Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study Design
Medical Records
Statistical Analysis
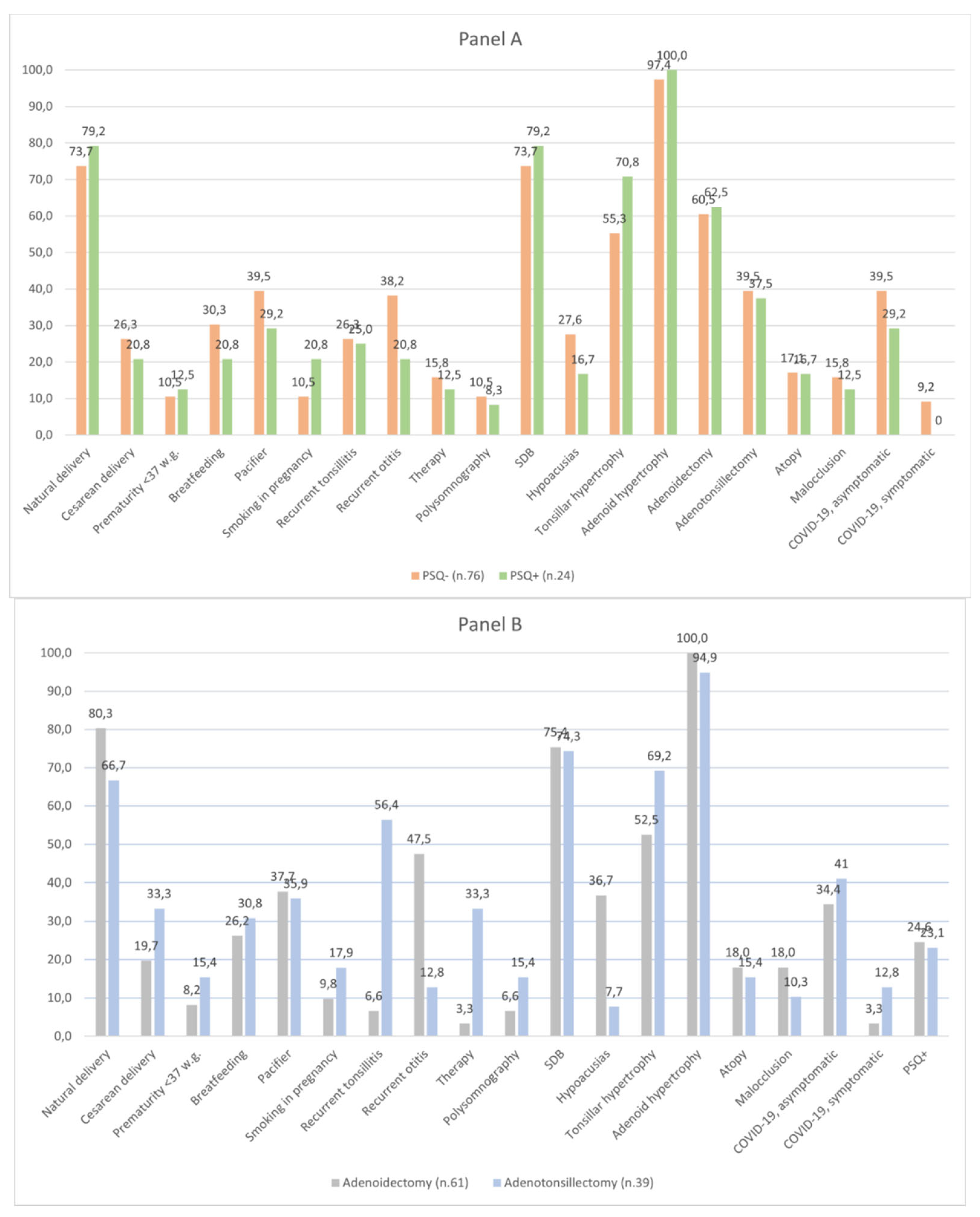
3. Results
| Continue variables | PSQ- (n.76) | PSQ+ (n.24) | |||
| Mean (S.D.) | C.I. 98% (lower-upper) | Mean (S.D.) | C.I. 98% (lower-upper) | Mann-Whitney test (p) | |
| PSQ (%), T1 | 16.2 (9.0) | -34.3 ÷ (-25.7) | 46.2 (10.0) | -34.7 ÷ (-25.4) | < 0.001 |
| Birth weight (grams), T-1 | 3249 (575) | -537 ÷ (- 21) | 3507 (677) | -570 ÷ 53 | 0.086 |
| Length (cm), T-1 | 50.57 (2.95) | -1.41 ÷ 1.46 | 50.54 (3.53) | -1.59 ÷ 1.64 | 0.267 |
| Age of intervention (years), T0 | 5.20 (1.6) | -0.70 ÷ 0.73 | 5.13 (1.44) | -0.68 ÷ 0.71 | 0.904 |
| Age follow-up (years), T1 | 7.13 (1.69) | -0.07 ÷ 1.47 | 6.42 (1.54) | -0.05 ÷ 1.44 | 0.061 |
| BMI z-score, T1 | 0.13 (1.56) | -1.28 ÷ 0.20 | 0.67 (1.67) | -1.32–0.24 | 0.086 |
| Physical score (%), T1 | 0.91 (0.09) | 0.05 ÷ 0.18 | 0.81 (0.23) | 0.01 ÷ 0.21 | 0.022 |
| School score (%), T1 | 0.86 (0.16) | 0.08 ÷ 0.26 | 0.68 (0.27) | 0.05 ÷ 0.29 | 0.003 |
| Variables entered | Dependent variable: PSQ (%), follow-up | T | Error std | Beta | t | P | 95% C.I. per B | |
| Birth weight, birth length, age at surgery, age at follow-up, BMI z-score at follow-up, physical quality of life, and school quality of life. | Lower limit | Upper limit | ||||||
| Surgery age (years), T0 | 3.503 | 1.667 | 0.337 | 2.101 | 0.038 | 0.193 | 6.812 | |
| Age at follow-up, T1 | -3.811 | 1.515 | -0.404 | -2.516 | 0.014 | -6.818 | -0.804 | |
| Physical results (%), T1 | -20.892 | 10.577 | -0.195 | -1.975 | 0.051 | -41.890 | 0.105 | |
| Educational attainment (%), Q1 | -30.891 | 7.680 | -0.399 | -4.022 | <0.0001 | -46.138 | -15.644 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690-702. [CrossRef]
- Zaffanello, M.; Lippi, G.; Arman, N.; Piazza, M.; Tenero, L.; Piacentini, G. Popularity of sleep disordered breathing in childhood: an analysis of worldwide search using Google Trends. Transl Pediatr 2019, 8, 383-390. [CrossRef]
- M, Z.; G, P.; S, L.G. Beyond the growth delay in children with sleep-related breathing disorders: a systematic review. Panminerva medica 2020, 62. [CrossRef]
- Lo Bue, A.; Salvaggio, A.; Insalaco, G. Obstructive sleep apnea in developmental age. A narrative review. Eur J Pediatr 2020, 179, 357-365. [CrossRef]
- Lagravère, M.O.; Zecca, P.A.; Caprioglio, A.; Fastuca, R. Metabolic effects of treatment in patients with obstructive sleep apnea: a systematic review. Minerva Pediatr 2019, 71, 380-389. [CrossRef]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2016. [CrossRef]
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children (Basel) 2022, 9. [CrossRef]
- Todd, C.A.; Bareiss, A.K.; McCoul, E.D.; Rodriguez, K.H. Adenotonsillectomy for Obstructive Sleep Apnea and Quality of Life: Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 2017, 157, 767-773. [CrossRef]
- Schupper, A.J.; Nation, J.; Pransky, S. Adenoidectomy in Children: What Is the Evidence and What Is its Role? Curr Otorhinolaryngol Rep 2018, 6, 64-73. [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576-584. [CrossRef]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int J Environ Res Public Health 2019, 16. [CrossRef]
- Tam, C.S.; Wong, M.; McBain, R.; Bailey, S.; Waters, K.A. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health 2006, 42, 277-282. [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. The cardiovascular risk in paediatrics: the paradigm of the obstructive sleep apnoea syndrome. Blood Transfus 2020, 1-9. [CrossRef]
- Lagravère, M.O.; Zecca, P.A.; Caprioglio, A.; Fastuca, R. Metabolic effects of treatment in patients with obstructive sleep apnea: a systematic review. Minerva pediatrica 2019, 71, 380-389. [CrossRef]
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children (Basel, Switzerland) 2022, 9, 1278.
- Todd, C.A.; Bareiss, A.K.; McCoul, E.D.; Rodriguez, K.H. Adenotonsillectomy for Obstructive Sleep Apnea and Quality of Life: Systematic Review and Meta-analysis. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2017, 157, 767–773 %U https://pubmed.ncbi.nlm.nih.gov/28675097/.
- Agrafiotis, M.; Galanou, A.; Fletsios, D.; Chassiotou, A.; Chloros, D.; Steiropoulos, P. Functional Comorbidity Index and health-related quality of life in patients with obstructive sleep apnea. Adv Respir Med 2022. [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, e714-755. [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical Practice Guideline: Tonsillectomy in Children (Update)-Executive Summary. Otolaryngol Head Neck Surg 2019, 160, 187-205. [CrossRef]
- Galluzzi, F.; Garavello, W. Impact of adenotonsillectomy in children with severe obstructive sleep apnea: A systematic review. Auris, nasus, larynx 2021, 48, 549–554 %U https://pubmed.ncbi.nlm.nih.gov/33109425/.
- Gozal, D.; Tan, H.L.; Kheirandish-Gozal, L. Treatment of Obstructive Sleep Apnea in Children: Handling the Unknown with Precision. J Clin Med 2020, 9. [CrossRef]
- Corrêa, C.C.; Weber, S.A.T.; Evangelisti, M.; Villa, M.P. Sleep Clinical Record application in Brazilian children and its comparison with Italian children. Sleep Med X 2019, 1, 100008. [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000, 1, 21-32. [CrossRef]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257-270. [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical care 2001, 39, 800–812 %U https://pubmed.ncbi.nlm.nih.gov/11468499/.
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2017, 35, 125-131. [CrossRef]
- Ferry, A.M.; Wright, A.E.; Ohlstein, J.F.; Khoo, K.; Pine, H.S. Efficacy of a Pediatric Sleep Questionnaire for the Diagnosis of Obstructive Sleep Apnea in Children. Cureus 2020, 12, e12244. [CrossRef]
- Umano, G.R.; Rondinelli, G.; Luciano, M.; Pennarella, A.; Aiello, F.; Mangoni di Santo Stefano, G.; Di Sessa, A.; Marzuillo, P.; Papparella, A.; Miraglia Del Giudice, E. Pediatric Sleep Questionnaire Predicts Moderate-to-Severe Obstructive Sleep Apnea in Children and Adolescents with Obesity. Children (Basel) 2022, 9. [CrossRef]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007, 133, 216-222. [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Seid, M.; Skarr, D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003, 3, 329-341. [CrossRef]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J Thorac Dis 2016, 8, E928-e931. [CrossRef]
- Imanguli, M.; Ulualp, S.O. Risk factors for residual obstructive sleep apnea after adenotonsillectomy in children. Laryngoscope 2016, 126, 2624-2629. [CrossRef]
- Huang, C.C.; Wu, P.W.; Chiu, C.H.; Lee, T.J.; Chen, C.L. Assessment of sleep-disordered breathing in pediatric otitis media with effusion. Pediatr Neonatol 2022, 63, 25-32. [CrossRef]
- Tatlıpınar, A.; Kınal, E. Links and risks associated with adenotonsillectomy and obesity. Pediatric Health Med Ther 2015, 6, 123-127. [CrossRef]
- Nosetti, L.; Zaffanello, M.; De Bernardi, F.; Piacentini, G.; Roberto, G.; Salvatore, S.; Simoncini, D.; Pietrobelli, A.; Agosti, M. Age and Upper Airway Obstruction: A Challenge to the Clinical Approach in Pediatric Patients. International Journal of Environmental Research and Public Health 2020, 17, E3531. [CrossRef]
- Wilhelm, C.P.; deShazo, R.D.; Tamanna, S.; Ullah, M.I.; Skipworth, L.B. The nose, upper airway, and obstructive sleep apnea. Ann Allergy Asthma Immunol 2015, 115, 96-102. [CrossRef]
- Shafiek, H.; Fawzy, N.; Mahmoud, M.I. Prevalence of obstructive sleep apnea in asthmatic school-aged children. European Respiratory Journal 2018, 52. [CrossRef]
- Alsufyani, N.; Isaac, A.; Witmans, M.; Major, P.; El-Hakim, H. Predictors of failure of DISE-directed adenotonsillectomy in children with sleep disordered breathing. J Otolaryngol Head Neck Surg 2017, 46, 37. [CrossRef]
- Nosetti, L.; Zaffanello, M.; De Bernardi di Valserra, F.; Simoncini, D.; Beretta, G.; Guacci, P.; Piacentini, G.; Agosti, M. Exploring the Intricate Links between Adenotonsillar Hypertrophy, Mouth Breathing, and Craniofacial Development in Children with Sleep-Disordered Breathing: Unraveling the Vicious Cycle. Children (Basel) 2023, 10. [CrossRef]
- Caruso, S.; Lisciotto, E.; Caruso, S.; Marino, A.; Fiasca, F.; Buttarazzi, M.; Sarzi Amadè, D.; Evangelisti, M.; Mattei, A.; Gatto, R. Effects of Rapid Maxillary Expander and Delaire Mask Treatment on Airway Sagittal Dimensions in Pediatric Patients Affected by Class III Malocclusion and Obstructive Sleep Apnea Syndrome. Life (Basel) 2023, 13. [CrossRef]
- Pegoraro, N.A.; Santos, C.M.D.; Colvara, B.C.; Rech, R.S.; Faustino-Silva, D.D.; Hugo, F.N.; Hilgert, J.B. Prevalence of malocclusion in early childhood and its associated factors in a primary care service in Brazil. Codas 2021, 34, e20210007. [CrossRef]
- Hansen, C.; Markström, A.; Sonnesen, L. Sleep-disordered breathing and malocclusion in children and adolescents-a systematic review. J Oral Rehabil 2022, 49, 353-361. [CrossRef]
- Gorlanova, O.; Thalmann, S.; Proietti, E.; Stern, G.; Latzin, P.; Kühni, C.; Röösli, M.; Frey, U. Effects of Breastfeeding on Respiratory Symptoms in Infancy. J Pediatr 2016, 174, 111-117.e115. [CrossRef]
- Jara, S.M.; Benke, J.R.; Lin, S.Y.; Ishman, S.L. The association between secondhand smoke and sleep-disordered breathing in children: a systematic review. Laryngoscope 2015, 125, 241-247. [CrossRef]
- Ramirez, F.D.; Groner, J.A.; Ramirez, J.L.; McEvoy, C.T.; Owens, J.A.; McCulloch, C.E.; Cabana, M.D.; Abuabara, K. Prenatal and Childhood Tobacco Smoke Exposure Are Associated With Sleep-Disordered Breathing Throughout Early Childhood. Acad Pediatr 2021, 21, 654-662. [CrossRef]
- Tan, Y.; Zhang, D.; Mei, H.; Mei, H.; Qian, Z.; Stamatakis, K.A.; Jordan, S.S.; Yang, Y.; Yang, S.; Zhang, B. Perinatal risk factors for obstructive sleep apnea syndrome in children. Sleep Med 2018, 52, 145-149. [CrossRef]
- Rapaport Pasternak, H.; Sheiner, E.; Goldbart, A.; Wainstock, T. Short and long interpregnancy interval and the risk for pediatric obstructive sleep apnea in the offspring. Pediatr Pulmonol 2021, 56, 1085-1091. [CrossRef]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998, 26, 257-268. [CrossRef]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Current Problems in Pediatric and Adolescent Health Care 2016. [CrossRef]
- Bucks, R.S.; Olaithe, M.; Eastwood, P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology 2013, 18, 61-70. [CrossRef]
- Testa, D.; Carotenuto, M.; Precenzano, F.; Russo, A.; Donadio, A.; Marcuccio, G.; Motta, G. Evaluation of neurocognitive abilities in children affected by obstructive sleep apnea syndrome before and after adenotonsillectomy. Acta Otorhinolaryngol Ital 2020, 40, 122-132. [CrossRef]
- Joosten, K.F.; Larramona, H.; Miano, S.; Van Waardenburg, D.; Kaditis, A.G.; Vandenbussche, N.; Ersu, R. How do we recognize the child with OSAS? Pediatr Pulmonol 2017, 52, 260-271. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





