritionnels-conseill%C3%A9s-pour-les-acides-gras-version-int%C3%A9grant-0 (accessed on 23 August 2021).
- AVIS et RAPPORT de l’Anses Sur l’Actualisation de La Base de Données Des Consommations Alimentaires et l’estimation Des Apports Nutritionnels Des Individus Vivant En France Par La Mise En Oeuvre de La 3ème Étude Individuelle Nationale Des Consommations Alimentaires (Etude INCA3) | Anses - Agence Nationale de Sécurité Sanitaire de l’alimentation, de l’environnement et Du Travail. Available online: https://www.anses.fr/fr/content/avis-et-rapport-de-lanses-sur-lactualisation-de-la-base-de-donn%C3%A9es-des-consommations (accessed on 23 August 2021).
- Dubuisson, C.; Carrillo, S.; Dufour, A.; Havard, S.; Pinard, P.; Volatier, J.-L. The French Dietary Survey on the General Population (INCA3). EFSA Support. Publ. 2017, 14, 1351E. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 Fatty Acids: Alternative Sources of Production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal Biofactories: A Promising Approach towards Sustainable Omega-3 Fatty Acid Production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Ji, L.; Song, X.; Kuang, C. Changes of Lipid Content and Fatty Acid Composition of Schizochytrium Limacinum in Response to Different Temperatures and Salinities. Process Biochem. 2007, 42, 210–214. [Google Scholar] [CrossRef]
- Cansell, M. Marine Phospholipids as Dietary Carriers of Long-Chain Polyunsaturated Fatty Acids. Lipid Technol. 2010, 22, 223–226. [Google Scholar] [CrossRef]
- Cansell, M.; Nacka, F.; Combe, N. Marine Lipid-Based Liposomes Increase in Vivo FA Bioavailability. Lipids 2003, 38, 551–559. [Google Scholar] [CrossRef]
- Couëdelo, L.; Termon, A.; Vaysse, C. Matrice lipidique et biodisponibilité de l’acide alpha-linolénique. OCL 2017, 24, D204. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A Randomised Cross-over Trial in Healthy Adults Indicating Improved Absorption of Omega-3 Fatty Acids by Pre-Emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K. Multiscale Structures of Lipids in Foods as Parameters Affecting Fatty Acid Bioavailability and Lipid Metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef]
- Fardet, A.; Souchon, I.; Dupont, D. Structure Des Aliments et Effets Nutritionnels; Syntheses; Editions Quae, 2013;
- Lawson, L.D.; Hughes, B.G. Human Absorption of Fish Oil Fatty Acids as Triacylglycerols, Free Acids, or Ethyl Esters. Biochem. Biophys. Res. Commun. 1988, 152, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into Plasma Phospholipids in Response to Different Omega-3 Fatty Acid Formulations - a Comparative Bioavailability Study of Fish Oil vs. Krill Oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- el Boustani, S.; Colette, C.; Monnier, L.; Descomps, B.; Crastes de Paulet, A.; Mendy, F. Enteral Absorption in Man of Eicosapentaenoic Acid in Different Chemical Forms. Lipids 1987, 22, 711–714. [Google Scholar] [CrossRef]
- Kling, D.F.; Johnson, J.; Rooney, M.; Davidson, M. Omega-3 Free Fatty Acids Demonstrate More Than 4-Fold Greater Bioavailability for EPA and DHA Compared with Omega-3-Acid Ethyl Esters in Conjunction with a Low-Fat Diet: The ECLIPSE Study†. J. Clin. Lipidol. 2011, 5, 231. [Google Scholar] [CrossRef]
- Destaillats, F.; Oliveira, M.; Bastic Schmid, V.; Masserey-Elmelegy, I.; Giuffrida, F.; Thakkar, S.K.; Dupuis, L.; Gosoniu, M.L.; Cruz-Hernandez, C. Comparison of the Incorporation of DHA in Circulatory and Neural Tissue When Provided as Triacylglycerol (TAG), Monoacylglycerol (MAG) or Phospholipids (PL) Provides New Insight into Fatty Acid Bioavailability. Nutrients 2018, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, C.; Destaillats, F.; Thakkar, S.K.; Goulet, L.; Wynn, E.; Grathwohl, D.; Roessle, C.; de Giorgi, S.; Tappy, L.; Giuffrida, F.; et al. Monoacylglycerol-Enriched Oil Increases EPA/DHA Delivery to Circulatory System in Humans with Induced Lipid Malabsorption Conditions1. J. Lipid Res. 2016, 57, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Cuenoud, B.; Rochat, I.; Gosoniu, M.L.; Dupuis, L.; Berk, E.; Jaudszus, A.; Mainz, J.G.; Hafen, G.; Beaumont, M.; Cruz-Hernandez, C. Monoacylglycerol Form of Omega-3s Improves Its Bioavailability in Humans Compared to Other Forms. Nutrients 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H. (Sabrina); Carne, A.; Bekhit, A.E. Marine Omega-3 (N-3) Phospholipids: A Comprehensive Review of Their Properties, Sources, Bioavailability, and Relation to Brain Health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Borgström, B. On the Interactions between Pancreatic Lipase and Colipase and the Substrate, and the Importance of Bile Salts. J. Lipid Res. 1975, 16, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Carrière, F.; Verger, R.; Lookene, A.; Olivecrona, G. Lipase Structures at the Interface between Chemistry and Biochemistry. EXS 1995, 73, 3–26. [Google Scholar]
- Valenzuela, A.; Valenzuela, V.; Sanhueza, J.; Nieto, S. Effect of Supplementation with Docosahexaenoic Acid Ethyl Ester and Sn-2 Docosahexaenyl Monoacylglyceride on Plasma and Erythrocyte Fatty Acids in Rats. Ann. Nutr. Metab. 2005, 49, 49–53. [Google Scholar] [CrossRef]
- Mu, H.; Porsgaard, T. The Metabolism of Structured Triacylglycerols. Prog. Lipid Res. 2005, 44, 430–448. [Google Scholar] [CrossRef]
- Banno, F.; Doisaki, S.; Shimizu, N.; Fujimoto, K. Lymphatic Absorption of Docosahexaenoic Acid given as Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2002, 48, 30–35. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Muriel Mundo, J.; McClements, D.J. Factors Impacting Lipid Digestion and Nutraceutical Bioaccessibility Assessed by Standardized Gastrointestinal Model (INFOGEST): Emulsifier Type. Food Res. Int. 2020, 137, 109739. [Google Scholar] [CrossRef]
- Couëdelo, L.; Joseph, C.; Abrous, H.; Chamekh-Coelho, I.; Vaysse, C.; Baury, A.; Guillemet, D. Effect of Gum Acacia on the Intestinal Bioavailability of N-3 Polyunsaturated Fatty Acids in Rats. Biomolecules 2022, 12, 975. [Google Scholar] [CrossRef]
- Sehl, A.; Couëdelo, L.; Chamekh-Coelho, I.; Vaysse, C.; Cansell, M. In Vitro Lipolysis and Lymphatic Absorption of N-3 Long-Chain PUFA in the Rat: Influence of the Molecular Lipid Species as Carrier. Br. J. Nutr. 2019, 122, 639–647. [Google Scholar] [CrossRef]
- Robert, C.; Buisson, C.; Couëdelo, L.; Meugnier, E.; Knibbe, C.; Loizon, E.; Fonseca, L.; Laugerette, F.; Vaysse, C.; Michalski, M.-C. Differential Metabolic Impact of Natural Food-Grade Emulsifiers Rich in Alpha-Linolenic Acid. Curr. Dev. Nutr. 2020, 4, nzaa045_094. [Google Scholar] [CrossRef]
- Sehl, A.; Couëdelo, L.; Vaysse, C.; Cansell, M. Intestinal Bioavailability of N-3 Long-Chain Polyunsaturated Fatty Acids Influenced by the Supramolecular Form of Phospholipids. Food Funct. 2020, 11, 1721–1728. [Google Scholar] [CrossRef]
- Amate, L.; Gil, A.; Ramírez, M. Dietary Long-Chain Polyunsaturated Fatty Acids from Different Sources Affect Fat and Fatty Acid Excretions in Rats. J. Nutr. 2001, 131, 3216–3221. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A Randomised Cross-over Trial in Healthy Adults Indicating Improved Absorption of Omega-3 Fatty Acids by Pre-Emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Haug, I.J.; Sagmo, L.B.; Zeiss, D.; Olsen, I.C.; Draget, K.I.; Seternes, T. Bioavailability of EPA and DHA Delivered by Gelled Emulsions and Soft Gel Capsules. Eur. J. Lipid Sci. Technol. 2011, 113, 137–145. [Google Scholar] [CrossRef]
- McClements, D.J.; Saliva-Trujillo, L.; Zhang, R.; Zhang, Z.; Zou, L.; Yao, M.; Xiao, H. Boosting the Bioavailability of Hydrophobic Nutrients, Vitamins, and Nutraceuticals in Natural Products Using Excipient Emulsions. Food Res. Int. 2016, 88, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Q.; Li, W.; Wright, A.J. Emulsification of Algal Oil with Soy Lecithin Improved DHA Bioaccessibility but Did Not Change Overall in Vitro Digestibility. Food Funct 2014, 5, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Redmon, J.B.; Wimmergren, N.; Donadio, J.V.; Bibus, D.M. Enhanced Absorption of N-3 Fatty Acids from Emulsified Compared with Encapsulated Fish Oil. J. Am. Diet. Assoc. 2009, 109, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Couëdelo, L.; Boué-Vaysse, C.; Fonseca, L.; Montesinos, E.; Djoukitch, S.; Combe, N.; Cansell, M. Lymphatic Absorption of α-Linolenic Acid in Rats Fed Flaxseed Oil-Based Emulsion. Br. J. Nutr. 2011, 105, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Devaraj, V.C.; Ramesh, M.; Lokesh, B.R. Lymphatic Transport of α-Linolenic Acid and Its Conversion to Long Chain n-3 Fatty Acids in Rats Fed Microemulsions of Linseed Oil. Lipids 2014, 49, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Armand, M. Lipases and Lipolysis in the Human Digestive Tract: Where Do We Stand? : Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Golding, M.; Wooster, T.J. The Influence of Emulsion Structure and Stability on Lipid Digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Couëdelo, L.; Amara, S.; Lecomte, M.; Meugnier, E.; Monteil, J.; Fonseca, L.; Pineau, G.; Cansell, M.; Carrière, F.; Michalski, M.C.; et al. Impact of Various Emulsifiers on ALA Bioavailability and Chylomicron Synthesis through Changes in Gastrointestinal Lipolysis. Food Funct. 2015, 6, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Sehl, A.; Couëdelo, L.; Vaysse, C.; Cansell, M. Intestinal Bioavailability of N-3 Long-Chain Polyunsaturated Fatty Acids Influenced by the Supramolecular Form of Phospholipids. Food Funct. 2020, 11, 1721–1728. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Knibbe, C.; Fonseca, L.; Buisson, C.; Errazuriz-Cerda, E.; Meugnier, E.; Loizon, E.; Vaysse, C.; Michalski, M.-C. Rapeseed Lecithin Increases Lymphatic Lipid Output and α-Linolenic Acid Bioavailability in Rats. J. Nutr. 2020, 150, 2900–2911. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M.-C. Vegetable Lecithins: A Review of Their Compositional Diversity, Impact on Lipid Metabolism and Potential in Cardiometabolic Disease Prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef]
- Lutz, O.; Meraihi, Z.; Mura, J.L.; Frey, A.; Riess, G.H.; Bach, A.C. Fat Emulsion Particle Size: Influence on the Clearance Rate and the Tissue Lipolytic Activity. Am. J. Clin. Nutr. 1989, 50, 1370–1381. [Google Scholar] [CrossRef]
- Lamothe, S.; Jolibois, É.; Britten, M. Effect of Emulsifiers on Linseed Oil Emulsion Structure, Lipolysis and Oxidation during in Vitro Digestion. Food Funct. 2020, 11, 10126–10136. [Google Scholar] [CrossRef]
- Bollman, J.L.; Cain, J.C.; Grindlay, J.H. Techniques for the Collection of Lymph from the Liver, Small Intestine, or Thoracic Duct of the Rat. J. Lab. Clin. Med. 1948, 33, 1349–1352. [Google Scholar] [PubMed]
- Lepage, G.; Roy, C.C. Improved Recovery of Fatty Acid through Direct Transesterification without Prior Extraction or Purification. J Lipid Res 1984, 25, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, P.; Fontecha, J.; Rodríguez-Alcalá, L.M. A High-Performance Direct Transmethylation Method for Total Fatty Acids Assessment in Biological and Foodstuff Samples. Talanta 2014, 128, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Sasaki, E.; Yasunami, H.; Nomiyama, S.; Nakayama, M.; Sugano, M.; Imaizumi, K.; Yazawa, K. Digestion and Lymphatic Transport of Eicosapentaenoic and Docosahexaenoic Acids given in the Form of Triacylglycerol, Free Acid and Ethyl Ester in Rats. Biochim. Biophys. Acta 1995, 1259, 297–304. [Google Scholar] [CrossRef]
- Lambert, M.S.; Botham, K.M.; Mayes, P.A. Modification of the Fatty Acid Composition of Dietary Oils and Fats on Incorporation into Chylomicrons and Chylomicron Remnants. Br. J. Nutr. 1996, 76, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Dey, T. kumar; Ghosh, S.; Ghosh, M.; Koley, H.; Dhar, P. Comparative Study of Gastrointestinal Absorption of EPA & DHA Rich Fish Oil from Nano and Conventional Emulsion Formulation in Rats. Food Res. Int. 2012, 49, 72–79. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring Food Emulsions in the Gastrointestinal Tract to Modify Lipid Digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured Emulsion-Based Delivery Systems: Controlling the Digestion and Release of Lipophilic Food Components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Carriere, F.; Barrowman, J.A.; Verger, R.; Laugier, R. Secretion and Contribution to Lipolysis of Gastric and Pancreatic Lipases during a Test Meal in Humans. Gastroenterology 1993, 105, 876–888. [Google Scholar] [CrossRef]
- Desnuelle, P. Pancreatic Lipase. Adv. Enzymol. Relat. Subj. Biochem. 1961, 23, 129–161. [Google Scholar] [PubMed]
- Borgström, B. On the Interactions between Pancreatic Lipase and Colipase and the Substrate, and the Importance of Bile Salts. J. Lipid Res. 1975, 16, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M. A Proposed Model for the Assembly of Chylomicrons. Atherosclerosis 2000, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.J.; Plonné, D.; Higgins, J.A. Intracellular Events in the Assembly of Chylomicrons in Rabbit Enterocytes. J. Lipid Res. 2000, 41, 1728–1739. [Google Scholar] [CrossRef]
- Lee, J.; Ridgway, N.D. Substrate Channeling in the Glycerol-3-Phosphate Pathway Regulates the Synthesis, Storage and Secretion of Glycerolipids. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 2020, 1865, 158438. [Google Scholar] [CrossRef]
- Sehl, A.; Couëdelo, L.; Vaysse, C.; Cansell, M. Intestinal Bioavailability of N-3 Long-Chain Polyunsaturated Fatty Acids Influenced by the Supramolecular Form of Phospholipids. Food Funct. 2020, 11, 1721–1728. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 ), A-DHA EE (
), A-DHA EE ( ), A-DHA PL Blend (
), A-DHA PL Blend ( ), A-DHA MG (….), A-DHA Emulsion (----). Data are presented as means ± standard deviation. a,b,c: fFor a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test).
), A-DHA MG (….), A-DHA Emulsion (----). Data are presented as means ± standard deviation. a,b,c: fFor a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test).
 ), A-DHA EE (
), A-DHA EE ( ), A-DHA PL Blend (
), A-DHA PL Blend ( ), A-DHA MG (….), A-DHA Emulsion (----). Data are presented as means ± standard deviation. a,b,c: fFor a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test).
), A-DHA MG (….), A-DHA Emulsion (----). Data are presented as means ± standard deviation. a,b,c: fFor a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test).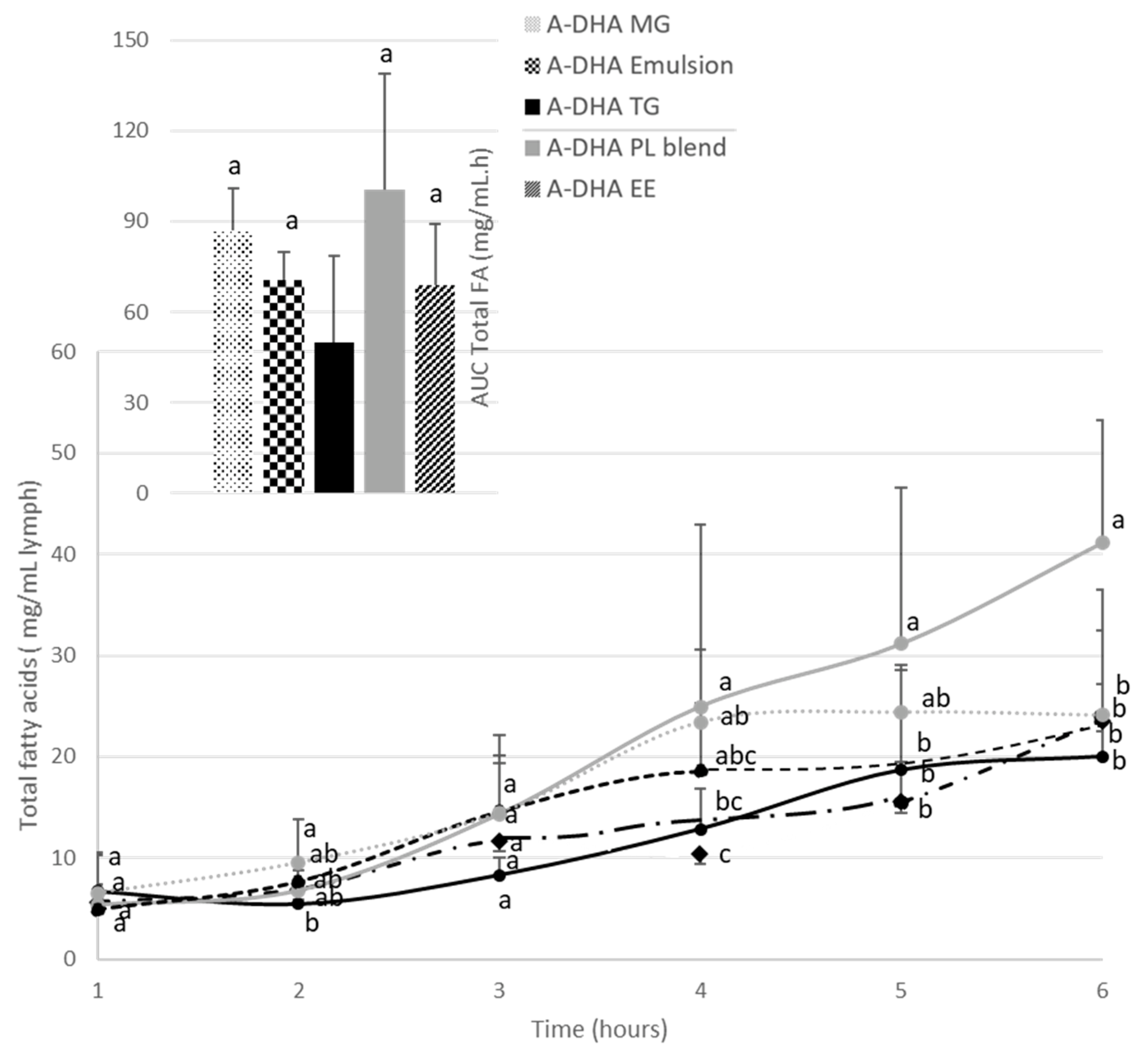
 ) , A-DHA EE (
) , A-DHA EE ( ), A-DHA PL Blend (
), A-DHA PL Blend ( ), A-DHA MG (….), A-DHA Emulsion (----) over a 6-hour period. Data are presented as means ± standard deviation. a,b,c: For a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test). DHA: docosahexaenoic acid.
), A-DHA MG (….), A-DHA Emulsion (----) over a 6-hour period. Data are presented as means ± standard deviation. a,b,c: For a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test). DHA: docosahexaenoic acid.
 ) , A-DHA EE (
) , A-DHA EE ( ), A-DHA PL Blend (
), A-DHA PL Blend ( ), A-DHA MG (….), A-DHA Emulsion (----) over a 6-hour period. Data are presented as means ± standard deviation. a,b,c: For a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test). DHA: docosahexaenoic acid.
), A-DHA MG (….), A-DHA Emulsion (----) over a 6-hour period. Data are presented as means ± standard deviation. a,b,c: For a time point, values are significantly different between the 6 groups (p<0.05; ANOVA, Mann Whitney post hoc test). DHA: docosahexaenoic acid.