Submitted:
14 February 2024
Posted:
15 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
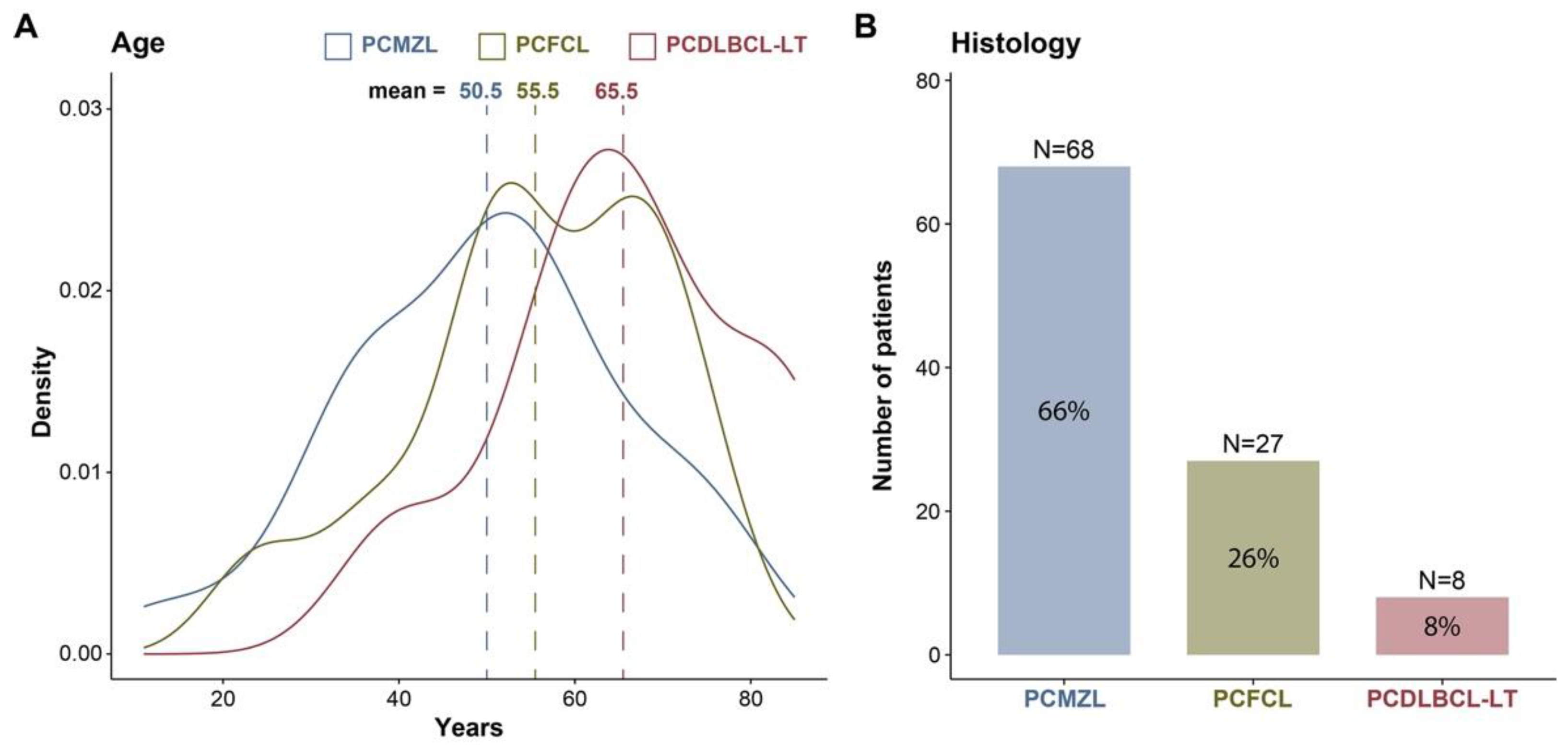
3.1. Epidemiology
3.2. Histology
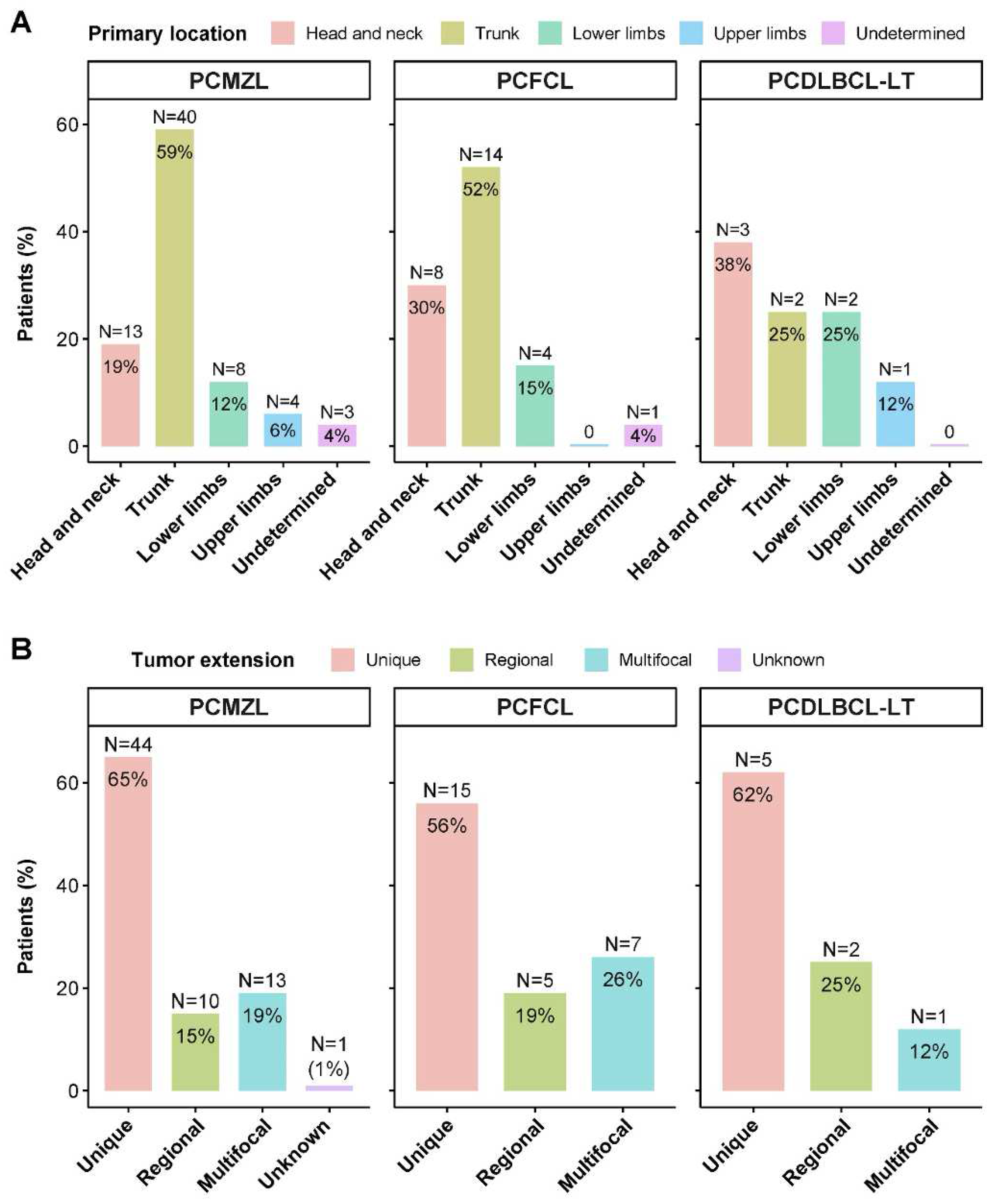
3.3. Location and Extension
3.4. Types of Treatments
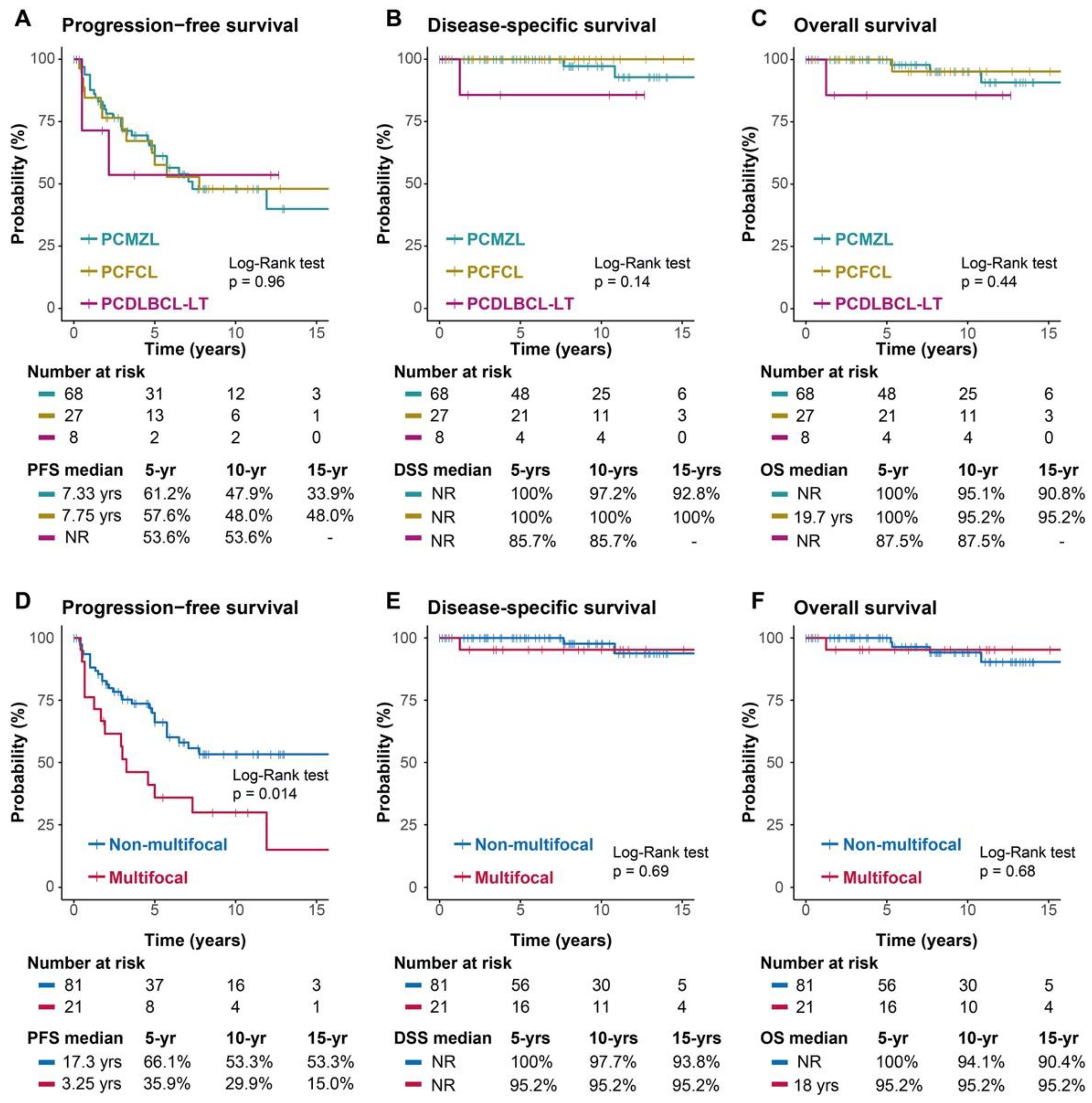
3.5. Response, Recurrence and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgements
Conflicts of interest
References
- Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113(21):5064-73. [CrossRef] [PubMed]
- Senff NJ, Hoefnagel JJ, Jansen PM, Vermeer MH, van Baarlen J, Blokx WA, et al. Reclassification of 300 primary cutaneous B-Cell lymphomas according to the new WHO-EORTC classification for cutaneous lymphomas: comparison with previous classifications and identification of prognostic markers. J Clin Oncol. 2007;25(12):1581-7. [CrossRef] [PubMed]
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;134(13):1112.
- Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997;90(1):354-71.
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768-85. [CrossRef]
- Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M, Committee EG. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv30-iv40. [CrossRef] [PubMed]
- Goyal N, O’Leary D, Carter JB, Comfere N, Sokumbi O, Goyal A. A Practical Review of the Presentation, Diagnosis, and Management of Cutaneous B-Cell Lymphomas. Dermatol Clin. 2023;41(1):187-208. [CrossRef]
- Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(2):479-84.
- Pham-Ledard A, Cowppli-Bony A, Doussau A, Prochazkova-Carlotti M, Laharanne E, Jouary T, et al. Diagnostic and prognostic value of BCL2 rearrangement in 53 patients with follicular lymphoma presenting as primary skin lesions. Am J Clin Pathol. 2015;143(3):362-73. [CrossRef]
- Guinard E, Alenezi F, Lamant L, Szablewski V, Tournier E, Laurent C, et al. Staging of primary cutaneous follicle centre B-cell lymphoma: bone marrow biopsy, CD10, BCL2 and t(14;18) are not relevant prognostic factors. Eur J Dermatol. 2019.
- Amitay-Laish I, Tavallaee M, Kim J, Hoppe RT, Million L, Feinmesser M, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176(4):1010-20.
- Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. 2022;140(11):1229-1253. Blood. 2023;141(4):437.
- Dumont M, Battistella M, Ram-Wolff C, Bagot M, de Masson A. Diagnosis and Treatment of Primary Cutaneous B-Cell Lymphomas: State of the Art and Perspectives. Cancers (Basel). 2020;12(6). [CrossRef]
- Swerdlow, SH. Cutaneous marginal zone lymphomas. Semin Diagn Pathol. 2017;34(1):76-84. [CrossRef]
- Cerroni L, Zöchling N, Pütz B, Kerl H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24(8):457-61. [CrossRef]
- Mandekou-Lefaki I, Delli FS, Kountouras J, Athanasiou E, Mattheou-Vakali G. Primary cutaneous MALT-type lymphoma and Helicobacter pylori: a possible relationship. J Eur Acad Dermatol Venereol. 2006;20(5):606-8. [CrossRef]
- May SA, Netto G, Domiati-Saad R, Kasper C. Cutaneous lymphoid hyperplasia and marginal zone B-cell lymphoma following vaccination. J Am Acad Dermatol. 2005;53(3):512-6.
- Guitart J, Deonizio J, Bloom T, Martinez-Escala ME, Kuzel TM, Gerami P, et al. High incidence of gastrointestinal tract disorders and autoimmunity in primary cutaneous marginal zone B-cell lymphomas. JAMA Dermatol. 2014;150(4):412-8. [CrossRef]
- Edinger JT, Kant JA, Swerdlow SH. Cutaneous marginal zone lymphomas have distinctive features and include 2 subsets. Am J Surg Pathol. 2010;34(12):1830-41. [CrossRef]
- Vermeer MH, Geelen FA, van Haselen CW, van Voorst Vader PC, Geerts ML, van Vloten WA, et al. Primary cutaneous large B-cell lymphomas of the legs. A distinct type of cutaneous B-cell lymphoma with an intermediate prognosis. Dutch Cutaneous Lymphoma Working Group. Arch Dermatol. 1996;132(11):1304-8.
- Pham-Ledard A, Prochazkova-Carlotti M, Andrique L, Cappellen D, Vergier B, Martinez F, et al. Multiple genetic alterations in primary cutaneous large B-cell lymphoma, leg type support a common lymphomagenesis with activated B-cell-like diffuse large B-cell lymphoma. Mod Pathol. 2014;27(3):402-11. [CrossRef]
- Mitteldorf C, Berisha A, Pfaltz MC, Broekaert SMC, Schön MP, Kerl K, et al. Tumor Microenvironment and Checkpoint Molecules in Primary Cutaneous Diffuse Large B-Cell Lymphoma-New Therapeutic Targets. Am J Surg Pathol. 2017;41(7):998-1004. [CrossRef]
- Pashtan I, Mauch PM, Chen YH, Dorfman DM, Silver B, Ng AK. Radiotherapy in the management of localized primary cutaneous B-cell lymphoma. Leuk Lymphoma. 2013;54(4):726-30. [CrossRef]
- Specht L, Dabaja B, Illidge T, Wilson LD, Hoppe RT, Group ILRO. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92(1):32-9. [CrossRef]
- Neelis KJ, Schimmel EC, Vermeer MH, Senff NJ, Willemze R, Noordijk EM. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74(1):154-8. [CrossRef]
- Izu-Belloso RM, García-Ruiz JC. Treatment of cutaneous lymphomas: an update. Actas Dermosifiliogr. 2012;103(8):694-707.
- Quéreux G, Brocard A, Peuvrel L, Nguyen JM, Knol AC, Dréno B. Systemic rituximab in multifocal primary cutaneous follicle centre lymphoma. Acta Derm Venereol. 2011;91(5):562-7. [CrossRef]
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, Santiago F, Rosales B, Servitje O, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167(1):174-9. [CrossRef]
- Leary DO, Goyal N, Rubin N, Goyal A. Characterization of Primary and Secondary Cutaneous B-Cell Lymphomas: A Population-Based Study of 4758 Patients. Clin Lymphoma Myeloma Leuk. 2022;22(4):e269-e78.
- Travaglino A, Varricchio S, Pace M, Russo D, Picardi M, Baldo A, et al. Borrelia burgdorferi in primary cutaneous lymphomas: a systematic review and meta-analysis. J Dtsch Dermatol Ges. 2020;18(12):1379-84. [CrossRef] [PubMed]
- Falkenhain-López D, Muniesa C, Estrach MT, Morillo-Andújar M, Peñate Y, Acebo E, et al. Primary Cutaneous Lymphoma Registry of the Spanish Academy of Dermatology and Venereology (AEDV): Data for the First 5 Years. Actas Dermosifiliogr. 2023;114(4):291-8. [CrossRef]
- Dobos G, de Masson A, Ram-Wolff C, Beylot-Barry M, Pham-Ledard A, Ortonne N, et al. Epidemiological changes in cutaneous lymphomas: an analysis of 8593 patients from the French Cutaneous Lymphoma Registry. Br J Dermatol. 2021;184(6):1059-67. [CrossRef]
- Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood. 2005;105(9):3671-8. [CrossRef]
- Menguy S, Beylot-Barry M, Parrens M, Ledard AP, Frison E, Comoz F, et al. Primary cutaneous large B-cell lymphomas: relevance of the 2017 World Health Organization classification: clinicopathological and molecular analyses of 64 cases. Histopathology. 2019;74(7):1067-80. [CrossRef]
- Olszewska-Szopa M, Sobas M, Laribi K, Bao Perez L, Drozd-Sokołowska J, Subocz E, et al. Primary cutaneous indolent B-cell lymphomas - a retrospective multicenter analysis and a review of literature. Acta Oncol. 2021;60(10):1361-8. [CrossRef]
- Oertel M, Elsayad K, Weishaupt C, Steinbrink K, Eich HT. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther Onkol. 2020;196(2):126-31. [CrossRef]
- Vitiello P, Sica A, Ronchi A, Caccavale S, Franco R, Argenziano G. Primary Cutaneous B-Cell Lymphomas: An Update. Front Oncol. 2020;10:651. [CrossRef]
- Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229-1253. Blood. 2023;141(4):437.
- Kraft RM, Ansell SM, Villasboas JC, Bennani NN, Wang Y, Habermann TM, et al. Outcomes in primary cutaneous diffuse large B-cell lymphoma, leg type. Hematol Oncol. 2021;39(5):658-63. [CrossRef]
- Krenitsky A, Klager S, Hatch L, Sarriera-Lazaro C, Chen PL, Seminario-Vidal L. Update in Diagnosis and Management of Primary Cutaneous B-Cell Lymphomas. Am J Clin Dermatol. 2022;23(5):689-706. [CrossRef]
| Characteristics | Total cases N = 103 |
PCMZL N = 68 |
PCFCL N = 27 |
PCDLBCL-LT N = 8 |
|
|---|---|---|---|---|---|
| Gender | Female | 53 (51%) | 32 (47%) | 18 (67%) | 3 (38%) |
| Male | 50 (49%) | 36 (53%) | 9 (33%) | 5 (62%) | |
| Age | Median (range) | 53 (40, 65) | 51 (38, 60) | 56 (50, 66) | 66 (58, 74) |
| ECOG | 0 | 90 (87%) | 62 (91%) | 23 (85%) | 5 (56%) |
| 1 | 9 (9%) | 5 (7%) | 2 (7%) | 2 (25%) | |
| 2 | 1 (1%) | 0 | 0 | 1 (12%) | |
| >= 3 | 0 | 0 | 0 | 0 | |
| Unknown | 3 (3%) | 1 (1.4%) | 2 (7%) | 0 | |
| Stage | I | 79 (77%) | 54 (79%) | 20 (74%) | 5 (62%) |
| II | 22 (21%) | 13 (19%) | 7 (26%) | 2 (25%) | |
| III | 0 | 0 | 0 | 0 | |
| IV | 2 (2%) | 1 (1%) | 0 | 1 (12%) | |
| Prior skin disease | Yes | 15 (14.6%) | 12 (18%) | 3 (11%) | 0 |
| No | 81 (78.6%) | 50 (74%) | 24 (89%) | 7 (88%) | |
| Unknown | 7 (6.8%) | 6 (9%) | 0 | 1 (12%) | |
| Tumour main location | Head and neck | 24 (23%) | 13 (19%) | 8 (30%) | 3 (38%) |
| Trunk | 56 (54%) | 40 (59%) | 14 (52%) | 2 (25%) | |
| Upper limbs | 14 (14%) | 8 (12%) | 4 (15%) | 2 (25%) | |
| Lower limbs | 5 (5%) | 4 (6%) | 0 | 1 (12%) | |
| Undetermined | 4 (4%) | 3 (4%) | 1 (4%) | 0 | |
| Tumour extension | Unique | 64 (62%) | 44 (65%) | 15 (56%) | 5 (62%) |
| Regional | 17 (17%) | 10 (15%) | 5 (19%) | 2 (25%) | |
| Multifocal | 21 (20%) | 13 (19%) | 7 (26%) | 1 (12%) | |
| Unknown | 1 (1%) | 1 (1%) | 0 | 0 | |
| Primary outcome | Complete response | 99 (96%) | 67 (99%) | 25 (93%) | 7 (88%) |
| No response | 4 (4%) | 1 (1%) | 2 (7%) | 1 (12%) | |
| Relapse | Yes | 45 (44%) | 30 (44%) | 12 (44%) | 3 (38%) |
| Number of relapses | 1-3 relapses | 35 (34%) | 24 (35%) | 9 (33%) | 2 (25%) |
| >3 relapses | 8 (7.8%) | 5 (7.4%) | 3 (11%) | 0 | |
| Unknown number | 2 (1.9%) | 1 (1.5%) | 0 | 1 (13%) | |
| Site of relapse | Local | 22 (21%) | 14 (21%) | 6 (22%) | 2 (25%) |
| Regional | 9 (8.7%) | 8 (12%) | 1 (3.7%) | 0 | |
| Distant | 10 (9.7%) | 6 (8.8%) | 4 (15%) | 0 | |
| Unknown | 4 (3.9%) | 2 (2.9%) | 1 (3.7%) | 1 (13%) | |
| Deaths | By lymphoma | 3 (3%) | 2 (3%) | 0 | 1 (12%) |
| By other causes | 4 (4%) | 2 (3%) | 2 (7%) | 0 |
| Treatments | Total cases N=103 |
PCMZL N=68 |
PCFCL N=27 |
PCDLBCL-LT N=8 |
|
|---|---|---|---|---|---|
| Only local | 73 (70.9%) | 51 (75%) | 19 (70.4%) | 3 (37.5%) | |
| Only S | 24 (23.3%) | 19 (28%) | 5 (18.5%) | 0 | |
| Only RT | 27 (26.2%) | 20 (29%) | 7 (25.9%) | 0 | |
| S + RT | 22 (21.4%) | 12 (18%) | 7 (25.9%) | 3 (37.5%) | |
| Only systemic | 14 (13.6%) | 9 (13.2%) | 3 (11.1%) | 2 (25%) | |
| Only CT or R-CT | 10 (9.7%) | 6 (8.8%) | 2 (7.4%) | 2 (25%) | |
| Only systemic rituximab | 4 (3.9%) | 3 (4.4%) | 1 (3.7%) | 0 | |
| Local + Systemic | 12 (11.6%) | 7 (8.8%) | 3 (11.1%) | 3 (37.5%) | |
| S + ST | 5 (4.8%) | 2 (2.9%) | 1 (3.7%) | 2 (25%) | |
| RT + ST | 6 (5.8%) | 3 (4.4%) | 2 (7.4%) | 1 (12.5%) | |
| S + RT + ST | 1 (1%) | 1 (1.5%) | 0 | 0 | |
| Other therapies | 4 (3.9%) | 2 (3%) | 1 (7.4%) | 0 | |
| RT + ILR | 1 (1%) | 1 (1.5%) | 0 | 0 | |
| Only ILR | 1 (1%) | 0 | 1 (3.7%) | 0 | |
| Only Intralesional corticoids | 1 (1%) | 0 | 1 (3.7%) | 0 | |
| Doxycycline | 1 (1%) | 1 (1.5%) | 0 | 0 |
| Treatments | Total cases N=103 |
PCMZL N=68 |
PCFCL N=27 |
PCDLBCL-LT N=8 |
|
|---|---|---|---|---|---|
| Any surgery | 52 (50%) | 34 (49%) | 13 (48%) | 5 (62%) | |
| Any CT | 26 (25%) | 15 (22%) | 6 (22%) | 5 (62%) | |
| CHOP | 4 (15%) | 2 (13%) | 1 (17%) | 1 (20%) | |
| R-CHOP | 8 (31%) | 4 (27%) | 1 (17%) | 3 (60%) | |
| R-CTX/R-CVP | 3 (12%) | 1 (7%) | 1 (17%) | 1 (20%) | |
| Rituximab | 7 (27%) | 4 (27%) | 3 (50%) | 0 | |
| Cyclophosphamide | 1 (4%) | 1 (7%) | 0 | 0 | |
| Chlorambucil | 2 (8%) | 2 (13%) | 0 | 0 | |
| CT not specified | 1 (4%) | 1 (7%) | 0 | 0 | |
| Any RT | 57 (55%) | 37 (54%) | 16 (59%) | 4 (50%) | |
| 30-35 Gy | 6 (11%) | 5 (14%) | 1 (6%) | 0 | |
| 36-40 Gy | 24 (42%) | 19 (51%) | 5 (31%) | 0 | |
| 41-45 Gy | 4 (7%) | 2 (5%) | 1 (6%) | 1 (25%) | |
| 46-50 Gy | 2 (4%) | 2 (5%) | 0 | 0 | |
| Dose not specified | 21 (37%) | 9 (24%) | 9 (56%) | 3 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





