1. Introduction
Non-ferrous metal smelting slag is a waste residue produced in the smelting of non-ferrous metal minerals and the large amount of waste slag generated leads to pollution to the environment. There are about 2010 million tons of zinc smelting slag stored in Guizhou Province, China. Romania has produced about 20.295 million tons of nickel slag since 2000-2010 [
1,
2,
3,
4]. Copper-nickel smelting slag has certain Pozzolanic activity, which has been used to prepare mine-filling cementitious material to solve the stability of current mining filling material and high processing cost [
5,
6,
7]. Materials such as fly ash, dolomite, and smelting slag have been used to replace cement to prepare composite cementitious materials [
8,
9,
10,
11].Moreover heavy metal elements in copper-nickel slag on the environmental impact of attracted the attention of scholars. Currently, it’s difficult to utilize copper slag, and more than 80% of copper slag is piled up. The soil of the copper-nickel tailings pond was polluted with heavy metals of Cd, Cu, Ni, and Cr [
12]. Copper slag can be used as a supplementary cementitious material for the preparation of cementitious materials, which meets the requirements of the strength of the building materials and cures heavy metal ions such as Cu, Pb, and Cr [
13,
14,
15,
16,
17,
18].
Palygorskite is a fibrous clay mineral, that belongs to the group of layered silicate minerals. In contrast to the arrangement of conventional layered silicate clay minerals, the silica-oxygen tetrahedra of palygorskite has an overall chain-like structure. The locally inverted tetrahedra are sandwiched with octahedral sheets to form a 2: 1 structure and pores, which are stacked to form fibrous particles. Due to its special structure, palygorskite is commonly used in the field of adsorption curing, such as curing heavy metal elements in sewage and soil [
19,
20]. Aconite is abundant and cheap, it can be modified by calcination and other methods and promote the early hydration reaction [
21,
22], improving the effect of the early mechanical properties of cementitious materials. palygorskite is mostly used in the construction field [
23,
24,
25,
26], but little research has been reported in the filling field.
In the previous research, the mechanical properties and structure of cementitious materials prepared from copper-nickel smelting slag (CNSS) were studied. The results show that the mechanically excited copper-nickel slag has good mechanical properties, which can meet the needs of mine filling [
27]. Based on the previous research, this paper investigates the effect of the addition of palygorskite on the mechanical properties of copper-nickel smelting slag cementitious materials and the curing properties of heavy metals, explores the new application of copper-nickel smelting slag preparation cementitious materials in the field of filling.
2. Materials and Methods
2.1. Materials
CNSS provied by Xinjiang Karatunk Mining Co. was grinded in a horizontal ball mill for 6 h. The chemical composition is shown in
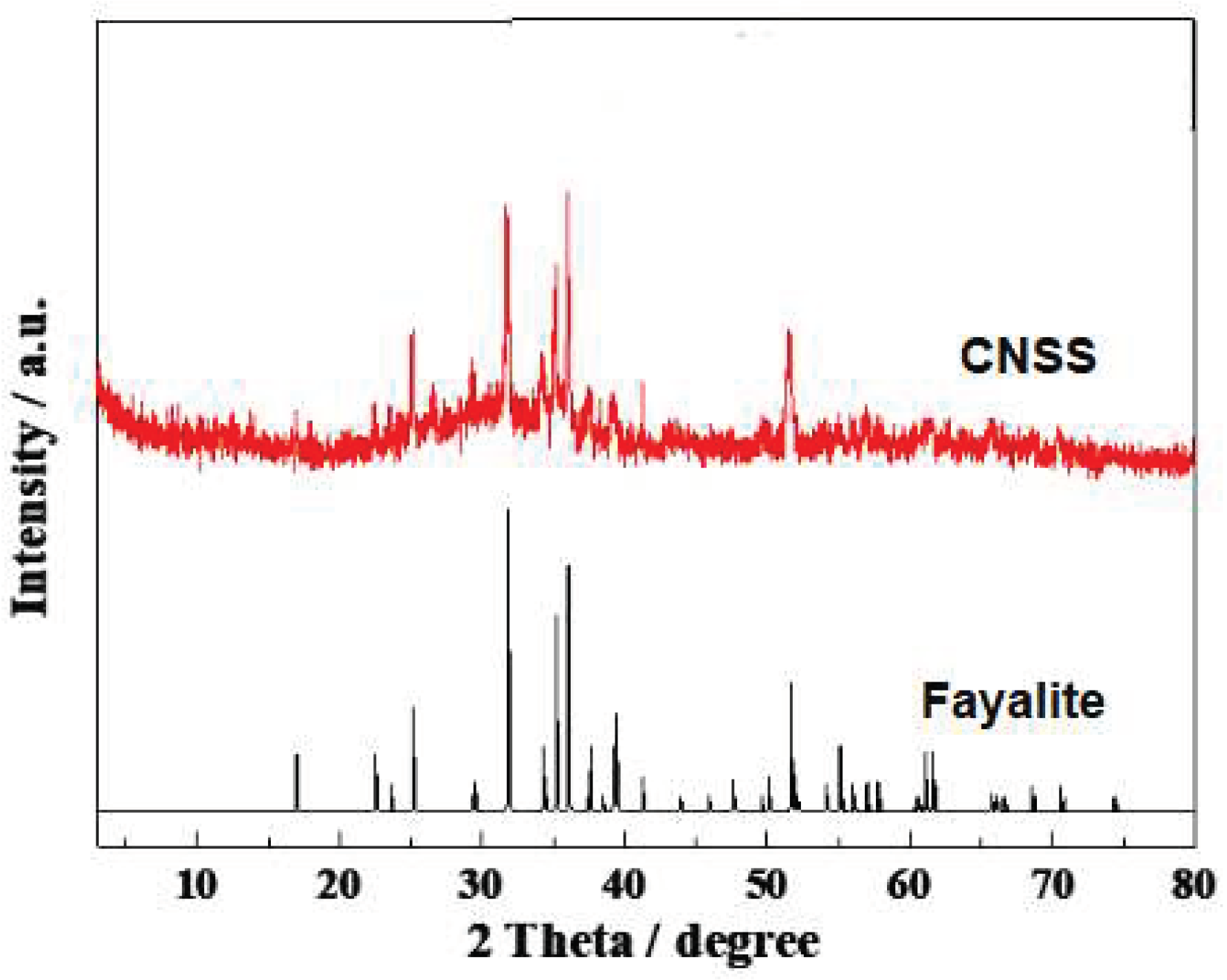
Table 1, and the primary ingredients are Fe2O3, SiO2, MgO, Al2O3 and some heavy metal elements including Fe, Cu, Co, Cr, Pb. CNSS is mainly composed of fayalite from XRD pattern in
Figure 1.
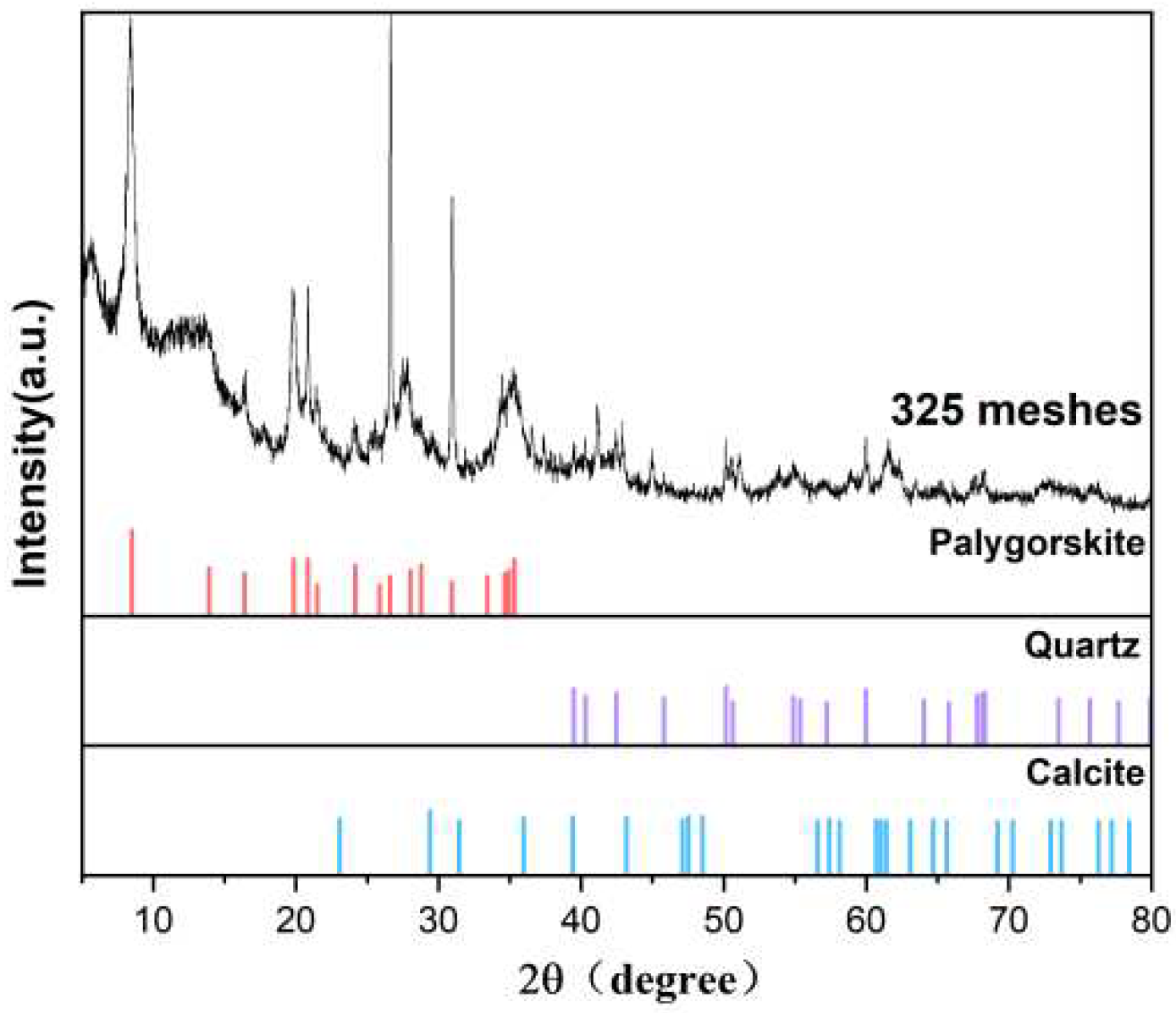
The palygorskite was purchased from Changzhou Dingbang Mineral Products Co., Ltd., and the origin was Xuyi, Jiangsu Province. The XRD characterization is shown in
Figure 2. The palygorskite contains quartz and calcium phases.
42.5 standard cement was used as the benchmark cement. produced by Fushun Cement Company Limited. Quartz sand was used as China ISO standard sand, produced by Xiamen Aisio Standard Sand Co.
Acetic acid (CH3COOH) of pure analytical grade was used for the test of heavy metal leaching from CNSS and cementitious materials.
2.2. Test Methods
The experimental conditions listed in
Table 2 were used. A certain amount of CNSS, cement, palygorskite and water were mixed, then added a certain amount of standard sand and stirred at high speed for 30s, stopped stirring for 90s and stirred at high speed for 60s. The fluidity and consistency were measured according to the cement mortar strength test method (GB-T 17671-2021 ). Subsequently, the mixed slurry was poured into the triple mold of 40mm × 40mm × 160mm and was kept at room temperature for 24 h. Finally placed it ina curing box with a temperature of 20 °C and a humidity of 95% for 3, 7, 14 and 28 days, and the compressive strength of the test specimen was tested. After the test, the test specimen was placed in absolute alcohol to stop hydration for 48 h, dried at 60 ° for 24 h, then broken and ground. The leaching experiment of harmful heavy metals was carried out according to the toxic leaching method of solid waste-extraction procedure for leaching toxicity-acetic acid buffer solution method (HJ / 300-2007), and the concentration of heavy metals in the leaching solution was measured by ICP.
2.3. Characterization
The compressive strength tests were conducted by an electrohydraulic servo pressure test machine under microcomputer controll manufactured (WAW-2000E). The fluidity and consistency of the mortar were tested by a fluidity shock mitigation table and grout consistency meter. X-ray diffraction (XRD) analyses were performed with CuKα radiation under 60 kV and 80 mA and a scanning speed at 5◦/min (D8 Advance, Bruker, Germany). SEM (scanning electron microscopy) tests were carried on a JSM-7610FPlus scanning electron microscope (Nihon Kohden). Pore size was measured by automatic mercury porosimeter (AutoporeIV 9500, Mack, USA). Thermal stability was tested by a thermogravimetric analyzer coupled with a differential scanning calorimeter (TGA-DSC) (TASDT Q600, TA Instruments, USA), with temperature range from room temperature to 800℃ at a rate of 10℃/min under a nitrogen atmosphere.The ion concentration of the leaching solution was tested by inductively coupled plasma optical emission spectrometry (ICAP7000, Thermo Fisher, USA).
3. Results and Discussion
3.1. Consistency and Fluidity of Mortar
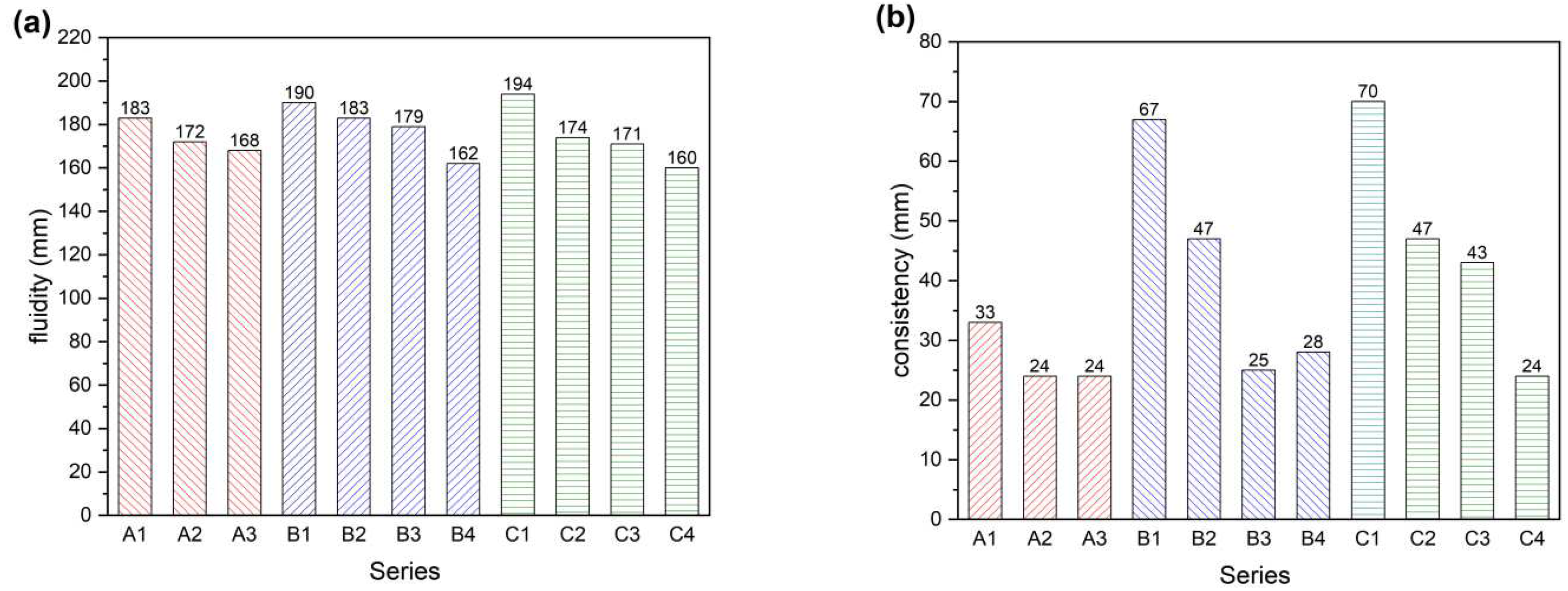
To investigate the effect of different palygorskite additions and smelting slag on the fluidity and consistency of cement, the experimental ratios designed in
Table 2 were used to test the components. The experimental results are shown in
Figure 3. When 1% and 2% of palygorskite were added, the fluidity of mortar relative to A1 decreased by 4.4% and 8.2%, and the consistency also has a corresponding trend of change. Therefore, the fluidity of mortar decreases with the amount of palygorskite substitution, the more palygorskite substitution, the worse the mortar fluidity [
28,
29].
With 50% and 60% of smelting slag added, the fluidity and consistency of mortar were improved compared with A1. With the addition of 1% and 2% palygorskite in B1, the fluidity of the mortar was slightly reduced by 3.7% and 5.8%. The consistency of C2 and C3 showed a reduction of 10.3% and 11.9% compared with C1. The data show that after replacing part of the cement with CNSS and adding palygorskite, the fluidity of the mortar decreases with the increasing amount of palygorskite replacement. A small amount of palygorskite can be added to meet the needs of improving the fluidity of mortar. After adding an amount of palygorskite, the copper and nickel smelting slag-based cementitious materials improved the flowability and pumping capacity of cement mortar and satisfied the needs of mine-filling pipeline transportation.
3.2. Mechanical Property Analysis
The test blocks were prepared according to the test ratios, and the compressive strength tests were carried out at 3d, 7d, 14d, and 28d under standard curing conditions.
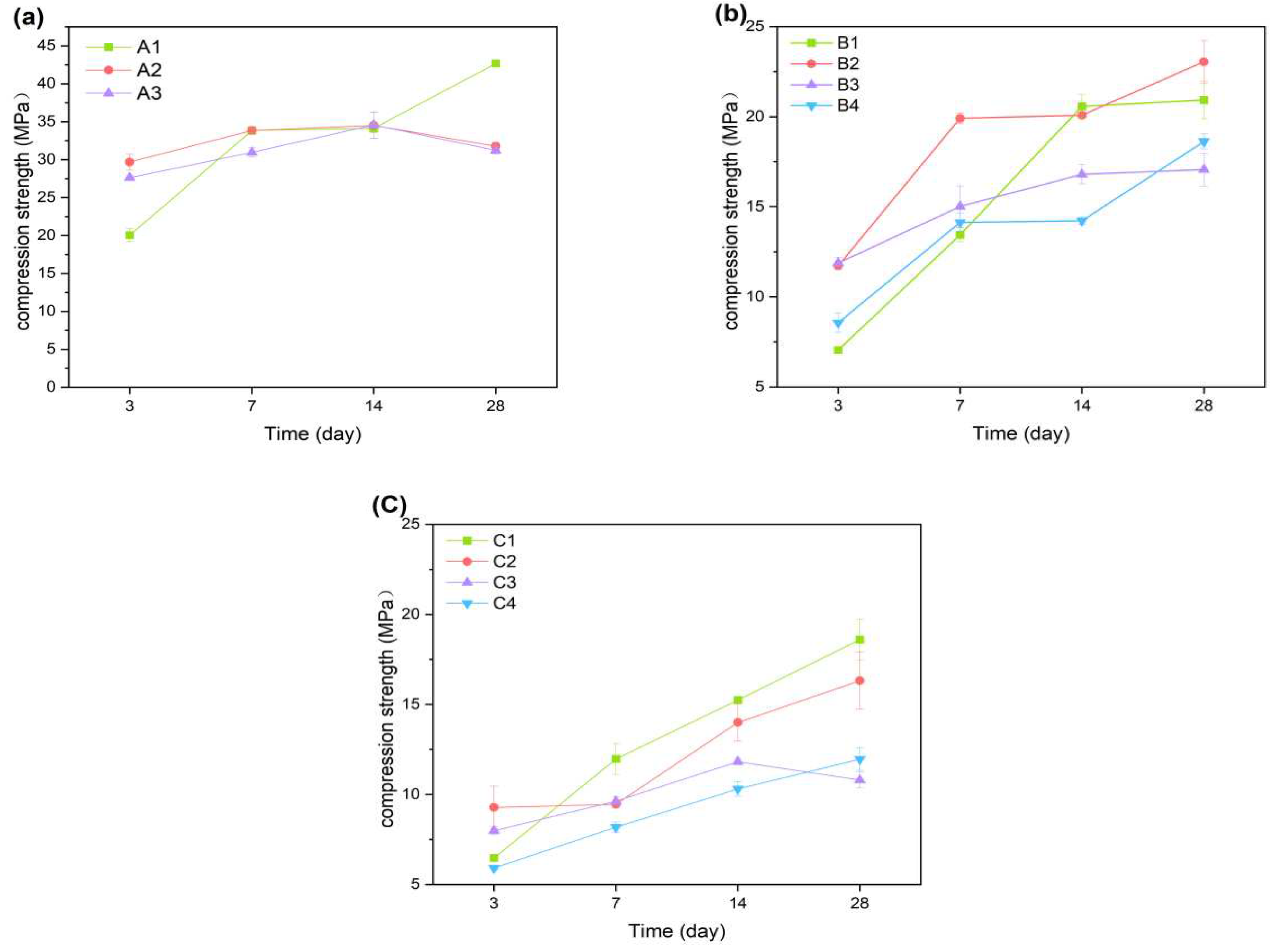
Figure 4a reveals the reinforcing effect of A1 on the cement compressive strength at both 7 and 28 days. As shown by the results, A1 shows a slight increase in compressive strength by 3.2%.With the curing time, the compressive strength increased more rapidly from 3d to 7d and 14d to 28d. The early hydration reaction on cement strength caused an increase of 13.78 Mpa for A1 in 3d to 7d. It can be concluded that the early hydration reaction plays an important role in influencing the compressive strength of the cementitious material. It can also promote the increase of compressive strength of the cementitious material in later standard curing stage. The compressive strength of A1 increased from 8.13Mpa from 14d to 28d. In 3d, the highest compressive strength of A3 was compared to A1 and A2 in 3d, while the strength of cement with palygorskite decreased after 28d.
As shown in
Figure 4b, with the incorporation of CNSS, the overall compressive strength of B1 decreased compared with A1. It can be seen from
Table 1 and
Figure 4 that the main phase in CNSS is fayalite with low pozzolanic activity. The early hydration reaction is slowed down [
30,
31]. The upward trend of compressive strength of C1 from 3d to 28d in
Figure 4c supports this view. With the further increase of CNSS content, the early hydration of cementitious materials was delayed.
The addition of palygorskite can significantly improve the early strength of cementitious materials. Compared with A1, the compressive strength of A2 increased by 9.64 Mpa at 3d. However, with the increase of palygorskite content, the 3d compressive strength of A3 was lower than that of A2. In
Figure 4b, the compressive strength of B1 decreases first and then increasescompared with B3, andwas still lower than B2. The compressive strength of C1 at 28d is higher than that of C2 and C3 added with palygorskite, showing a similar law.
During early hydration, the addition of palygorskite can promote the hydration of the cementitious materials. The compressive strength of gellable composite materials was increased by adding 1% palygorskite. There was a decrease in the compressive strength of cementitious materials with the addition of CNSS. Adding any material with volcanic ash activity affects the early hydration process. The replacement of cement by low-calcium volcanic ash materials, such as fly ash, reduces the early compressive strength of the cementitious material (typically up to 28 days). The addition of excessive palygorskite is not conducive to the development of the reticular structure of the hydration products of cement. It leads to difficulties in increasing the compressive strength in the late stages of hydration [
29].
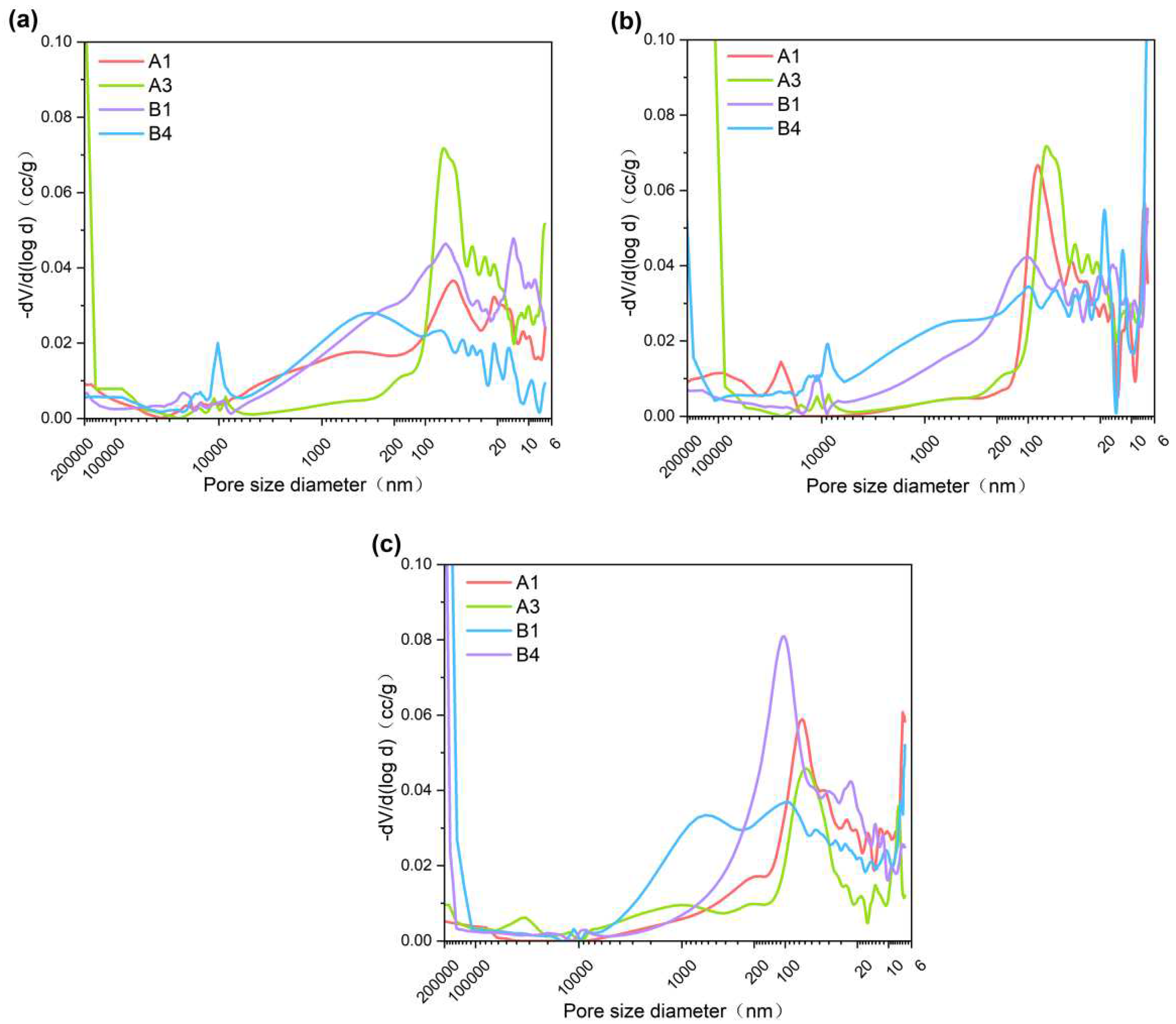
3.3. Porosity
The influence of palygorskite and CNSS on the pore size distribution of cement can be qualitatively observed in
Figure 5. As the palygorskite content and curing age increased, the peak value of the pore size distribution curve of A1 and B1 shifted towards smaller pore sizes. These results indicate that palygorskite content and curing age significantly impact the internal pore structure and promote the early reaction of hydration. Palygorskite can adsorb a large amount of water, fill the pores of the cementitious material, and solidify heavy metal ions mainly in the form of adsorption [
32,
33]. The generation of hydration products reduces the pores in the cementitious material, but the previously filled pores are subject to more harmful pores (> 200 nm) due to water loss. In
Figure 5b, B4 appeared the pores larger than 200,000nm. The same phenomenon was observed in
Figure 5c. It is speculated that the addition of palygorskite in B4 causes the early hydration of cementitious materials at 7d. However, macropores higher than 200,000 nm were also produced, which was caused by both CNSS reaction and water loss of palygorskite.
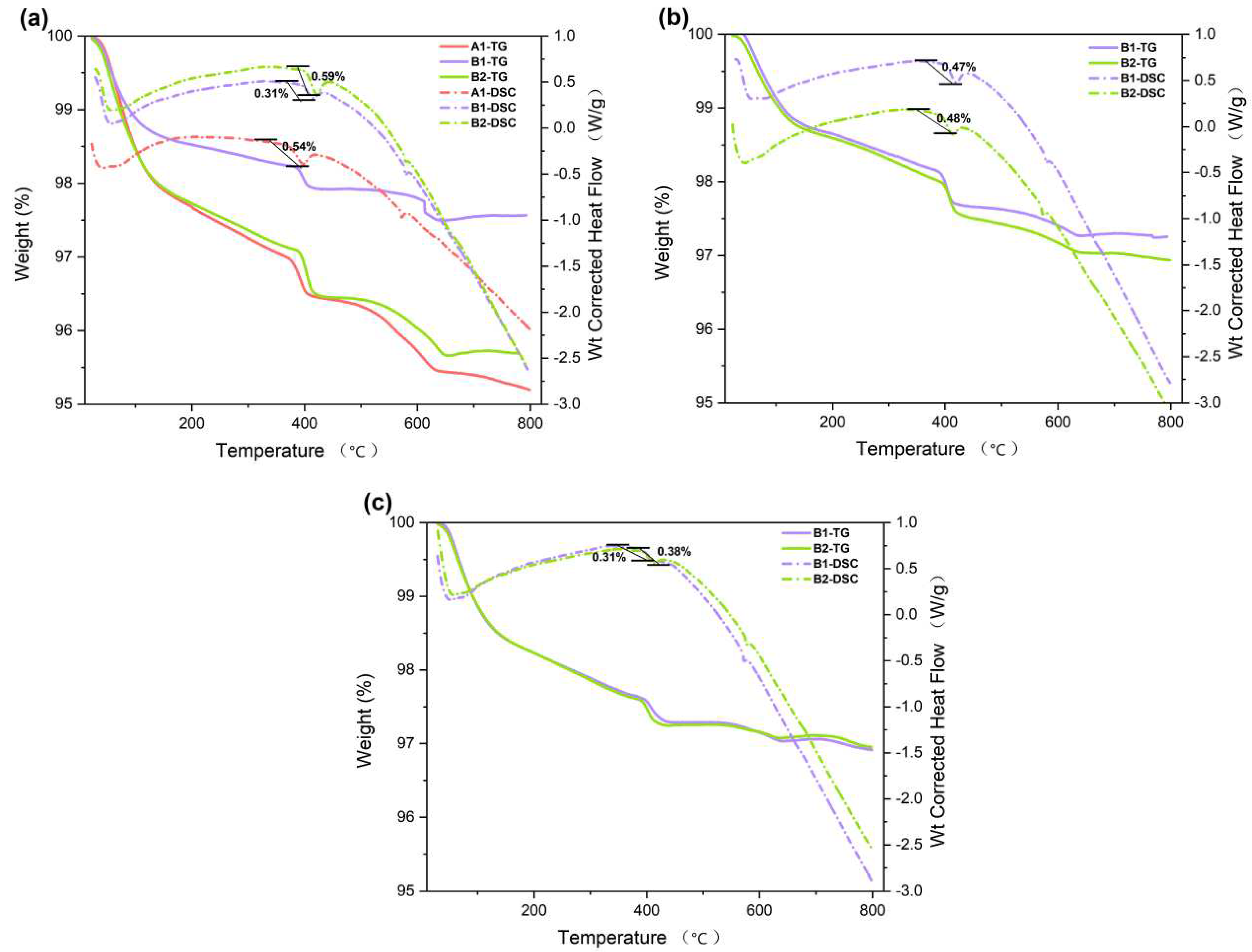
3.4. TGA-DSC
Through the TGA-DSC test of the cementitious material, the composition of the cementitious material can be analyzed [
34,
35,
36]. The effect of TGA-DSC is illustrated in Figure A, where 3 notable changes in the mass can be seen. Particularly in 80-150℃, 400-450℃, and 600℃. The mass loss obtained by TGA between 80℃ and 150℃ is mainly caused by the water loss of ettringite and C-S-H gel. Above approximately 400℃, a mass decrease is observed as Ca (OH)
2 is oxidized. As shown by the results, the addition of palygorskite promotes the early hydration of cementitious materials and creates more hydration products.
As shown in
Figure 3b, the mass loss of A1 in 400℃-450℃ is 3.2%, whereas the results for B and C decreased by 0.47% and 0.49%. A1 produced more hydration product Ca (OH)
2. The same phenomenon of increased hydration product was observed in the DSC curves of both 7d and 28d. In
Figure 6b, the weight loss of B1 was 0.47%, while B2 had a weight loss of 0.49%, indicating a slight difference. In
Figure 6c, the weight loss of B1 and B2 was 0.31% and 0.38% respectively. The addition of palygorskite can enhance the hydration of cementitious materials with CNSS, especially during the early stage of hydration. The peak of 600℃-700℃ is attributed to the decomposition of CaCO
3.
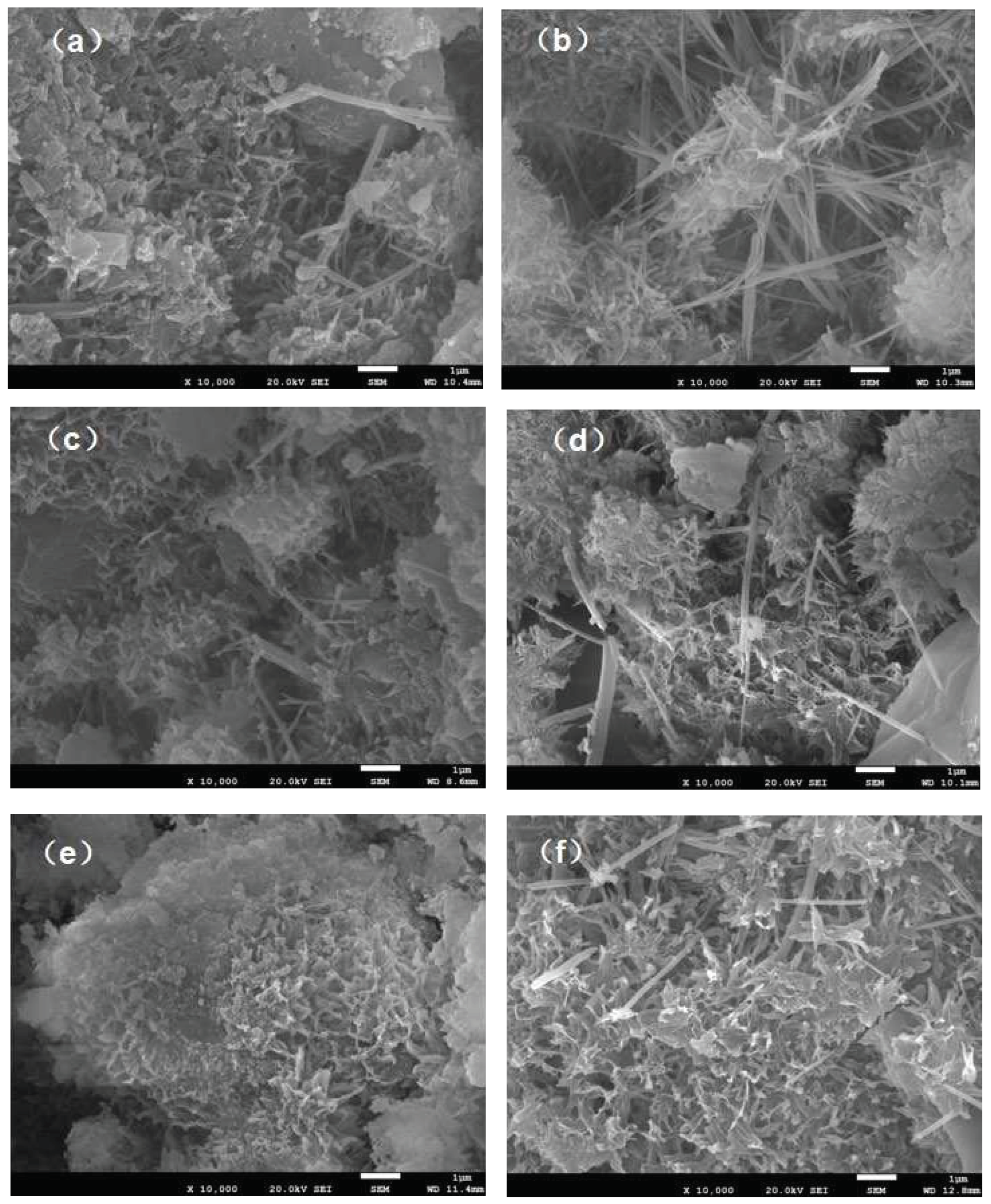
3.5. Surface Morphology Analysis
To investigate the strength change rule of cementitious materials, and understand the microscopic growth condition inside the cementitious materials, we analyzed the samples by SEM. As shown in
Figure 7, B1 and B2 are visible at 3d, 7d, and 28d with microforms of calcium silicate hydrate (C-S-H) composed of fine grains, and calcite alumina (AFt) with microforms of needles. In addition, large block substances can be observed, suggesting that they may be portlandite (CH) crystals [
37,
38,
39]. As can be seen from
Figure 6a,b, compared to B1, B2 with the addition of palygorskite has a higher degree of early hydration, and more hydration products can be found. The CSH gel forms a spatial mesh structure, which fills the pores of the material.It can be clearly observed from
Figure 7b–d that the process of pore reduction is due to the cementation of hydration products. It is speculated that the hydration products of copper-nickel smelting slag-cement cementitious materials are basically similar to cement cementitious materials. The needle-like AFt crystal and the flocculent C-S-H gel are cross-linked to form a spatial network structure, and the bulk CH crystal is filled to form a whole, resulting in strength. This may be one of the reasons why the early compressive strength of B2 is higher than that of B1. Appropriate amount of palygorskite added can promote the early hydration of cement. The microscopic hydration products in the overall image of B2 are higher than those of B1. It can be conclude that the promotion effect of the appropriate amount of palygorskite on the hydration of cementitious materials may not only be early, but also have a beneficial effect on the hydration reaction as a whole.
3.7. Analysis of Heavy Metal Curing Efficiency
Heavy metal leaching tests were first carried out on CNSS to analyze the efficiency of cement blended with copper-nickel smelting slag in curing heavy metals. It can be seen from
Table 1 that CNSS contains more heavy metal ions, Cu and Ni. Through the leaching test for CNSS, the concentration of Cu
2+ in the leach solution was 6.05 mg/L, and Ni
2+ was 3.98 mg/L. Subsequently, the solidification effect of 28d cementitious material on heavy metals was investigated. It can be seen from
Figure 8 that the curing rate of Ni
2+ is higher when palygorskite is not added. With the increase in the amount of palygorskite, the curing rate of B1-B4 increased first and then decreased. C1-C4 also has the same trend, and the curing rate after adding palygorskite is greater than that of B1.This is due to the fact that a moderate amount of palygorskite promotes early hydration of the cementitious material, generating more hydration products while reducing the pore size.
In summary, cementitious materials can remarkably cure heavy metal Ni2+. The ability of the cementitious material to cure the heavy metal Ni2+ increased in the early stage. It was similar in the later stage after the addition of an appropriate amount of palygorskite. This is because palygorskite promotes the hydration of the cementitious material. The ability of cementitious materials to solidify heavy metal Cu2+ is weak. After palygorskite was added, the ability of cementing material to solidify heavy metal Cu2+ was also improved. In general, B2 has the most vital ability to immobilize heavy metals.
4. Conclusions
In this paper, a type of mineral-based composite cementitious material was prepared by adding a certain amount of palygorskite. The effects of macroscopic properties such as fluidity and compressive strength were analyzed and tested, and its ability to solidify heavy metals was explored. The conclusions are reached through investigating the influence of CNSS and palygorskite on the hydration process of cementitious materials.
- (1)
The addition of CNSS to OPC causes an increase in the fluidity of the mortar, while the addition of palygorskite reduces the fluidity or even causes it to stop. The fluidity of mortar can be changed by adding a small amount of palygorskite.
- (2)
CNSS will affect the early hydration of cementitious materials and reduce the compressive strength. palygorskite can strengthen the early compressive strength of cementitious materials, but too much palygorskite will affect the late hydration of cementitious materials. The strength of the cementitious material mixed with 50 CNSS with 1% palygorskite was tested to be the best.
- (3)
The porosity and thermogravimetric of cementitious materials were analyzed. An appropriate amount of palygorskite promotes the hydration process of the cementitious materials to which CNSS has been added. The surface morphology of the SEM also proves it.
- (4)
The ability of cementing material to solidify heavy metal Cu2+ is poor, and the ability to solidify Ni2+ is better. Adding 1% palygorskite can improve the ability of cementitious materials to solidify heavy metals.
Acknowledgments
The authors would like to acknowledge the financial support from the project of the National Key Research and Development Program of China (Grant No. 2022YFE0129200).
References
- Iluţiu-Varvara, D.-A.; Aciu, C. Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability 2022, 14, 5488. [Google Scholar] [CrossRef]
- Kanneboina, Y.Y.; T., J.S.; Kabeer, K.I.S.A.; Bisht, K. Valorization of Lead and Zinc Slags for the Production of Construction Materials - A Review for Future Research Direction. Constr. Build. Mater. 2023, 367, 130314. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Liu, K.; Mei, H.; Xia, Y.; Dong, Y. An Efficient Approach to Utilize Copper Smelting Slag: Separating Nonferrous Metals and Reducing Iron Oxide at High Temperature. Waste Manag. 2023, 172, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Z.; Zhang, F.; Gao, Y.; Wu, Q. Chloride and Heavy Metal Binding Capacities of Hydrotalcite-like Phases Formed in Greener One-Part Sodium Carbonate-Activated Slag Cements. J. Clean. Prod. 2020, 253, 120047. [Google Scholar] [CrossRef]
- Kurniati, E.O.; Pederson, F.; Kim, H.-J. Application of Steel Slags, Ferronickel Slags, and Copper Mining Waste as Construction Materials: A Review. Resour. Conserv. Recycl. 2023, 198, 107175. [Google Scholar] [CrossRef]
- Lu, K.; Sun, W.; Gao, T.; Li, Z.; Zhao, J.; Cheng, H. Preparation of New Copper Smelting Slag-Based Mine Backfill Material and Investigation of Its Mechanical Properties. Constr. Build. Mater. 2023, 382, 131228. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Lv, G.; Liao, L.; Zhang, D. Experimental Studies on Chemical Activation of Cementitious Materials from Smelting Slag of Copper and Nickel Mine. Materials 2019, 12, 303. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of Microwave-Activated Coal Gangue Powder as Auxiliary Cementitious Material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Ben Haha, M.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Portland Metakaolin Cement Containing Dolomite or Limestone – Similarities and Differences in Phase Assemblage and Compressive Strength. Constr. Build. Mater. 2017, 157, 214–225. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, L.; Guo, Z.; Hou, C.; Yao, S.; Zhang, F.; Fu, C.; Zhang, H. Mechanistic Study of Fly Ash Activity Enhanced by High Temperature to Strengthen Cementitious Materials. Constr. Build. Mater. 2024, 416, 135026. [Google Scholar] [CrossRef]
- Adesanya, E.; Sreenivasan, H.; Kantola, A.M.; Telkki, V.-V.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Ladle Slag Cement – Characterization of Hydration and Conversion. Constr. Build. Mater. 2018, 193, 128–134. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, N.; Shen, W.; Wei, X.; Li, F.; Ma, W.; Mao, F.; Wu, P. Relationship between Mineralogical Phase and Bound Heavy Metals in Copper Smelting Slags. Resour. Conserv. Recycl. 2022, 178, 106098. [Google Scholar] [CrossRef]
- Alp, İ.; Deveci, H.; Süngün, H. Utilization of Flotation Wastes of Copper Slag as Raw Material in Cement Production. J. Hazard. Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Huang, Z. Value-Added Utilization of Copper Slag to Enhance the Performance of Magnesium Potassium Phosphate Cement. Resour. Conserv. Recycl. 2022, 180, 106212. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, Y.; Feng, Y.; Zhang, Q.; Liu, Y. Utilization of Modified Copper Slag Activated by Na2SO4 and CaO for Unclassified Lead/Zinc Mine Tailings Based Cemented Paste Backfill. J. Environ. Manage. 2021, 290, 112608. [Google Scholar] [CrossRef] [PubMed]
- Edwin, R.S.; Gruyaert, E.; De Belie, N. Valorization of Secondary Copper Slag as Aggregate and Cement Replacement in Ultra-High Performance Concrete. J. Build. Eng. 2022, 54, 104567. [Google Scholar] [CrossRef]
- Zhao, Q.; Pang, L.; Wang, D. Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review. Materials 2022, 15, 3803. [Google Scholar] [CrossRef]
- He, R.; Zhang, S.; Zhang, X.; Zhang, Z.; Zhao, Y.; Ding, H. Copper Slag: The Leaching Behavior of Heavy Metals and Its Applicability as a Supplementary Cementitious Material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Ren, J.; Dai, L.; Tao, L. Stabilization of Heavy Metals in Sewage Sludge by Attapulgite. J. Air Waste Manag. Assoc. 2021, 71, 392–399. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, L.; Zhang, P.; Jiao, B.; Shiau, Y.; Li, D. Solidification of Chromium-Containing Sludge with Attapulgite Combined Alkali Slag. Environ. Sci. Pollut. Res. 2022, 29, 13580–13591. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Z.; Ma, Y.; Xiang, J.; Sun, X.; Li, J.; Zuo, X. Mechanism of Attapulgite Processed by Calcination and Grinding on Hydration Process and Mechanical Properties of Cementitious Materials. Case Stud. Constr. Mater. 2023, 18, e02091. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, M.; Fan, J.; Duan, P.; Zhang, Z. Exploration of the Compressive Strength and Microscopic Properties of Portland Cement Taking Attapulgite and Montmorillonite Clay as an Additive. Materials 2023, 16, 1794. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q. Effect of Mineral Admixtures on the Structural Build-up of Cement Paste. Constr. Build. Mater. 2018. [Google Scholar] [CrossRef]
- Mirza, Z.A.; Khalid, N.N. Flexural Performance of Reinforced Concrete Beams Containing Treated Attapulgite as Lightweight Aggregate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 988, 012049. [Google Scholar] [CrossRef]
- Abdulrasool, A.T.; Mohammed, S.S.; Kadhim, N.R.; Kadhim, Y.N. Effect Of Attapulgite as Internal Curing in High-Performance Concrete with Variable Temperature Curing to Enhance Mechanical Properties. IOP Conf. Ser. Earth Environ. Sci. 2022, 961, 012054. [Google Scholar] [CrossRef]
- Abbas, M.L.; Abbas, W.A.; Güneyisi, E. Shrinkage and Thermo-Mechanical Properties of Concretes Incorporated with Different Substitutions of Natural Aggregates by Cold Bonded Calcined Attapulgite Lightweight Aggregates. J. Build. Eng. 2023, 79, 107921. [Google Scholar] [CrossRef]
- Na, H.; Lv, G.; Wang, L.; Liao, L.; Zhang, D.; Guo, L.; Li, W. A New Expansion Material Used for Roof-Contacted Filling Based on Smelting Slag. Sci. Rep. 2021, 11, 2607. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Tian, Z.; Bu, J.; Zuo, X.; Li, J.; Xiang, J.; Fan, H. Characterization of the Structural Build-up of Cementitious Suspensions Containing Attapulgite from the Perspective of Flocculent Structure. Constr. Build. Mater. 2023, 373, 130867. [Google Scholar] [CrossRef]
- Luan, X.; Li, J.; Yang, Z. Effects of Attapulgite Addition on the Mechanical Behavior and Porosity of Cement-Based Porous Materials and Its Adsorption Capacity. Mater. Chem. Phys. 2020, 239, 121962. [Google Scholar] [CrossRef]
- Li, Z.; Gao, X.; Lu, D.; Dong, J. Early Hydration Properties and Reaction Kinetics of Multi-Composite Cement Pastes with Supplementary Cementitious Materials (SCMs). Thermochim. Acta 2022, 709, 179157. [Google Scholar] [CrossRef]
- Yun-hong, C.; Si-hui, Y.; Jing-yu, Z.; Xiao-hui, S. Test Research on Hydration Process of Cement-Iron Tailings Powder Composite Cementitious Materials. Powder Technol. 2022, 399, 117215. [Google Scholar] [CrossRef]
- Du, Y.; Du, Y.; Ma, W.; Zhao, X.; Ma, M.; Cao, L.; Du, D. Application of Dirty-Acid Wastewater Treatment Technology in Non-Ferrous Metal Smelting Industry: Retrospect and Prospect. J. Environ. Manage. 2024, 352, 120050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, M.; Yan, J.; Zhou, M.; Duan, P.; Zhang, Z.; Liu, J. Assessment of Early Hydration and Microstructures of Portland Cement Incorporating Calcined Attapulgite. Arab. J. Sci. Eng. 2023, 48, 12891–12902. [Google Scholar] [CrossRef]
- Qiu, J.; Luan, X.; Cheng, K.; Guan, X.; Yang, M.; Xiao, Z. Study on the Modification Effect and Mechanism of Tailings Powder on Coal Gangue-Based Mining Cementitious Filling Material. Environ. Sci. Pollut. Res. 2023, 30, 46038–46057. [Google Scholar] [CrossRef] [PubMed]
- Bernal, S.A.; Juenger, M.C.G.; Ke, X.; Matthes, W.; Lothenbach, B.; De Belie, N.; Provis, J.L. Characterization of Supplementary Cementitious Materials by Thermal Analysis. Mater. Struct. 2017, 50, 26. [Google Scholar] [CrossRef]
- Maier, M.; Beuntner, N.; Thienel, K.-C. Mineralogical Characterization and Reactivity Test of Common Clays Suitable as Supplementary Cementitious Material. Appl. Clay Sci. 2021, 202, 105990. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, C. Experimental Study of Electric Furnace Ferronickel Slag as a Supplementary Cementitious Material in Massive High-Strength Concrete. J. Therm. Anal. Calorim. 2022, 147, 4983–4993. [Google Scholar] [CrossRef]
- Basquiroto De Souza, F.; Sagoe-Crentsil, K.; Duan, W. A Century of Research on Calcium Silicate Hydrate (C–S–H): Leaping from Structural Characterization to Nanoengineering. J. Am. Ceram. Soc. 2022, 105, 3081–3099. [Google Scholar] [CrossRef]
- Kumar, A.; Walder, B.J.; Kunhi Mohamed, A.; Hofstetter, A.; Srinivasan, B.; Rossini, A.J.; Scrivener, K.; Emsley, L.; Bowen, P. The Atomic-Level Structure of Cementitious Calcium Silicate Hydrate. J. Phys. Chem. C 2017, 121, 17188–17196. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).