Introduction
Thyroid surgery rates have tripled over the past 3 decades making it one of the most frequently performed procedures within general surgery. Moreover, the increase in number of the incidence of papillary cancer, especially low risk, has contributed to an increase in number of thyroid surgeries performed (1,2).
Surgical treatment of thyroid diseases is associated with the possibility of serious postoperative complications having a significant impact on the patient’s quality of life. The recurrent laryngeal nerve (RLN) palsy and the external branch of the superior laryngeal nerve (EBSLN) palsy are, next to hypoparathyroidism and bleeding after operation, one of the most common complications. While unilateral RLN injury usually results in hoarseness and changes in the timbre of the voice, the bilateral injury may result in complete loss of voice, shortness of breath, stridor and life-threatening acute respiratory failure. This condition often requires a tracheostomy. According to Jeannon and al. about 1 in 10 patients experience temporary recurrent laryngeal nerve injury after thyroid surgery, with longer lasting voice problems in up to 1 in 25 (3). Whereas the EBSLN injury is the most underestimated vocal folds paralysis in the statistics of complications of thyroid surgery. The frequency of this injury is estimated at 0.3-58% during the procedure, and its detection is practically impossible during postoperative laryngoscopy, therefore complications related to this nerve are often omitted. Its unilateral injury leads to a changes in the voice timbre and significantly weakens its strength. In turn, bilateral injury may cause a hoarse, monotonous voice, which quickly weakens with vocalization. Its injury is particularly burdensome for people working with voice (4). The frequency of vocal cord paralysis depends on the type of surgery (primary/secondary surgery), thyroid pathology (non-cancerous goiter/thyroid cancer/Graves’ disease), the extent of the surgery (partial/total removal of the thyroid gland), and the experience of the surgeon himself (3)
For years, the gold standard for preventing laryngeal nerve injury during thyroid surgery has been intraoperative identification of the laryngeal nerves. Visual identification of the RLN and the EBSLN allows only to confirm anatomical integrity of the laryngeal nerves, without the possibility of assessing their function. The introduction of neuromonitoring into thyroid surgery, enabling both confirmation of anatomical integrity and assessment of laryngeal nerve function, was a milestone that has begun a new era in thyroid surgery, which has become much safer and more precise over the last three decades. The use of the laryngeal nerve monitoring provides a chance to avoid the most serious complication in thyroid surgery, which is bilateral vocal fold paresis, by enabling operator to finish the surgery after unilateral nerve injury detection (staged thyroidectomy) (4,5,6,7,8)
Intraoperative neuromonitoring is an increasingly accepted tool for identifying both the RLN and the EBSLN. In 2011, Randolph et al. published recommendations for the identification of the RLN, and in 2013, Barczyński et al. together with the International Neural Monitoring Study Group proposed guidelines on the method and technique of identifying the RLN using the neuromonitoring method (5,6). In 2018, further international neural monitoring guidelines for optimal use of IONM appeared: part I -regarding staging bilateral thyroid surgery with monitoring loss of signal and part II- for optimal recurrent laryngeal nerve management for invasive thyroid cancer (7,8). In 2021 consensus statement about informed consent for intraoperative neural monitoring in thyroid and parathyroid surgery has been published (6). For over three decades, intraoperative RLN neuromonitoring as a standardized method has been increasingly used around the world. The benefits of using neuromonitoring in the clinical, educational and legal aspects determine the widespread acceptance of this method by both experienced surgeons and doctors undergoing specialization (5,6,7,8,10,11,12).
Thyroid Surgery
Surgical treatment of thyroid diseases has always been associated with the risk of voice disorders, which affect proper functioning of a person in a society, and for many also their professional life. Voice disorders after thyroid surgery are the result of unintentional injury of the RLN or the EBSLN, which are located in the immediate vicinity of the thyroid gland(4).
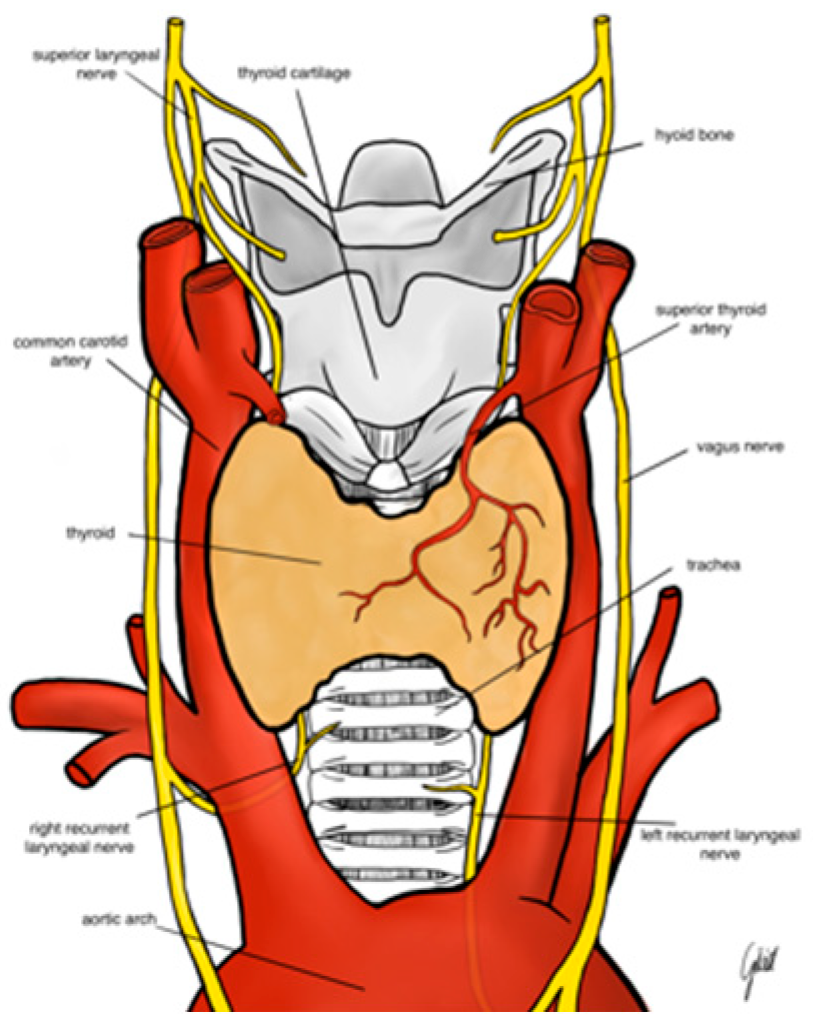
The RLN branches from the vagus nerve at the level of the arch of the aorta on the left and the right subclavian artery on the right. The right nerve crosses the undersurface of the right subclavian artery and ascends in the neck to extend to the right tracheoesophageal groove. Usually, the nerve crosses superficially or deeply to the inferior thyroid artery or between its branches (
Figure 1). The left RLN runs around the arch of the aorta and ascends more vertically in the left tracheoesophageal groove. The mean diameter of the RLN is about 1-3 mm; the left nerve is 12 cm in length and is longer than the right nerve, which is usually about 7 cm long. The nerve carries motor, sensory and parasympathetic fibers. There are many anatomic relations and variations which makes the nerve prone to injury during thyroidectomy. Anatomical variations of the RLN include: different course of the RLN at the level of the inferior thyroid artery hilum, the ligament of Berry, different course along tracheoesophageal groove, the Zuckerkandle’s tubercle and non-recurrent laryngeal nerve. Moreover, RLN branching is observed in 90% of the cases and it is another risk factor for RLN injury. Most often RLN divides into an anterior and posterior branch. The anterior branch supplies motor fibers to intrinsic laryngeal muscles. The posterior branch usually supplies sensory fibers to the trachea, esophagus, hypopharynx. The non-recurrent RLN is extremely rare anatomical variant which occurs in 0.5-1% of the cases on the right side and in less than 0.04% of the cases on left side. The non-recurrent RLN runs directly from the vagus nerve (usually at the level of the inferior thyroid artery) to the larynx. The left non-recurrent RLN is associated with dextrocardia and situs inversus (
Figure 2). Detailed knowledge of the RLN’s anatomy is crucial for surgeon to locate the nerve. Usually, the inferior thyroid artery, the trachoesophageal groove and the laryngeal entry point are landmarks for RLN identification (13,14)
The EBSLN is very close to the superior thyroid pedicle and is of a particular risk of injury during dissection of the vessels. The superior laryngeal nerve (SLN) is one of the first branches of the vagus nerve. The SLN divides into an internal and external branch, which descend dorsally to the carotid sheath and then extend to the larynx (
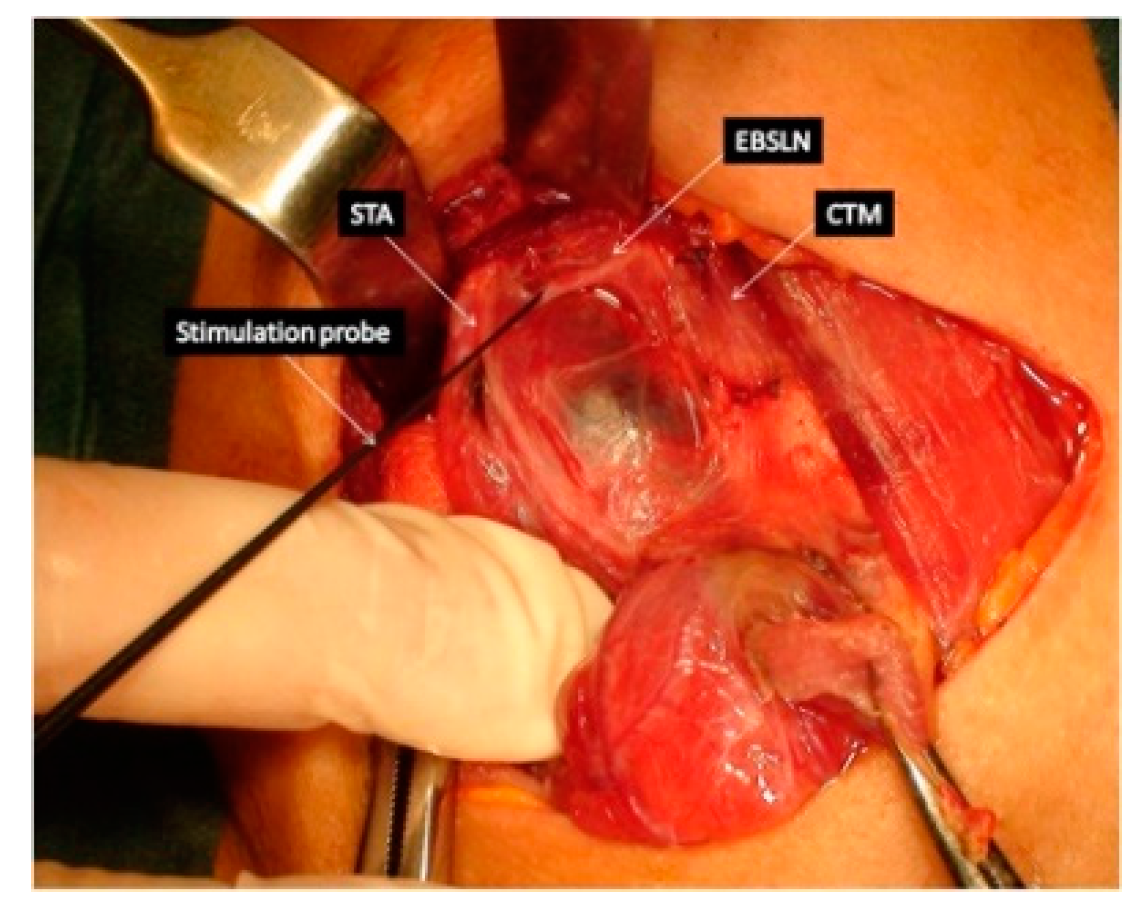
Figure 1). After the EBSLN travels down the lateral surface of the larynx on the inferior pharyngeal constrictor muscle, the EBSLN typically bifurcates into two branches at the level of the cricoid cartilage, entering separately at the pars recta and pars obliqua of the cricothyroid muscle bellies. The sternothyroid -laryngeal triangle named Jolls-space is a landmark for EBSLN localization (
Figure 3). The EBSLN is usually dorsal to the superior thyroid artery and superficial to the inferior pharyngeal constrictor muscle. The EBSLN is 0.8 mm wide and 8-8.9 cm long (6,13).
The Carnea classification is the most common and recognized anatomical classification of EBSLN based on the potential risk of injury to the nerve during thyroidectomy. Type 1: the EBSLN crosses the superior thyroid artery more than 1 cm above the upper edge of the thyroid superior pole and is common in 68% patients with small goiter and in 23% of patients with large goiter. Type 2A: the nerve crosses the superior vessels less than 1 cm above the upper edge of the superior pole; recognized in 18% of patients with small goiter and 15% of patients with large goiter. Type 2B: the EBSLN crosses the superior thyroid vessels below the upper edge of the superior thyroid pole. Usually, type 2B occurs in 14% of patients with small goiter and 54% with large goiter. The most prone to injury is type 2B (
Figure 2). Visual identification of the EBSLN may be impossible in up to 20% of patients when nerve is located deep within the fascia of the inferior constrictor muscle (15).
The most common mechanisms of recurrent laryngeal nerve’s injury include traction (e.g. during extraction of the retrosternal goiter); incision or electrocoagulation are much less frequent mechanisms. Jeannon et al., showed, based on a review of 27 studies analyzing over 25,000 thyroid surgeries, that the average frequency of RLN paralysis was 9.8%. The complication rate ranged from 2.3-26% (3).This large discrepancy is due to many factors; secondary operations on the thyroid gland, large goiter, retrosternal goiter, as well as operations for thyroid cancer and Graves’ disease have a higher rate of complications than procedures for multinodular goiter (16). Moreover, the rate of RLN injury depends on the number of laryngeal examinations after thyroidectomy which are not routinely performed in ever surgery units.
Unilateral RLN palsy most often results in hoarseness, voice timbre or swallowing disorders, while bilateral injury may result in shortness of breath and acute respiratory failure, which may be life-threatening. This condition often requires a tracheostomy. In more than 20% of patients, vocal cord paralysis resulting from RLN injury may be asymptomatic, therefore laryngoscopy examination is the only objective tool for the proper assessment of the percentage of the RLN palsy and should always be performed both before and after thyroid surgery (4,5).
The injury of the EBSLN can occur in up to 58% of patients (17,18,19).
In the case of the EBSLN injury - the most underestimated complication in thyroid surgery - the patient is unable to produce high-pitched sounds, the voice weakens during modulation, which is important for people working with voice(4,6). Clinically the patient with EBSLN injury presents a hoarse or weak voice. These symptoms are the results of the dysfunction of the cricothyroid muscle which is innervated by the EBSLN (6). The injury of the nerve is difficult to identify in a routine postoperative laryngoscopy and both the Voice Handicap Index (VHI) and the Voice-Related Quality of Life instrument (V-RQOL) are the validated instruments to assess the quality of voice and the risk for EBSLN injury (4).
Already in 1938, one of the pioneers of thyroid surgery, Frank Lahey, observed on the basis of over 3,000 thyroidectomies performed that routine identification of RLN during thyroid surgery reduces the frequency of its injury (20). Currently, visual identification of the RLN is the gold standard in thyroid surgery, and for over 30 years this method has been complemented by the use of intraoperative neuromonitoring of the RLN and the EBSLN during the procedure. The advantage of the neuromonitoring of the laryngeal nerves over visual visualization alone is the ability to assess not only the preserved anatomical integrity of the nerve, but also its function during surgery (5,6,7,8).
Technique for Monitoring the Laryngeal Nerves (RLN and EBSLN)
Intraoperative neuromonitoring involves electromyographic response from the vocal muscles after electrical stimulation of the laryngeal nerves (RLN and EBSLN), which motorically innervate the vocal folds. Monitoring of the laryngeal nerves during thyroid surgery was first used by Shedd in 1966 (21).The paper published in “Annals of Surgery” presented the RLN and SLN monitoring in an animal model. In the same year the author translated this study to the human population and showed how to monitor RLN and SLN during thyroid surgery with the use of an endolaryngeal balloon (21). However, it is most likely that Riddle was the first surgeon who identified the RLN using laryngeal palpation with stimulation of the RLN. In 1970 he published a work based on 23 years of his own experience (from 1946 to 1986) (22). Finally, in 1986, Galivan and Galivan showed that palpation of the posterior cricoarytenoid muscle combined with nerve stimulation 0,5-2,0 mA is a safe technique for RLN identification and assessment during thyroid surgeries (23). Over the following years, various IONM techniques were used including laryngeal palpation, glottic pressure monitoring, glottic observation and intralaryngeal hookwire electrodes (24). Both the variety of techniques used and the lack of standards meant that this technique did not come into common use until the beginning of the 21st century. There was a need of standardization of IONM technique to ensure that the results generated are repeatable, reliable and clinically meaningful (24).
In 2006, the International Neural Monitoring Study Group (INMSG) was established to serve the emerging field of neurophysiologic monitoring of laryngeal nerves in neck endocrine surgery. It brings experts together to collaborate on improving the quality of the IONM. The result of their cooperation was standardization of the technique of RLN and EBSLN monitoring during thyroid and parathyroid surgery (5,6). In 2018, the INMSG published a two-part consensus guideline regarding the staged bilateral thyroid surgery in case of loss of signal (Part I) and the optimal RLN monitoring for invasive thyroid cancer (Part II) (7,8). Since then, the era of the IONM in thyroid surgery has begun, starting its widespread use around the world.
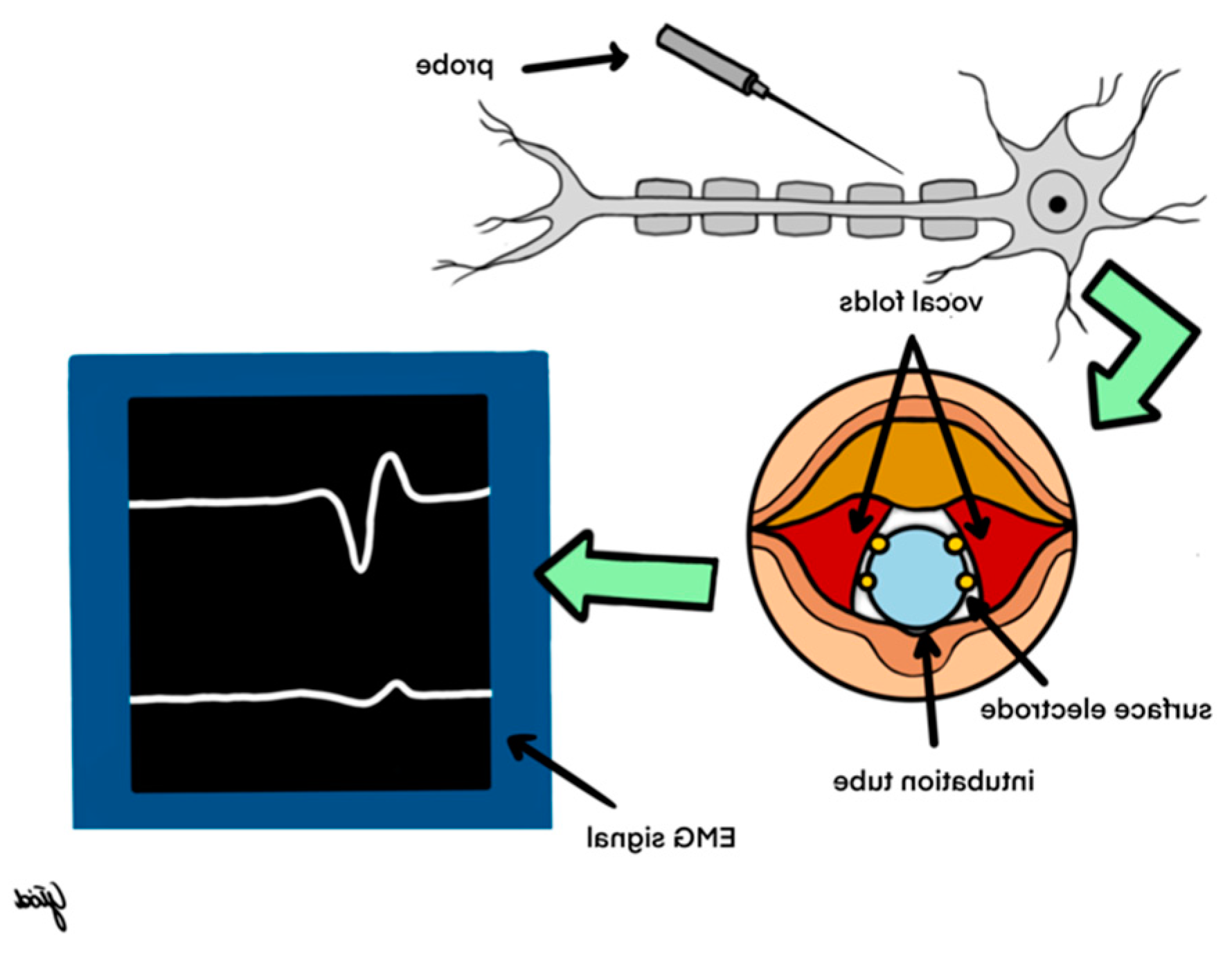
To this day, the most common system for intraoperative neuromonitoring is based on the use of an endotracheal tube with built-in surface electrodes. When the patient is intubated, the tube is precisely placed between the vocal folds (
Figure 4). During thyroid surgery using an electrical probe (mono- or bipolar), the nerve is stimulated with a current of 0.5-2.0 mA. After the latency period, a contraction of the vocal muscles occurs, which is detected by surface electrodes placed on the endotracheal tube and transmitted to the receiving part - the neuromonitor, which reflects a contraction in the form of an electromyographic (EMG) wave. The amplitude of the EMG wave above 200 µV indicates the proper functioning of the nerve, and its absence at a current intensity of 1-2 mA indicates the so-called loss of signal (LOS) and laryngeal nerve injury. Detailed knowledge of the LOS resolution algorithm is essential for the surgeon to properly predict postoperative nerve function. The algorithm has been discussed in detail in the recommendations regarding the use of neuromonitoring in thyroid surgery(5,6)
Cooperation with an anesthesiologist plays an important role in performing thyroid procedures using neuromonitoring of the laryngeal nerves. It is important to correctly place the appropriately selected endotracheal tube so that the surface electrodes adhere to the vocal folds. To obtain a response from the vocal muscles during laryngeal nerve stimulation, it is necessary to use short-acting muscle relaxants during intubation (5).
During nerve stimulation, modern devices generate both an acoustic signal and an electromyographic signal (EMG wave) on the monitor. The EMG wave recording proving the response from the laryngeal nerves should be archived and attached as a printout to the medical documentation.
Monitoring of the laryngeal nerves is a standardized technique. This means that in all centers where this method is used, there is an adopted scheme of thyroid surgery using neuromonitoring, which includes laryngeal examinations performed before (L1) and after (L2) surgery, as well as identification and assessment of vagus nerve (V1) and laryngeal nerve (R1) activity, both before and after thyroid lobe removal (V2 and R2, respectively)2(5).A laryngeal examination performed before surgical treatment makes it possible to diagnose even discrete disorders of their functioning, which may often be asymptomatic; Moreover, correct phonation does not always indicate the absence of disorders in the functioning of the vocal folds. Therefore, a laryngeal examination performed after thyroid surgery is more important, as approximately 30% of vocal fold paralysis may occur with properly maintained phonation1. Stimulation of the vagus nerve before identifying the RLN is performed to verify the correct positioning of the endotracheal tube with built-in surface electrodes, which determines the optimal use of the neuromonitoring technique. Stimulation of the vagus nerve after resection of the thyroid gland is the most sensitive way to assess the function of the RLN and excludes the possibility of its potential injury throughout its entire course (from its branching from the vagus nerve to its entry into the larynx) (4,5).
Actually, we have different options for RLN monitoring: intermittent intraoperative neuromonitoring (I-IONM) using handled probe versus continuous intraoperative neuromonitoring (C-IONM) using temporary implantable vagus electrode (5, 16, 24). The latest introduced technique is Time Trend Monitoring which seems to be a transitional technique in between I-IONM and C-IONM (25).
Conventional intermittent IONM - the most common technique, could only provide intermittent RLN evaluation, allowing the nerve to be at risk of injury between the stimulations. The C-IONM overcomes this limitation of I-IONM by offering real-time RLN monitoring using temporary vagus electrode stimuli. This technique involves placing a special receiving electrode on the vagus nerve at the beginning of the procedure, which allows continuous monitoring of the RLN function. The advantage of this method over intermittent stimulation is that continuous stimulation can detect impending injury to the RLN, e.g. during goiter extraction from behind the sternum (possibility to avoid excessive traction)(26,27). The use of C-IONM allows the surgeon to perform a corrective action like stopping or reversing underlying maneuvers, thus may be able to avoid a permanent injury (16,24,26,27,28,29,30). Phelan et al. (2012), under the consideration of EMG adverse events, has coupled amplitude decline and latency incline to identify mild combined events (mCE) and severe combined events (sCE). The mCE is defined as an amplitude decrease of 50-70% with latency increase of 5-10% and sCE as an amplitude decrease of >70% and a latency increase of >10%. He discovered that the postoperative vocal fold palsy was not connected with mCE, whereas the sCE could result in LOS and postoperative vocal fold paresis (29). In this way C-IONM is able to reduce not only bilateral vocal folds paresis as I-IONM, but also is able to prevent unilateral permanent traction-related nerve injury. The natural evolution from I-IONM to C-IONM is Nerve Trend
TM. In 2020, NIM Vital equipment (Medtronic, Jackson-ville, FL) was introduced to the market offering NerveTrend
TM EMG reporting which enables nerve condition tracking throughout a procedure, even when using I-IONM. It is operator-dependent and not automatic as in C-IONM. This new IONM technique is used in the same manner as in the I-IONM arm and the EMG trending includes amplitude and latency changes from initial vagal baseline of the NIM. NerveTrend
TM mode operates at 3-5 min intervals or in cases of difficult maneuvers to assure an almost real-time EMG tracing and therefore allows tailoring surgical approach by modification of surgical maneuvers in case of occurrence of sCEs (yellow zone) in order not to end up with the LOS (red zone) as shown in (
Figure 5)(25).
In 2013, Barczyński published guidelines for IONM recording of EBSLN during thyroid and parathyroid surgery (6). Before these recommendations there were only few reports in the literature describing how to identify and preserve the EBSLN during superior pole dissection and the identification of the EBSLN was out of interest of many surgeons (15,31,32). There are two ways to monitor EBSLN: I- evaluation of the cricothyroid twitch response, which is present in all patients and II- EMG glottic response of vocal cord depolarization identified by the surface ET electrodes. The second maneuver is present in about 70-100% of patients depending on type of ET used. Transverse division of the superior edge of the sternothyroid muscle and gentle traction of the superior thyroid pole into a lateral and caudal direction, followed by blunt dissection within the avascular plane of sternothyroid–laryngeal triangle, allows for improving exposure of the EBSLN which is usually descending parallel to superior thyroid artery and is lying on the fascia or between the fibers of the inferior constrictor muscle before its termination within the cricothyroid muscle.
Approximately 20% of the EBSLN could not be identified by visualization alone because the nerve has a subfascial or intramuscular course within the inferior constrictor muscle. Nerve stimulation can objectively identify the EBSLN, leading to a visible cricothyroid muscle twitch in all (100%) cases. Application of IONM should not be limited to the RLN only but should be also expanded for EBSLN mapping, identification, and functional testing during thyroidectomy (6).
Loss of Signal during Thyroidectomy and Decision Making
The definition of loss of signal was proposed in guidelines from 2011 (5). Part I of the INMSG guidelines discusses the use of IONM in bilateral thyroid surgery in case of loss of signal and the incorporation in surgical strategy. The INMSG recommends that neural monitoring information should be obtained and utilized in the strategy of a planned bilateral procedure by staging the surgery in the setting of ipsilateral LOS. This algorithm should be shared and discussed with the patient during the preoperative informed consent process. It is important because approximately 30% of patients with bilateral vocal fold palsy require tracheostomy. This strategy may prevent bilateral vocal fold palsy allowing the surgery to be staged (7). The second recommendation from Part I states that a surgeon should prioritize concern for the obvious significant medical and psychological morbidity of bilateral VCP and possible tracheotomy (even temporary) over perceived surgical convenience, the routine of doing the “planned procedure” or the potential perceived impact on surgical reputation by openly acknowledging the surgical complication of ipsilateral loss of signal. The full benefit of neural monitoring information in this surgical setting is appreciated through both optimization of the patient’s quality of life as well as surgical cost (7,33). The Part I guidelines were published in conjunction with the INMSG Guidelines Part II regarding optimal management of the recurrent laryngeal nerve that is adherent to or invaded by cancer using preoperative glottic function through preoperative laryngeal examination as well as intraoperative monitoring electromyography signal (7,8). To sum up, in case of loss of signal without electromyography recovery, the surgeon should consider postponing the contralateral procedure to limit risk of bilateral cord paralysis and tracheostomy.
Clinical Aspect of the Use of Neuromonitoring in Thyroid Surgery
The IONM should be utilized during thyroid and parathyroid surgery for many reasons. Neuromonitoring during thyroid surgery facilitates the identification of the RLN and the EBSLN and the precise dissection of these nerves from the surrounding tissues (34). It should be noted that the identification of laryngeal nerves is difficult, especially for a young surgeons, as this skill is acquired over many years (35). This is partly due to the highly variable anatomy of the nerve course in relation to the surrounding tissues. The IONM is excellent tool to identify anatomical variations such as extralaryngeal branches, diverse relations with inferior thyroid artery and non-RLN as well. Moreover, the RLN could be distorted in difficult operations, for example in secondary thyroid operations or huge retrosternal goiter. Neuromonitoring usually confirms the previously performed visual identification of the laryngeal nerves, but in particularly difficult cases, electrophysiological assessment precedes their visual recognition. During a neuromonitoring assisted surgery, a mapping technique is often used, which involves identifying the nerve in the surgical field by moving the stimulation probe at small, regular intervals (1-2 mm) along the trachea (5). The signal of the evoked potential in the area of the nerve guides the operator to its location and allows proper determination of its course. The mapping technique is particularly applicable during secondary procedures on the thyroid gland where scar tissues were formed (36). The effectiveness of identifying the RLN using neuromonitoring is 98-100% and is statistically significantly higher compared to visual identification, which has been confirmed by numerous multicenter studies (37,38,39).
In thyroid surgery, the basis for a properly performed operation is the complete removal of the pathological tissue (thyroidectomy is performed when changes in the thyroid gland affect both lobes, lobectomy - in the case of changes located in one lobe only). Subtotal thyroid resection should not be performed due to the need for radicalization in the event of a postoperative diagnosis of thyroid cancer and due to the possibility of goiter recurrence. Secondary procedures on the thyroid gland have a much higher rate of complications, especially injury to the laryngeal nerves (36,40).Neuromonitoring enables precise dissection of the laryngeal nerves from the surrounding tissues and significantly increases the radicalness of the surgeries performed. Barczyński et al. showed that the average iodine 131I uptake after total thyroidectomy using intraoperative neuromonitoring compared to procedures during which no neuromonitoring was used was 0.67% ± 0.39% vs 1.59% ± 0.69 % (p < 0.001), and the percentage of patients with iodine uptake below 1% increased by as much as 45% when neuromonitoring was used (41). The use of a careful dissection technique in the area of Zuckerkandl’s tubercle and Berry’s ligament with the use of neuromonitoring undoubtedly contributed to obtaining such good results (14,41).
Neuromonitoring allows to predict postoperative RLN activity already during the operation, which practically eliminated the risk of bilateral damage (5,7,8,41,42). Introduction of neuromonitoring in thyroid surgery established the concept of two-stage thyroidectomy. It involves refraining from removal of the second lobe of the thyroid gland if nerve injury is suspected on the side already operated on (presence of signal loss on the originally operated side). This procedure is intended to protect the patient against bilateral RLN paresis and possible tracheostomy (41). LOS troubleshooting algorithms have been developed to assist surgeon using IONM in identifying the true LOS and determining optimal course for any remaining parts of the surgical procedure. The introduction of C-IONM made it possible to avoid unilateral vocal fold paresis due to traction. Moreover, the IONM is able to precisely identify the type of RLN injury; type I (focal nerve injury) and type II (global nerve injury) (5).
Additionally, undeniable applicability value is improving rate of identification of EBSLN.
IONM in the Reduction of RLN Injury
Since IONM was introduced into thyroid surgery, there has been an ongoing discussion about the superiority of IONM over visualization alone. Generally, in the vast majority of publications lower rates of RLN injury are noted with IONM, but the differences are not statistically significant (43,44,45,46). Dralle et al. pointed out that to reach an adequately powered study would require 9 million patients per arm for benign goiter and 40 thousand patients per arm for thyroid malignancy surgery to detect a statistically significant difference (43). Moreover, another limitation of many publications is the heterogeneity of the studies including variability in laryngeal examinations practice, type and extend of the surgical procedure and surgeon’s experience (4,24,16). The use of neuromonitoring reduces the number of postoperative vocal cord paralysis, which was first confirmed by Barczyński et al. in a randomized clinical trial involving the assessment of 2,000 laryngeal nerves at risk of injury during 1,000 thyroid surgeries(44).The authors showed that the number of transient nerve palsies in people operated on with the use of neuromonitoring was 2.9% and 0.9% lower in the high-risk group (surgery for thyroid cancer with central lymphadenectomy, large, retrosternal goiter) and low-risk patients (multinodular goiter), respectively, compared to the number of this complication in patients undergoing a procedure during which only visual identification was used. In another large study analyzing over 850 cases of secondary thyroid operation, Barczynski et al. found statistically significant reduction (2.6% vs. 2.4%) in transient paralysis rates between patient operated with vs. without I-IONM (45). One of the first meta-analyses by Pisanu et al. from 2014 shoved no statistically significant difference in the incidence of RLN palsy when using IONM versus visualization alone during thyroidectomy, although in the IONM group the rates of transient and permanent paresis were lower compared to visualization alone: 2.62% vs. 2.72% and 0.79% vs. 0.92% respectively. This retrospective, observational study included 23,512 patients must be approached with caution as it was based mostly on non-randomized studies (46). In 2017, Brandon published a high-quality PRISMA-compliant systematic review of overlapping meta-analyses summarizing current state of I-IONM for prevention of RLN injury during thyroidectomy and found that to date I-IONM had not achieved a significant level of RLN injury reduction (47). The meta-analysis by Yang et al., from 2017 showed benefits of reducing RLNP rate by using IONM, but without statistical significance for persistent RLN palsy (48). Interesting meta-analysis on intra-operative neuro-monitoring in high-risk thyroidectomy was published in 2017 by Wong et al. The authors compare the use of IONM with RLN visual identification alone during high-risk thyroidectomies: reoperations, thyroid surgery for malignancy, thyrotoxicosis and retrosternal goiter. Use of IONM decreased the rate of overall RLN palsy during reoperation (7.6% vs. 4.5%) and the result was statistically significant (49). In 2023, Wojtczak et al. proved the superiority of IONM over visualization alone during thyroidectomy by analyzing risk factors in thyroid surgery. The authors proved that risk factors for complications in thyroid surgery are not significant for any increase in the rate of vocal fold paralysis as long as surgery is performed with IONM, in contrast to thyroid surgery performed only with visualization alone (50). In the context of the reduction of RLN injury it is worth noting that various nerve monitoring techniques influence the rate of complications. In 2021, Schneider et al. proved the superiority of continuous over intermittent intraoperative nerve monitoring in preventing vocal cord palsy. Based on numbers of the nerves at risk (5208 versus 5024 nerves), continuous IONM had a 1,7-fold lower early postoperative vocal cord palsy rate than intermittent monitoring (1.5 vs 2.5 %). This translated into a 30-fold lower permanent vocal cord palsy rate (0.02 vs 0.6%). In multivariable logistic regression analysis, continuous IONM independently reduced early postoperative vocal cord palsy 1,8-fold (odds ratio (OR) 0.56) and permanent vocal cord palsy 29.4-fold (OR 0.034) compared with intermittent IONM. One permanent vocal cord palsy per 75.0 early vocal cord palsies was observed with continuous IONM, compared with one per 4.2 after intermittent IONM. Therefore, early postoperative vocal cord palsies were 17.9-fold less likely to become permanent with continuous than intermittent IONM (51). In 2023, the first randomized control trial by Barczyński and Konturek was published, compering Nerve Trend vs. conventional I-IONM in prevention of recurrent laryngeal nerve events during bilateral surgery. The study included 264 patients: 132 operated with Nerve Trend TM vs 132 operated with conventional I-IONM. On the first postoperative day RLN injury was less frequent in Nerve Trend TM group (1.89% nerves at risk) vs. I-IONM group (4.65%), but the results were not statistically significant (p=0.67). Moreover, the study showed that staged thyroidectomy was not performed in Nerve Trend TM group (0%) vs. 4.54% of patients in conventional I-IONM group and the results were statistically significant (p=0.029). This study confirms hypothesis that the use of NerveTrendTM mode results in reduced RLN injury on postoperative day 1 and significant decrease of need for a staged thyroidectomy (25).
It is much easier to prove the superiority of IONM over visualization alone in preventing EBSLN injury. There are many publications proving that the use of IONM significantly improved the identification rate of the EBSLN during thyroidectomy, as well as reduced the risk of early phonation changes after thyroidectomy (44,52,53,54).
Educational Value of Intraoperative Neuromonitoring. Research Studies
Neuromonitoring is of particular value to both young surgeons undergoing training and experienced surgeons who perform relatively few thyroid surgeries. Efficient performance of thyroid procedures by a surgeon requires more than 100 thyroidectomies per year (55).Neuromonitoring enables young surgeons to learn about the anatomical variants of both nerves and their anatomical variability resulting from various factors (displacement of the trachea, retrosternal goiter, infiltration of thyroid cancer into the surrounding tissues). Moreover, one should not forget about possible anomalies, such as the identification of a non-recurrent laryngeal nerve - this is a very rare development anomaly detected on the right side in approximately 0.5% of patients(34).
Understanding the mechanisms of the RLN injury during procedures influences the development of surgical techniques for safe thyroidectomy, both for younger surgeons and those with extensive experience(5).
With the introduction of neuromonitoring to thyroid surgery, numerous studies began in the field of neurophysiology and neuropathology of laryngeal nerves, which resulted in deepening our knowledge in these fields. Serpell et al. observed that in case of the RLN branching in the extralaryngeal section, RLN motor fibers responsible for adduction and abduction of the vocal folds are most often located in the anterior branch of this nerve (42).However, proper use of IONM requires appropriate training. In 2021, the INMSG published document which describes the minimum training required for learning practical application of IONM. The training includes both basic and advanced courses made by specialized accreditation center (56).
Medical and Legal Aspects of the Neuromonitoring Use in Thyroid Surgery
The use of intraoperative neuromonitoring provide a possibility to obtain medical documentation confirming the proper function of the RLN after the completed surgical procedure. This documentation may be archived as evidence of the utmost care in identifying and maintaining RLN activities during the operation.
Moreover, knowledge about the functioning of the vocal folds in the immediate postoperative period facilitates and directs the conversation with the patient in the event of hoarseness in the first days after the procedure. It allows the differentiation of voice disorders dependent on the RLN injury from abnormalities that may be caused by other factors (difficult intubation, postoperative swelling of the glottis, iatrogenic injury to the larynx, etc.).
The implementation of the neuromonitoring method in thyroid surgery has undoubtedly influenced the development of standards of conducting this type of procedures. It is also an important element of intraoperative quality control of surgical treatment (5,6,57).In 2021, the INMSG published recommended informed consent for intraoperative neural monitoring in thyroid and parathyroid surgery. The INMSG consensus statement outlines the general and specific considerations regarding the surgical use of IONM and provides essential recommended standard elements of informed consent for the use of IONM thereby assisting surgeons and patients in the informed consent process and in shared decision making prior to thyroid or parathyroid surgery (9).
Prospects for the Development of Neuromonitoring Over the World.
Over the past three decades we observed the increasing interest in IONM all over the world. It is undoubtedly the fastest developing technique in head and neck surgery (…). According to the EUROCRINE register IONM was used in 85.2% of thyroid surgeries in Europe in 2022, but most of the operations were done with I-IONM (87.1%) whereas C-IONM was used in only 12.9% of thyroid operations (58). In Germany, intraoperative neuromonitoring is used in over 100 % of thyroid surgeries. The German Association of Endocrine Surgery Guidelines recommend to use IONM in all cases of thyroid and parathyroid surgery (44, 45). Moreover, in Germany there is the highest number of thyroid operations performed with C-IONM reaching about 17% (60). The rest of American and international guidelines recommending the use the IONM pointed the utility in neural identification, reduction of transient nerve paralysis, prognostication of nerve function and avoidance of bilateral cord paralysis (10,61,62,63,64,65,66).
Publication by Allen L.F et al. from 2019, revealed increased prevalence of neural monitoring during thyroidectomy according global surgical survey carried out among 1.015 respondents. The survey showed that 83% of respondents are using IONM: 65,1% always and 18,1% selectively. In these centers, where IONM was used selectively the main indications were reoperative (secondary) thyroid surgeries (95.1%) and the cases with preoperative vocal cord paralysis (59.8%). Another conclusion from these study was that surgeons ≤45 years of age and those with ≤15 years of practice used IONM more than their peers (P < 0.001). Thyroid surgery volume, fellowship training and type of practice had no bearing on the IONM use (11).
In Poland, the country where authors work, over 30,000 thyroid surgeries are performed annually. It is the fourth most frequently performed general surgery procedure. Among over 300 surgical departments offering the treatment of thyroid diseases in 2011, only a few centers had equipment for intraoperative identification of the RLN, therefore only 1-2% of thyroid surgeries in Poland were neuromonitoring assisted. Centers with neuromonitoring equipment used it primarily in patients with an increased risk of postoperative complications. After the first decade of neuromonitoring use in Poland, this method has become extremely popular - currently 50% of thyroid surgeries are neuromonitoring assisted. The use of neuromonitoring is not reimbursed by the National Health Fund, but this situation may soon improve due to the active work of the Polish Research Group for Neuromonitoring, established in 2010, which operates within the Polish Club of Endocrine Surgery. Its members stated that the endocrine surgery centers should be provided with neuromonitoring equipment. They also expressed a need of conducting a routine training program in the field of standardized neuromonitoring technique of laryngeal nerves and the use of this method during surgery of selected thyroid diseases.
Conclusions
Neuromonitoring of the laryngeal nerves, both the recurrent nerves and the external branch of the superior laryngeal nerve, has become standard in thyroid surgery. The use of the basic intermittent neuromonitoring technique (i-IONM) facilitates not only the identification of the nerve but also enables the assessment of its functional integrity, and not only its anatomical integrity, as in the case of the visual nerve identification method. In the event of loss of the neuromonitoring signal, it is possible to modify the surgical procedure, i.e. postpone the operation on the opposite side, if the loss of signal on the first operated side persists during the procedure, which is essential in the prevention of bilateral RLN damage (so-called staged thyroidectomy). Continuous neuromonitoring (c-IONM) enables the surgeon to recognize impending RLN injury during surgery (in the most common traction mechanism), correct surgical maneuvers to avoid nerve damage, and verify the recovery of RLN function after intraoperative electromyographic signal loss. Hence, the c-IONM technique has the potential to prevent not only bilateral but also unilateral injury to the recurrent laryngeal nerve. In centers that do not have the ability to use c-IONM, NerveTrend mode may be helpful in reducing the risk of unilateral nerve damage and limiting the indications for staged thyroid surgery. In turn, EBSLN neuromonitoring is important for improving the quality of life of patients undergoing thyroid surgery, increasing the chance of maintaining the timbre and register of the voice after surgery.
Author Contributions
Conceptualization, B.W.; Validation, M.G.; Formal analysis, M.B.; Data curation, K.K., K.S-S, K.S; Writing—original draft, B.W.; Project administration, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank all the staff at the study center who contributed to this work.
Conflicts of Interest
The authors declare no conflict of tnterest. The Infermedica Sp. z o.o. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Agency for Healthcare Research and Quality. Statistical brief 86. Healthcare Cost and Utilization Project (HCUP). http://www.hcup-us.ahrq.gov/reports/statbriefs/sb86.jsp. Published 2010. Accessed June 4, 2012.
- Rusinek, D.; Chmielik, E.; Krajewska, J.; Jarzab, M.; Oczko-Wojciechowska, M.; Czarniecka, A.; Jarzab, B. Current Advances in Thyroid Cancer Management. Are We Ready for the Epidemic Rise of Diagnoses? Int. J. Mol. Sci. 2017, 22,18(8):1817. [CrossRef]
- Jeannon, J.P.; Orabi, A.A.; Bruch, G.A.; Abdalsalam, H.A.; Simo, R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: A systematic review. Int. J. Clin. Pract. 2009, 63, 624–629. [CrossRef]
- Chandrasekhar, S.S.; Randolph, G.W.; Seidman, M.D.; Rosenfeld, R.M.; Angelos, P.; Barkmeier-Kraemer, J.; Benninger, M.S.; Blumin, J.H.; Dennis, G.; Hanks, J.; et al. Clinical Practice Guideline: Improving voice outcomes after thyroid surgery. Otolaryngol.— Head Neck Surg. 2013, 148, S1–S37. [CrossRef]
- Randolph, G.W.; Dralle, H.; Abdullah, H. International Intraoperative Monitoring Study Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope 2011, 122, S1–S16. [CrossRef]
- Barczyński,M.;Randolph,G.W.;Cernea,C.R.;Dralle,H.;Dionigi,G.;Alesina,P.F.;Mihai,R.;Finck,R.;Finck,C.;Lombardi,D.; et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International neural monitoring study Group standards guideline statement. Laryngoscope 2013, 123, S1–S14. [CrossRef]
- Schneider, R.; Randolph, G.W.; Dionigi, G.;Wu, C.W.; Barczynski, M.; Chiang, F.Y.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Cernea, C.R.;et al. International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope 2018, 128, S1-S17. [CrossRef]
- Wu, C.W.; Dionigi, G.; Barczynski, M.; Chiang, F.Y.; Dralle, H.; Schneider, R.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Brooks, J.A.; et al. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018, 128, S18-S27. [CrossRef]
- Wu, C.W.; Huang, T.Y.; Randolph, G.W.; Barczyński, M.; Schneider, R.; Chiang, F.Y.; Silver Karcioglu, A.; Wojtczak, B.; Frattini, F.; Gualniera, P.; et al. Informed Consent for Intraoperative Neural Monitoring in Thyroid and Parathyroid Surgery - Consensus Statement of the International Neural Monitoring Study Group. Front Endocrinol 2021, 7,12,795281. [CrossRef]
- Pardal-Refoyo, J.L.; Parente-Arias, P.; Arroyo-Domingo, M.M.; Maza-Solano, J.M.; Granell-Navarro, J.; Martínez-Salazar, J.M.; Moreno-Luna, R.; Vargas-Yglesias, E. Recommendations on the use of neuromonitoring in thyroid and parathyroid surgery. Acta Otorrinolaringol Esp 2018, 69, 231-242. [CrossRef]
- Feng, A.L.; Puram, S.V.; Singer, M.C.; Modi, R.; Kamani, D.; Randolph, G.W. Increased prevalence of neural monitoring during thyroidectomy: Global surgical survey. Laryngoscope 2020, 130, 1097-1104. [CrossRef]
- Bartsch, D.K.; Dotzenrath, C.; Vorländer, C.; Zielke, A.; Weber, T.; Buhr, H.J.; Klinger, C.; Lorenz, K. The StuDoQ/Thyroid Study Group TSS. Current Practice of Surgery for Benign Goitre-An Analysis of the Prospective DGAV StuDoQ|Thyroid Registry. J Clin Med 2019 8,8(4):477. [CrossRef]
- Randolph, G. The Recurrent and Superior Laryngeal Nerve, Springer 2016. [CrossRef]
- Wojtczak, B.; Kaliszewski, K.; Sutkowski, K.; Bolanowski, M.; Barczyński, M. A functional assessment of anatomical variants of the recurrent laryngeal nerve during thyroidectomies using neuromonitoring. Endocrine 2018, 59, 82-89; PMID: 29119329; PMCID: PMC5765187. [CrossRef]
- Cernea, C.R.; Ferraz, A.R.; Nishio, S.; Dutra, A. Jr.; Hojaij, F.C.; dos Santos, L.R. Surgical anatomy of the external branch of the superior laryngeal nerve. Head Neck 1992, 14, 380-3. PMID: 1399571. [CrossRef]
- Fundakowski, C.E.; Hales, N.W.; Agrawal, N.; Barczyński, M.; Camacho, P.M.; Hartl, D.M.; Kandil, E.; Liddy, W.E.; McKenzie, T.J., Morris, J.C. Surgical management of the recurrent laryngeal nerve in thyroidectomy: American Head and Neck Society Consensus Statement. Head Neck 2018, 40,663-675. PMID: 29461666. [CrossRef]
- Bevan, K.; Griffiths, M.V.; Morgan, M. H. Cricothyroid muscle paralysis: its recognition and diagnosis. J Laryngol Otol 1989,103,191–195. [CrossRef]
- Jansson, S.; Tisell, L.E.;Hagne, I.; Sanner, E.; Stenborg, R.; Svensson, P. Partial laryngeal nerve lesions before and after thyroid surgery. World J Surg 1988,12,522–527.
- Teitelbaum, B.J.;Wenig, B.L. Superior laryngeal nerve injury from thyroid surgery. Head Neck 1995, 17, 36–40. [CrossRef]
- Lahey, F.H. Routine dissection and demonstration recurrent laryngeal nerve in subtotal thyroidectomy. Surg Gynecol Obstet 1938, 66, 775–777.
- Shedd, D.P.; Burget, G.C. Identification of the recurrent laryngeal nerve. Arch Surg. 1966, 92, 861–864; PMID: 5933254. [CrossRef]
- Riddell, V. Thyroidectomy: prevention of bilateral recurrent nerve palsy. Results of identification of the nerve over 23 consecutive years (1946-69) with a description of an additional safety measure. Br J Surg 1970, 57, 111. [CrossRef]
- Gavilán, J.; Gavilan, C. Recurrent laryngeal nerve. Identification during thyroid and parathyroid surgery. Archives of otolaryngology Head and Neck surgery 1986,112, 1286-8. [CrossRef]
- Sinclair,C.F.; Kamani,D.; Gregory W. Randolph, G.W. The evolution and progress of standard procedures for intraoperative nerve monitoring. Annals of Thyroid, 2019, 4, 1-12. [CrossRef]
- Barczyński, M.; Konturek, A. Clinical validation of NerveTrend versus conventional i-IONM mode of NIM Vital in prevention of recurrent laryngeal nerve events during bilateral thyroid surgery: A randomized controlled trial. Head Neck 2023, PMID: 38095022. [CrossRef]
- Schneider, R.; Machens, A.; Randolph, G.; Kamani,D.; Lorenz, K.; Dralle, H.; et al. Impact of continuous intraoperative vagus stimulation on intraoperative decision making in favor of or against bilateral surgery in benign goiter. Best Pract Res Clin Endocrinol Metab 2019, 33, 4 101285. Epub 2019 Jun 6. PMID: 31221571. [CrossRef]
- Stankovic, P.; Wittlinger, J.; Georgiew, R.; et al. Continuous intraoperative neuromonitoring (cIONM) in head and neck surgery – a review. HNO 2020, 68, 86-92. [CrossRef]
- Lamadé, W.; Meyding-Lamadé, U.; Buchhold, C.;et al. First continuous nerve monitoring in thyroid gland surgery. Chirurg 2000, 71, 551-7. [CrossRef]
- Phelan, E.; Schneider, R.; Lorenz, K.; et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014,124,1498-505. [CrossRef]
- Schneider, R.;Randolph, G.W.; Sekulla, C.; et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013, 35,1591-8. [CrossRef]
- Kark, A.E.; Kissin, M.W.; Auerbach, R.; et al. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br Med J 1984, 289, 1412-5. [CrossRef]
- Lekacos, N.L.; Miligos, N.D.; Tzardis, P.J.; et al. The superior laryngeal nerve in thyroidectomy. Am Surg 1987, 53, 610-2.
- Al-Qurayshi, Z.; Kandil, E.; Randolph, G.W. Cost-effectiveness of intraopera- tive nerve monitoring in avoidance of bilateral recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. Br J Surg 2017,104, 1523–1531. [CrossRef]
- Cossa, A.; Castagnola, G.; Romeo, G.; et al. Utility of intraoperative neuromonitoring in detecting recurrent nerve’s anatomical anomalies during thyroidectomy. Endocrine 2020, 70, 194-7. [CrossRef]
- Wojtczak, B.; Sutkowski, K.; Kaliszewski, K.; et al. Experience with intraoperative neuromonitoring of the recurrent laryngeal nerve improves surgical skills and outcomes of non-monitored thyroidectomy. Langenbecks Arch Surg 2017, 402,709-17. [CrossRef]
- Wojtczak, B.; Sutkowski, K.; Kaliszewski, K.; et al. Thyroid reoperation using intraoperative neuromonitoring. Endocrine 2017,58, 458-66. [CrossRef]
- Wojtczak, B.; Kaliszewski, K.; Sutkowski, K.; Głód, M.; Barczyński, M. The learning curve for intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Langenbecks Arch Surg. 2017, 402, 701-708; PMID: 27178203; PMCID: PMC5437179. [CrossRef]
- Schneider, R.; Machens, A.; Lorenz, K.; et al. Intraoperative nerve monitoring in thyroid surgery-shifting current paradigms. Gland Surg 2020, 9, S120-8. [CrossRef]
- Deniwar, A.; Bhatia, P.; Kandil, E. Electrophysiological neuromonitoring of the laryngeal nerves in thyroid and parathyroid surgery: a review. World J Exp Med 2015, 5,120-3. [CrossRef]
- Lynch, J.; Parameswaran, R. Management of unilateral recurrent laryngeal nerve injury after thyroid surgery: a review. Head Neck 2017, 39, 1470-8. [CrossRef]
- Barczyński, M.; Konturek, A.; Stopa, M.; et al. Clinical value of intraoperative neuromonitoring of the recurrent laryngeal nerves in improving outcomes of surgery for well-differentiated thyroid cancer. Pol Przegl Chir 2011, 83,196-203. [CrossRef]
- Serpell, J.W.; Yeung, M.J.; Grodski, S. The motor fibers of the recurrent laryngeal nerve are located in the anterior extralaryngeal branch. Ann Surg 2009, 249, 648-52. [CrossRef]
- Dralle, H.; Sekulla, C.; Haerting, J.; Timmermann, F.; Neumann, H.J.; Kruse, E.; Grond, S.; Muhlig, H.P.; Richter, C.; Voss, J.; et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 2004, 136, 1310–1322. [CrossRef]
- Barczyński, M.; Konturek, A.; Cichoń, S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br. J. Surg. 2009, 96, 240–246. [CrossRef]
- Barczyński, M.; Konturek, A.; Pragacz, K.; et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a ret-rospective cohort study. World J Surg 2014, 38, 599-606. [CrossRef]
- Pisanu, A.; Porceddu, G.; Podda, M.; Cois, A.; Uccheddu, A. Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 2014,188,152-161. [CrossRef]
- Henry, B.M.; Graves, M.J.; Vikse, J.; et al. The current state of inter- mittent intraoperative neural monitoring for prevention recurrent laryngeal nerve injury during thyroidectomy: a PRISMA-compliant systematic review of overlapping meta analyses. Langenbecks Arch Surg 2017, 402, 663-673. [CrossRef]
- Yang, S.; Zhou, L.; Lu, Z.; Ma, B.; Ji, Q.; Wang, Y. Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. Int J Surg 2017, 39, 104-113. Epub 2017 Jan 25. PMID: 28130189. [CrossRef]
- Wong, K.P.; Mak, K.L.; Wong, C.K.; Lang, B.H. Systematic review and meta-analysis on intra-operative neuro-monitoring in high-risk thyroidectomy. Int J Surg 2017, 38, 21-30. Epub 2016 Dec 26. PMID: 28034775. [CrossRef]
- Wojtczak, B.; Marciniak, D.; Kaliszewski, K.; Sutkowski, K.; Głód, M.; Rudnicki, J.; Bolanowski, M.; Barczyński, M. Proving the Superiority of Intraoperative Recurrent Laryngeal Nerve Monitoring over Visualization Alone during Thyroidectomy. Biomedicines 2023, 11, 880. PMID: 36979859; PMCID: PMC10045399. [CrossRef]
- Schneider, R.; Machens, A.; Sekulla, C.; Lorenz, K.; Elwerr, M.; Dralle, H. Superiority of continuous over intermittent intraoperative nerve monitoring in preventing vocal cord palsy. Br J Surg. 2021, 108,566-573. PMID: 34043775. [CrossRef]
- Masuoka, H.; Miyauchi, A.; Higashiyama, T,; Yabuta, T.; Fukushima, M.; Ito, Y.; Kihara, M.; Kobayashi, K.; Yamada, O.; Nakayama, A.; Miya, A. Prospective randomized study on injury of the external branch of the superior laryngeal nerve during thyroidectomy comparing intraoperative nerve monitoring and a conventional technique. Head Neck. 2015, 10,1456-60. [CrossRef]
- Aygün, N.; Uludağ, M.; İşgör, A. Contribution of intraoperative neuromonitoring to the identification of the external branch of superior laryngeal nerve. Turk J Surg 2017, 33, 169-174. PMID: 28944328; PMCID: PMC5602307. [CrossRef]
- Yuan, Q.; Zheng, L.; Hou, J.; Zhou, R.; Xu, G.; Li, C.; Wu, G. Visual identification and neuromonitoring vs. no sighting the external branch of the superior laryngeal nerve in thyroid surgery: a randomized clinical trial. Updates Surg 2022, 74, 727-734. Epub 2021 Jul 29. PMID: 34327667. [CrossRef]
- Lorenz, K.; Raffaeli, M.; Barczyński, M.; et al. Volume, outcomes and quality standards in thyroid surgery: an evidence-based analysis – European Society of Endocrine Surgeons (ESES) positional statement. Langenbecks Arch Surg 2020, 405, 401-25. [CrossRef]
- Wu, C.W.; Randolph, G.W.; Barczyński, M.; Schneider, R.; Chiang, F.Y.; Huang, T.Y.; Karcioglu, A.S.; Konturek, A.; Frattini, F.; Weber, F.; et al. Training Courses in Laryngeal Nerve Monitoring in Thyroid and Parathyroid Surgery- The INMSG Consensus Statement. Front Endocrinol 2021, 12, 705346. PMID: 34220726; PMCID: PMC8253252. [CrossRef]
- Zhang, D.; Pino, A.; Caruso, E.; et al. Neural monitoring in thyroid surgery is here to stay. Gland Surg 2020, 9, S43-6. [CrossRef]
-
www.eurocrine.eu.
- Musholt, T.J.; Clerici, T.; Dralle, H. et al. German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease. Langenbecks Arch Surg 2011, 396, 639–649. [CrossRef]
- Bartsch, D.K.; Dotzenrath, C.; Vorländer, C.; Zielke, A.; Weber, T.; Buhr, H.J.; Klinger, C.; Lorenz, K.; and the StuDoQ/Thyroid Study Group Current Practice of Surgery for Benign Goitre—An Analysis of the Prospective DGAV StuDoQ|Thyroid. J Clin Med 2019, 8, 477. PMID: 30965665; PMCID: PMC651792. [CrossRef]
- Bryan, R.; Haugen, E.; Alexander, E.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, E.Y.; Pacini, F.; Randolph,G.W.; Sawka J.; et.al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016 (1):1-133. [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M. et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg. 2020, 3, 21-93. PMID: 32079830. [CrossRef]
- BC, Jr.; Tolley, N.S.; Bartel, T.B.; Bilezikian, J.P.; Bodenner, D.; Camacho, P.; Cox, J.P.D.T.; Dralle, H.; Jackson, J.E.; Morris, J.C.; et.al. AHNS Series: Do you know your guidelines? Optimizing outcomes in reoperative parathyroid surgery: Definitive multidisciplinary joint consensus guidelines of the American Head and Neck Society and the British Association of Endocrine and Thyroid Surgeons. Head Neck 2018, 40,1617-1629. Epub 2018 Aug 2. PMID: 30070413. [CrossRef]
- Del Rio, P.; Polistena, A.; Chiofalo, M.G.; De Pasquale, L.; Dionigi, G.; Docimo, G.; Graceffa, G.; Iacobone, M.; Medas, F.; Pezzolla, A.; et al. Management of surgical diseases of thyroid gland indications of the United Italian Society of Endocrine Surgery (SIUEC). Updates Surg. 2023, 75(6),1393-1417. Epub 2023 May 18. PMID: 37198359; PMCID: PMC10435599. [CrossRef]
- Krajewska, J.; Chmielik, E.; Dedecjus, M.; Jarząb, B.; Hubalewska-Dydejczyk, A.; Karbownik-Lewińska, M.; Kos-Kudła, B.; Lewiński, A.; Ruchała, M. Diagnosis and treatment of thyroid cancer in adult patients - Recommendations of Polish Scientific Societies and the National Oncological Strategy. Update of the 2022 Update [Diagnostyka i leczenie raka tarczycy u chorych dorosłych - Rekomendacje Polskich Towarzystw Naukowych oraz Narodowej Strategii Onkologicznej. Aktualizacja na rok 2022 - uzupełnienie]. Endokrynol Pol 2022, 73(5), 799-802. PMID: 37067538. [CrossRef]
- Sun, H.; Tian, W.; Chinese Thyroid Association, College of Surgeons, Chinese Medical Doctor Association; Chinese Research Hospital Association Thyroid Disease Committee. Chinese guidelines on intraoperative neuromonitoring in thyroid and parathyroid surgery (2023 edition). Gland Surg. 2023 12(8),1031-1049. Epub 2023 Aug 2. PMID: 37701297; PMCID: PMC10493630. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).