Submitted:
15 February 2024
Posted:
15 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalysts Characterization
2.3. Catalytic soot combustion tests
3. Results and discussion
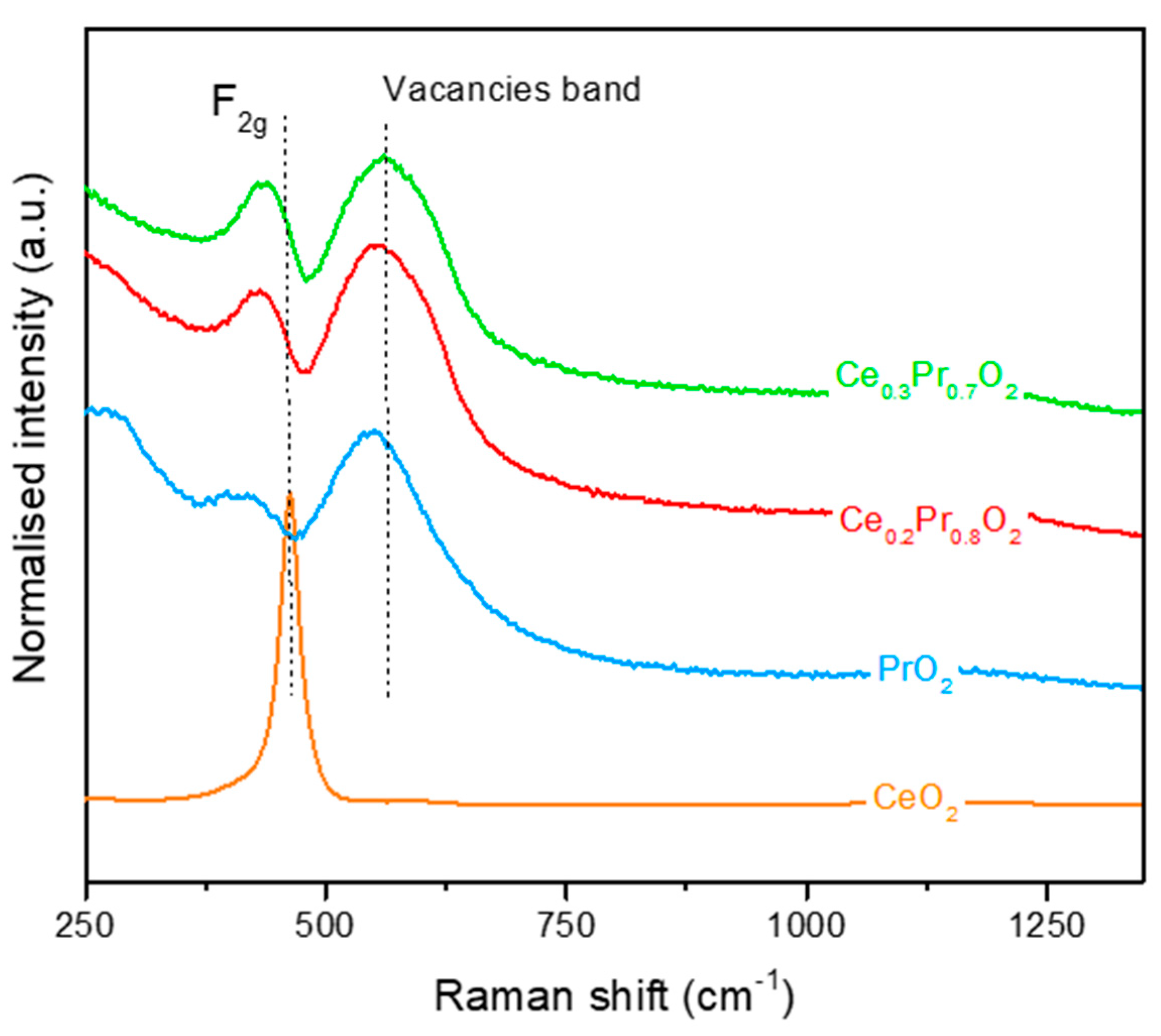
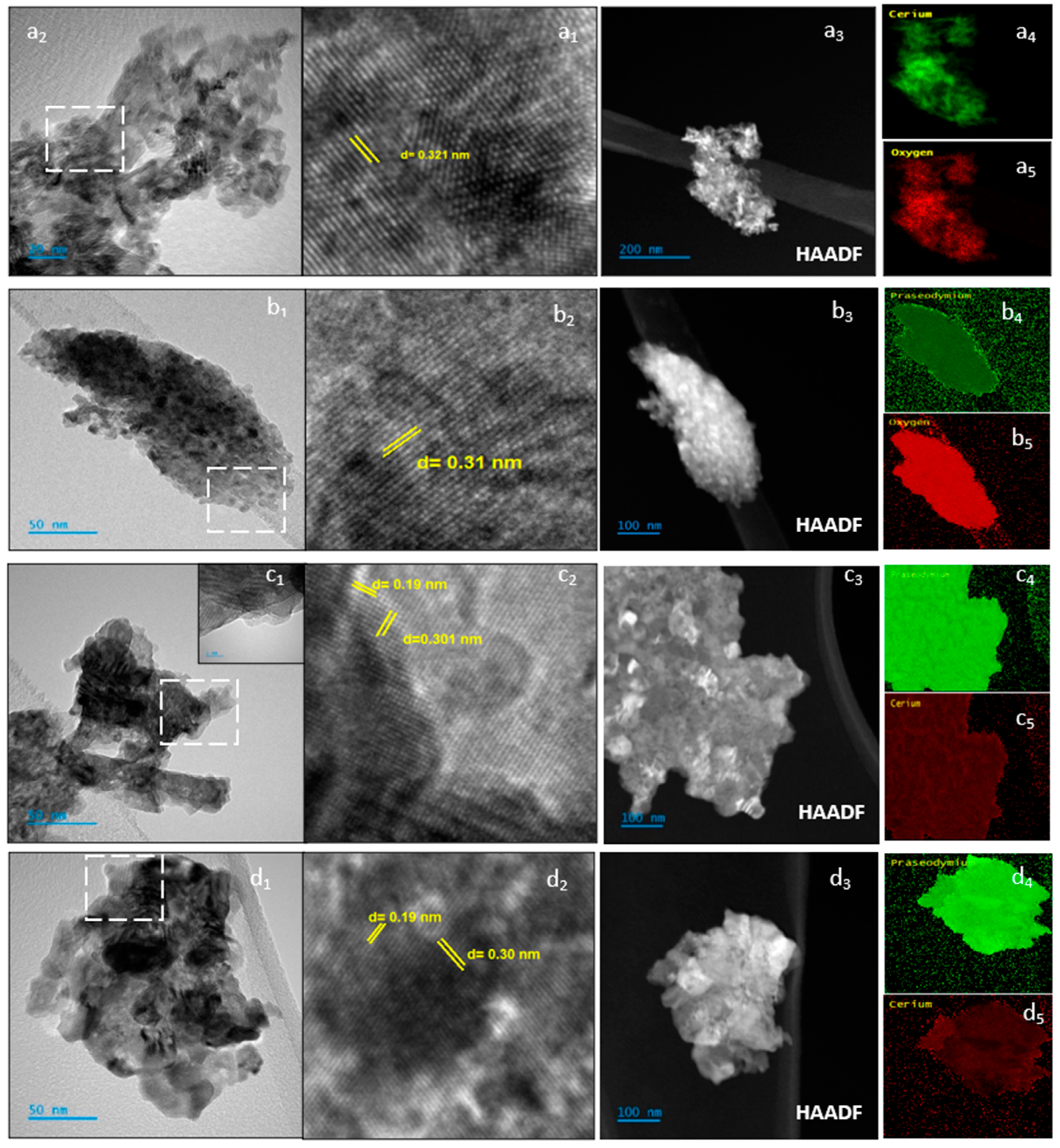
3.1. Catalysts characterization
3.1.1. Structural and textural parameters.
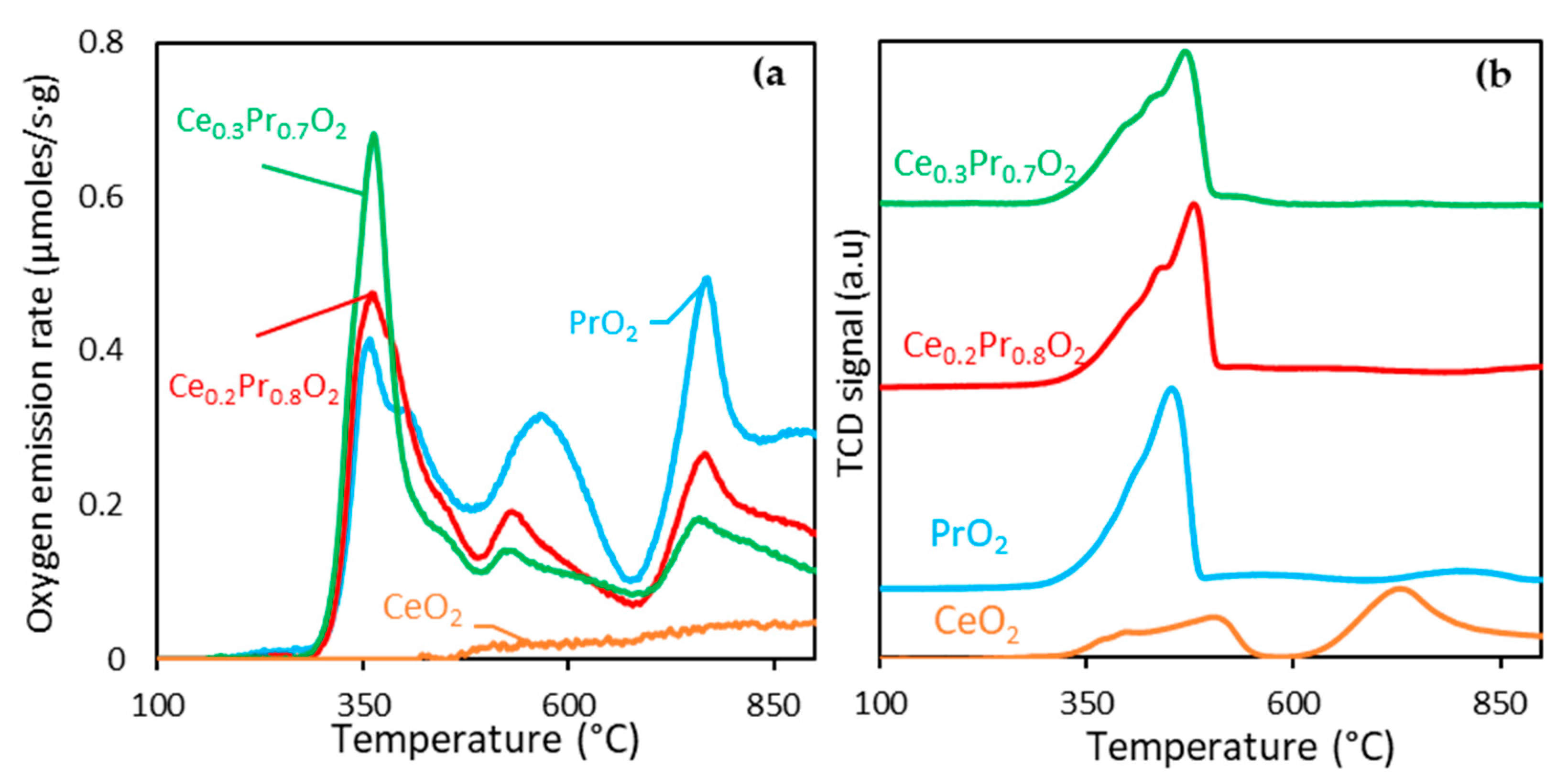
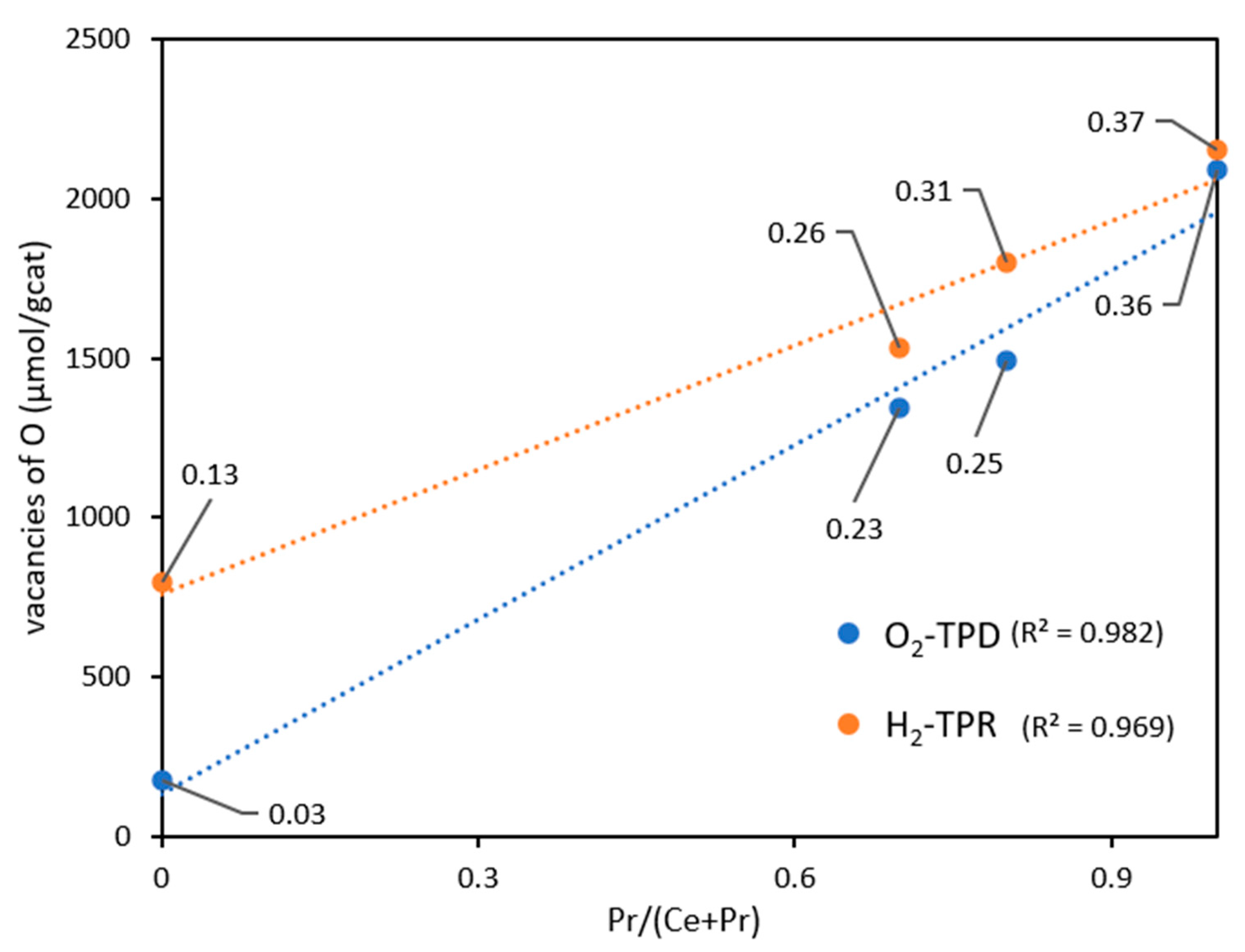
3.1.1. Comparison of the oxygen lability of the catalysts under different environments
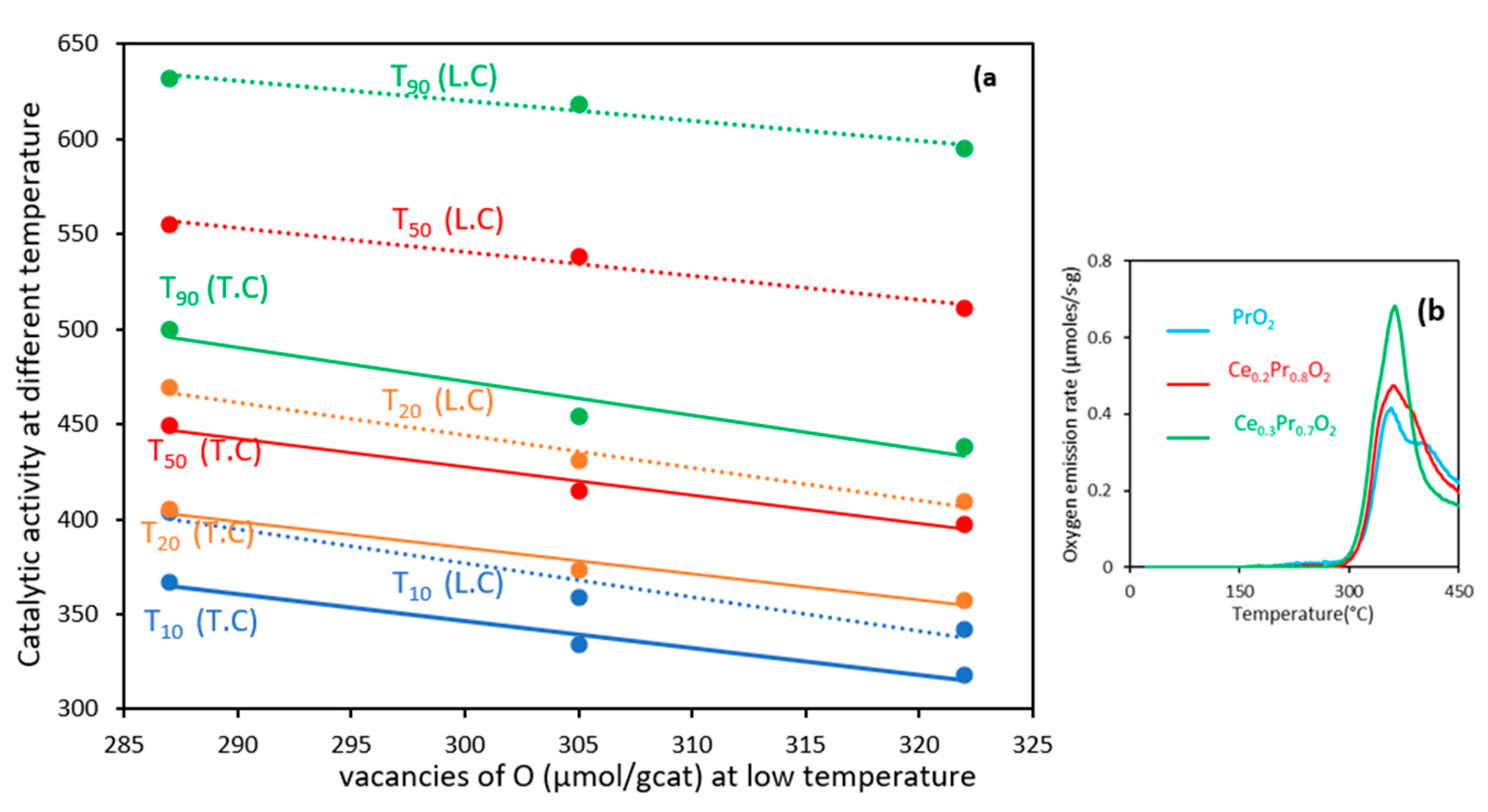
3.2. Catalytic performances of soot combustion under 5% of O2 /He atmosphere
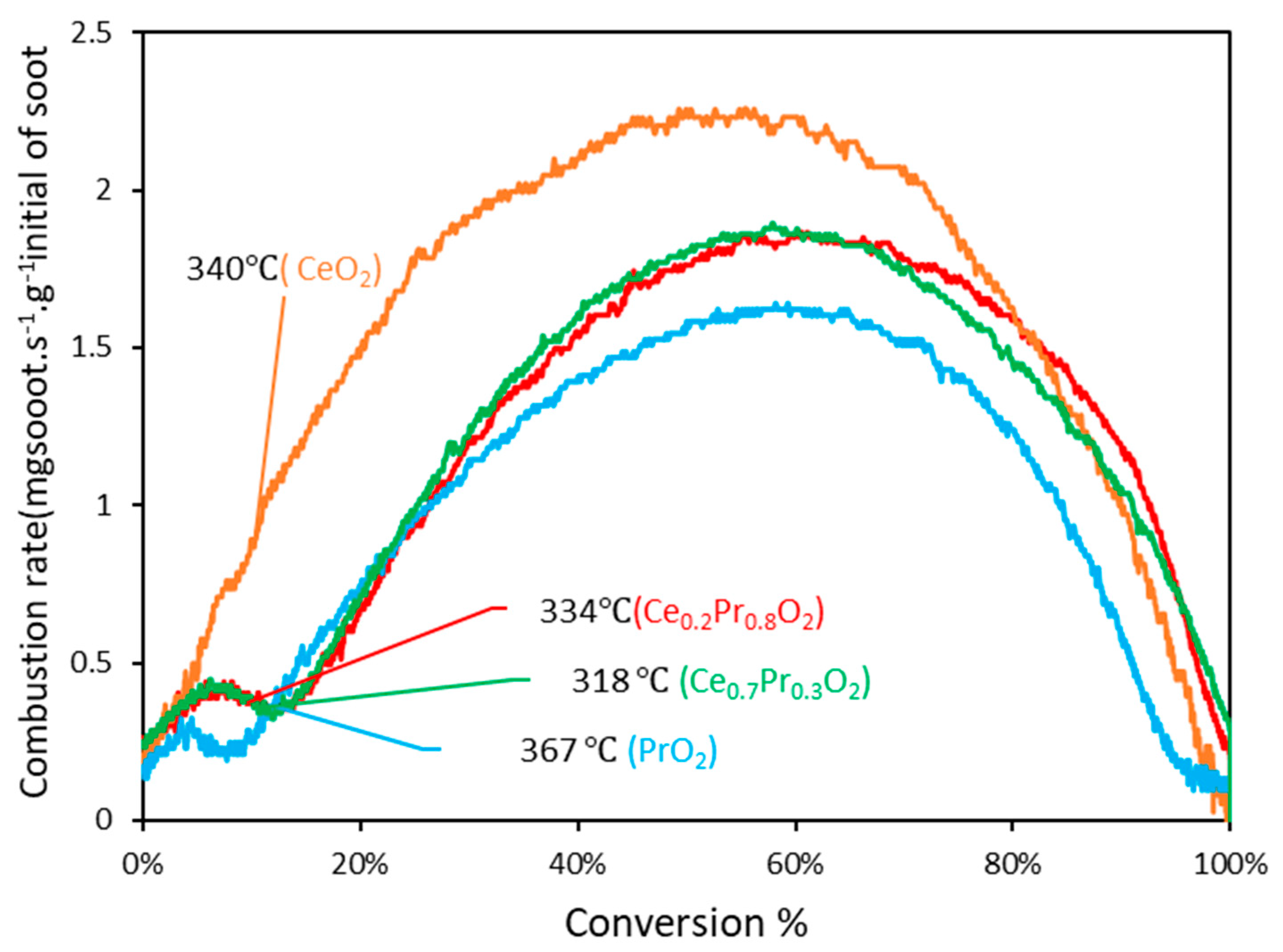
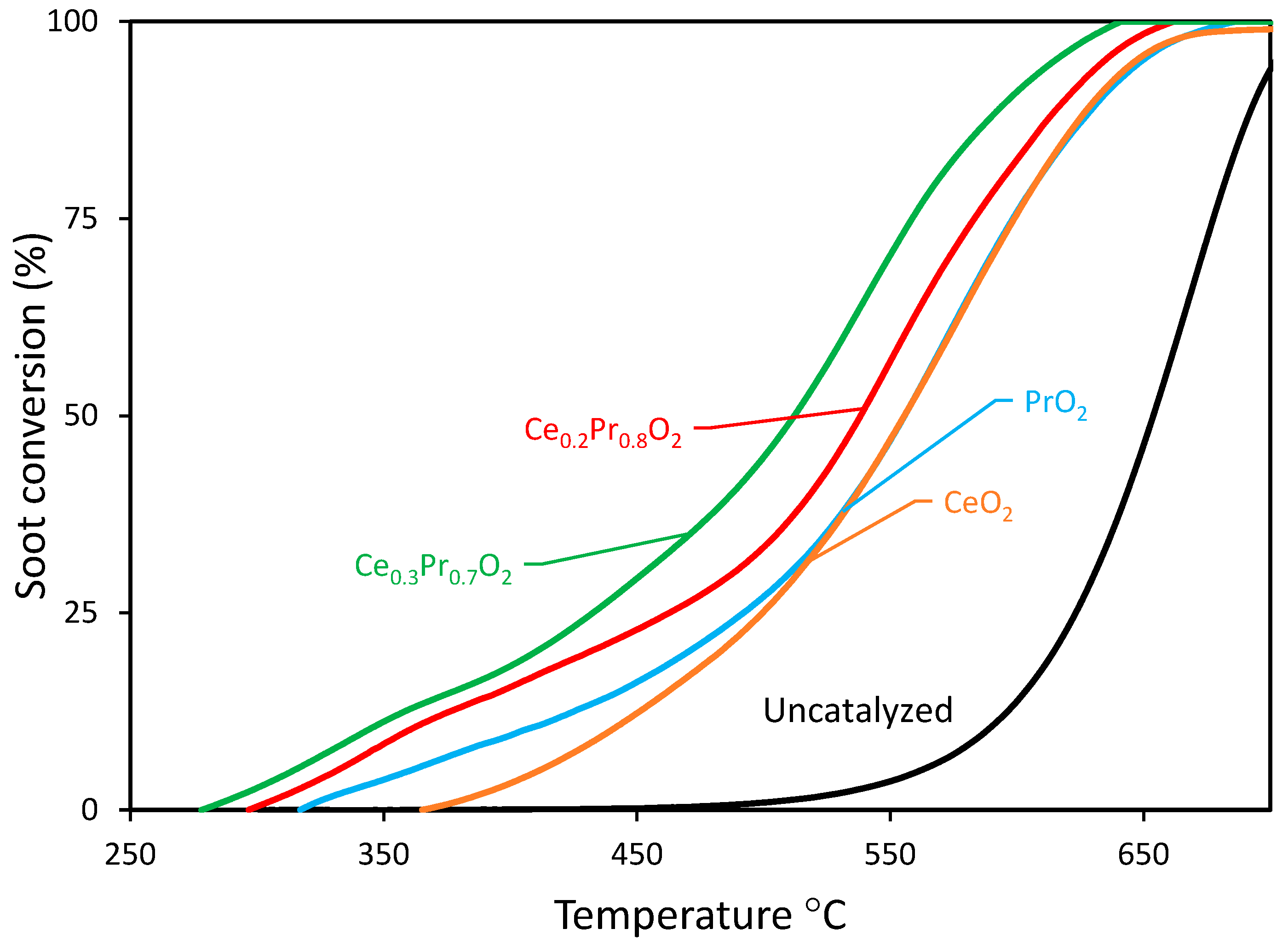
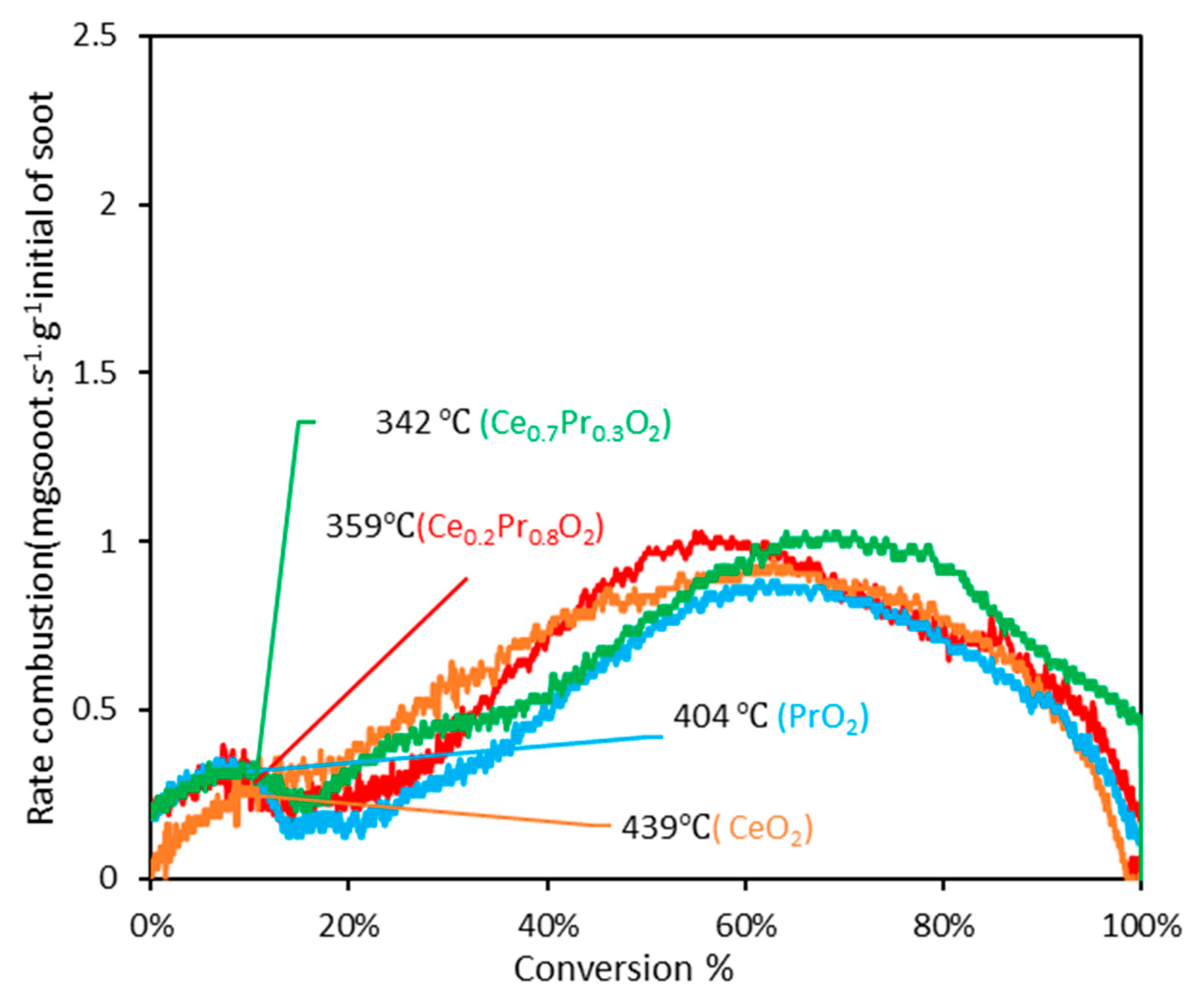
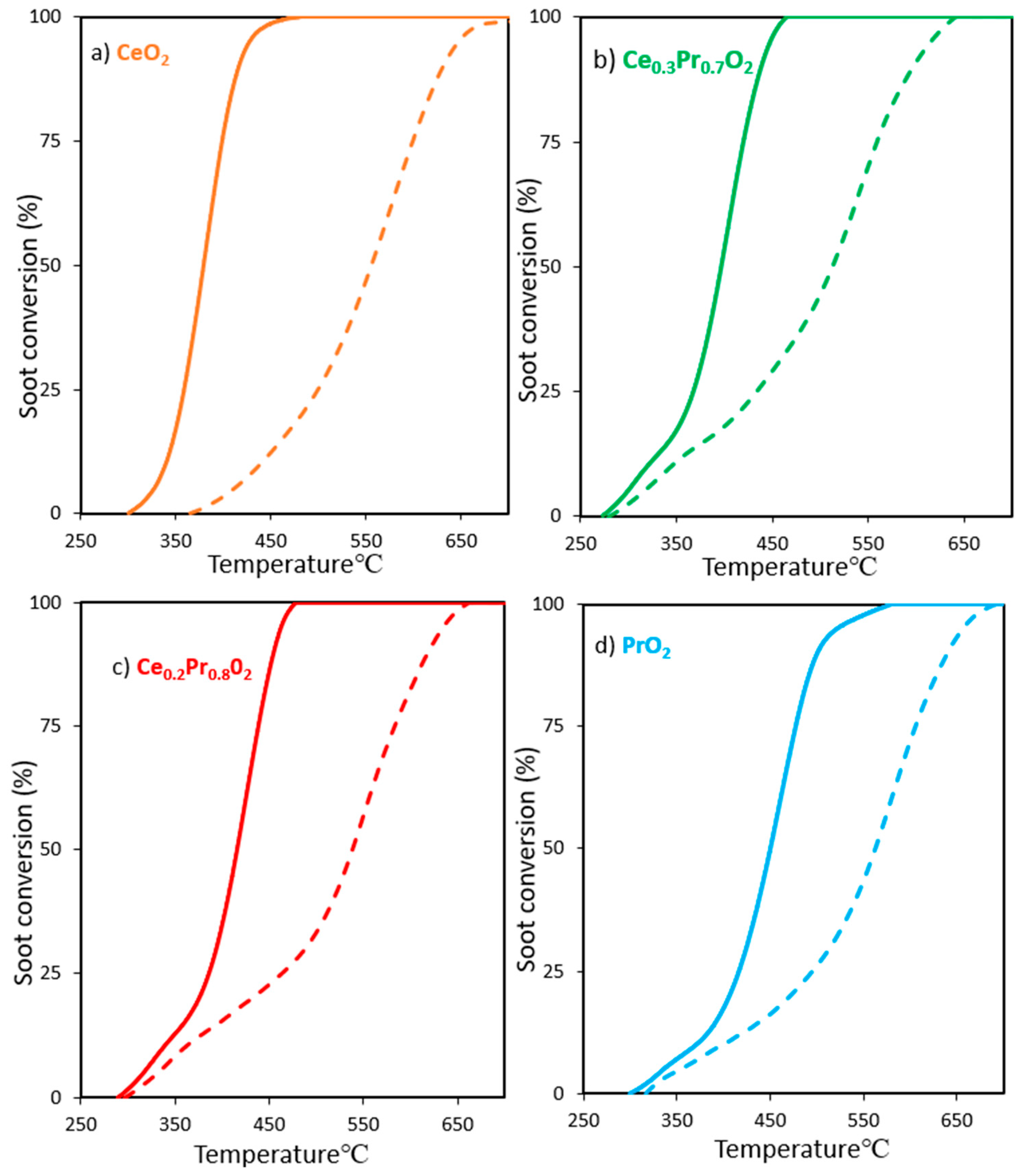
3.2.1. Experiments under tight contact mode.
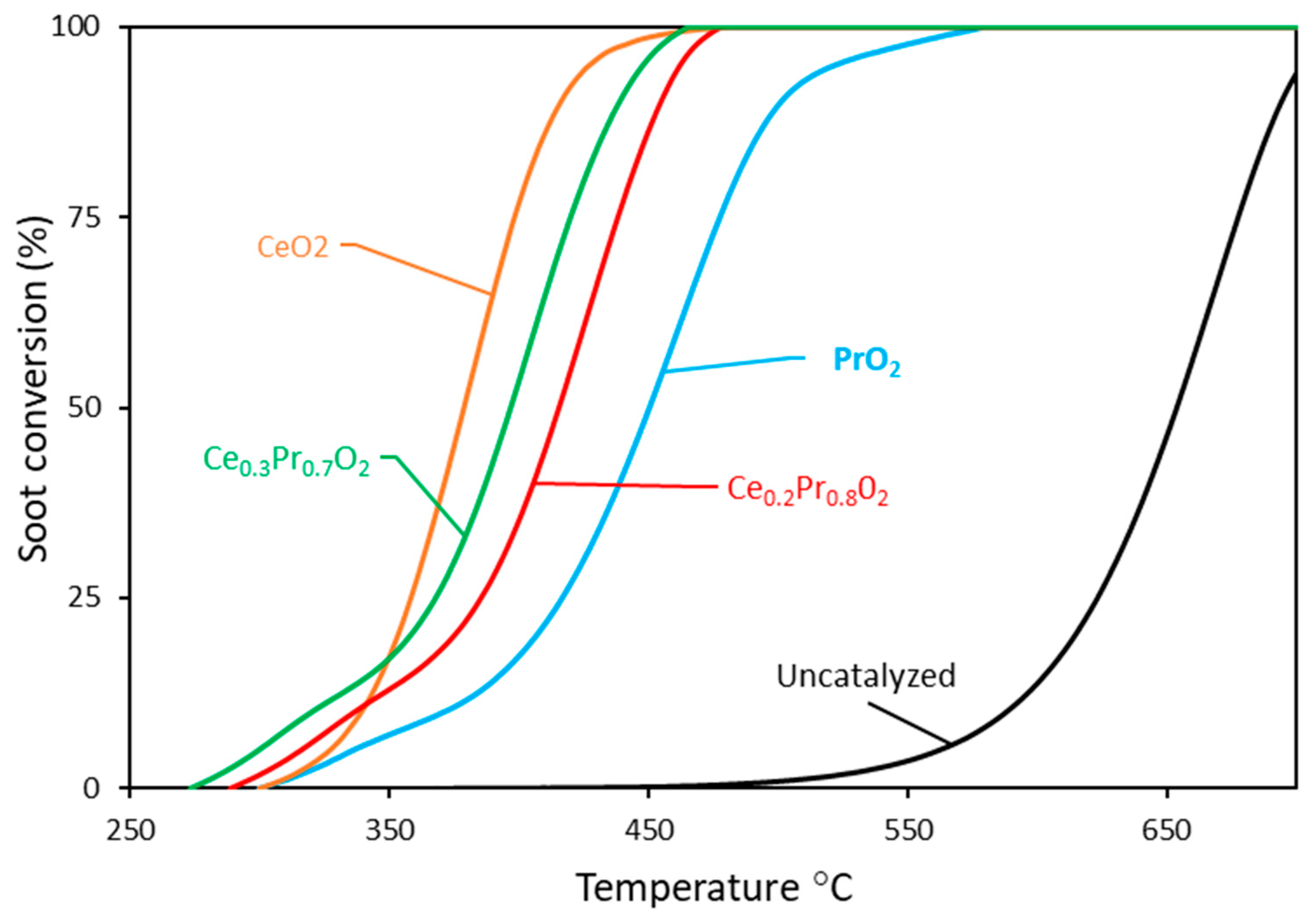
| Sample | Temperature (℃) | |||
|---|---|---|---|---|
| 10%a | 20% a | 50% a | 90% a | |
| T.C L.C | T.C L.C | T.C. L.C | T.C L.C | |
| CeO2 | 340(0.89) b 439(0.24) b | 355(1.46) b 483(0.43) b | 382(2.23) b 556(0.83) b | 415(0.80) b 630(0.51) b |
| Ce0.3Pr0.7O2 | 318(0.36) b 342(0.30) b | 357(0.71) b 409(0.32) b | 397(1.78) b 511(0.78) b | 438(1.07) b 595(0.69) b |
| Ce0.2Pr0.8O2 | 334(0.38) b 359(0.28) b | 373(0.63) b 431(0.27) b | 415(1.74) b 538(0.94) b | 454(1.20) b 618(0.58) b |
| PrO2 | 367(0.24) b 404(0.31) b | 405(0.72) b 469(0.15) b | 449(1.54) b 555(0.72) b | 500(0.63) b 632(0.58) b |
| 1Ag/YSZbc | 380 420 | - - | 430 530 | - - |
3.2.2. Experiments under loose contact mode.
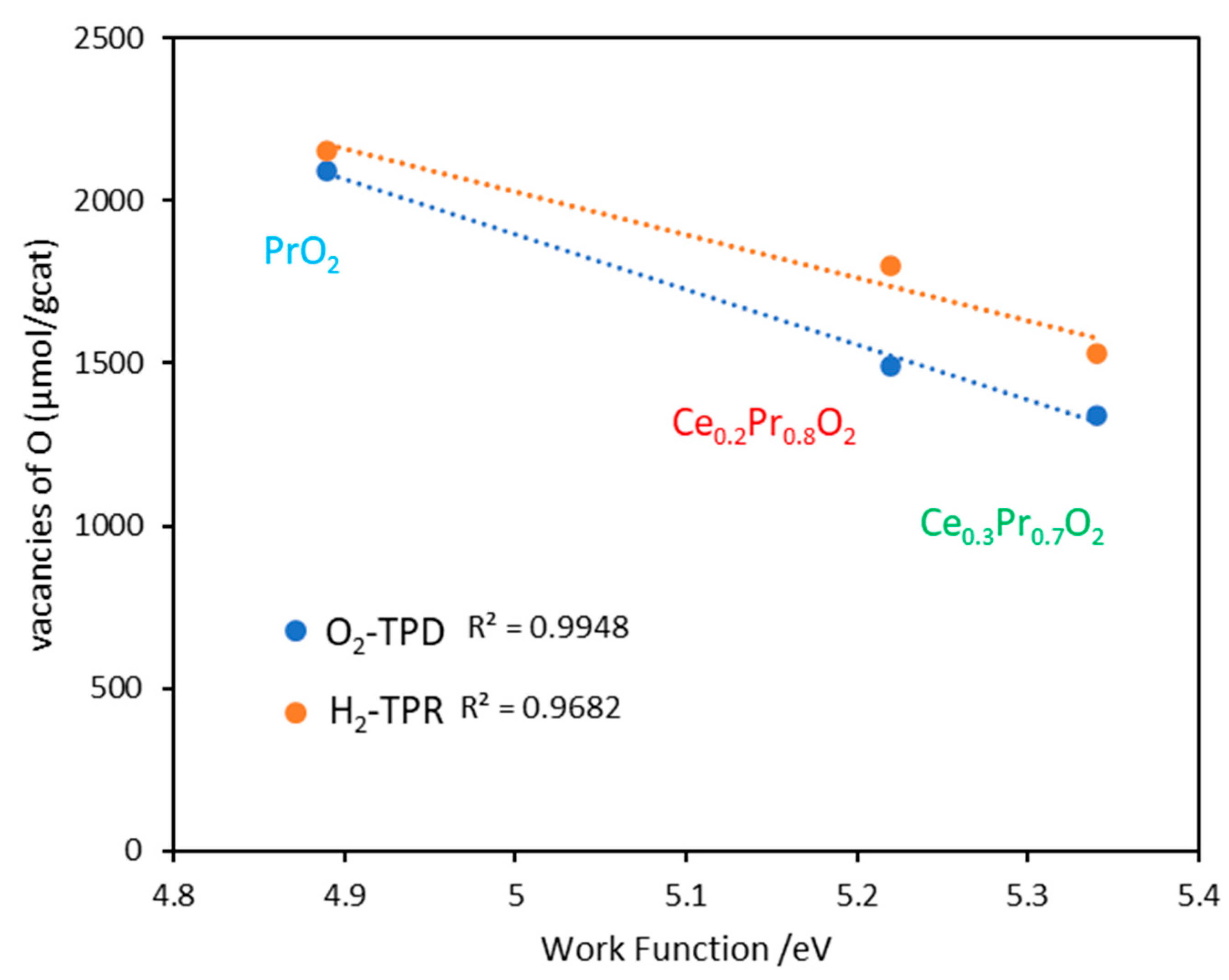
3.3. Work function measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakagoshi, Y.; Mori, K.; Tanaka, K.; Furuta, Y.; Aoki, T.; Yoshioka, F.; Kato, K. New Generation Diesel Particulate Filter for Future Euro7 Regulation; SAE International: Warrendale, PA, 2023. [Google Scholar]
- Commission Proposes New Euro 7 Standards. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_6495 (accessed on 15 November 2023).
- Lou, D.; Chen, Y.; Zhang, Y.; Wan, P.; Tan, P.; Hu, Z.; Fang, L.; Wang, T. Study on Soot Oxidation Characteristics of Ce and La Modified Pt-Pd CDPF Catalysts; SAE International: Warrendale, PA, 2023; 01-0390. [Google Scholar]
- Yu, D.; Yu, X.; Zhang, C.; Wang, L.; Fan, X.; Zhao, Z.; Wei, Y.; Liu, J.; Gryboś, J.; Leszczyński, B.; et al. Layered Na2Mn3O7 Decorated by Cerium as the Robust Catalysts for Efficient Low Temperature Soot Combustion. Appl. Catal. B Environ. 2023, 338, 123022. [Google Scholar] [CrossRef]
- Euro 7: MEPs back new rules to reduce road transport emissions | Nyheter | Europaparlamentet. Available online: https://www.europarl.europa.eu/news/sv/press-room/20231009IPR06746/euro-7-meps-back-new-rules-to-reduce-road-transport-emissions (accessed on 31 October 2023).
- Nain Singh, G.; Singh Bharj, R. Experimental Study of Filtration Behavior of Diesel Particulate Filter in a Diesel Engine to Meet BS-VI Emission Norms in INDIA. J. Phys. Conf. Ser. 2019, 1276, 012078. [Google Scholar] [CrossRef]
- Serve, A.; Boreave, A.; Cartoixa, B.; Pajot, K.; Vernoux, P. Synergy between Ag Nanoparticles and Yttria-Stabilized Zirconia for Soot Oxidation. Appl. Catal. B Environ. 2019, 242, 140–149. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, D.; Peng, C.; Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research Progress on Preparation of 3DOM-Based Oxide Catalysts and Their Catalytic Performances for the Combustion of Diesel Soot Particles. Appl. Catal. B Environ. 2022, 319, 121946. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic Diesel Particulate Filters with Highly Dispersed Ceria: Effect of the Soot-Catalyst Contact on the Regeneration Performance. Appl. Catal. B Environ. 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Lisi, L.; Landi, G.; Di Sarli, V. The Issue of Soot-Catalyst Contact in Regeneration of Catalytic Diesel Particulate Filters: A Critical Review. Catalysts 2020, 10, 1307. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Nanostructured Ceria-Praseodymia Catalysts for Diesel Soot Combustion. Appl. Catal. B Environ. 2016, 197, 125–137. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Bueno-López, A.; García-García, A. Study of Ce/Pr Ratio in Ceria-Praseodymia Catalysts for Soot Combustion under Different Atmospheres. Appl. Catal. Gen. 2020, 590, 117339. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; Guillén-Hurtado, N.; Fernández-García, S.; Chen, X.; Calvino-Gámez, J.J.; García-García, A. Ceria-Praseodymia Mixed Oxides: Relationships Between Redox Properties and Catalytic Activities Towards NO Oxidation to NO2 and CO-PROX Reactions. Top. Catal. 2016, 59, 1065–1070. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Zoccoli, M.; Giménez-Mañogil, J.; García-García, A. Lattice Oxygen Activity in Ceria-Praseodymia Mixed Oxides for Soot Oxidation in Catalysed Gasoline Particle Filters. Appl. Catal. B Environ. 2019, 245, 706–720. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; Fallah, J.E.; Hilaire, L.; Normand, F.L.; Quéméré, E.; et al. Reduction of CeO2 by Hydrogen. Magnetic Susceptibility and Fourier-Transform Infrared, Ultraviolet and X-Ray Photoelectron Spectroscopy Measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Borchert, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P.; et al. Electronic and Chemical Properties of Nanostructured Cerium Dioxide Doped with Praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A. Catalytic Soot Combustion─General Concepts and Alkali Promotion. ACS Catal. 2023, 13, 3395–3418. [Google Scholar] [CrossRef]
- Ruiz, M.L.; Lick, I.D.; Ponzi, M.I.; Castellón, E.R.; Jiménez-López, A.; Ponzi, E.N. Thermal Decomposition of Supported Lithium Nitrate Catalysts. Thermochim. Acta 2010, 1–2, 21–26. [Google Scholar] [CrossRef]
- Rajendran, M.; Mallick, K.K.; Bhattacharya, A.K. Combustion Synthesis, Powder Characteristics and Crystal Structure of Phases in Ce-Pr-O System. J. Mater. Sci. 1998, 33, 5001–5006. [Google Scholar] [CrossRef]
- Frizon, V.; Bassat, J.-M.; Pollet, M.; Durand, E.; Hernandez, J.; Pajot, K.; Vernoux, P.; Demourgues, A. Tuning the Pr Valence State To Design High Oxygen Mobility, Redox and Transport Properties in the CeO2–ZrO2–PrOx Phase Diagram. J. Phys. Chem. C 2019, 123, 6351–6362. [Google Scholar] [CrossRef]
- Fahed, S.; Pointecouteau, R.; Aouine, M.; Boréave, A.; Gil, S.; Meille, V.; Bazin, P.; Toulemonde, O.; Demourgues, A.; Daturi, M.; et al. Pr-Rich Cerium-Zirconium-Praseodymium Mixed Oxides for Automotive Exhaust Emission Control. Appl. Catal. Gen. 2022, 644, 118800. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-Ray Studies of Ce1-xRExO2-y, Where RE=La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno-López, A.; Makkee, M.; Moulijn, J.A. Potential Rare Earth Modified CeO2 Catalysts for Soot Oxidation: I. Characterisation and Catalytic Activity with O2. Appl. Catal. B Environ. 2007, 75, 189–200. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; Guillén-Hurtado, N.; Fernández-García, S.; Chen, X.; Calvino-Gámez, J.J.; García-García, A. Ceria-Praseodymia Mixed Oxides: Relationships Between Redox Properties and Catalytic Activities Towards NO Oxidation to NO2 and CO-PROX Reactions. Top. Catal. 2016, 59, 1065–1070. [Google Scholar] [CrossRef]
- Luo, M.F.; Yan, Z.L.; Jin, L.Y.; He, M. Raman Spectroscopic Study on the Structure in the Surface and the Bulk Shell of CexPr1-xO2-δ Mixed Oxides. J. Phys. Chem. B 2006, 110, 13068–13071. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thrimurthulu, G.; Katta, L.; Yamada, Y.; Park, S.E. Structural Characteristics and Catalytic Activity of Nanocrystalline Ceria-Praseodymia Solid Solutions. J. Phys. Chem. C 2009, 113, 15882–15890. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.; Jayachandran, M.; Subramanian, B.; Bajaj, H.C.; Panda, A.B. Facile Low-Temperature Synthesis of Ceria and Samarium-Doped Ceria Nanoparticles and Catalytic Allylic Oxidation of Cyclohexene. 2011, 7628–7637. [CrossRef]
- Carlos, J.; Munuera, M. Ceria-Based Catalysts for Exhaust Aftertreatment Systems in Last Generation Diesel and Gasoline Engines. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2022. [Google Scholar]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. Nanostructured Equimolar Ceria-Praseodymia for NOx-Assisted Soot Oxidation: Insight into Pr Dominance over Pt Nanoparticles and Metal–Support Interaction. Appl. Catal. B Environ. 2018, 226, 147–161. [Google Scholar] [CrossRef]
- Fan, L.; Xi, K.; Zhou, Y.; Zhu, Q.; Chen, Y.; Lu, H. Design Structure for CePr Mixed Oxide Catalysts in Soot Combustion. RSC Adv. 2017, 7, 20309–20319. [Google Scholar] [CrossRef]
- Ballauri, S.; Sartoretti, E.; Hu, M.; D’Agostino, C.; Ge, Z.; Wu, L.; Novara, C.; Giorgis, F.; Piumetti, M.; Fino, D.; et al. Praseodymium Doping in Ceria-Supported Palladium Nanocatalysts as an Effective Strategy to Minimize the Inhibiting Effects of Water during Methane Oxidation. Appl. Catal. B Environ. 2023, 320, 121898. [Google Scholar] [CrossRef]
- Sinev, M.Yu.; Graham, G.W.; Haack, L.P.; Shelef, M. Kinetic and Structural Studies of Oxygen Availability of the Mixed Oxides Pr1-xMxOy (M = Ce, Zr). J. Mater. Res. 1996, 11, 1960–1971. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot Oxidation over CeO2 and Ag/CeO2: Factors Determining the Catalyst Activity and Stability during Reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A.; Azambre, B. Contributions of Surface and Bulk Heterogeneities to the NO Oxidation Activities of Ceria-Zirconia Catalysts with Composition Ce0.76Zr 0.24O2 Prepared by Different Methods. Phys. Chem. Chem. Phys. 2010, 12, 13770–13779. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. Hydrothermal Synthesis and Properties of Terbium- or Praseodymium-Doped Ce1−xSmxO2−x/2 Solid Solutions. Solid State Ion. 1998, 113–115, 305–310. [Google Scholar] [CrossRef]
- Knauth, P.; Tuller, H.L. How Unique Are the Microstructure and the Electrical Properties of Nanocrystalline Ceramics? 1999; Volume 548, pp. 429–442. [Google Scholar]
- Koichiro Harada†, Tetsuya Oishi‡, Seiji Hamamoto‡, and Tatsumi Ishihara Lattice Oxygen Activity in Pr- and La-Doped CeO2 for Low-Temperature Soot Oxidation.The Journal of Physical Chemistry C Available online:. [CrossRef]
- Madier, Y.; Descorme, C.; Le Govic, A.M.; Duprez, D. Oxygen Mobility in CeO2 and CexZr(1-x)O2 Compounds: Study by CO Transient Oxidation and 18O/16O Isotopic Exchange. J. Phys. Chem. B 1999, 103, 10999–11006. [Google Scholar] [CrossRef]
- Logan, A.D.; Shelef, M. Oxygen Availability in Mixed Cerium/Praseodymium Oxides and the Effect of Noble Metals. J. Mater. Res. 1994, 9, 468–475. [Google Scholar] [CrossRef]
- Letichevsky, S.; Tellez, C.A.; Avillez, R.R.D.; Silva, M.I.P.D.; Fraga, M.A.; Appel, L.G. Obtaining CeO2–ZrO2 Mixed Oxides by Coprecipitation: Role of Preparation Conditions. Appl. Catal. B Environ. 2005, 58, 203–210. [Google Scholar] [CrossRef]
- Trovarelli, A.; Boaro, M.; Rocchini, E.; De Leitenburg, C.; Dolcetti, G. Some Recent Developments in the Characterization of Ceria-Based Catalysts. J. Alloys Compd. 2001, 323–324, 584–591. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, J. New Directions in Solid State Chemistry; Cambridge University Press, 1997; ISBN 978-0-521-49559-2. [Google Scholar]
- Aneggi, E.; Llorca, J.; Trovarelli, A.; Aouine, M.; Vernoux, P. In Situ Environmental HRTEM Discloses Low Temperature Carbon Soot Oxidation by Ceria–Zirconia at the Nanoscale. Chem. Commun. 2019, 55, 3876–3878. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, G.; Lang, Y.; Chen, R.; Jia, L.; Yue, J.; Shen, M.; Du, C.; Shan, B. Promoting Soot Combustion Efficiency by Strengthening the Adsorption of NOx on the 3DOM Mullite Catalyst. J. Catal. 2020, 384, 96–105. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Bueno-López, A.; García-García, A. Catalytic Performances of Ceria and Ceria-Zirconia Materials for the Combustion of Diesel Soot under NO x/O 2 and O 2. Importance of the Cerium Precursor Salt. Appl. Catal. Gen. 2012, 437–438, 166–172. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Trovarelli, A. On the Role of Lattice/Surface Oxygen in Ceria–Zirconia Catalysts for Diesel Soot Combustion. Catal. Today 2012, 181, 108–115. [Google Scholar] [CrossRef]
- Serve, A.; Boreave, A.; Cartoixa, B.; Pajot, K.; Vernoux, P. Impact of the Support on the Catalytic Activity of Ag Nanoparticles for Soot Combustion. Catal. Today 2021, 363, 93–104. [Google Scholar] [CrossRef]
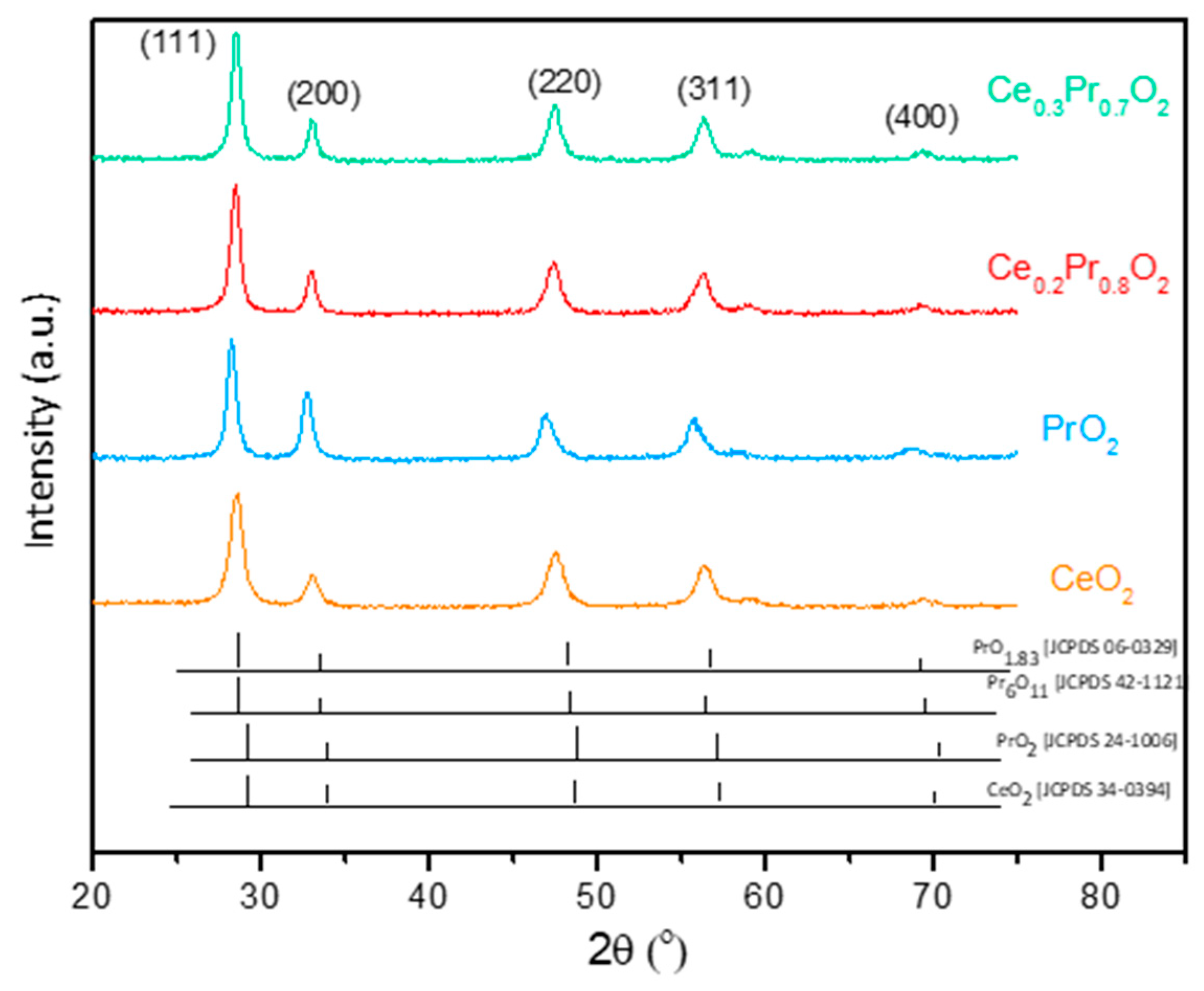
|
Sample |
Lattice parameter a (nm) | Average crystal size (nm) |
SBET (m2/g) |
Vp (cm3/g) |
F2g band position (cm-1) |
Intensity ratio of the vacancies band/F2g band |
|---|---|---|---|---|---|---|
| CeO2 Ce0.3Pr0.7O2 Ce0.2Pr0.8O2 PrO2 |
0.5415 0.5418 0.5420 0.5467 |
11.1 12.2 12.0 11.5 |
81 42 31 6 |
0.221 0.059 0.030 0.012 |
463.1 438.6 431.8 428.3 |
- 1.09 1.15 1.20 |
| Sample | Ce (%) | Pr (%) | O (%) | C* (%) |
|---|---|---|---|---|
| CeO2 Ce0.3Pr0.7O2 Ce0.2Pr0.8O2 PrO2 |
25.03 4.94 3.52 - |
- 14.77 14.98 13.66 |
55.45 50.02 47.38 43.01 |
19.50 30.25 34.10 43.31 |
| Sample | Pr+3 (%) | Ce+3 (%) | Ce/Pr surface | Ce/Pr nominal | O/(Ce+Pr) |
|---|---|---|---|---|---|
| CeO2 Ce0.3Pr0.7O2 Ce0.2Pr0.8O2 PrO2 |
0.0 48.3 44.9 44.7 |
36.3 31.1 34.1 0.0 |
- 0.26 0.23 - |
- 0.43 0.25 - |
2.21 2.53 2.56 2.67 |
| Sample | O2 emitted (μmol/gcat) | δ1 | 1formula after reduction | H2 consumption (µmol/gcat) | δ2 | 2formula after reduction |
|---|---|---|---|---|---|---|
| CeO2 Ce0.3Pr0.7O2 Ce0.2Pr0.8O2 PrO2 |
88 671/(322)* 745/(305)* 1045/(287)* |
0.03 0.23 0.25 0.36 |
CeO1.97 Ce0.3Pr0.7 O1.77 Ce0.2Pr0.8O1.74 PrO1.64 |
779 1530.0 1797.0 2155.6 |
0.13 0.26 0.31 0.37 |
CeO1.87 Ce0.3Pr0.7O1.74 Ce0.2Pr0.8O1.69 PrO1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





