Submitted:
15 February 2024
Posted:
15 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. DNA extraction and HPV testing
2.3. HPV6 and HPV11 genomic variant characterization
2.4. Statistical analysis
3. Results
3.1. Characteristics of study participants and follow-up visits
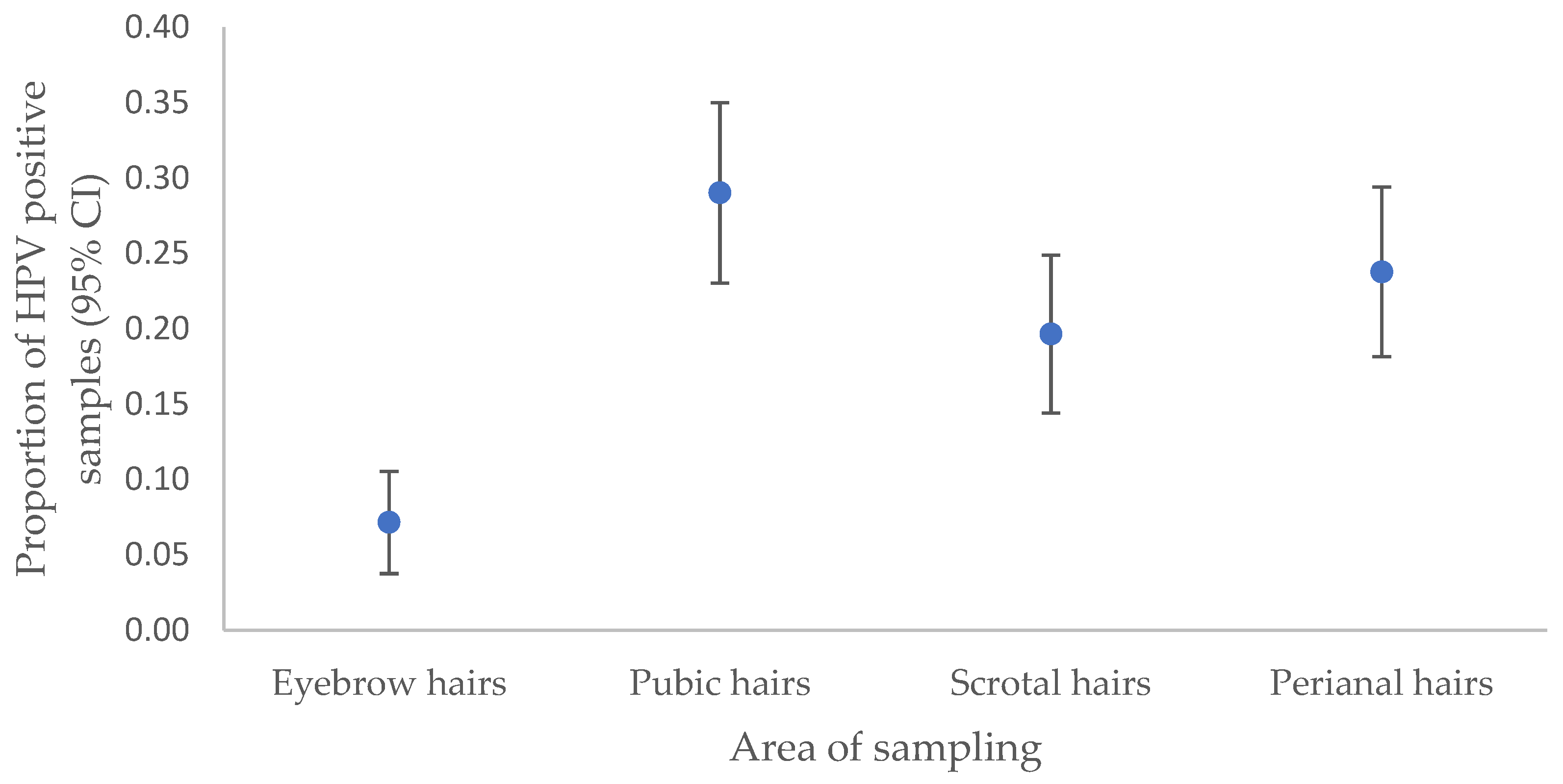
3.2. HPV infection in anogenital warts and corresponding hair samples
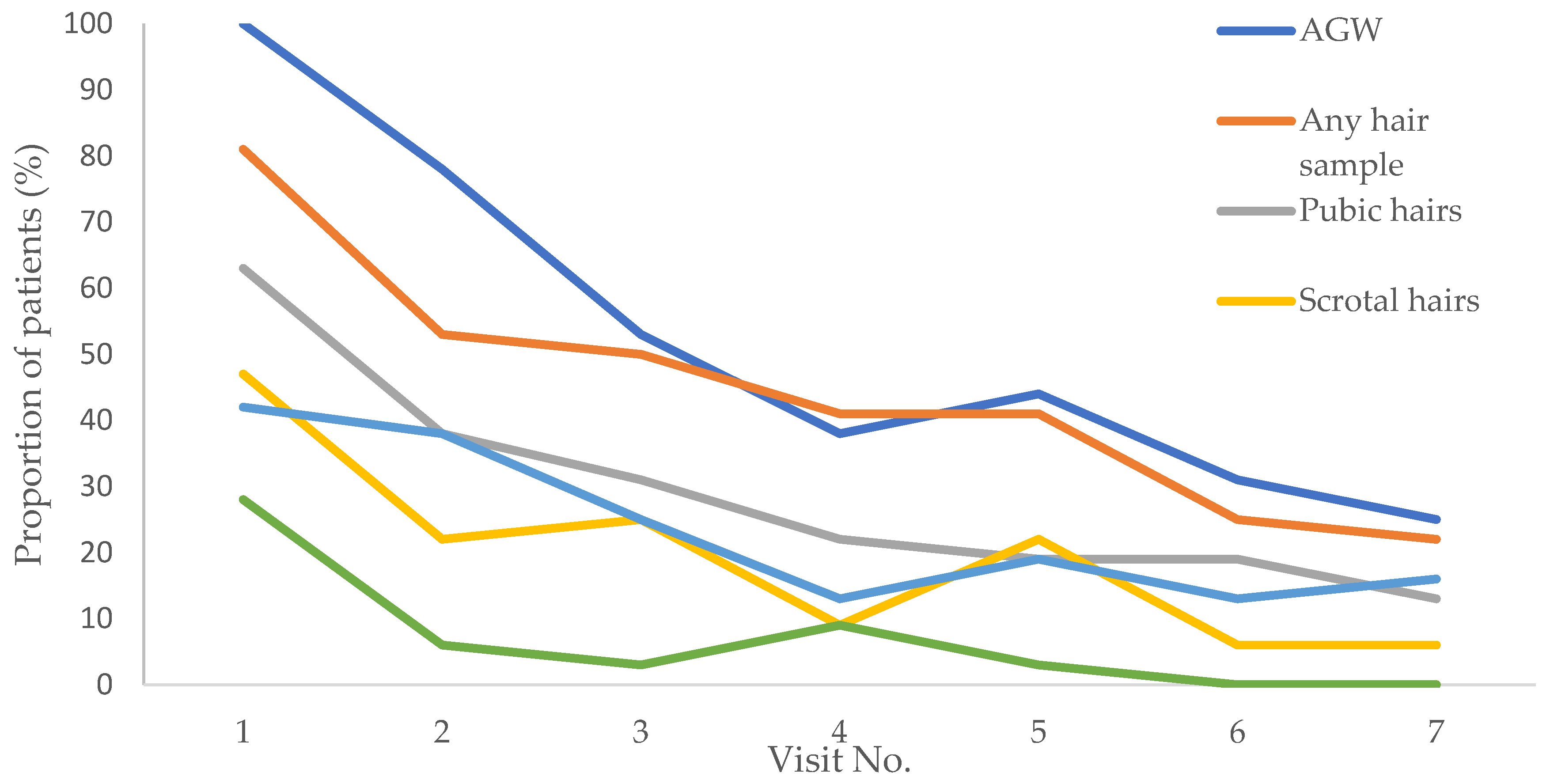
3.3. Dynamic of HPV6/11 infection in hair samples
| Hair sampling area/age/lifestyle factors | OR (95% CI) | P |
| Perianal hairs | 1 | |
| Eyebrow hairs | 0.17 (0.08–0.34) | < 0.001 |
| Pubic hairs | 1.42 (0.68–2.98) | 0.354 |
| Scrotal hairs | 0.71 (0.37–1.34) | 0.29 |
| Follow-up time | 0.84 (0.78–0.91) | < 0.001 |
| Patients’ age at enrolment | 1.06 (1.04–1.08) | < 0.001 |
| Non-cigarette smoking | 1 | |
| Cigarette smoking | 3.04 (1.49–6.22) | 0.002 |
| Shaving – no | 1 | |
| Shaving – yes | 2.34 (1.13–4.82) | 0.022 |
3.4. Recurrence of anogenital warts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacey, C.J.; Lowndes, C.M.; Shah, K.V. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006, 24, 35–41. [Google Scholar] [CrossRef]
- Potočnik, M.; Kocjan, B.; Seme, K.; Poljak, M. Distribution of human papillomavirus (HPV) genotypes in genital warts from males in Slovenia. Acta Dermatovenerol. Alp. Pannonica Adriat. 2007, 16, 91–96. [Google Scholar]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al.; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009, 10, 321–322. [CrossRef]
- Garland, S.M.; Steben, M.; Sings, H.L.; James, M.; Lu, S.; Railkar, R.; Barr, E.; Haupt, R.M.; Joura, E.A. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009, 199, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.L.; Winder, D.M.; Vaughan, K.; Hanna, N.; Levy, J.; Sterling, J.C.; Stanley, M.A.; Goon, P.K. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J. Med. Virol. 2011, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Komloš, K.F.; Kocjan, B.J.; Košorok, P.; Luzar, B.; Meglič, L.; Potočnik, M.; Hočevar-Boltežar, I.; Gale, N.; Seme, K.; Poljak, M. Tumor-specific and gender-specific pre-vaccination distribution of human papillomavirus types 6 and 11 in anogenital warts and laryngeal papillomas: a study on 574 tissue specimens. J. Med. Virol. 2012, 84, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill. 2018, 23, 1700737. [Google Scholar] [CrossRef]
- Grulich, A.E.; de Visser, R.O.; Smith, A.M.; Rissel, C.E.; Richters, J. Sex in Australia: sexually transmissible infection and blood-borne virus history in a representative sample of adults. Aust. N. Z. J. Public Health 2003, 27, 234–241. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Tran, T.N.; Sparen, P.; Tryggvadottir, L.; Munk, C.; Dasbach, E.; Liaw, K.L.; Nygård, J.; Nygård, M. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J. Infect. Dis. 2007, 196, 10, 1447–1454. [Google Scholar] [CrossRef]
- Dinh, T.H.; Sternberg, M.; Dunne, E.F.; Markowitz, L.E. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999--2004. Sex. Transm. Dis. 2008, 35, 357–360. [Google Scholar] [CrossRef]
- Munk, C.; Nielsen, A.; Liaw, K.L.; Kjaer, S.K. Genital warts in men: a large population-based cross-sectional survey of Danish men. Sex. Transm. Infect. 2012, 88, 640–644. [Google Scholar] [CrossRef]
- Jeynes, C.; Chung, M.C.; Challenor, R. 'Shame on you'--the psychosocial impact of genital warts. Int. J. STD. AIDS 2009, 20, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Lee Mortensen, G.; Larsen, H.K. Quality of life of homosexual males with genital warts: a qualitative study. BMC Res. Notes 2010, 4, 280. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Pista, A.; Lisboa, C.; Santo, I.; Azevedo, L.; Cunha, M.J.; HERCOLES Study Group. Epidemiology of human papillomavirus on anogenital warts in Portugal - The HERCOLES study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1342–1348. [CrossRef]
- O'Mahony, C.; Gomberg, M.; Skerlev, M.; Alraddadi, A.; de Las Heras-Alonso, M.E.; Majewski, S.; Nicolaidou, E.; Serdaroğlu, S.; Kutlubay, Z.; Tawara, M.; et al. Position statement for the diagnosis and management of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A.; Mitao, M.; Nagai, N.; Silverstein, S.J.; Crum, C.P. Latent papillomavirus and recurring genital warts. N. Engl. J. Med. 1985, 313, 784–788. [Google Scholar] [CrossRef]
- Boxman, I.L.; Hogewoning, A.; Mulder, L.H.; Bouwes Bavinck, J.N.; ter Schegget, J. Detection of human papillomavirus types 6 and 11 in pubic and perianal hair from patients with genital warts. J. Clin. Microbiol. 1999, 37, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Kocjan, B.J.; Potocnik, M.; Seme, K. Anogenital hairs are an important reservoir of alpha-papillomaviruses in patients with genital warts. J. Infect. Dis. 2009, 199, 1270–1274. [Google Scholar] [CrossRef]
- Wang, Y.B.; Han, T.; Zhao, C.X. [Prevalence of human papillomavirus in the pubic hair follicles of healthy men and male patients with genital warts]. Zhonghua Nan. Ke. Xue. 2010, 16, 783–785. Chinese.
- Lacey, C.J.; Woodhall, S.C.; Wikstrom, A.; Ross, J. 2012 European guideline for the management of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e263–e270. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Kocjan, B.J.; Hošnjak, L.; Pižem, J.; Beltram, M.; Gale, N.; Drnovšek-Olup, B.; Poljak, M. Morphological characteristics of conjunctival squamous papillomas in relation to human papillomavirus infection. Br. J. Ophthalmol. 2015, 99, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, B.J.; Seme, K.; Poljak, M. Detection and differentiation of human papillomavirus genotypes HPV-6 and HPV-11 by FRET-based real-time PCR. J. Virol. Methods 2008, 153, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jelen, M.M.; Chen, Z.; Kocjan, B.J.; Burt, F.J.; Chan, P.K.; Chouhy, D.; Combrinck, C.E.; Coutlée, F.; Estrade, C.; Ferenczy, A.; et al. Global genomic diversity of human papillomavirus 6 based on 724 isolates and 190 complete genome sequences. J. Virol. 2014, 88, 7307–7316. [Google Scholar] [CrossRef]
- Jelen, M.M.; Chen, Z.; Kocjan, B.J.; Hošnjak, L.; Burt, F.J.; Chan, P.K.S.; Chouhy, D.; Combrinck, C.E.; Estrade, C.; Fiander, A.; et al. Global Genomic Diversity of Human Papillomavirus 11 Based on 433 Isolates and 78 Complete Genome Sequences. J. Virol. 2016, 90, 5503–5513. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Danielewski, J.A.; Garland, S.M.; McCloskey, J.; Hillman, R.J.; Tabrizi, S.N. Human papillomavirus type 6 and 11 genetic variants found in 71 oral and anogenital epithelial samples from Australia. PLoS One 2013, 8, e63892. [Google Scholar] [CrossRef]
- Flores-Díaz, E.; Sereday, K.A.; Ferreira, S.; Sirak, B.; Sobrinho, J.S.; Baggio, M.L.; Galan, L.; Silva, R.C.; Lazcano-Ponce, E.; Giuliano, A.R.; et al.; HIM Study group. HPV-6 Molecular Variants Association With the Development of Genital Warts in Men: The HIM Study. J. Infect. Dis. 2017, 215, 559–565. [CrossRef]
- Flores-Díaz, E.; Sereday, K.A.; Ferreira, S.; Sirak, B.; Sobrinho, J.S.; Baggio, M.L.; Galan, L.; Silva, R.C.; Lazcano-Ponce, E.; Giuliano, A.R.; et al; The Him Study Group. HPV-11 variability, persistence and progression to genital warts in men: the HIM study. J. Gen. Virol. 2017, 98, 2339–2342. [CrossRef]
- Measso do Bonfim, C.; Simão Sobrinho, J.; Lacerda Nogueira, R.; Salgado Kupper, D.; Cardoso Pereira Valera, F.; Lacerda Nogueira, M.; Villa, L.L.; Rahal, P.; Sichero, L. Differences in Transcriptional Activity of Human Papillomavirus Type 6 Molecular Variants in Recurrent Respiratory Papillomatosis. PLoS One 2015, 10, e0132325. [Google Scholar] [CrossRef]
- Szinai, M.; Nagy, Z.; Máté, P.; Kovács, D.; Laczkó, L.; Kardos, G.; Sápy, T.; Szűcs, A.; Szarka, K. Comparative analysis of human papillomavirus type 6 complete genomes originated from head and neck and anogenital disorders. Infect. Genet. Evol. 2019, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Steben, M.; Garland, S.M. Genital warts. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lazcano-Ponce, E.; Villa, L.L.; Flores, R.; Salmeron, J.; Lee, J.H.; Papenfuss, M.R.; Abrahamsen, M.; Jolles, E.; Nielson, C.M.; el, al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Villa, L.L.; Lin, H.Y.; Fulp, W.J.; Lazcano-Ponce, E.; Salmerón, J.; Abrahamsen, M.E.; Papenfuss, M.R.; Quiterio, M.; Giuliano, A.R. A prospective analysis of smoking and human papillomavirus infection among men in the HPV in Men Study. Int. J. Cancer 2014, 134, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Ingles, D.J.; Lin, H.Y.; Fulp, W.J.; Sudenga, S.L.; Lu, B.; Schabath, M.B.; Papenfuss, M.R.; Abrahamsen, M.E.; Salmeron, J.; Villa, L.L.; et al. An analysis of HPV infection incidence and clearance by genotype and age in men: The HPV Infection in Men (HIM) Study. Papillomavirus Res. 2015, 1, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Anic, G.M.; Lee, J.H.; Villa, L.L.; Lazcano-Ponce, E.; Gage, C.; José C Silva, R.; Baggio, M.L.; Quiterio, M.; Salmerón, J.; Papenfuss, M.R.; et al. Risk factors for incident condyloma in a multinational cohort of men: the HIM study. J. Infect. Dis. 2012, 205, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Sudenga, S.L.; Ingles, D.J.; Pierce Campbell, C.M.; Lin, H.Y.; Fulp, W.J.; Messina, J.L.; Stoler, M.H.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; et al. Genital Human Papillomavirus Infection Progression to External Genital Lesions: The HIM Study. Eur. Urol. 2016, 166–173. [Google Scholar] [CrossRef]
- Kocjan, B.J.; Poljak, M.; Seme, K.; Potocnik, M.; Fujs, K.; Babic, D.Z. Distribution of human papillomavirus genotypes in plucked eyebrow hairs from Slovenian males with genital warts. Infect. Genet. Evol. 2005, 5, 255–259. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Sirak, B.; Abrahamsen, M.; Silva, R.J.C.; Baggio, M.L.; Galan, L.; Cintra, R.C.; Lazcano-Ponce, E.; Villa, L.L. Genital Wart Recurrence Among Men Residing in Brazil, Mexico, and the United States. J. Infect. Dis. 2019, 219, 703–710. [Google Scholar] [CrossRef]
- Pamnani, S.J.; Sudenga, S.L.; Rollison, D.E.; Ingles, D.J.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; Huang, Y.; Borenstein, A.; Giuliano, A.R. Recurrence of Genital Infections With 9 Human Papillomavirus (HPV) Vaccine Types (6, 11, 16, 18, 31, 33, 45, 52, and 58) Among Men in the HPV Infection in Men (HIM) Study. J. Infect. Dis. 2018, 218, 1219–1227. [Google Scholar] [CrossRef]
- Widschwendter, A.; Böttcher, B.; Riedl, D.; Coban, S.; Mutz-Dehbalaie, I.; Matteucci Gothe, R.; Ciresa-König, A.; Marth, C.; Fessler, S. Recurrence of genitals warts in pre-HPV vaccine era after laser treatment. Arch. Gynecol. Obstet. 2019, 300, 661–668. [Google Scholar] [CrossRef]
- Alberts, C.J.; Schim van der Loeff, M.F.; Papenfuss, M.R.; da Silva, R.J.; Villa, L.L.; Lazcano-Ponce, E.; Nyitray, A.G.; Giuliano, A.R. Association of Chlamydia trachomatis infection and herpes simplex virus type 2 serostatus with genital human papillomavirus infection in men: the HPV in men study. Sex. Transm. Dis. 2013, 40, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Trčko, K.; Hošnjak, L.; Kušar, B.; Zorec, T.M.; Kocjan, B.J.; Križmarić, M.; Seme, K.; Miljković, J.; Luzar, B.; Poljak, M. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum. Open Forum Infect. Dis. 2018, 5, ofy298. [Google Scholar] [CrossRef]
- Anic, G.M.; Messina, J.L.; Stoler, M.H.; Rollison, D.E. , Stockwell, H., Villa, L.L., Lazcano-Ponce, E., Gage, C., Silva, R.J., Baggio, M.L.; et al. Concordance of human papillomavirus types detected on the surface and in the tissue of genital lesions in men. J. Med. Virol. 2013, 85, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.G.; Winder, D.M.; Ball, S.L.; Vaughan, K.; Sonnex, C.; Stanley, M.A.; Sterling, J.C.; Goon, P.K. Detection of specific HPV subtypes responsible for the pathogenesis of condylomata acuminata. Virol. J. 2013, 10, 137. [Google Scholar] [CrossRef] [PubMed]
| Controls (n=32) | Patients (n=32) | OR (95%CI) | P | |
|---|---|---|---|---|
| Mean age (years) ± SD | 30.7± 8 | 30.8± 10 | 1 (0.9; 1.1) | 0.955 |
| ≥14 years of education | 12 (37.5) | 13 (40.6) | 1.1 (0.4; 3.1) | 0.798 |
| Currently employed | 21 (65.6) | 20 (62.5) | 0.9 (0.3; 2.4) | 0.795 |
| Marital status | ||||
| Married | 7 (21.9) | 4 (12.5) | 1 | |
| Cohabiting | 10 (31.3) | 15 (46.9) | 2.6 (0.6; 11.4) | 0.197 |
| Single | 15 (46.9) | 13 (40.6) | 1.5 (0.4; 6.4) | 0.570 |
| Cigarette smoking | 14 (43.8) | 19 (59.4) | 1.9 (0.7; 5.1) | 0.213 |
| History of skin disease | ||||
| No | 20 (62.5) | 15 (46.9) | 1 | |
| Yes | 7 (21.9) | 14 (43.8) | 2.7 (0.9; 8.2) | 0.088 |
| Unsure | 5 (15.6) | 3 (9.4) | 0.8 (0.2; 3.9) | 0.782 |
| Current STI other than AGW | 0.034* | |||
| No | 29 (90.6) | 22 (68.8) | ||
| Yes | 0 | 3 (9.4) | ||
| Unsure | 3 (9.4) | 7 (21.9) | ||
| Past STIb | 0.329 | |||
| No | 30 (93.8) | 27 (84.4) | ||
| Yes | 2 (6.3) | 4 (12.5) | ||
| Unsure | 0 | 1 (3.1) | ||
| Ever tested for STI | 8 (25) | 8 (25) | 1 (0.3; 3.1) | 1 |
| Mean age at first sexual intercourse (years) ± SD | 17.2 ± 2.2 | 17.7 ± 2 | 1.1 (0.9; 1.4) | 0.373 |
| Currently sexually active | 26 (81.3) | 23 (71.9) | 0.6 (0.2; 1.9) | 0.379 |
| Partners with present history of AGW | ||||
| No | 32 (100) | 20 (62.5) | <0.001* | |
| Yes | 0 | 5 (15.6) | ||
| Unsure | 0 | 7 (21.9) | ||
| Lifetime No. of sexual partners | ||||
| 1 | 6 (18.8) | 3 (9.4) | 1 | |
| <5 | 6 (18.8) | 8 (25) | 2.7 (0.5; 15.3) | 0.270 |
| 6–10 | 9 (28.1) | 10 (31.3) | 2.2 (0.4; 11.6) | 0.344 |
| >10 | 11 (34.4) | 11 (34.4) | 2 (0.4; 10.1) | 0.401 |
| No. of sexual partners in the past year | 0.202* | |||
| 0 | 0 | 3 (9.4) | ||
| 1 | 22 (68.8) | 18 (56.3) | ||
| 1–5 | 8 (25) | 9 (28.1) | ||
| >5 | 2 (6.3) | 2 (6.3) | ||
| Sexual orientation | 0.220* | |||
| MSW | 30 (93.7) | 27 (84.4) | ||
| MSM | 2 (6.3) | 3 (9.4) | ||
| MSWM | 0 | 2 (6.3) | ||
| Condom use | ||||
| Never | 4 (12.5) | 4 (12.5) | 1 | |
| Occasionally | 22 (68.8) | 24 (75) | 1.1 (0.2; 4.9) | 0.910 |
| Always | 6 (18.8) | 4 (12.5) | 0.7 (0.1; 4.4) | 0.672 |
| Circumcised | 2 (6.7) | 8 (25) | 4.7 (0.9; 24.1) | 0.066 |
| Shaving of anogenital region | 18 (58.1) | 18 (56.3) | 0.9 (0.3; 2.5) | 0.884 |
| HPV type, lineage and sublineage | No. (%) of samples |
|---|---|
| HPV6 | 28 (87.5) |
| HPV6 A* | 2 (7.1) |
| HPV6 B | 26 (92.9) |
| HPV6 B1 | 17 (65.4) |
| HPV6 B2 | 5 (19.2) |
| HPV6 B3 HPV6 B untypable |
2 (7.7) 2 (7.7) |
|
HPV11 HPV11A2 |
3 (9.4) 3 (100.0) |
| HPV40 | 1 (3.1) |
| Previous visit | AGW | OR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| YES | NO | |||||
| n | % | n | % | |||
| Hairs YES | 70 | 37.6% | 26 | 14.0% | 10.06 (5.11–19.8) | <0.0001 |
| Hairs NO | 19 | 10.2% | 71 | 38.2% | ||
| AGW YES | 73 | 39.5% | 35 | 18.9% | 11.30 (5.41–23.58) | <0.0001 |
| AGW NO | 12 | 6.5% | 65 | 35.1% | ||
| Baseline | After 11 months* | P | ||
|---|---|---|---|---|
| No | Yes | |||
| HPV6 | No | 5 (62.5) | 3 (37.5) | 0.001 |
| Yes | 19 (79.2) | 5 (20.8) | ||
| HPV 6 A | No | 29 (100) | 0 (0) | 0.48 |
| Yes | 2 (100) | 0 (0) | ||
| HPV 6 B | No | 7 (77.8) | 2 (22.2) | 0.001 |
| Yes | 17 (77.3) | 5 (22.7) | ||
| HPV 6 B1 | No | 13 (92.9) | 1 (7.1) | 0.003 |
| Yes | 12 (80) | 3 (20) | ||
| HPV 6 B2 | No | 25 (96.2) | 1 (3.8) | 1 |
| Yes | 1 (33.3) | 2 (66.7) | ||
| HPV 6 B3 | No | 27 (100) | 0 (0) | 0.48 |
| Yes | 2 (100) | 0 (0) | ||
| HPV 11 A2 | No | 30 (100) | 0 (0) | 1 |
| Yes | 1 (50) | 1 (50) | ||
| Hair sampling area/follow-up time | OR (95% CI) | P |
|---|---|---|
| Perianal region | 1 | |
| Eyebrows | 0.36 (0.14; 0.92) | 0.034 |
| Pubis | 1.48 (0.68; 3.25) | 0.324 |
| Scrotum | 0.83 (0.38; 1.82) | 0.634 |
| Follow-up time | 0.86 (0.78; 0.96) | 0.007 |
| Perianal region | 1 | |
| Eyebrows | 0.81 (0.65; 1.00) | 0.055 |
| Pubis | 0.99 (0.89; 1.11) | 0.874 |
| Scrotum | 0.97 (0.88; 1.07) | 0.566 |
| HPV type, lineage and sublineage |
AGW recurrence | P | ||
|---|---|---|---|---|
| No (n (%)) | Yes (n (%)) | |||
| HPV6 | No | 1 (4.5) | 0 (0) | 0.425 |
| Yes | 21 (95.5) | 8 (100) | ||
| HPV6 A | No | 18 (90.0) | 7 (100) | 0.263 |
| Yes | 2 (10.0) | 0 (0) | ||
| HPV6 B | No | 3 (15.0) | 0 (0) | 0.165 |
| Yes | 17 (85.0) | 7 (100) | ||
| HPV6 B1 | No | 8 (42.1) | 2 (33.3) | 0.700 |
| Yes | 11 (57.9) | 4 (66.7) | ||
| HPV6 B2 | No | 16 (84.2) | 4 (66.7) | 0.369 |
| Yes | 3 (15.8) | 2 (33.3) | ||
| HPV6 B3 | No | 17 (89.5) | 6 (100) | 0.283 |
| Yes | 2 (10.5) | 0 (0) | ||
| HPV11 A2* | No | 20 (90.9) | 7 (87.5) | 0.787 |
| Yes | 2 (9.1) | 1 (12.5) | ||
| HPV40** | Yes | 1 (100) | 0 (0) | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





