Submitted:
14 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
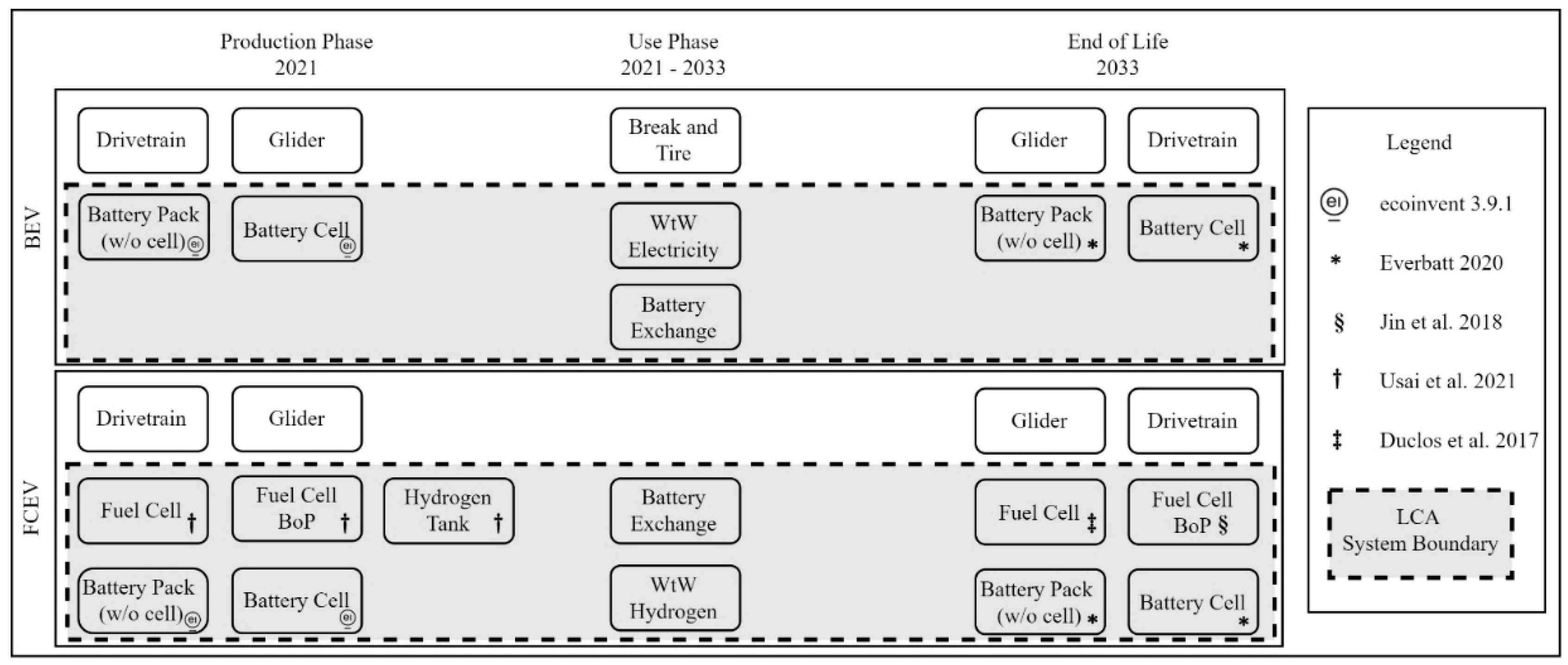
2.1. Inventory Analysis of Batteries, Fuel Cell and Hydrogen Tank
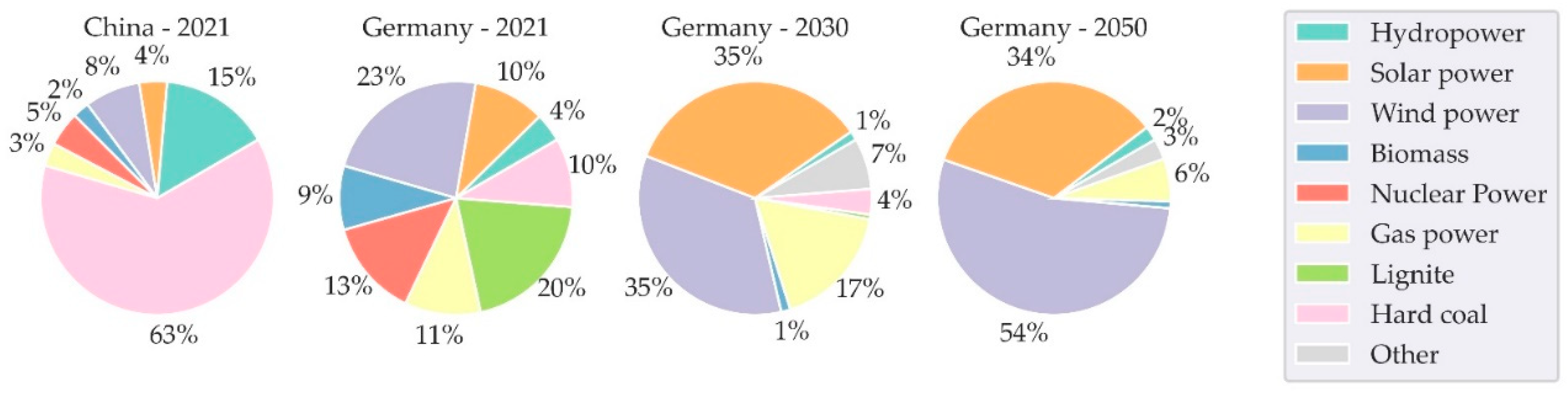
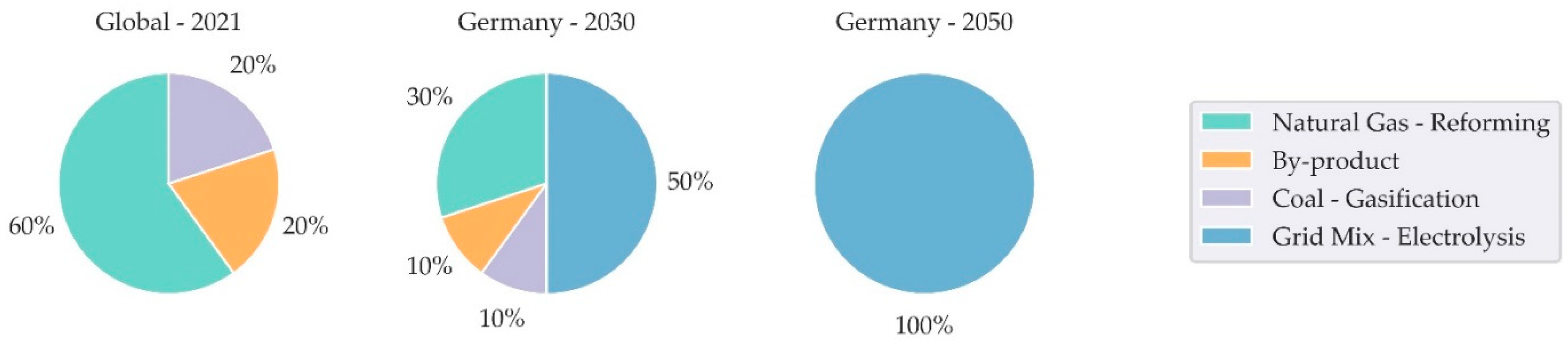
2.2. Inventory Analysis Grid Mix and Hydrogen
2.3. Selection of Typical Vehicles
2.3.1. Passenger Cars
2.3.2. Heavy-Duty Trucks
2.3.3. City Buses
3. Results
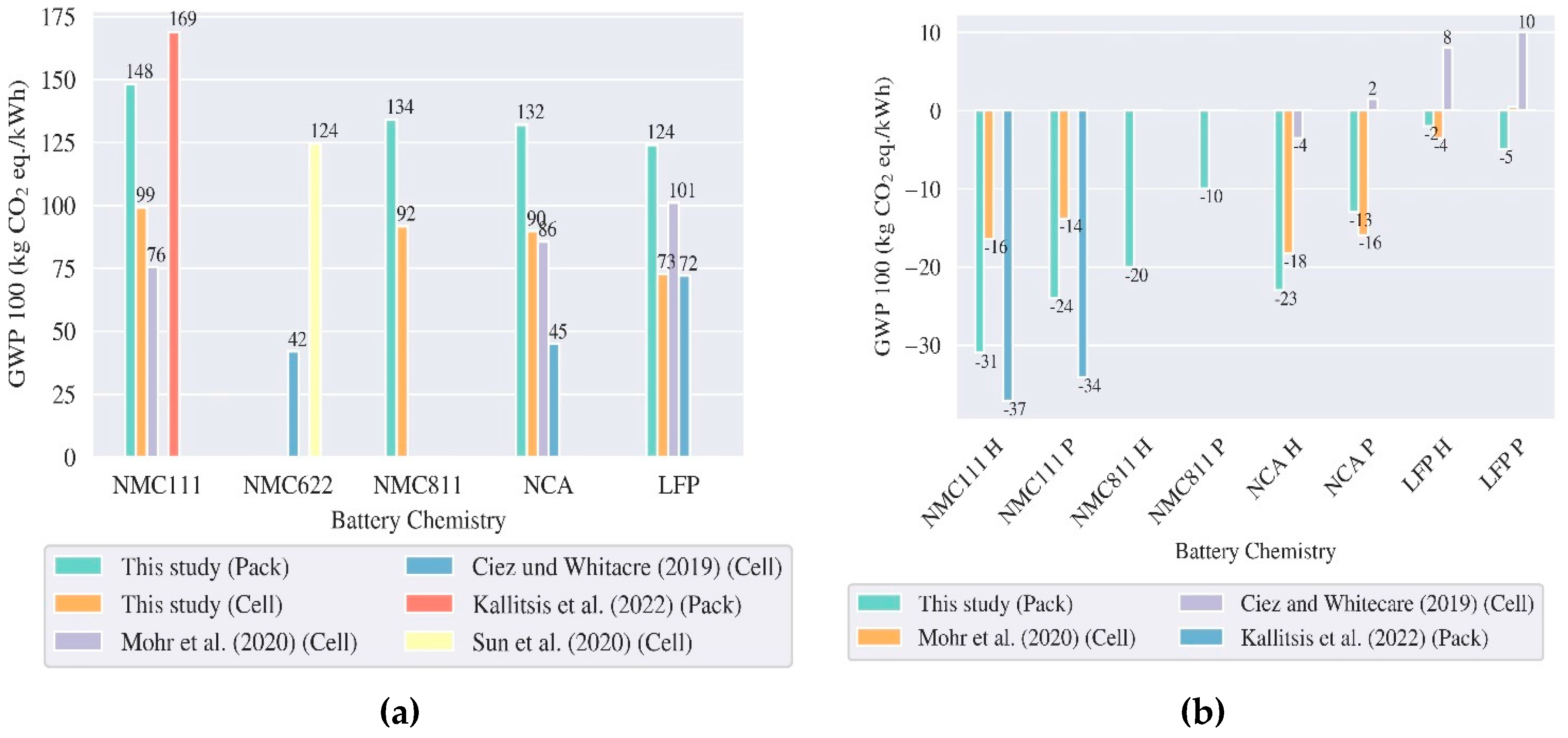
3.1. LCA Batteries – Production and End of Life
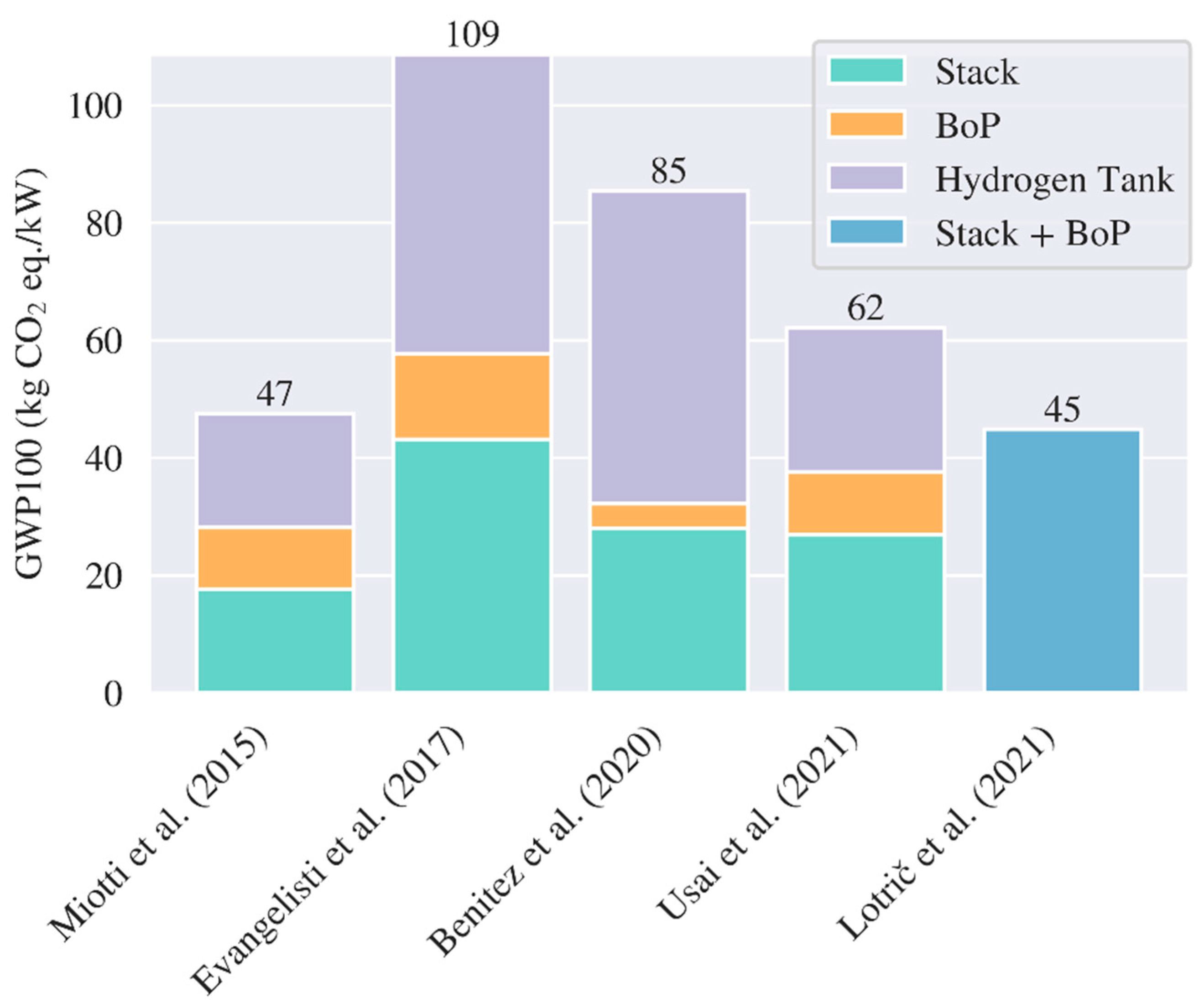
3.2. LCA Fuel Cell and Hydrogen Tank – Production and End of Life
3.3. LCA Vehicles (w/o Glider, Motor, Brake and Tire)
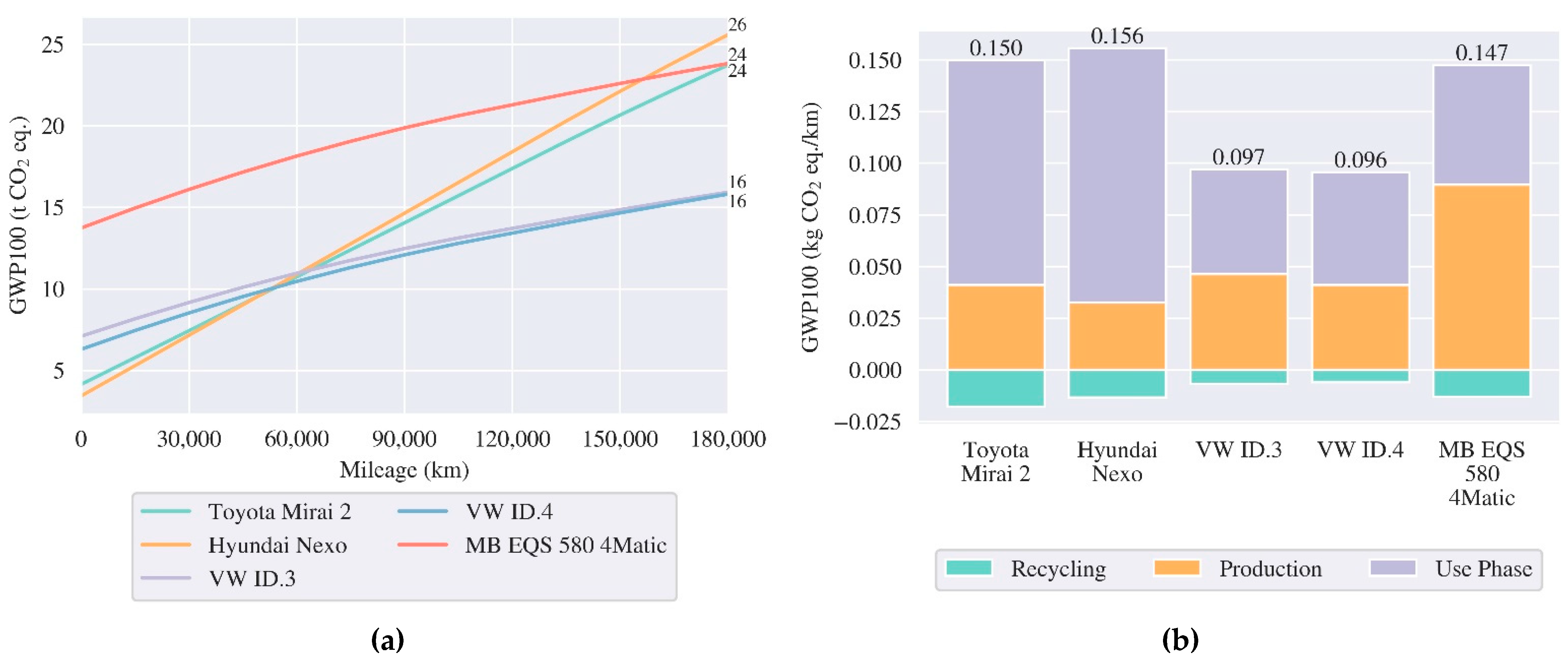
3.3.1. Passenger Cars
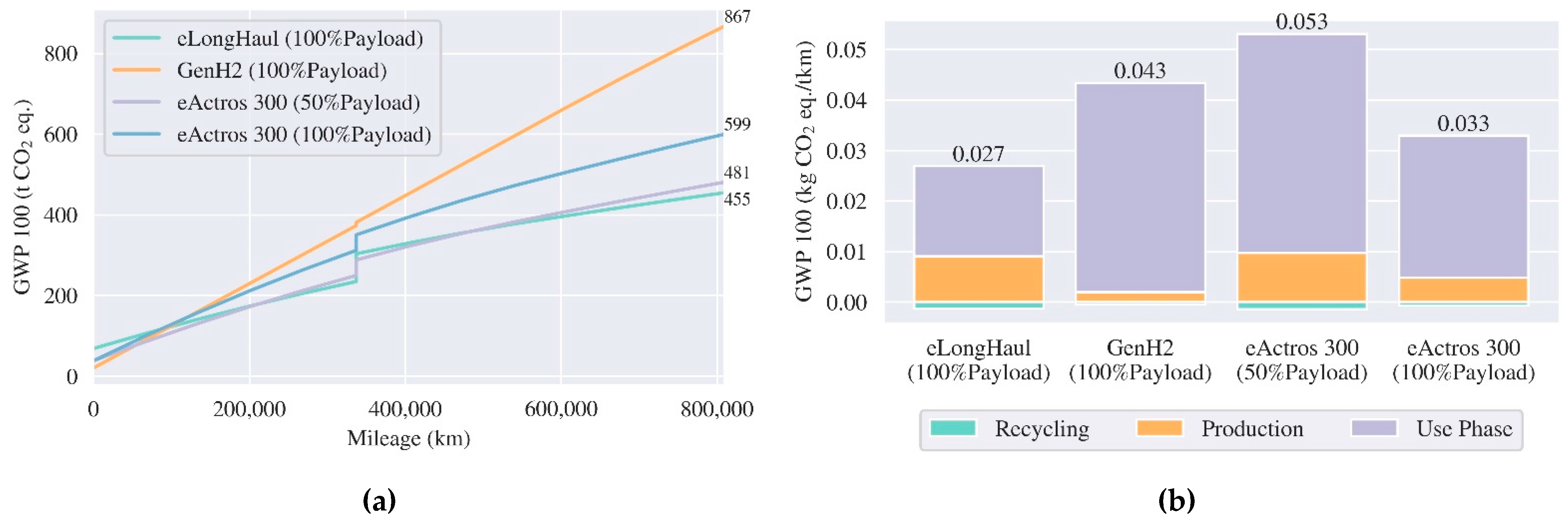
3.3.2. Heavy-Duty Trucks
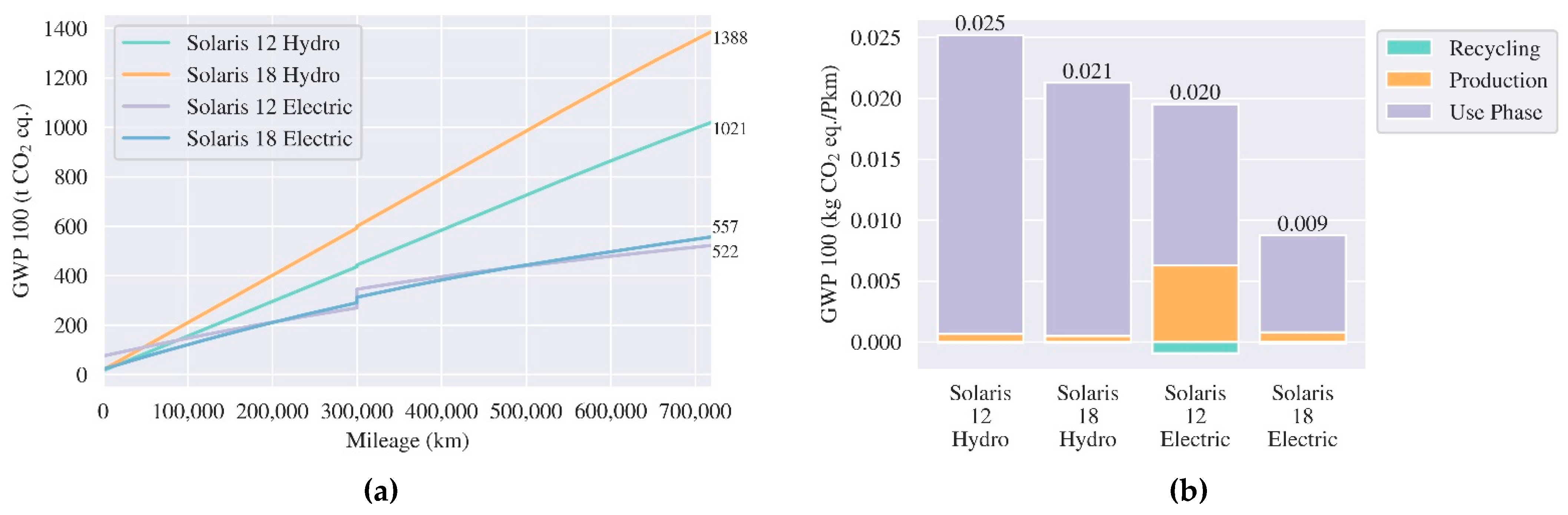
3.3.3. City Buses
5. Discussion
5.1. Comparison with other Studies
5.2. Comparative Life Cycle Assessment
5.3. Limitations
6. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Global Hydrogen Review 2021. Available online: https://iea.blob.core.windows.net/assets/5bd46d7b-906a-4429-abda-e9c507a62341/GlobalHydrogenReview2021.pdf (accessed on 6 February 2024).
- Statista. Mobility Market Insights - Electric Vehicles Worldwide. Available online: https://www.statista.com/outlook/mmo/electric-vehicles/worldwide (accessed on 6 February 2024).
- Statista. Projected fuel cell electric vehicle deployment worldwide between 2020 and 2030. Available online: https://www.statista.com/statistics/1272505/fuel-cell-electric-vehicle-global-deployment/ (accessed on 6 February 2024).
- Gregor Fröhlich. Well-to-Wheel – Verbrenner versus E-Mobil. Available online: https://energiewende.eu/well-to-wheel/#Wasserstoff_Brennstoffzellenfahrzeug (accessed on 6 February 2024).
- Mori, M.; Stropnik, R.; Sekavčnik, M.; Lotrič, A. Criticality and Life-Cycle Assessment of Materials Used in Fuel-Cell and Hydrogen Technologies. Sustainability 2021, 13, 3565. [Google Scholar] [CrossRef]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Raugei, M.; Steubing, B.; Hartwell, A.; Anderson, P.A.; Heidrich, O. Life cycle assessment of lithium-ion battery recycling using pyrometallurgical technologies. Journal of Industrial Ecology 2021, 25, 1560–1571. [Google Scholar] [CrossRef]
- Kallitsis, E.; Korre, A.; Kelsall, G.H. Life cycle assessment of recycling options for automotive Li-ion battery packs. Journal of Cleaner Production 2022, 371, 133636. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhang, Z.; Meng, F.; Yang, J. Life cycle assessment of lithium nickel cobalt manganese oxide (NCM) batteries for electric passenger vehicles. Journal of Cleaner Production 2020, 273, 123006. [Google Scholar] [CrossRef]
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. Journal of Industrial Ecology 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat Sustain 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Tao, Y.; Rahn, C.D.; Archer, L.A.; You, F. Second life and recycling: Energy and environmental sustainability perspectives for high-performance lithium-ion batteries. Sci. Adv. 2021, 7, eabi7633. [Google Scholar] [CrossRef]
- Dai, Q.; Spangenberger, J.; Ahmed, S.; Gaines, L.; Kelly, J.C.; Wang, M. EverBatt: A Closed-loop Battery Recycling Cost and Environmental Impacts Model. Available online: https://publications.anl.gov/anlpubs/2019/07/153050.pdf (accessed on 6 February 2024).
- ecoinvent. Home - ecoinvent. Available online: https://ecoinvent.org/ (accessed on 6 February 2024).
- GREET | Argonne National Laboratory. Available online: https://www.anl.gov/topic/greet (accessed on 6 February 2024).
- Miotti, M.; Hofer, J.; Bauer, C. Integrated environmental and economic assessment of current and future fuel cell vehicles. Int J Life Cycle Assess 2015, 22, 94–110. [Google Scholar] [CrossRef]
- Usai, L.; Hung, C.R.; Vásquez, F.; Windsheimer, M.; Burheim, O.S.; Strømman, A.H. Life cycle assessment of fuel cell systems for light duty vehicles, current state-of-the-art and future impacts. Journal of Cleaner Production 2021, 280, 125086. [Google Scholar] [CrossRef]
- Evangelisti, S.; Tagliaferri, C.; Brett, D.J.; Lettieri, P. Life cycle assessment of a polymer electrolyte membrane fuel cell system for passenger vehicles. Journal of Cleaner Production 2017, 142, 4339–4355. [Google Scholar] [CrossRef]
- Benitez, A.; Wulf, C.; Palmenaer, A. de; Lengersdorf, M.; Röding, T.; Grube, T.; Robinius, M.; Stolten, D.; Kuckshinrichs, W. Ecological assessment of fuel cell electric vehicles with special focus on type IV carbon fiber hydrogen tank. Journal of Cleaner Production 2020, 278, 123277. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Kuštrin, I.; Mori, M. Life-cycle assessment of hydrogen technologies with the focus on EU critical raw materials and end-of-life strategies. International Journal of Hydrogen Energy 2021, 46, 10143–10160. [Google Scholar] [CrossRef]
- Duclos, L.; Chattot, R.; Dubau, L.; Thivel, P.-X.; Mandil, G.; Laforest, V.; Bolloli, M.; Vincent, R.; Svecova, L. Closing the loop: life cycle assessment and optimization of a PEMFC platinum-based catalyst recycling process. Green Chem. 2020, 22, 1919–1933. [Google Scholar] [CrossRef]
- Duclos, L.; Lupsea, M.; Mandil, G.; Svecova, L.; Thivel, P.-X.; Laforest, V. Environmental assessment of proton exchange membrane fuel cell platinum catalyst recycling. Journal of Cleaner Production 2017, 142, 2618–2628. [Google Scholar] [CrossRef]
- Stropnik, R.; Lotrič, A.; Bernad Montenegro, A.; Sekavčnik, M.; Mori, M. Critical materials in PEMFC systems and a LCA analysis for the potential reduction of environmental impacts with EoL strategies. Energy Science & Engineering 2019, 7, 2519–2539. [Google Scholar]
- Lombardi, L.; Tribioli, L.; Cozzolino, R.; Bella, G. Comparative environmental assessment of conventional, electric, hybrid, and fuel cell powertrains based on LCA. Int J Life Cycle Assess 2017, 22, 1989–2006. [Google Scholar] [CrossRef]
- Idris. M; Koestoer R. H. Environmental life cycle assessment of conventional and electric vehicles: lessons learned from selected countries. JIMESE 2023, 1. [Google Scholar]
- Joshi, A.; Sharma, R.; Baral, B. Comparative life cycle assessment of conventional combustion engine vehicle, battery electric vehicle and fuel cell electric vehicle in Nepal. Journal of Cleaner Production 2022, 379, 134407. [Google Scholar] [CrossRef]
- Rüdisüli, M.; Bach, C.; Bauer, C.; Beloin-Saint-Pierre, D.; Elber, U.; Georges, G.; Limpach, R.; Pareschi, G.; Kannan, R.; Teske, S.L. Prospective life-cycle assessment of greenhouse gas emissions of electricity-based mobility options. Applied Energy 2022, 306, 118065. [Google Scholar] [CrossRef]
- Sacchi, R.; Bauer, C.; Cox, B.L. Does Size Matter? The Influence of Size, Load Factor, Range Autonomy, and Application Type on the Life Cycle Assessment of Current and Future Medium- and Heavy-Duty Vehicles. Environ. Sci. Technol. 2021, 55, 5224–5235. [Google Scholar] [CrossRef]
- Booto, G.K.; Aamodt Espegren, K.; Hancke, R. Comparative life cycle assessment of heavy-duty drivetrains: A Norwegian study case. Transportation Research Part D: Transport and Environment 2021, 95, 102836. [Google Scholar] [CrossRef]
- Grazieschi, G.; Zubaryeva, A.; Sparber, W. Energy and greenhouse gases life cycle assessment of electric and hydrogen buses: A real-world case study in Bolzano Italy. Energy Reports 2023, 9, 6295–6310. [Google Scholar] [CrossRef]
- Analy Castillo Munoz, M.S.; Brian Tarroja, P.; and Scott Samuelsen, P. Life Cycle Assessment of Environmental and Economic Impacts of Deploying Alternative Urban Bus Powertrain Technologies in the South Coast Air Basin, 2019.
- DIN EN ISO 14044 - Umweltmanagement – Ökobilanz – Anforderungen und Anleitungen 2021.
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: a harmonised life cycle impact assessment method at midpoint and endpoint level. Int J Life Cycle Assess 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Ekvall, T.; Björklund, A.; Sandin, G.; Jelse, K. Modeling recycling in life cycle assessment. Available online: https://www.lifecyclecenter.se/wp-content/uploads/2020_05_Modeling-recyling-in-life-cycle-assessment-1.pdf (accessed on 6 February 2024).
- Dai, Q.; Dunn, J.; : Kelly, C.; Elgowainy, A. Update of Life Cycle Analysis of Lithium-ion Batteries in the GREET 2017 Model. Available online: https://greet.es.anl.gov/files/Li_battery_update_2017 (accessed on 6 February 2024).
- NPROXX. Type 4 Pressure Vessels. Available online: https://www.nproxx.com/capabilities/type-4-pressure-vessels/ (accessed on 6 February 2024).
- Jin, H.; Afiuny, P.; Dove, S.; Furlan, G.; Zakotnik, M.; Yih, Y.; Sutherland, J.W. Life Cycle Assessment of Neodymium-Iron-Boron Magnet-to-Magnet Recycling for Electric Vehicle Motors. Environ. Sci. Technol. 2018, 52, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Strom-Report. Strommix & Stromerzeugung. Available online: https://strom-report.de/strom/ (accessed on 6 February 2024).
- IEA. China - Countries & Regions - IEA. Available online: https://www.iea.org/countries/china (accessed on 30 March 2023).
- Öko-Institut e.V. - Institut für angewandte Ökologie, Wuppertal Institut, Prognos AG. Klimaneutrales Deutschland 2045: Wie Deutschland seine Klimaziele schon vor 2050 erreichen kann. Available online: https://www.agora-verkehrswende.de/veroeffentlichungen/klimaneutrales-deutschland-2045-langfassung/ (accessed on 6 February 2024).
- Burchart, D.; Gazda-Grzywacz, M.; Grzywacz, P.; Burmistrz, P.; Zarębska, K. Life Cycle Assessment of Hydrogen Production from Coal Gasification as an Alternative Transport Fuel. Energies 2022, 16. [Google Scholar] [CrossRef]
- Di Xu; Dong, L.; Ren, J. Chapter 2 - Introduction of hydrogen routines. In HYDROGEN ECONOMY: Supply chain, life cycle analysis and energy transition; Scipioni, A., Manzardo, A., Ren, J., Eds.; ELSEVIER ACADEMIC PRESS: [S.l.], 2023; pp. 45–65. ISBN 978-0-323-99514-6. [Google Scholar]
- Bareiß, K.; La Rua, C. de; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Applied Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Göhlich, D.; Fay, T.-A.; Jefferies, D.; Lauth, E.; Kunith, A.; Zhang, X. Design of urban electric bus systems. Des. Sci. 2018, 4. [Google Scholar] [CrossRef]
- Ferrara, A.; Jakubek, S.; Hametner, C. Cost-optimal design and energy management of fuel cell electric trucks. International Journal of Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Kim, H.; Hartmann, N.; Zeller, M.; Luise, R.; Soylu, T. Comparative TCO Analysis of Battery Electric and Hydrogen Fuel Cell Buses for Public Transport System in Small to Midsize Cities. Energies 2021, 14, 4384. [Google Scholar] [CrossRef]
- Koroma, M.S.; Costa, D.; Philippot, M.; Cardellini, G.; Hosen, M.S.; Coosemans, T.; Messagie, M. Life cycle assessment of battery electric vehicles: Implications of future electricity mix and different battery end-of-life management. Sci. Total Environ. 2022, 831, 154859. [Google Scholar] [CrossRef] [PubMed]
- Menter, J.; Fay, T.-A.; Grahle, A.; Göhlich, D. Long-Distance Electric Truck Traffic: Analysis, Modeling and Designing a Demand-Oriented Charging Network for Germany. WEVJ 2023, 14, 205. [Google Scholar] [CrossRef]
- Mercedes-Benz. Der eActros und seine Services. Available online: https://www.mercedes-benz-trucks.com/de_DE/emobility/world/our-offer/eactros-and-services.html (accessed on 6 February 2024).
- Mercedes-Benz. Mercedes-Benz eActros LongHaul feiert Weltpremiere als eActros 600 im Oktober – Produktionswerke bereiten Serienfertigung vor. Available online: https://www.daimlertruck.com/newsroom/pressemitteilung/mercedes-benz-eactros-longhaul-feiert-weltpremiere-als-eactros-600-im-oktober-produktionswerke-bereiten-serienfertigung-vor-52260702 (accessed on 6 February 2024).
- Mercedes-Benz. Mercedes-Benz Trucks gibt auf der IAA Transportation 2022 in Hannover einen Ausblick auf den wasserstoffbasierten GenH2 Truck. Available online: https://www.daimlertruck.com/newsroom/pressemitteilung/mercedes-benz-trucks-gibt-auf-der-iaa-transportation-2022-in-hannover-einen-ausblick-auf-den-wasserstoffbasierten-genh2-truck-52032506 (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Solaris Urbino 12 hydrogen Technische Daten. Available online: https://www.solarisbus.com/public/assets/Biuro_prasowe/UITP_PR/Technische_Daten_Solaris_Urbino_12_hydrogen_de.pdf (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Emissionsfreie Antriebe Produktkatalog 2023. Available online: https://www.solarisbus.com/public/assets/content/pojazdy/Katalogi_2023/DE_Zeroemisyjne_1920_x_1080.pdf (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Solaris Urbino 18 hydrogen Technische Daten. Available online: https://www.busplaner.de/sites/default/files/public/data-news/02-technische-daten-urbino-18-hydrogen.pdf (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Hydrogen. Available online: https://www.solarisbus.com/de/fahrzeuge/zero-emissions/hydrogen (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Technische Daten – Solaris Urbino 18,75 electric. Available online: https://www.solarisbus.com/public/assets/Biuro_prasowe/2022_10_12_Transexpo/Technische_Daten_Urbino_1875_electric_Transexpo_DE.pdf (accessed on 6 February 2024).
- Solaris Bus & Coach sp. z o.o. Technische Daten – Solaris Urbino 12 electric. Available online: https://www.solarisbus.com/public/assets/Biuro_prasowe/2022_10_12_Transexpo/Technische_Daten_Urbino_12_electric_Transexpo_DE.pdf (accessed on 6 February 2024).
- Jefferies, D.; Göhlich, D. A Comprehensive TCO Evaluation Method for Electric Bus Systems Based on Discrete-Event Simulation Including Bus Scheduling and Charging Infrastructure Optimisation. WEVJ 2020, 11, 56. [Google Scholar] [CrossRef]
- Cigarini, F.; Fay, T.-A.; Artemenko, N.; Göhlich, D. Modeling and Experimental Investigation of Thermal Comfort and Energy Consumption in a Battery Electric Bus. WEVJ 2021, 12, 7. [Google Scholar] [CrossRef]
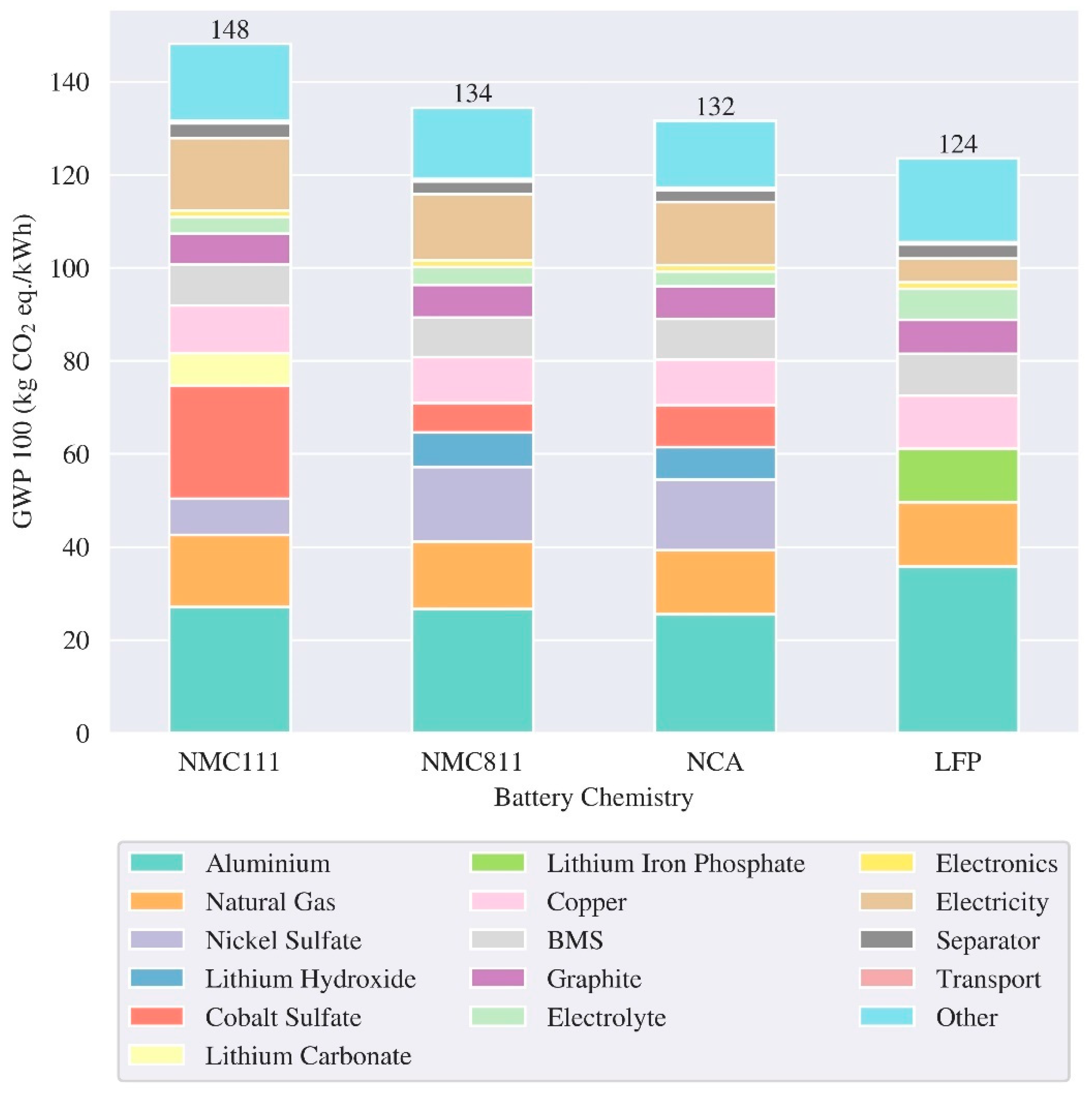
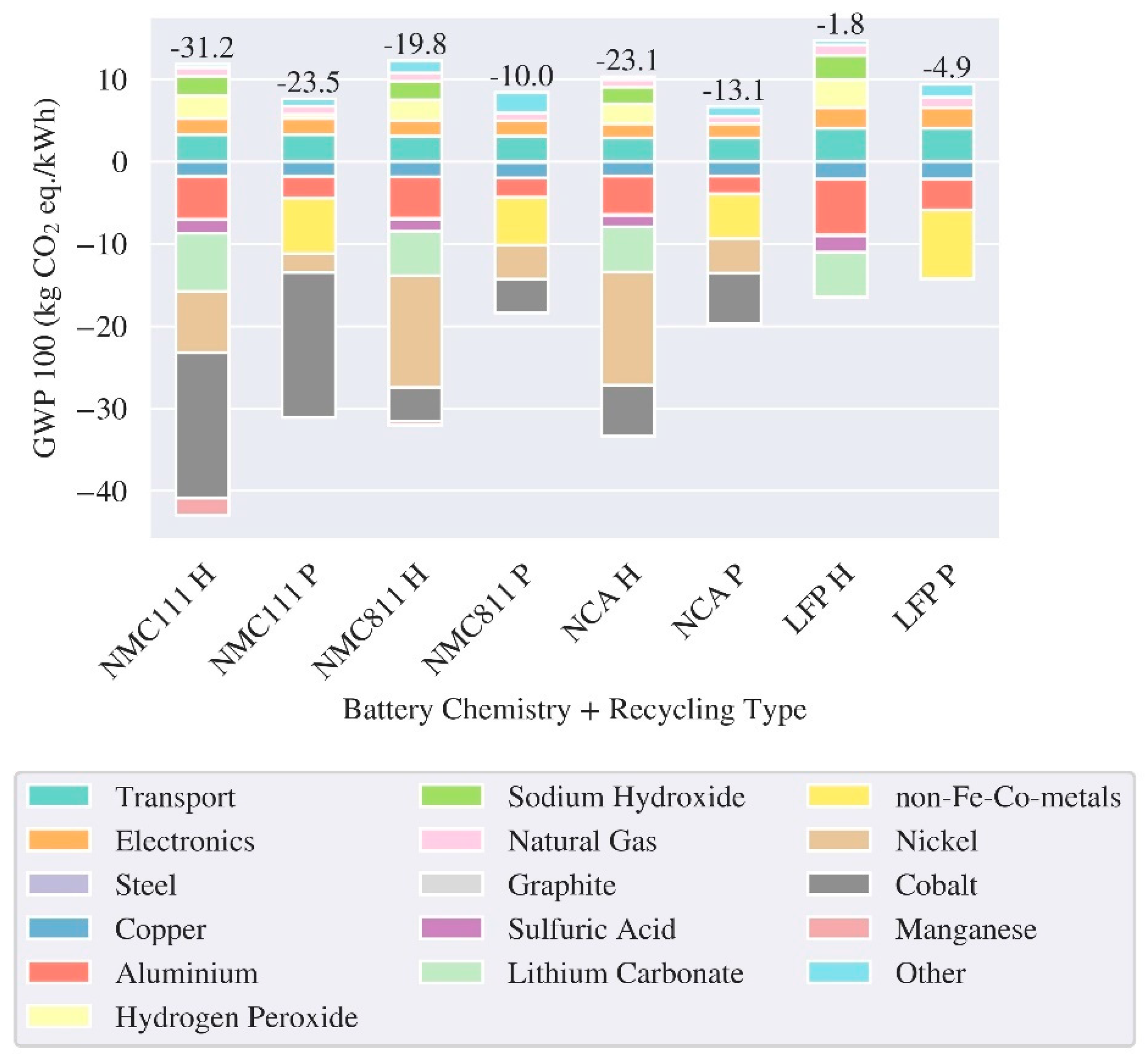
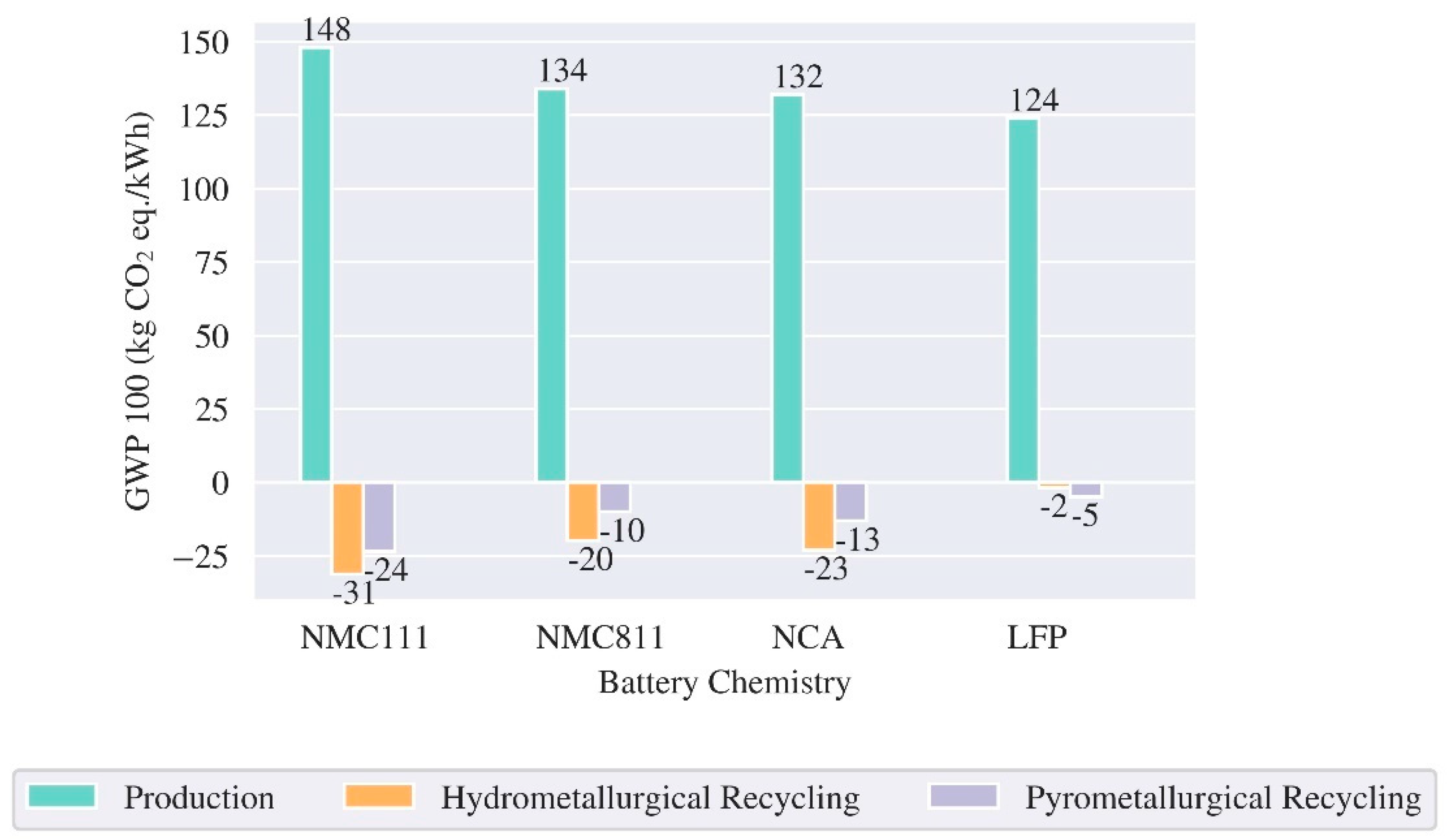
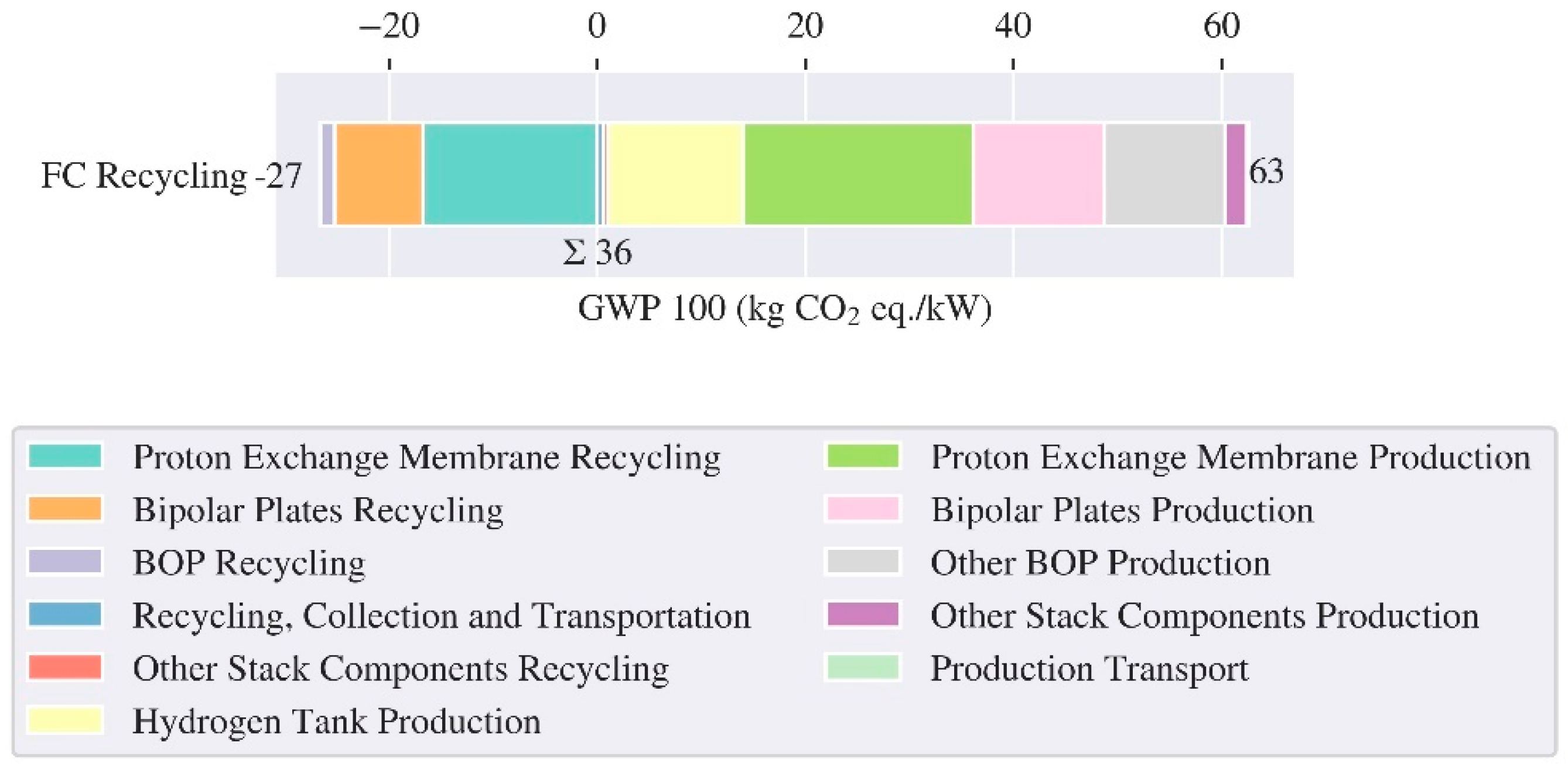
| Reference | Cell/ Pack level | Region | Cathode chemistry | Energy density (Wh/kg) | GWP (kg CO2 eq./kWh) | ||
| Production | Recycling | ||||||
| Hydro. | Pyro. | ||||||
| Dai et al. (2019, [6]) | Pack | USA | NMC111 | 143 | 72.9 | - | - |
| Rajaeifar et al. (2021, [7]) | Pack | UK | NMC111 | - | - | - | -0.77 (/kg) |
| Kallitsis et al. (2022, [8]) | Pack | China NA Europe |
NMC111 | 105 | 168.8 133.8 124.1 |
-65.1 -45.1 -37.1 |
-59 -40.6 -34.1 |
| Sun et al. (2020, [9]) | Pack | China | NMC622 | 115 | 124.5 | -30.9 | - |
| Mohr et al. (2020, [10]) | Cell |
- |
NMC111 NCA LFP |
170 174 108 |
75.5 85.6 101 |
-16.4 -18.3 -3.52 |
-13.8 -15.9 0.45 |
| Ciez and Whitacre (2019, [11]) | Cell | USA | NMC622 NCA LFP |
210 190 100 |
42 49 45 |
-5 -3.5 8 |
3 1.5 10 |
| Battery Chemistry | Energy density, cell (Wh/kg) |
Energy density, pack (Wh/kg) |
| NMC111 [6] | 197 | 143 |
| NMC811 [35] | 209 | 149 |
| NCA [35] | 224 | 158 |
| LFP [35] | 159 | 116 |
| Process |
Electricity Production (kg CO2 eq./kWh) |
Hydrogen Production (kg CO2 eq./kg) |
||||||
| Year | 2021 | 2021 | 2030 | 2050 | 2020 | 2030 | 2050 | |
| Region | China | Germany | Germany | Germany | Global | Germany | Germany | |
| GWP | 0.740 | 0.490 | 0.251 | 0.094 | 13.103 | 13.224 | 5.184 | |
| Type | FCEV | FCEV | BEV | BEV | BEV |
| Reference Vehicle |
Toyota Mirai 2 [37] |
Hyundai Nexo [38] | VW ID.3 [39] |
VW ID.4 [40] |
MB EQS 580 4Matic [41] |
| Range (km) | 650 | 666 | 426 | 346 | 672 |
| SFC ((kg or kWh)/100 km) | 0.84 | 0.95 | 15.5 | 16.7 | 17.7 |
| Battery size (kWh) | 0.95 | 1.56 | 62 | 55 | 120 |
| Fuel Cell size (kW) | 128 | 95 | 0 | 0 | 0 |
| Hydrogen Tank (kg) | 6.2 | 6.33 | 0 | 0 | 0 |
| Passenger Capacity (P) | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Vehicle weight (kg) | 1950 | 1948 | 1805 | 1966 | 2585 |
| Vehicle Class | Coupé | SUV | Coupé | SUV | Coupé |
| Type | BEV | BEV | FCEV |
| Reference Vehicles | Daimler eActros 300 + Trailer [49] | Daimler eLongHaul [50] |
Daimler GenH2 [51] |
| Assumed Payload (%) | 50 (100) | 100 | 100 |
| Max. Payload (t) | 23 | 22 | 25 |
| Range (km) | 220 (170) | 500 | 1000 |
| SFC ((kg or kWh)/100 km) | 153* (198*) | 120* | 8* |
| Battery size (kWh) | 336 | 600 | 70 |
| Fuel Cell Power (kW) | - | - | 300 |
| Hydrogen Tank (kg) | - | - | 80 |
| Type | FCEV | FCEV | BEV | BEV |
| Reference Vehicles | Solaris Urbino Hydrogen [52,53,54,55] | Solaris Urbino Electric [56,57,58] | ||
| Vehicle Length (m) | 12 | 18 | 12 | 18 |
| Range (km) | 350 | 350 | 300 | 60 |
| SFC ((kg or kWh)/100 km) | 10.7* | 14.6* | 158 | 218 |
| Battery size (kWh) | 60 | 60 | 658 | 193 |
| Fuel Cell Power (kW) | 70 | 100 | - | - |
| Hydrogen Tank (kg) | 37 | 51 | - | - |
| Passenger Capacity (P) | 87 | 140 | 60 | 138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).