Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
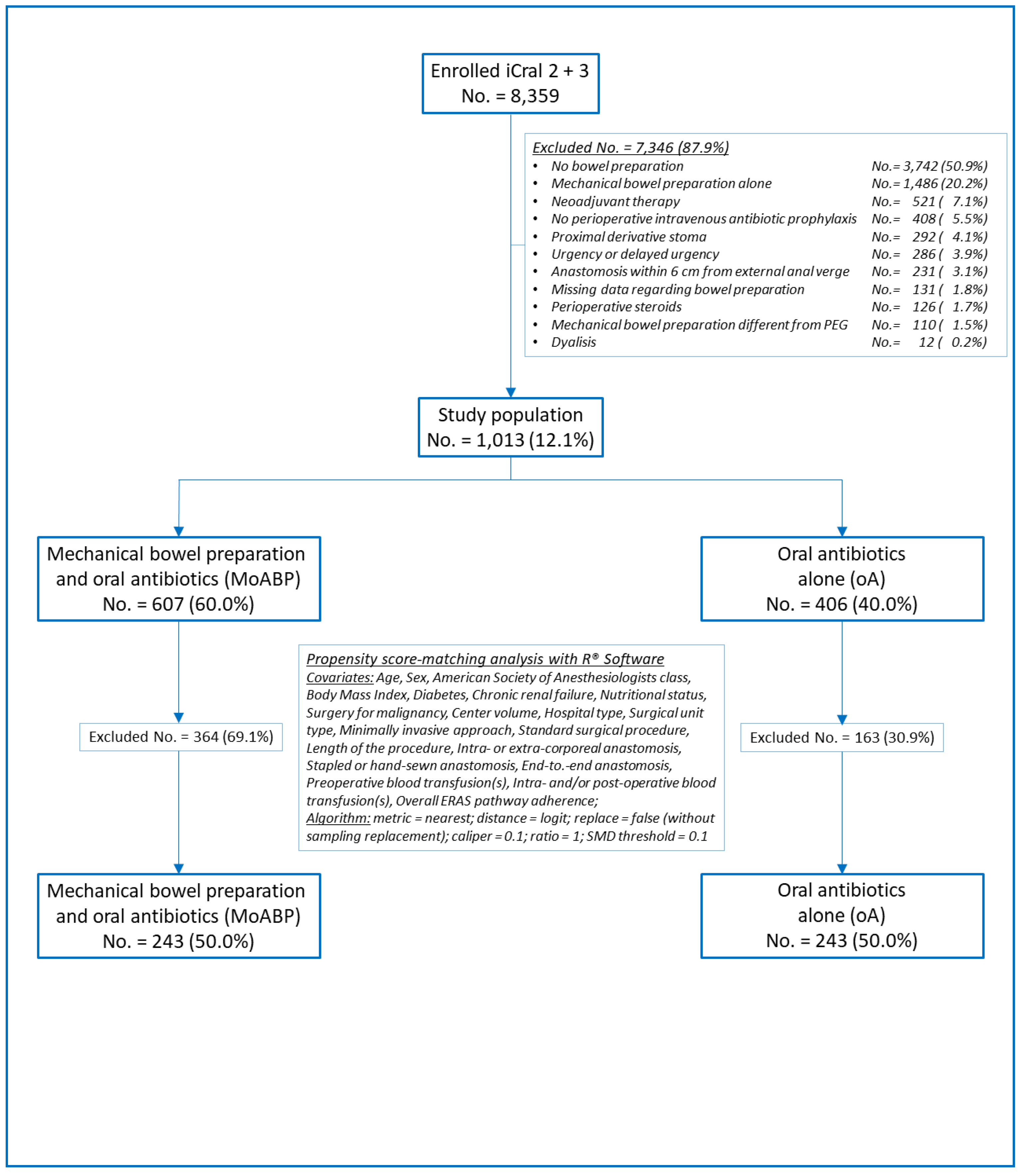
2.1. Study design
2.2. Patient population and data collection
2.3. Outcomes
2.4. Statistical analysis
 values (each 0.1 increment of
values (each 0.1 increment of  values representing a 10%-odds of differential assignment to treatment due to any unobserved variable). Sidak-Bonferroni’s adjustment for multiple comparisons was applied, setting α = 0.025, because the two primary outcomes were not independent and were selected based on literature evidence [2].
values representing a 10%-odds of differential assignment to treatment due to any unobserved variable). Sidak-Bonferroni’s adjustment for multiple comparisons was applied, setting α = 0.025, because the two primary outcomes were not independent and were selected based on literature evidence [2].3. Results
| Overall (No. 1013) | MoABP (No. 607) | oA (No.406) | |||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | *OR (95%CI) | |
| AL | 37 | 3.7 | 21 | 3.5 | 16 | 3.9 | 1.14 (0.59-2.22) p=.689 |
| SSIs | 32 | 3.2 | 17 | 2.8 | 15 | 3.7 | 1.33 (0.66-2.70) p=.425 |
| OM | 239 | 23.6 | 135 | 22.2 | 104 | 25.6 | 1.20 (0.90-1.62) p=.215 |
| MM | 61 | 6.0 | 30 | 4.9 | 31 | 7.6 | 1.59 (0.95-2.67) p=.077 |
| Reoperation | 49 | 4.8 | 27 | 4.5 | 22 | 5.4 | 1.23 (0.69-2.19) p=.480 |
| Before PSM | After PSM | ||||||||
| MoABP No. 609 |
oA No.406 |
MoABP No. 243 |
oA No.243 |
||||||
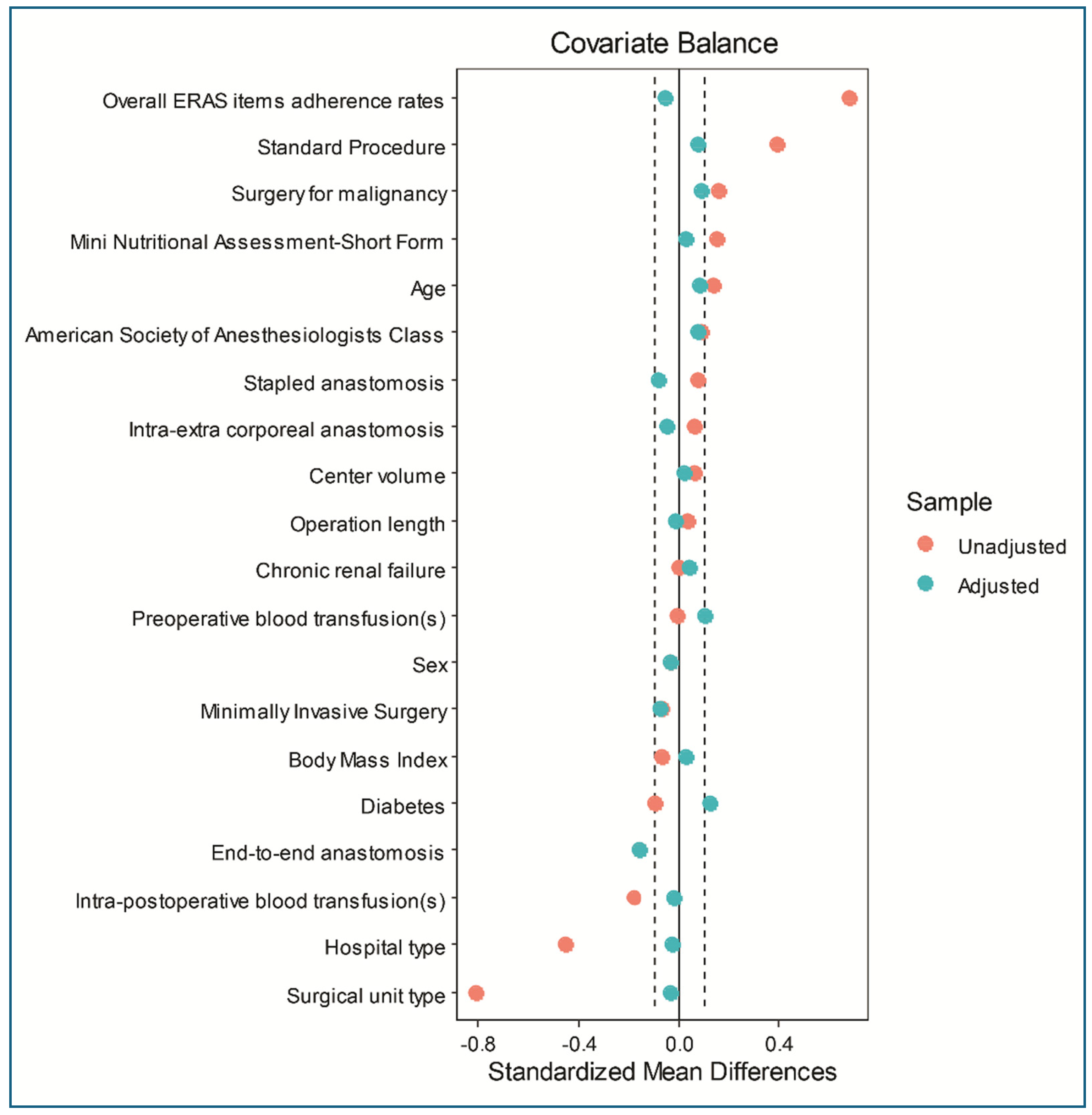
| Covariates | Pattern | *p | **SMD | *p | **SMD | ||||
| Age | ≤ 69 years | 324 | 283 | .039 | 0.14 | 128 | 118 | .414 | 0.08 |
| > 69 years | 189 | 217 | .039 | -0.14 | 115 | 125 | .414 | -0.08 | |
| Sex | Male | 323 | 209 | .633 | 0.03 | 129 | 125 | .785 | 0.03 |
| Female | 284 | 197 | .633 | -0.03 | 114 | 118 | .785 | -0.03 | |
| ASA class | I-II | 407 | 255 | .186 | 0.09 | 165 | 156 | .444 | 0.08 |
| III | 200 | 151 | .186 | -0.09 | 78 | 87 | .444 | -0.08 | |
| Body Mass Index | ≤24.67 Kg/m2 | 295 | 212 | .287 | -0.07 | 124 | 121 | .856 | 0.02 |
| > 24.67 Kg/m2 | 312 | 194 | .287 | 0.07 | 119 | 122 | .856 | -0.02 | |
| Diabetes | Yes | 81 | 42 | .182 | 0.09 | 21 | 30 | .236 | -0.12 |
| No | 526 | 364 | .182 | -0.09 | 222 | 213 | .236 | 0.12 | |
| Chronic renal failure | Yes | 27 | 18 | 1.00 | 0.00 | 10 | 12 | .827 | -0.04 |
| No | 580 | 388 | 1.00 | -0.00 | 233 | 231 | .827 | 0.04 | |
| MNA-SF | ≤ 13 | 433 | 260 | .017 | 0.16 | 165 | 162 | .847 | 0.03 |
| > 13 | 174 | 146 | .017 | -0.16 | 78 | 81 | .847 | -0.03 | |
| Malignancy | Yes | 427 | 312 | .027 | -0.15 | 167 | 176 | .426 | -0.08 |
| No | 180 | 94 | .027 | 0.15 | 76 | 67 | .426 | 0.08 | |
| Mini-invasive surgery | Yes | 545 | 355 | .288 | 0.07 | 221 | 215 | .455 | 0.08 |
| No | 62 | 51 | .288 | -0.07 | 22 | 28 | .455 | -0.08 | |
| Standard procedures | Yes | 488 | 371 | .000 | -0.32 | 208 | 213 | .594 | -0.06 |
| No | 119 | 35 | .000 | 0.32 | 35 | 30 | .594 | 0.06 | |
| Anastomosis 1 | Intracorporeal | 432 | 300 | .381 | -0.06 | 177 | 172 | .687 | 0.05 |
| Extracorporeal | 175 | 106 | .381 | 0.06 | 66 | 71 | .687 | -0.05 | |
| Anastomosis 2 | Stapled | 514 | 354 | .304 | -0.07 | 212 | 205 | .436 | 0.08 |
| Handsewn | 93 | 52 | .304 | 0.07 | 31 | 38 | .436 | -0.08 | |
| Anastomosis 3 | End-to-end | 293 | 164 | .016 | 0.16 | 116 | 97 | .010 | 0.16 |
| Other shape | 314 | 242 | .016 | -0.16 | 127 | 146 | .010 | -0.16 | |
| Operation length | ≤160′ | 291 | 201 | .671 | -0.03 | 131 | 133 | .927 | -0.02 |
| ˃160′ | 316 | 205 | .671 | 0.03 | 112 | 110 | .927 | 0.02 | |
| Hospital type | Met/Ac | 516 | 257 | .000 | 0.51 | 178 | 175 | .839 | 0.03 |
| Local/Regional | 91 | 149 | .000 | -0.51 | 65 | 68 | .839 | -0.03 | |
| Unit type | Col/Onc | 144 | 22 | .000 | 0.54 | 24 | 22 | .877 | 0.03 |
| General | 463 | 384 | .000 | -0.54 | 219 | 221 | .877 | -0.03 | |
| Center volume | Low | 221 | 136 | .377 | 0.06 | 65 | 63 | .918 | 0.02 |
| High | 386 | 270 | .377 | -0.06 | 178 | 180 | .918 | -0.02 | |
| Preoperative BT(s) | Yes | 26 | 17 | 1.00 | 0.00 | 8 | 13 | .372 | -0.10 |
| No | 581 | 389 | 1.00 | -0.00 | 235 | 230 | .372 | 0.10 | |
| Intra/Post-operative BT(s) | Yes | 43 | 15 | .033 | 0.15 | 15 | 14 | 1.00 | 0.02 |
| No | 564 | 391 | .033 | -0.15 | 228 | 229 | 1.00 | -0.02 | |
| ERAS adherence | ≤78.95% | 450 | 166 | .000 | 0.71 | 140 | 147 | .580 | -0.06 |
| ˃ 78.95% | 157 | 240 | .000 | -0.71 | 103 | 96 | .580 | 0.06 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix
References
- Yeo B, Harley V, Goodbody F, Pope FM, Herschell G, Wild RB, Haig A. A discussion on intestinal antiseptics. BMJ 1899;2:1250-1257.
- Willis MA, Toews I, Soltau SLV, Kal JC, Meerpohl JJ, Vilz TO. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst Rev 2023;2:CD014909.
- Poth EJ, Ross CA. The clinical use of phthalylsulfathiazole. J Lab Clin Med 1944; 29:785– 808.
- Lloyd-Davies OV, Morgan CN, Goligher JC. The treatment of carcinoma of the colon. In British Surgical Practice: Progress volume; Carling ER and Ross JP, Eds. London: Butterworth, UK, 1953; p. 71.
- Nichols RL, Condon RE, Gorbach SL, Nyhus LM. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg 1972;176:227-232. [CrossRef]
- Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178(4):453-462. [CrossRef]
- Nichols RL, Smith JW, Garcia RY, Waterman RS, Holmes JW. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis 1997;24:609-619. [CrossRef]
- Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014;2014(5):CD001181. [CrossRef]
- Global guidelines for the prevention of surgical site infection, 2nd ed. Geneva: World Health Organization; 2018.
- Guenaga KF, Matos D, Castro AA, Atallah AN, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2005;(1):CD001544. [CrossRef]
- Guenaga KF, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;9:CD001544.
- Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS©) Society Recommendations: 2018. World J Surg 2019;43:659-695. [CrossRef]
- Ficari F, Borghi F, Catarci M, et al. Enhanced recovery pathways in colorectal surgery: a consensus paper by the Associazione Chirurghi Ospedalieri Italiani (ACOI) and the PeriOperative Italian Society (POIS). G Chir 2019;40(4 Suppl.):1-40.
- Markell KW, Hunt BM, Charron PD, Kratz RJ, Nelson J, Isler JT, Steele SR, Billingham RP. Prophylaxis and management of wound infections after elective colorectal surgery: a survey of the American Society of Colon and Rectal Surgeons membership. J Gastrointest Surg 2010;14:1090-1098. [CrossRef]
- Toneva GD, Deierhoi RJ, Morris M, et al. Oral antibiotic bowel preparation reduces length of stay and readmissions after colorectal surgery. J Am Coll Surg 2013;216:756–763. [CrossRef]
- Kim EK, Sheetz KH, Bonn J, et al. A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg 2014;259:310–314.
- Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg 2015;261:1034–1040. [CrossRef]
- Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337.
- Garfinkle R, Abou-Khalil J, Morin N, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum 2017; 60: 729–737. [CrossRef]
- Koller SE, Bauer KW, Egleston BL, et al. Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann Surg 2018;267:734–742. [CrossRef]
- Midura EF, Jung AD, Hanseman DJ, et al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018;163:528–534. [CrossRef]
- Klinger AL, Green H, Monlezun DJ, et al. The role of bowel preparation in colorectal surgery. Ann Surg 2019;269:671–677. [CrossRef]
- Holubar SD, Hedrick T, Gupta R, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 2017;6:4. [CrossRef]
- Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761–784. [CrossRef]
- Migaly J, Bafford AC, Francone TD, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8. [CrossRef]
- McChesney SL, Zelhart MD, Green RL, et al. Current U.S. Pre-operative bowel preparation trends: a 2018 survey of the American Society of Colon and Rectal Surgeons Members. Surg Infect (Larchmt) 2020; 21: 1-8. [CrossRef]
- Willis MA, Keller PS, Sommer N, et al. Adherence to fast-track measures in colorectal surgery - a survey among German and Austrian surgeons. Int J Colorectal Dis 2023;38:80. [CrossRef]
- Catarci M, Guadagni S, Masedu F, et al. Mechanical bowel preparation in elective colorectal surgery: a propensity score-matched analysis of the Italian colorectal anastomotic leakage (iCral) study group prospective cohorts. Updates Surg 2024;76(1):107-117. [CrossRef]
- Antoniou SA, Huo B, Tzanis AA, et al. EAES, SAGES, and ESCP rapid guideline: bowel preparation for minimally invasive colorectal resection. Surg Endosc 2023;37: 9001-9012. [CrossRef]
- Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann Surg 2019;270: 43–58.
- Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019; 394: 840-848. [CrossRef]
- Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5(8):729-738. [CrossRef]
- Preoperative oral antibiotics in colon surgery (letters to the editor). Lancet Gastroenterol Hepatol 2020;5(9):800-803.
- Pellino_G, Solís-Peña_A, KraP_M, Huguet_BM, Espín-Basany_E. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: Protocol for an international randomized controlled trial (ORALEV2). Colorectal Dis 2021;23(8):2173–2181.
- Futier E, Jaber S, Garot M, et al.; COMBINE study group. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial. BMJ 2022;379:e071476. [CrossRef]
- Assistance Publique - Hôpitaux de Paris. Mechanical Bowel Preparation and Oral Antibiotics Before Rectal Cancer Surgery (PREPACOL2). NCT03491540. ClinicalTrials.gov – NIH – US National Library of Medicine, Available at: https://clinicaltrials.gov/ct2/show/NCT03491540 (last accessed January 11, 2024).
- Assistance Publique - Hôpitaux de Paris. Mechanical Bowel Preparation and Oral Antibiotics Before Colon Cancer Surgery (COLONPREP). NCT03475680. ClinicalTrials.gov – NIH – US National Library of Medicine, Available at: https://clinicaltrials.gov/ct2/show/NCT03475680 (last accessed January 11, 2024).
- Catarci M, Ruffo G, Viola MG, et al. ERAS program adherence-institutionalization, major morbidity and anastomotic leakage after elective colorectal surgery: the iCral2 multicenter prospective study. Surg Endosc 2022;36:3965-3984. [CrossRef]
- Italian ColoRectal Anastomotic Leakage (iCral) study group. Patient-reported outcomes, return to intended oncological therapy and enhanced recovery pathways after colorectal surgery: a prospective multicenter observational investigation by the Italian ColoRectal Anastomotic Leakage (iCral 3) study group. Ann Surg Open 2023;4: e267.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12(12):1495-1499. [CrossRef]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205-213.
- Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 2016; 46: 668-685. [CrossRef]
- Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010; 147: 339-351. [CrossRef]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [CrossRef]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [CrossRef]
- Bujang MA, Sa’at N, Sidik TMITAB, Joo LC. Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population: Emphasis on the Accuracy Between Statistics and Parameters Based on Real Life Clinical Data. Malays J Med Sci 2018; 25: 122-130. [CrossRef]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46: 399-424. [CrossRef]
- Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983; 70: 41-55.
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol 2006; 163: 1149-1156. [CrossRef]
- Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009; 13: 782.
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150-161. [CrossRef]
- Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007; 15: 199-236. [CrossRef]
- Rosenbaum PR. The power of a sensitivity analysis and its limit. In: Design of Observational studies, 2nd Ed. Springer Series in Statistics. Springer Nature Switzerland AG, 2020. pp. 317-336.
- Zmora O, Mahajna A, Bar-Zakai B, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg 2003; 237:363–367.
- Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita H, Ogimi T. al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Dig Surg 2020; 37:192-198. [CrossRef]
- Cannon JA, Altom LK, Deierhoi RJ, Moris M, Richman JS, Vick CC, Itani KMF, Hawn MT. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum 2012; 55:1160–1166. [CrossRef]
- Schardey HM, Rogers S, Schopf SK, Ahnen T, Wirth U. Are gut bacteria associated with the development of anastomotic leaks ? A review of experimental and clinical studies. Coloproctology 2017; 39: 94–100. [CrossRef]
- Fry DE. Antimicrobial Bowel Preparation for Elective Colon Surgery. Surg Infect (Larchmt) 2016;17(3):269-274. [CrossRef]
- Poth EJ. Historical development of intestinal antisepsis. World J Surg 1982; 6: 153–159. [CrossRef]
- Cao Y, Shang F, Jin M, et al. Changes in Bacteroides and the microbiota in patients with obstructed colorectal cancer: retrospective cohort study. BJS Open 2023;7(6):zrad105. [CrossRef]
- Shogun BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014; 2: 35. [CrossRef]
- Ljungqvist O, Lobo DN. Bowel Preparation for Colorectal Surgery: Have All Questions Been Answered? JAMA Surg 2022; 157: 41-42.
- Sell NM, Francone TD. Anastomotic Troubleshooting. Clin Colon Rectal Surg 2021; 34: 385–390.
- Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol 2017; 14: 43-54. [CrossRef]
- Kirby A, Santoni N. Antibiotic resistance in Enterobacteriaceae: What impact on the efficacy of antibiotic prophylaxis in colorectal surgery? J Hosp Infect 2015; 89: 259-263.
- Haak BW, Lankelma JM, Hugenholtz F, Belzer C, de Vos WM, Wiersinga WJ. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J Antimicrob Chemother 2019;74(3):782-786. [CrossRef]
- Hajjar R, Santos MM, Dagbert F, Richard CS. Current evidence on the relation between gut microbiota and intestinal anastomotic leak in colorectal surgery. Am J Surg 2019;218:1000-1007. [CrossRef]
- Correia S, Poeta P, Hébraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand? J Med Microbiol 2017;66(5):551-559.
- Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, Leavitt A, Carmeli Y. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis 2006;42(7):925-934.
- Sartelli M, Coccolini F, Labricciosa FM, Al Omari AH, Bains L, Baraket O, Catarci M, Cui Y, Ferreres AR, Gkiokas G, Gomes CA, Hodonou AM, Isik A, Litvin A, Lohsiriwat V, Kotecha V, Khokha V, Kryvoruchko IA, Machain GM, O’Connor DB, Olaoye I, Al-Omari JAK, Pasculli A, Petrone P, Rickard J, Sall I, Sawyer RG, Téllez-Almenares O, Catena F, Siquini W. Surgical Antibiotic Prophylaxis: A Proposal for a Global Evidence-Based Bundle. Antibiotics (Basel) 2024;13(1):100. [CrossRef]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007;449(7164):804-10. [CrossRef]
- Yao XI, Wang X, Speicher PJ, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst 2017; 109:djw323.
- Simoneau G, Pellegrini F, Debray TPA, Rouette J, Muñoz J, Platt RW, Petkau J, Bohn J, Shen C, de Moor C, Karim ME. Recommendations for the use of propensity score methods in multiple sclerosis research. Multiple Sclerosis Journal 2022; 28: 1467–1480. [CrossRef]
- García-Granero E, Navarro F, Cerdán Santacruz C, et al. Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery 2017;162:1006-1016. [CrossRef]
| Overall | MoABP | oA | ||||||
| No. 1,013 | No. 607 | No. 406 | ||||||
| Variables | Pattern | No. | % | No. | % | No. | % | *p |
| Age (years) | ≤ 69 | 513 | 50.6 | 324 | 53.4 | 189 | 46.5 | .033 |
| > 69 | 500 | 49.4 | 283 | 46.6 | 217 | 53.5 | ||
| Sex | Male | 532 | 52.5 | 323 | 53.2 | 209 | 51.5 | .588 |
| Female | 481 | 47.5 | 284 | 46.8 | 197 | 48.5 | ||
| ASA class | I-II | 662 | 65.3 | 407 | 67.0 | 255 | 62.8 | .164 |
| III | 351 | 34.7 | 200 | 33.0 | 151 | 37.2 | ||
| Body Mass Index (Kg/m2) | ≤ 24.67 | 507 | 50.1 | 295 | 48.6 | 212 | 52.2 | .259 |
| > 24.67 | 506 | 49.9 | 312 | 51.4 | 194 | 47.8 | ||
| Diabetes | Yes | 123 | 12.1 | 81 | 13.3 | 42 | 10.3 | .152 |
| No | 890 | 87.9 | 526 | 86.7 | 364 | 89.7 | ||
| Chronic renal failure | Yes | 45 | 4.4 | 27 | 4.5 | 18 | 4.4 | .991 |
| No | 968 | 95.6 | 580 | 95.5 | 388 | 95.6 | ||
| MNA-SF | ≤ 13 | 693 | 68.4 | 433 | 71.3 | 260 | 64.0 | .014 |
| > 13 | 320 | 31.6 | 174 | 28.7 | 146 | 36.0 | ||
| Malignancy | Yes | 739 | 73.0 | 427 | 70.4 | 312 | 76.9 | .022 |
| No | 274 | 27.0 | 180 | 29.6 | 94 | 23.1 | ||
| Diverticular disease | 167 | 60.9 | 107 | 59.4 | 60 | 63.8 | 167 | |
| Endometriosis | 2 | 0.8 | 0 | 0 | 2 | 2.2 | ||
| Polyps | 35 | 12.8 | 17 | 9.5 | 18 | 19.1 | ||
| IBD | 28 | 10.2 | 22 | 12.2 | 6 | 6.4 | ||
| Other | 42 | 15.3 | 34 | 18.9 | 8 | 8.5 | ||
| Mini-invasive surgery | No | 113 | 11.1 | 62 | 10.2 | 51 | 12.6 | .245 |
| Yes | 900 | 88.9 | 545 | 89.8 | 355 | 87.4 | ||
| Laparoscopic | 826 | 81.5 | 509 | 93.4 | 317 | 89.3 | ||
| Robotic | 32 | 3.2 | 17 | 3.1 | 15 | 4.2 | ||
| Converted | 42 | 4.2 | 19 | 3.5 | 23 | 6.5 | ||
| Standard procedure | Yes | 859 | 84.8 | 488 | 80.4 | 371 | 91.4 | .000 |
| Right colectomy | 407 | 47.4 | 199 | 40.8 | 208 | 56.1 | 407 | |
| Left colectomy | 356 | 41.4 | 223 | 45.7 | 133 | 35.9 | ||
| Anterior resection | 96 | 11.2 | 66 | 13.5 | 30 | 8.1 | ||
| No | 154 | 15.2 | 119 | 19.6 | 35 | 8.6 | ||
| Transverse colectomy | 28 | 18.2 | 18 | 15.1 | 10 | 28.6 | ||
| Splenic flexure colectomy | 26 | 16.9 | 14 | 11.8 | 12 | 34.3 | ||
| Hartmann reversal | 16 | 10.4 | 12 | 10.1 | 4 | 11.4 | ||
| (Sub) total colectomy | 23 | 14.9 | 19 | 16.0 | 4 | 11.4 | ||
| Other | 61 | 39.6 | 56 | 47.0 | 5 | 14.3 | ||
| Anastomosis 1 | Intracorporeal | 732 | 72.3 | 432 | 71.2 | 300 | 73.4 | .343 |
| Extracorporeal | 281 | 27.7 | 175 | 28.8 | 106 | 26.1 | ||
| Anastomosis 2 | Stapled | 868 | 85.7 | 514 | 84.7 | 354 | 87.2 | .263 |
| Handsewn | 145 | 14.3 | 93 | 15.3 | 52 | 12.8 | ||
| Anastomosis 3 | End-to-end | 457 | 45.1 | 293 | 48.3 | 164 | 40.4 | .014 |
| Other shape | 556 | 54.9 | 314 | 51.7 | 242 | 59.6 | ||
| Operation length | ≤ 160′ | 521 | 51.4 | 316 | 52.1 | 205 | 50.5 | .625 |
| ˃ 160′ | 492 | 48.6 | 291 | 47.9 | 201 | 49.5 | ||
| Hospital type | Met./Ac. | 773 | 76.3 | 516 | 85.0 | 257 | 63.3 | .000 |
| Local/Regional | 240 | 23.7 | 91 | 15.0 | 149 | 36.7 | ||
| Unit type | Colorectal/Oncologic | 166 | 16.4 | 144 | 23.7 | 22 | 5.4 | .000 |
| General | 847 | 83.6 | 463 | 76.3 | 384 | 94.6 | ||
| Center volume | < 4 cases/month | 357 | 35.2 | 221 | 36.4 | 136 | 33.5 | .342 |
| ≥ 4 cases/month | 656 | 64.8 | 386 | 63.6 | 270 | 66.5 | ||
| Preoperative BT(s) | Yes | 43 | 4.2 | 26 | 4.3 | 17 | 4.2 | .941 |
| No | 970 | 95.8 | 581 | 95.7 | 389 | 95.8 | ||
| Intra/postoperative BT(s) | Yes | 58 | 5.7 | 43 | 7.1 | 15 | 3.7 | .023 |
| No | 955 | 94.3 | 564 | 92.9 | 391 | 96.3 | ||
| ERAS adherence (%) | ≤ 78.95 | 616 | 60.8 | 450 | 74.1 | 166 | 40.9 | .000 |
| ˃ 78.95 | 397 | 39.2 | 157 | 25.9 | 240 | 59.1 | ||
| Nutritional screening | 711 | 70.2 | 410 | 67.6 | 301 | 74.1 | ||
| Prehabilitation | 411 | 40.6 | 183 | 30.2 | 228 | 56.2 | ||
| Counseling | 747 | 73.7 | 471 | 77.6 | 276 | 68.0 | ||
| Immune enhancing nutrition | 330 | 32.6 | 113 | 18.6 | 217 | 53.5 | ||
| Antithrombotic prophylaxis | 938 | 92.6 | 550 | 90.6 | 388 | 95.6 | ||
| Preoperative carbohydrates load | 582 | 57.5 | 326 | 53.7 | 256 | 63.1 | ||
| No preanesthesia | 741 | 73.2 | 448 | 73.8 | 293 | 72.2 | ||
| Standard anesthesia protocol | 980 | 96.7 | 584 | 96.2 | 396 | 97.5 | ||
| Normothermia | 974 | 96.2 | 576 | 94.9 | 398 | 98.0 | ||
| Goal-directed or restrictive fluid therapy | 898 | 88.7 | 539 | 88.8 | 359 | 88.4 | ||
| Postoperative nausea/vomit prophylaxis | 935 | 92.3 | 543 | 89.5 | 392 | 96.6 | ||
| Multimodal analgesia | 975 | 96.3 | 573 | 94.4 | 402 | 99.0 | ||
| No nasogastric tube | 882 | 87.1 | 491 | 80.9 | 391 | 96.3 | ||
| Minimally invasive surgery | 900 | 88.9 | 545 | 89.8 | 355 | 87.4 | ||
| No drains | 420 | 41.5 | 178 | 29.3 | 242 | 59.6 | ||
| Urinary catheter < 24-48 hours | 864 | 85.3 | 484 | 79.7 | 380 | 93.6 | ||
| Early mobilization | 842 | 83.1 | 469 | 77.3 | 373 | 91.9 | ||
| Early oral feeding | 726 | 71.7 | 374 | 61.6 | 352 | 86.7 | ||
| Pre-discharge check | 848 | 83.7 | 503 | 82.9 | 345 | 85.0 | ||
| Oral Antibiotic(s) | Administration schedule | oA (No. 406) | MoABP (No. 607) | *p | ||
| No. | % | No. | % | |||
| Metronidazole (500 mg) Paromomycin (250 mg) |
Started 2 days preop., TID Started 2 days preop., BID |
118 | 29.1 | 29 | 4.8 | .006 |
| Metronidazole (500 mg) Cefazolin (2000 mg) |
Started 1 day preop., TID Started 1 day preop., OD |
76 | 18.7 | 50 | 8.2 | .102 |
| Metronidazole (500 mg) Trimethoprim (160 mg) + Sulfamethoxazole (800 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
68 | 16.7 | 61 | 10.0 | .267 |
| Metronidazole (500 mg) Neomicin + Bacitracin (300 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
47 | 11.6 | 6 | 0.9 | .419 |
| Metronidazole (500 mg) Amoxicilline (1000 mg) |
Started 3 days preop., BID Started 3 days preop., BID |
25 | 6.2 | 5 | 0.8 | .623 |
| Metronidazole (250 mg) Ciprofloxacin (500 mg) |
Started 1 day preop., TID Started 1 day preop., BID |
20 | 4.9 | 21 | 3.5 | .823 |
| Metronidazole (500 mg) Rifaximin (400 mg) |
Started 7 days preop., TID Started 7 days preop., BID |
5 | 1.2 | 9 | 1.5 | .963 |
| Metronidazole (250 mg) Amoxicilline (1000 mg) |
Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 50 | 8.2 | n.e. |
| Paramomycin (250 mg) | Started 4 days preop., QID | 44 | 10.8 | 0 | 0 | n.e. |
| Paromomycin (1000 mg) | Started 1 day preop., OD | 0 | 0 | 37 | 6.1 | n.e. |
| Metronidazole (250 mg) Rifaximin (200 mg) |
Started 1 day preop., TID Started 1 day preop., BID |
3 | 0.8 | 0 | 0 | n.e. |
| Metronidazole (500 mg) Rifaximin (200 mg) |
Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 68 | 11.2 | n.e. |
| Metronidazole (1000 mg) Rifaximin (400 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
0 | 0 | 11 | 1.8 | n.e. |
| Metronidazole (500 mg) Paromomycin (500 mg) Rifaximin (400 mg) |
Started 1 day preop., BID Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 126 | 20.8 | n.e. |
| Rifaximin (400 mg) | Started 1 day preop., TID | 0 | 0 | 102 | 16.8 | n.e. |
| Amoxicilline (1000 mg) | Started 3 days preop., TID | 0 | 0 | 17 | 2.8 | n.e. |
| Neomicin + Bacitracin (300 mg) | Started 1 day preop., TID | 0 | 0 | 15 | 2.5 | n.e. |
| Propensity score-matched analysis | ||||||||
| MoABP No. 243 | oA No. 243 | *Sensitivity | ||||||
| Endpoint | No. | % | No. | % | OR (95%CI) | p | Γ | **p |
| Anastomotic leakage | 6 | 2.5 | 14 | 5.8 | 3.77 (1.22-11.67) | .021 | 1.0 | .057 |
| SSIs | 7 | 2.9 | 9 | 3.7 | 1.02 (0.31 - 3.29) | .977 | ||
| Overall morbidity | 49 | 20.2 | 64 | 26.3 | 1.52 (0.96 - 3.40) | .075 | ||
| Major Morbidity | 9 | 3.7 | 25 | 10.3 | 4.55 (1.82-11.38) | .001 | 1.4 | .038 |
| Reoperation | 5 | 2.1 | 16 | 6.6 | 5.05 (1.55-16.49) | .007 | 1.3 | .037 |
| MoABP No. 243 | oA No. 243 | |||||
| Adverse events | OM (%) | MM (%) | OM (%) | MM (%) | *p (OM) | *p (MM) |
| Anastomotic leakage | 6 (2.5) | 4 (1.6) | 14 (5.8) | 12 (4.9) | .068 | .042 |
| sdiSSIs | 2 (0.8) | 0 (0) | 6 (2.5) | 4 (1.6) | .154 | .045 |
| Deepwound dehiscence | 1 (0.4) | 1 (0.4) | 2 (0.8) | 2 (0.8) | .562 | .562 |
| Abdominal collection/abscess | 4 (1.7) | 1 (0.4) | 3 (1.2) | 3 (1.2) | .703 | .315 |
| Small bowel obstruction | 7 (2.9) | 5 (2.1) | 4 (1.6) | 3 (1.2) | .360 | .476 |
| Anastomotic bleeding | 2 (0.8) | 1 (0.4) | 8 (3.3) | 1 (0.4) | .055 | 1.00 |
| Abdominal bleeding | 2 (0.8) | 1 (0.4) | 1 (0.4) | 1 (0.4) | .562 | 1.00 |
| Small bowel perforation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | n.e. | n.e. |
| Trocar/wound site bleeding | 1 (0.4) | 0 (0) | 1 (0.4) | 0 (0) | 1.00 | n.e. |
| Anemia | 6 (2.5) | 0 (0) | 9 (3.7) | 1 (0.4) | .431 | .317 |
| Paralytic ileus | 9 (3.7) | 0 (0) | 8 (3.3) | 0 (0) | .805 | n.e. |
| Fever | 6 (2.5) | 0 (0) | 8 (3.3) | 0 (0) | .588 | n.e. |
| DVT/PE | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | .317 | n.e. |
| Neurologic | 1 (0.4) | 1 (0.4) | 1 (0.4) | 0 (0) | 1.00 | .317 |
| Pneumonia and pulmonary failure | 5 (2.1) | 0 (0) | 7 (2.9) | 2 (0.8) | .559 | .156 |
| Urinary retention | 1 (0.4) | 0 (0) | 2 (0.8) | 0 (0) | .562 | n.e. |
| Urinary tract infection | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | .317 | n.e. |
| Acute renal failure | 0 (0) | 0 (0) | 4 (1.6) | 0 (0) | .062 | n.e. |
| Acute mesenteric ischemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | n.e. | n.e. |
| Acute peptic ulcer/erosive gastritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | n.e. | n.e. |
| Cardiac dysfunction and failure | 2 (0.8) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 1.00 | .562 |
| Other | 14 (5.8) | 1 (0.4) | 7 (2.9) | 2 (0.8) | .118 | .562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





