1. Introduction
The polysaccharide, chitin, is a β-(1,4)-linked polymer of
N-acetyl-d-glucosamine, found commonly in the marine environment, and forming a key component of the exoskeletons of many crustaceans [
1]. These chitinous exoskeletons can be broken down in the environment by chitinolytic bacteria, usually after moulting or post-mortem, in the process of nutrient recycling [
2]. The condition commonly known as “black spot” [
3], but also as black necrosis [
4] or shell disease [
5,
6], is associated with the breakdown of chitin by chitinolytic bacteria in live crustaceans and is characterised by the formation of black necrotic lesions on the body of the animal extending through the cuticle of the shell [
2]. Black spot can be contained, to an extent, by the immune response of the crab, or the moulting of the infected exoskeleton, but mortality can occur due to the necrosis associated with severe infection, or the prevention of moulting due to adhering of the old exoskeleton to the new at the site of the lesions [
7,
8,
9]. Black spot is found globally and is seen in many species of crustaceans [
10]
, however the focus of this study is on the commercially important edible crab,
Cancer pagurus. Found over a relatively large geographic area from Norway to Portugal [
11,
12]
C. pagurus inhabits rocky shores in a variety of habitats (e.g. bedrock, coarse sand, kelp holdfasts) from intertidal areas to depths of around 400m [
13,
14]. A narrow temperature (4 - 15°C) and salinity (17 PSU) range may play a role in choice of habitat [
13,
15,
16]. A commercially important species throughout its distribution with catch increasing in the UK from 10,000 t in 1950 to 32,500 t in 2014 [
17,
18]. Currently worth £13.8 million in Scotland alone in 2015, with increasing landing trends this number is expected to increase year-on-year [
19]. Annual landings for the UK were in excess of 31,632 tonnes worth £74.3 million in 2019 [
20,
21].
The chitinolytic bacteria responsible for black spot has been isolated and characterised by Vogan
et al. [
2] and a review by Getchell [
22] found several genera responsible for black spot lesions including
Vibrio,
Aeromonas,
Pseudomonas,
Alteromonas,
Flavobacterium,
Spirillum,
Moraxella,
Pasteurella and
Photobacterium. Increased prevalence of black spot within
C. pagurus populations has been linked to degraded environmental conditions and areas of increased pollution [
23,
24] or physical damage to the crabs’ exoskeletons linked to mechanical fishing gear, sediment scouring and aggression between individuals [
8,
25]. Additional factors previously considered to affect the incidence rates of black spot include the depth at capture, seasonality, difference between sexes and
Sacculina carcini infestations, but no clear relationships have been determined [
24,
25].
Around 20-30% of the populations targeted by fisheries (Ayres and Edwards., 1982) including prawns [
5], lobsters [
5], and crabs [
10,
26] are susceptible to black spot lesions. This poses a financial loss to fishers, as crabs displaying excessive carapace disease are typically rejected due to its effect on the quality of the meat [
27].
The primary aims of this study were to assess the prevalence of black spot on both a fished (>140mm carapace width (Minimum Landing Size (MLS)) and juvenile non-fished population (≤139mm carapace width) of C. pagurus within a voluntary marine reserve. Secondary aims are to determine the percentage coverage of black spot lesions and determine if differences exist between crabs of different size and sex. The final aim is to compare infection rates with other geographical areas around the UK.
2. Materials and Methods
Intermoult crabs were collected from within the Berwickshire Marine Reserve (BMR) (St Abbs, Berwickshire, UK). Large (>121mm) and medium (80 – 120mm) crabs were caught by local commercial fishing boats using double and single eye parlour creels. Small (≤79mm) crabs were collected via intertidal shore surveys within the BMR. Collection and analysis of both methods described above were conducted from May 2018 – May 2019.
The weight (g), carapace width (CW) (mm), sex, and condition index [
28] was obtained prior to photographs being taken of the top and underside of the crabs next to a marked scale. Images were loaded individually into ImageJ (Version 1.52a), the scale was set using a known distance (scale in the image or known carapace width of the crab), black necrotic areas were then measured using ImageJ, and the total coverage assessed compared to the area of the crab’s body (
Figure 1).
Analysis was conducted comparing the prevalence of the disease in males and females separately, the significance of the location of disease and the significance of the size of afflicted crabs. For size analysis, crabs were first grouped both by MLS (⩾150mm) and undersized (<150mm), and then further sub categorized into size groups of small (≤79mm), medium (80 – 120mm) and large (>121mm) as per Scott
et al. [
28] which was subsequently based upon sexual maturity. The location of black spot was divided into sections based on the crab’s anatomy; carapace, chelae, telson and pereopods.
2.2. Statistics
Data of disease occurrence was analysed using log linear analysis. The effects of variables were compared using chi-square tests. All other data is expressed as mean ± standard error (SEM) and were tested using non-parametric tests to distinguish where significant differences lay. Mann-Whitney U-test was used where only 2 sample groups existed and Kruskall-Wallis test was used for multiple group comparison. Frequency data were analysed using a log-linear model with disease occurrence as the dependent variable and size, and sex as independent variables. Results were tested at a significance level of p<0.05. Statistical analysis was conducted using STATISTICA (version 7.1, StatSoft In. 2005).
3. Results
Crab Sex
Loglinear analysis showed significant differences in the frequency of black spot between female and male crabs (p<0.01). Presence of black spot in male crabs was at a ratio of 3:1, healthy: infected, compared to females with a ratio of 5:1. In males 26.21% of crabs were infected, while in female crabs the rate of infection was 16.43%.
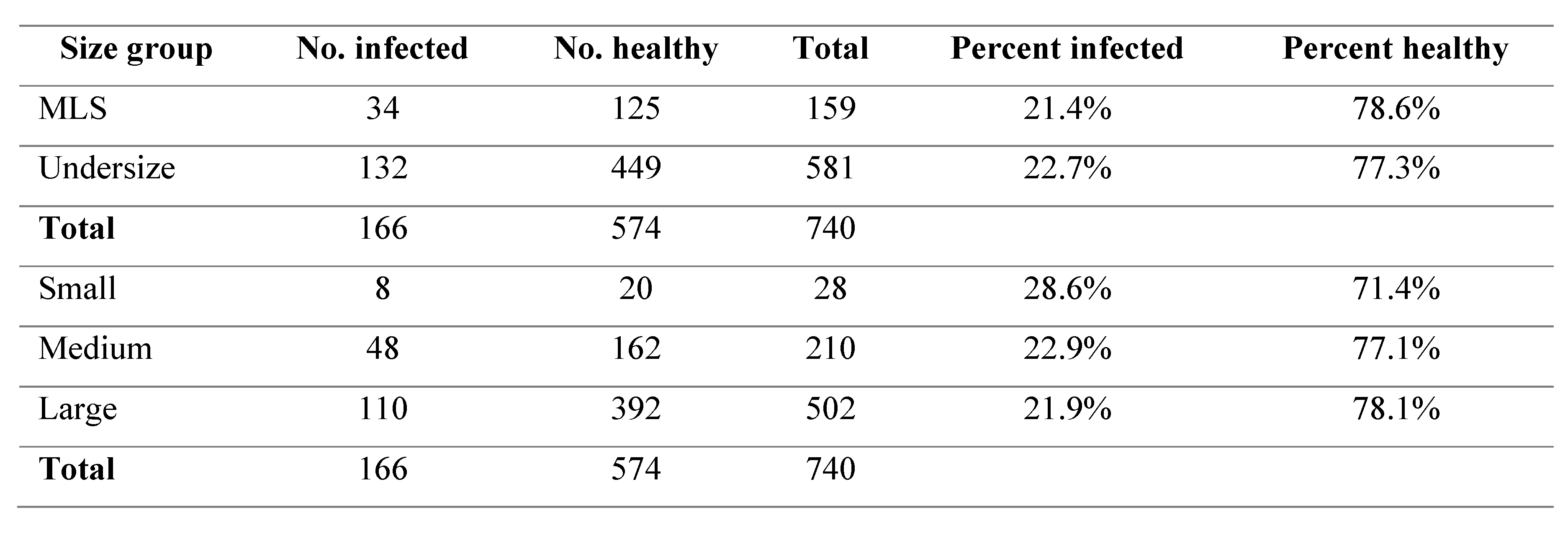
Crab Size
There were no significant differences found in the prevalence of black spot in either the comparison of undersize to MLS or in comparison of the small, medium and large size classifications (p>0.05). The percentage of black spot occurrence ranged from 21.4 – 28.6% throughout all the size groups (
Table 1).
Table 1.
Black spot infection rates between difference size groups.
Table 1.
Black spot infection rates between difference size groups.
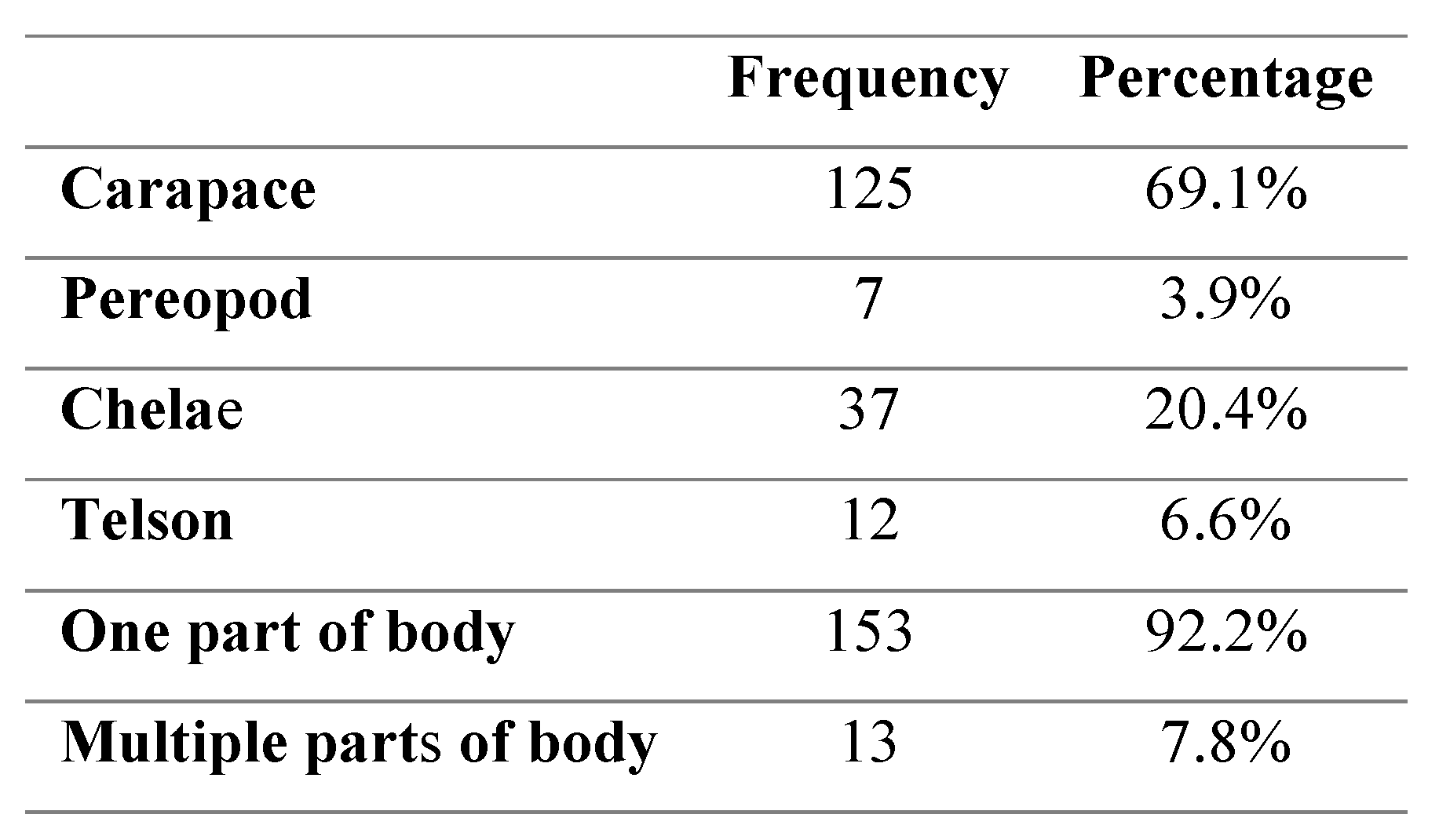
Location of Black Spot
The location of visible blackspot lesions was found to be significantly more frequent on the carapace and chelae of crabs in comparison to the telson and pereopod areas (p<0.01) (
Table 2). There were no significant differences in the location of black spot in relation to crab size (p>0.05) or in relation to sex (p>0.05). The presence of black spot in multiple areas of the crabs’ body was found to be significantly higher in males (p<0.01) than females.
Table 2.
Frequency and percentage of crab body sections infected with black spot.
Table 2.
Frequency and percentage of crab body sections infected with black spot.
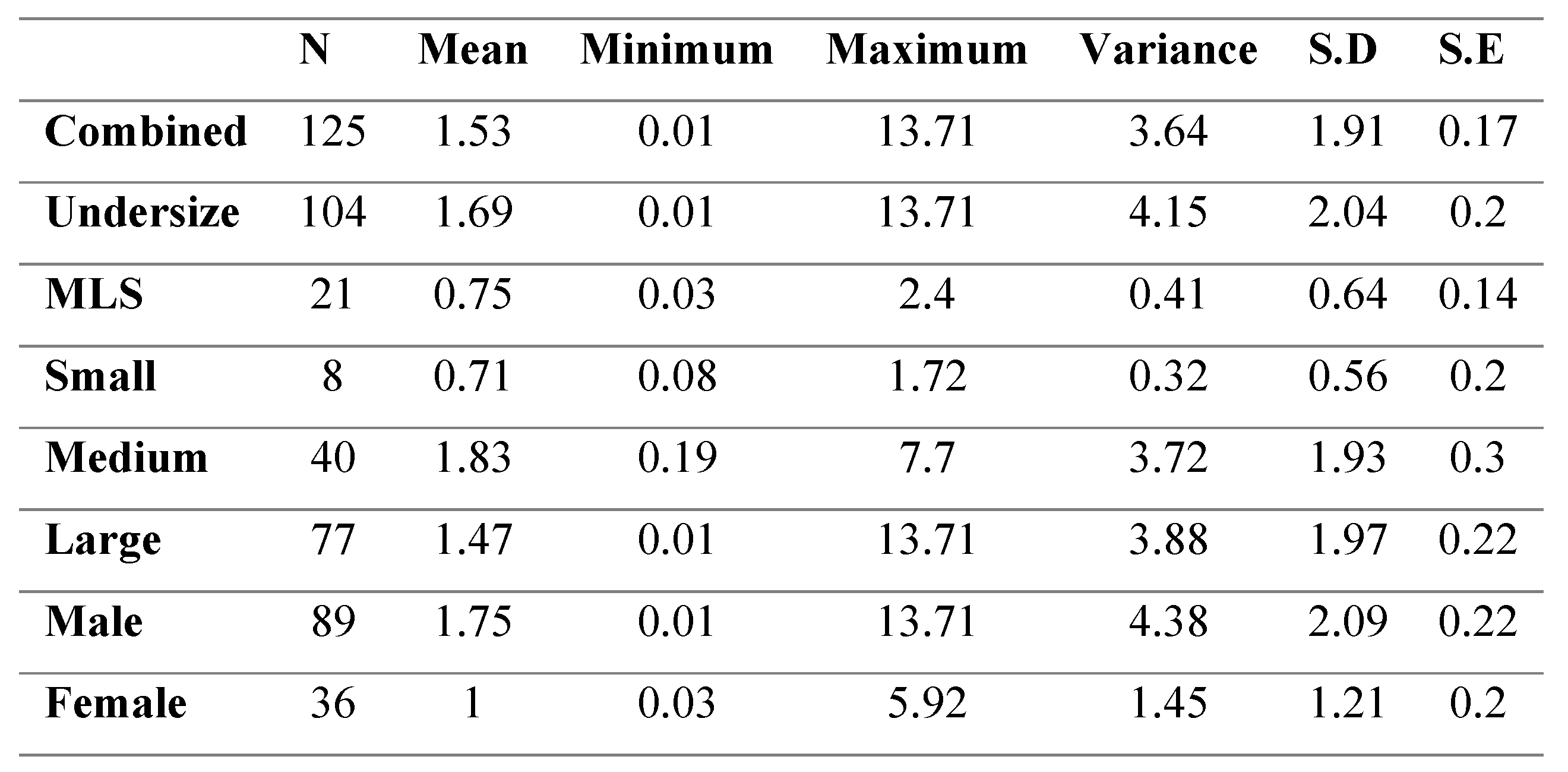
Degree of Black Spot Infection
Male crabs had significantly higher black spot coverage compared to female crabs (p<0.05). There was no significant difference found in the relative black spot area of the small, medium and large class sizes (p>0.05) (
Table 3). Undersize crabs, however, were found to have significantly larger relative black spot areas in comparison to MLS crabs (p<0.05).
Table 3.
Percent coverage of black spot across size groups in infected C. pagurus.
Table 3.
Percent coverage of black spot across size groups in infected C. pagurus.
4. Discussion
Results from this study highlight that male crabs tend to exhibit a significantly higher rate of infection of black spot lesions, significantly more body sections infected, larger overall area and larger lesions when black spot is present. These findings are consistent with those obtained in past studies, finding an average of 61.3% of male crabs were infected with the disease, compared to 40.1% of females [
8,
9]. This increase in prevalence and size of black spot is hypothesised to be due to the increased aggression demonstrated by male crabs [
29,
30] and leading to exoskeleton damage and increased risk of chitinolytic bacterial infections [
8,
9]. Female crabs of sexual maturity (~110mm) may be ‘berried’ (carrying eggs under abdomen) for 7 to 9 months [
31]. These female crabs limit movement and reduce foraging thus lessening their chance of scouring damage as well as reducing the likelihood of them being caught by baited traps [
31]. Berried crabs are also thought to avoid burrowing backwards when carrying eggs, something seen in male and un-berried female crabs, potentially preventing excess scouring damage [
8,
9]. Haig
et al. [
32] found that an infection rate of 9.6% with 7.27% of the sampled females (mean CW = 115mm) and 12.87% of sampled males (mean CW = 123mm) showing signs of black spot disease. This study also highlighted that crabs from Scotland and Isle of Man populations had a higher infection rate with 26.35% and 12% respectively.
A study by Ayres and Edwards [
3] found that the occurrence of black spot was a naturally occurring condition that was found more commonly in older animals when moult frequency is reduced. In this same study it was determined that black spot was more common in areas that were lightly fished than in established, heavily fished areas. This is thought to be due to the removal of older individuals with higher infection rates.
No significant relationship was found between crab size and the prevalence of black spot infection within this study. This, however, contradicts previous literature that found greatest prevalence of black spot with increasing crab size [
8,
9]. This study found undersized crabs to possess a significantly larger relative area of black spot infection when compared to crabs of legal catch size (>140mm). To date there has been limited black spot assessments that have incorporated juveniles, with studies instead focusing on the commercially fished populations. These size biased surveys may partly explain why higher infection rates were found in juveniles than older crabs as previously reported. Juvenile crabs inhabit areas that have significantly higher biomass such as kelp forests and algal beds [
33,
34,
35] and as such experience higher rates of predation, competition for food and shelter, and due to the nature of intertidal areas, more significant changes in water movement, salinity and temperature. These factors could also play a role in the increased black spot found within juveniles by increasing the likelihood of carapace damage via intra and interspecific conflict, high periods of wave activity or via anthropogenic means such as fishing and infrastructures such as harbours. Changes in salinity and temperature have been found to cause physiological stress which reduces immunocompetence [
36]. In a recent studies exposure to electromagnetic fields (EMF) was found to result in increased physiological stress within both juvenile and adult
C. pagurus [
37,
38]. This suggests that higher levels of black spot may occur around renewable energy devices such as offshore wind farms, due to increased physiological stress from EMF. Scour protection zones around wind farm turbine bases have been shown to create 2.5 times more habitat than is lost by the array installation [
39]. Holes drilled into the scour protection areas have been shown to increase the carrying capacity per turbine to around 65,000kg of fish [
39]. The addition of holes of various sizes, transforms the scour protection blocks into artificial reefs which can enhance the abundance and diversity of species, including commercially important crustaceans [
40,
41]. This increase in abundance, combined with the previously described attraction to EMF from nearby power cables [
37] could increase conflict resulting in carapace damage and lower immunocompetence which may ultimately lead to an increase in black spot prevalence.
5. Conclusions
Black spot infection rates found within this study confirmed previous findings that males tend to show higher rates of infection, more parts of the body infected and greater overall black spot coverage than females. Size was not found to be a significant factor in black spot infection rates, however undersized crabs did show significantly higher coverage of black spot, predicted to be due to MSL crabs being removed from the habitat by fishing. When black spot was detected, there were higher rates of lesion on the carapace and chelae than anywhere else on the crab’s body. The Berwickshire Marine Reserve is situated in an area with excellent water quality, with salinities, temperature, Nitrate, Phosphate, Ammonia and Silicate concentrations monitored throughout the year by Marine Scotland and St Abbs Marine Station. As such relatively low infection rates of 21.4-28.6% do not appear to be a cause for immediate concern. Previous studies have reported significantly higher rates of infection within local populations (55% total sampled crabs and 88% MLS crabs), which may have far reaching impacts both financially in terms of fishery rejection and ecologically by impacting the reproduction of females.
Author Contributions
Conceptualization, K.S. and P.H.; methodology, K.S and P.H.; validation, K.S. and P.H.; formal analysis, P.H.; investigation, K.S. and P.H.; resources, K.S.; data curation, K.S. and P.H.; writing—original draft preparation, K.S.; writing—review and editing, K.S. and P.H.; visualization, K.S. and P.H.; supervision, K.S.; project administration, K.S. and P.H.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research adhered to the legal requirements of the country (U.K.) in which the work was carried out, and all institutional guidelines of St Abbs Marine Station. Informal ethical review and approval of this research was conducted by authors and trustees of St Abbs Marine Station using published literature and information obtained from Animals (Scientific Procedures) Act 1986 (ASPA). Animal collection was conducted with the local commercial fishing fleet. Derogations were granted by Marine Scotland over the sampling period to allow the landing of individuals below the Scottish MCRS of 150 mm. The surveys were in accordance with terms of Section 9 of the Sea Fish Conservation Act 1967, Article 25 of Council Regulation No. 2019/1241, the specified crustaceans (prohibition on landing, sale and carriage) (Scotland): order 2017 No.455 and the undersized edible crabs (Scotland) order 200 No.228. At present crustaceans and cephalopods remain outside the protections of the Animal Welfare Act, the Act which makes it an offence to cause unnecessary suffering to protected animals. The experiment does not need ethical approval.
Data Availability Statement
The data collected and analysed in this paper is not available publicly. Readers can inquire and request the raw data files from the first author (kevin.scott@marinestation.co.uk).
Acknowledgments
We would like to thank the staff and volunteers at St Abbs Marine Station for help with the sample collection and analysis. We would also like to thank the local inshore fishermen for their knowledge and the Nesbitt-Cleland Trust for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swiontek Brzezinska, M.; Jankiewicz, U.; Burkowska, A.; Walczak, M. Chitinolytic Microorganisms and Their Possible Application in Environmental Protection. Current Microbiology 2014, 68, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vogan, C.L.; Costa-Ramos, C.; Rowley, A.F. Shell disease syndrome in the edible crab, Cancer pagurus – isolation, characterization and pathogenicity of chitinolytic bacteria. Microbiology 2002, 148, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Ayres, P.A.; Edwards, E. Notes on the Distribution of “Black Spot” Shell Disease in Crustacean Fisheries. Chemistry and Ecology 1982, 1, 125–130. [Google Scholar] [CrossRef]
- Perkins, E. Some aspects of the biology of Carcinus maenas (L.). Trans. Dumfriesshire Galway Nat. Hist. Antiq. Soc 1967, 44, 47–56. [Google Scholar]
- Cook, D.W.; Lofton, S.R. Chitinoclastic bacteria associated with shell disease in Penaeus shrimp and the blue crab (Callinectes sapidus). Journal of Wildlife Diseases 1973, 9, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Roald, S.O.; Aursjø, J.; Håstein, T. Occurrence of shell disease in lobsters, Homarus gammarus (L.), in the southern part of Oslofjord, Norway. Fiskeridirektoratets Skrifter Serie Havundersokelser 1981, 17, 153–160. [Google Scholar]
- Fisher, W.S.; Nilson, E.H.; Steenbergen, J.F.; Lightner, D.V. Microbial diseases of cultured lobsters: A review. Aquaculture 1978, 14, 115–140. [Google Scholar] [CrossRef]
- Vogan, C.L.; Llewellyn, P.; Rowley, A.F. Epidemiology and dynamics of shell disease in the edible crab Cancer pagurus: a preliminary study of Langland Bay, Swansea, UK. Diseases of Aquatic Organisms 1999, 35, 81–87. [Google Scholar] [CrossRef]
- King, N.G.; Duncan, P.F.; Kennington, K.; Wootton, E.C.; Jenkins, S.R. Characterisation of Shell Disease Syndrome in the Brown Crab, Cancer Pagurus, in a Discrete Irish Sea Fishery. Journal of Crustacean Biology 2014, 34, 40–46. [Google Scholar] [CrossRef]
- Claire, L.V.; Carolina, C.-R.; Andrew, F.R. A histological study of shell disease syndrome in the edible crab Cancer pagurus. Diseases of Aquatic Organisms 2001, 47, 209–217. [Google Scholar] [CrossRef]
- Heraghty, N. Investigating the Abundance, Distribution and Habitat Use of Juvenile Cancer Pagurus (l.) of the Intertidal Zone Around Anglesey and Llyn Peninsula, North Wales (UK). Bangor University (Marine Biology), 2013.
- MarLIN, BIOTIC - Biological Traits Information Catalogue. 2006.
- Neal, K.; Wilson, E. Cancer pagurus Edible crab. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [online]. Plymouth: Marine Biological Association of the United Kingdom: 2008.
- Bakke, S.; Buhl-Mortensen, L.; Buhl-Mortensen, P. Some observations of Cancer pagurus Linnaeus, 1758 (Decapoda, Brachyura) in deep water. Crustaceana 2019, 92, 95–105. [Google Scholar] [CrossRef]
- Wanson, S.; Pequeux, A.; Gilles, R. Osmoregulation in the stone crab Cancer pagurus. Marine biology letters 1983, 4, 321–330. [Google Scholar]
- Cuculescu, M.; Hyde, D.; Bowler, K. Thermal tolerance of two species of marine crab, Cancer pagurus and Carcinus maenas. Journal of Thermal Biology 1998, 23, 107–110. [Google Scholar] [CrossRef]
- Bannister, R.C.A. On the Management of Brown Crab Fisheries; Shellfish Association of Great Britain: 2009.
- Fish and Shellfish Stocks: 2015 Edition. 2015.
- Consultation on landing controls for the Scottish crab and lobster fisheries. 2016.
- Moran-Quintana, M.; Motova, A.; Witteveen, A. UK Economic Fleet Estimates and Fleet Enquiry Tool; SR750; 2020.
- UK sea fisheries annual statistics report 2019. 2020.
- Getchell, R. Bacterial shell disease in crustaceans: a review. 8, 1–6.
- Sindermann, C.J. Shell disease of crustaceans in the New York Bight. 1989.
- Powell A., R. A.F. Unchanged prevalence of shell disease in the edible crab Cancer pagurus four years after decommissioning of a sewage outfall at Langland Bay, UK. Diseases of Aquatic Organisms 2005, 68, 83–87. [Google Scholar] [CrossRef]
- Comely, C.A.; Ansell, A.D. The occurrence of black necrotic disease in crab species from the West of Scotland. Ophelia 1989, 30, 95–112. [Google Scholar] [CrossRef]
- Stentiford, G.D. Diseases of the European edible crab (Cancer pagurus): a review. ICES Journal of Marine Science 2008, 65, 1578–1592. [Google Scholar] [CrossRef]
- McIntosh, N. A study of black necrosis on the edible crab Cancer pagurus. 1963, 13.
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Baseline measurements of physiological and behavioural stress markers in the commercially important decapod Cancer pagurus (L.). Journal of Experimental Marine Biology and Ecology 2018, 507, 1–7. [Google Scholar] [CrossRef]
- Warner, G.F. The Biology of Crabs; Elek Science: London, 1977; p. 202. [Google Scholar]
- Edwards, E. The edible crab and its fishery in British waters; Fishing News: 1979.
- Bennett, D.B.C. Factors in the life history of the edible crab (Cancer pagurus L.) that influence modelling and management.; ICES-CIEM: 1995; pp. 89-98.
- Haig, J.A.; Bakke, S.; Bell, M.C.; Bloor, I.S.M.; Cohen, M.; Coleman, M.; Dignan, S.; Kaiser, M.J.; Pantin, J.R.; Roach, M.; et al. Reproductive traits and factors affecting the size at maturity of Cancer pagurus across Northern Europe. ICES Journal of Marine Science 2016, 73, 2572–2585. [Google Scholar] [CrossRef]
- Mann, K.H. Seaweeds: Their Productivity and Strategy for Growth. Science 1973, 182, 975–981. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Miller, G.L. Ecology, 4th ed.; W.H.Freeman & Co Ltd: New York, 2000; p. 833. [Google Scholar]
- Park, C. The environment: principles and applications. 2001, 704. [CrossRef]
- Evans, L.H. Lobster health and disease: basic concepts. In Proceedings of the International symposium on lobster health management, 1999; pp. 19-21.
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Understanding the effects of electromagnetic field emissions from Marine Renewable Energy Devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Marine Pollution Bulletin 2018, 131, 580–588. [Google Scholar] [CrossRef]
- Scott, K.; Harsanyi, P.; Easton, B.A.A.; Piper, A.J.R.; Rochas, C.M.V.; Lyndon, A.R. Exposure to Electromagnetic Fields (EMF) from Submarine Power Cables Can Trigger Strength-Dependent Behavioural and Physiological Responses in Edible Crab, Cancer pagurus (L.). Journal of Marine Science and Engineering 2021, 9, 776. [Google Scholar] [CrossRef]
- Burdon Daryl, A.L., Karine Laffont, Ben; Wilson, M.E., Rafael Perez-Dominguez Offshore and Coastal Renewable Energy: Potential ecological benefits and impacts of large-scale offshore and coastal renewable energy; PML Applications Ltd, SAMS, IECS: May 2009 2009.
- Bortone, S.A.; Martin, T.; Bundrick, C.M. Factors Affecting Fish Assemblage Development on a Modular Artificial Reef in a Northern Gulf of Mexico Estuary. Bulletin of Marine Science 1994, 55, 319–332. [Google Scholar]
- Kawasaki, H.; Sano, M.; Shibuno, T. The relationship between habitat physical complexity and recruitment of the coral reef damselfish, Pomacentrus amboinensis: an experimental study using small-scale artificial reefs. Ichthyological Research 2003, 50, 0073–0077. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).