Submitted:
15 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
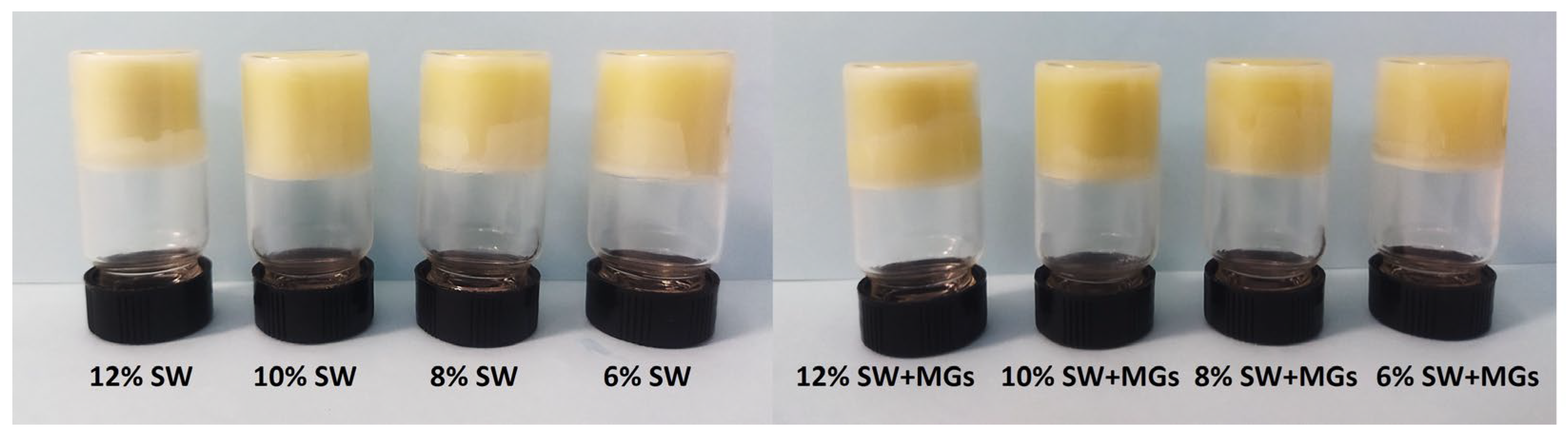
2.1. Oleogel Appearance
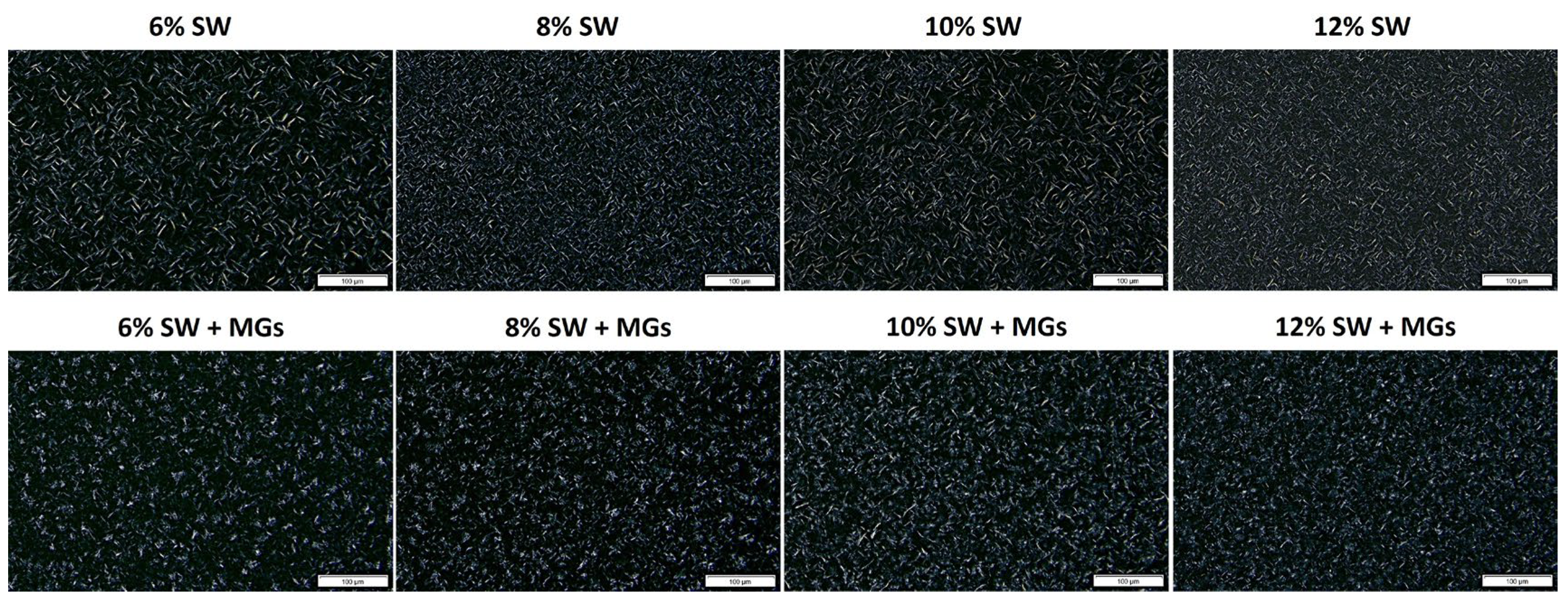
2.2. Microstructural Assessmnet
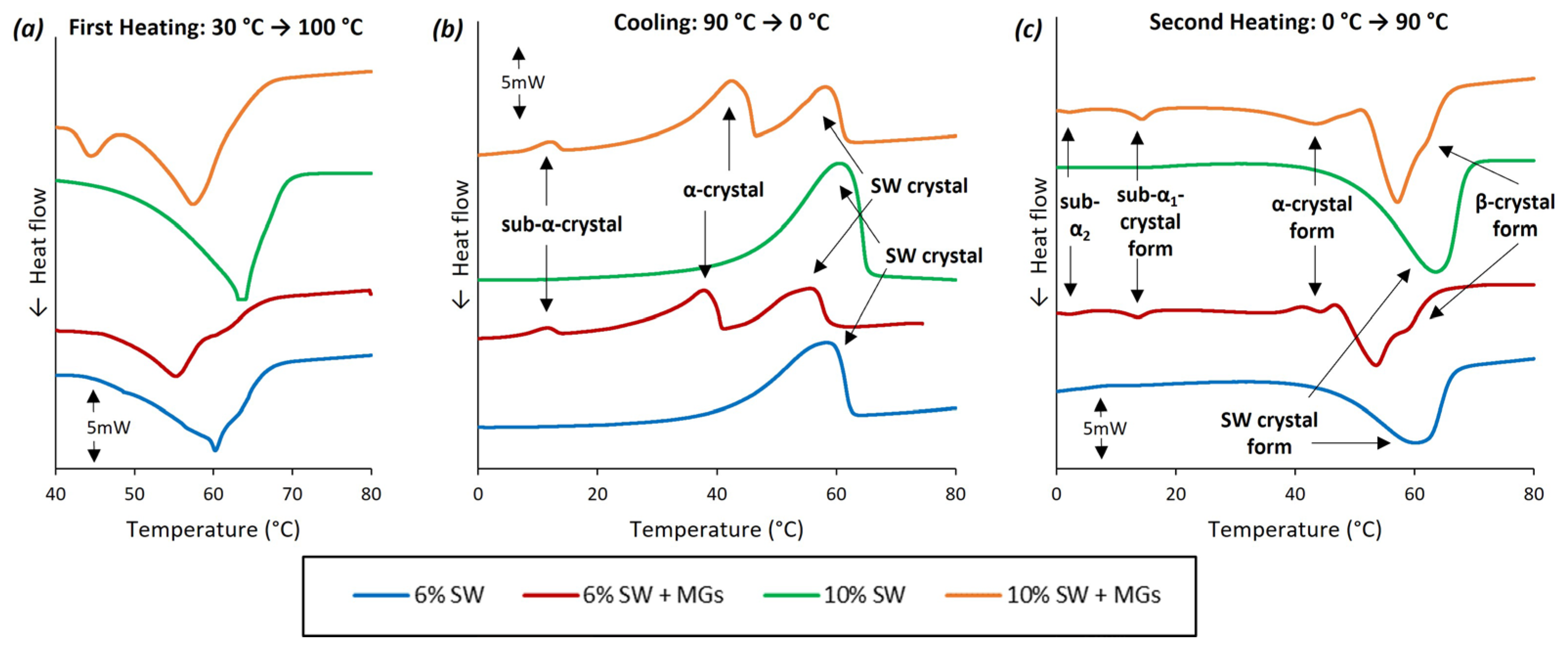
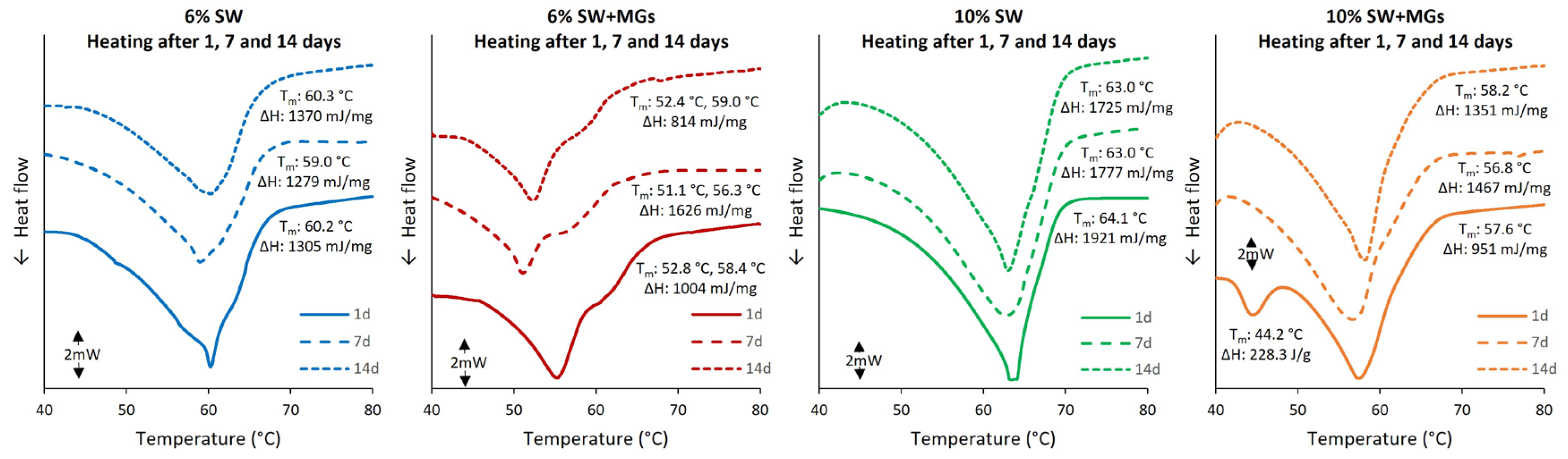
2.3. Thermal analysis
2.4. Texture Analysis
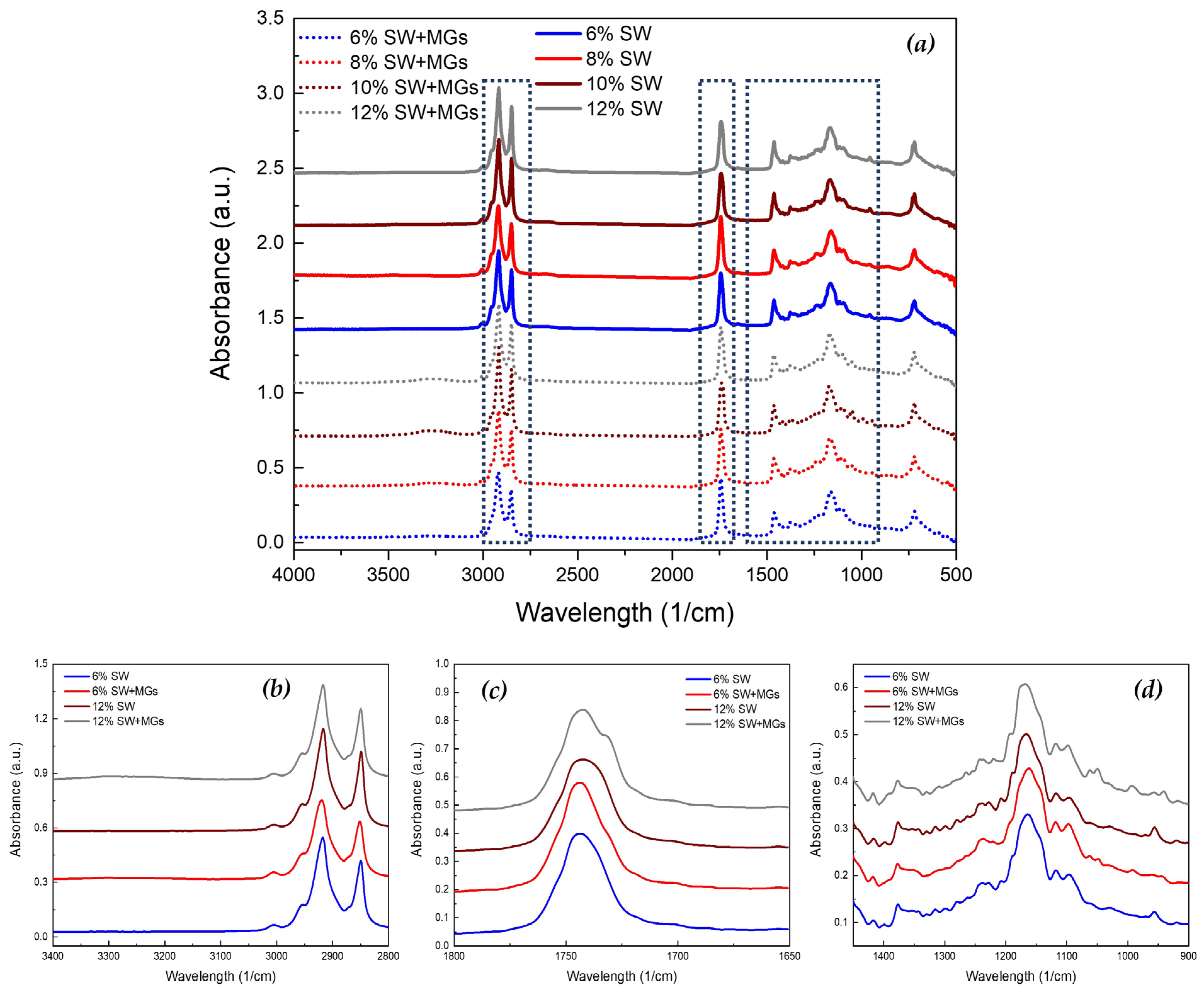
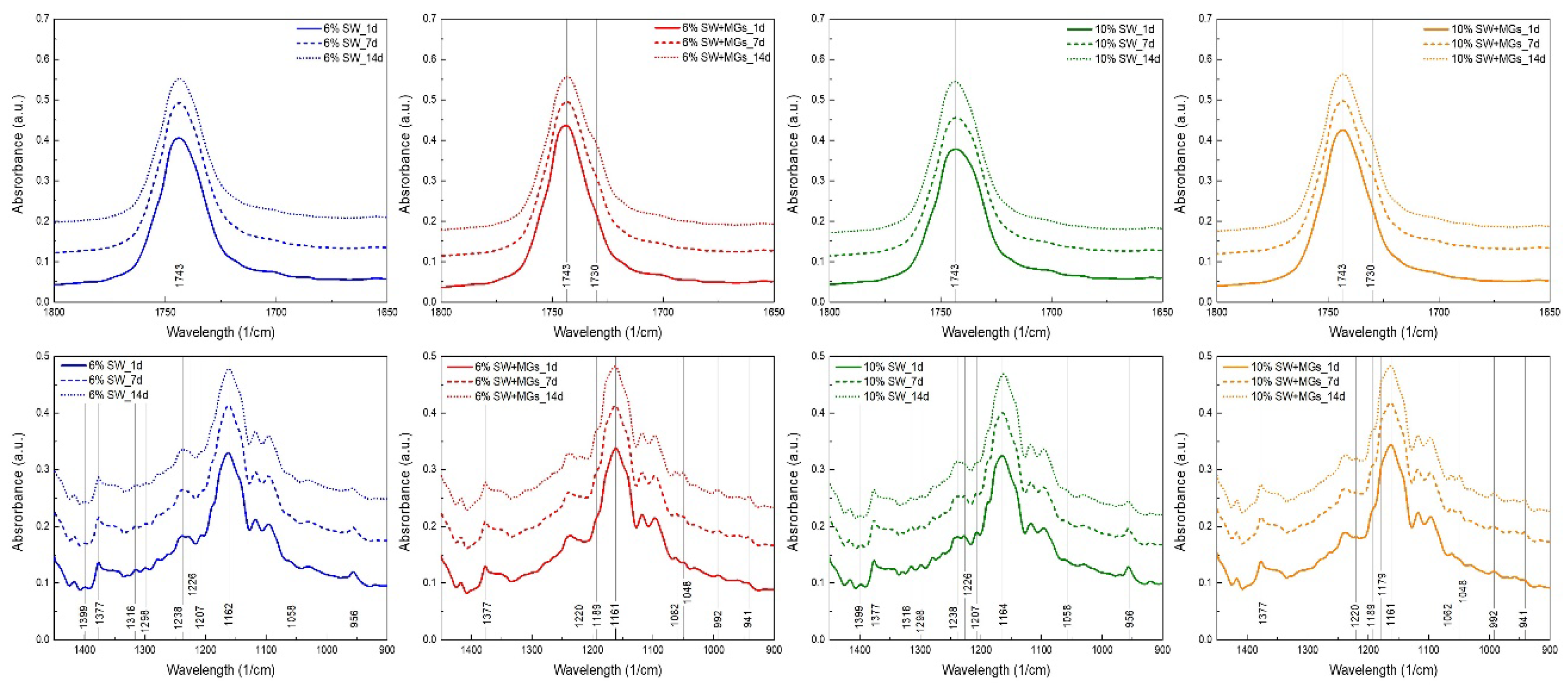
2.5. FTIR Analysis
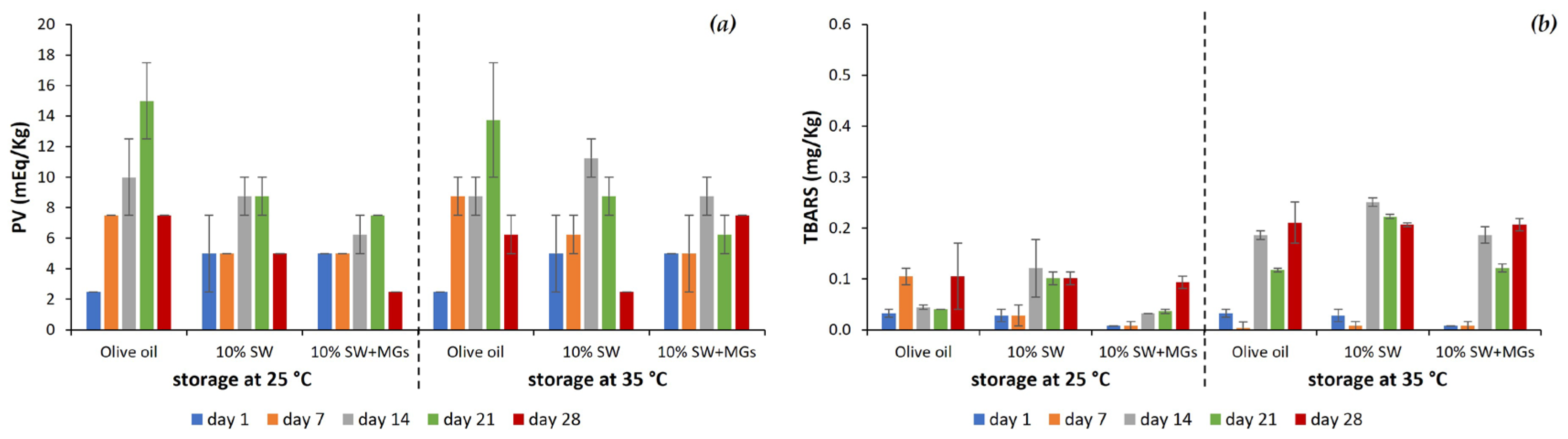
2.6. Oxidative Stability
3. Conclusions
4. Materials and Methods
4.1. Raw Materials and Oleogels Preparation
4.2. Color Measurement
4.3. Polarized Optical Microscopy
4.4. Differential Scanning Calorimetry (DSC)
4.5. Texture Analysis
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Oxidative Stability of Oleogels
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a Promising Structured Oil for Decreasing Saturated Fatty Acid Concentrations: Production and Food-Based Applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for Development of Health-Promoting Food Products. Food Sci. Hum. Wellness. 2020, 9, 31–39. [Google Scholar] [CrossRef]
- WHO Healthy Diet; 2020; Available online:. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 29 April 2020).
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper; 2010; pp. 1–166. [Google Scholar]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health Effects of Trans-Fatty Acids: Experimental and Observational Evidence. European Journal of Clinical Nutrition 2009, 63, S5–S21. [Google Scholar] [CrossRef] [PubMed]
- Zampouni, K.; Soniadis, A.; Dimakopoulou-Papazoglou, D.; Moschakis, T.; Biliaderis, C.; Katsanidis, E. Modified Fermented Sausages with Olive Oil Oleogel and NaCl–KCl Substitution for Improved Nutritional Quality. LWT 2022, 158, 113172. [Google Scholar] [CrossRef]
- Silva, R.C.; Ferdaus, Md.J.; Foguel, A.; da Silva, T.L. Oleogels as a Fat Substitute in Food: A Current Review. Gels 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Bin Sintang, M.D.; Rimaux, T.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Oil Structuring Properties of Monoglycerides and Phytosterols Mixtures. Eur. J. Lipid Sci. Technol. 2017, 119, 1500517. [Google Scholar] [CrossRef]
- Perța-Crișan, S.; Ursachi, C.-Ștefan; Chereji, B.-D.; Tolan, I.; Munteanu, F.-D. Food-Grade Oleogels: Trends in Analysis, Characterization, and Applicability. Gels 2023, 9. [CrossRef]
- Co, E.D.; Marangoni, A.G. Organogels: An Alternative Edible Oil-Structuring Method. J. Am. Oil Chemists Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of Oleogels Based on Edible Lipid Materials. Curr. Opin. Colloid. Interface. 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible Oil Structuring: An Overview and Recent Updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef]
- Banaś, K.; Harasym, J. Natural Gums as Oleogelators. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Golodnizky, D.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The Role of the Polar Head Group and Aliphatic Tail in the Self-Assembly of Low Molecular Weight Molecules in Oil. Food Struct. 2021, 30, 100240. [Google Scholar] [CrossRef]
- Hwang, H.-S. A Critical Review on Structures, Health Effects, Oxidative Stability, and Sensory Properties of Oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. Edible Oleogels: Structure and Health Implications. Elsevier, 2018; ISBN 0-12-814271-5. [Google Scholar]
- Prodromidis, P.; Katsanidis, E.; Biliaderis, C.G.; Moschakis, T. Effect of Tween 20, Emulsification Temperature and Ultrasonication Intensity on Structured Emulsions with Monoglycerides. Food Hydrocoll. 2024, 151, 109772. [Google Scholar] [CrossRef]
- Palla, C.A.; Dominguez, M.; Carrín, M.E. Recent Advances on Food-Based Applications of Monoglyceride Oleogels. J. Am. Oil Chemists Soc. 2022, 99, 985–1006. [Google Scholar] [CrossRef]
- Fayaz, G.; Polenghi, O.; Giardina, A.; Cerne, V.; Calligaris, S. Structural and Rheological Properties of Medium-Chain Triacylglyceride Oleogels. J. Food Sci. Technol. 2021, 56, 1040–1047. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A.; Katsanidis, E. Crystalline Microstructure and Physicochemical Properties of Olive Oil Oleogels Formulated with Monoglycerides and Phytosterols. LWT 2022, 154, 112815. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial Replacement of Animal Fat by Oleogels Structured with Monoglycerides and Phytosterols in Frankfurter Sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Physical Properties of Candelilla Wax, Monoacylglycerols, and Fully Hydrogenated Oil Oleogels. J. Am. Oil Chemists Soc. 2018, 95, 797–811. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6. [Google Scholar] [CrossRef]
- Yilmaz, E.; Demirci, Ş. Preparation and Evaluation of Virgin Olive Oil Oleogels Including Thyme and Cumin Spices with Sunflower Wax. Gels 2021, 7. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Giannakaki, F.; Katsanidis, E. Structural and Physical Characteristics of Mixed-Component Oleogels: Natural Wax and Monoglyceride Interactions in Different Edible Oils. Gels 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and Rheology of Oleogels Made from Rice Bran Wax and Rice Bran Oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Kim, M.-J.; Lee, S.Y.; Lee, J. Physicochemical Properties and Oxidative Stability of Oleogels Made of Carnauba Wax with Canola Oil or Beeswax with Grapeseed Oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Singh, A.; Prabhakar, P.K.; Meghwal, M.; Upadhyay, A. Optimization and Characterization of Soybean Oil-Carnauba Wax Oleogel. LWT 2022, 157, 113108. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Rice Bran Wax in Bulk and Organogels. J. Am. Oil Chemists Soc. 2009, 86, 1163. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and Textural Properties of Organogels Developed by Candelilla Wax in Safflower Oil. J. Am. Oil Chemists Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Sánchez-Becerril, M.; Marangoni, A.G.; Perea-Flores, M.J.; Cayetano-Castro, N.; Martínez-Gutiérrez, H.; Andraca-Adame, J.A.; Pérez-Martínez, J.D. Characterization of the Micro and Nanostructure of the Candelilla Wax Organogels Crystal Networks. Food Struct. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels 2022, 8. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Frolova, Y.; Sobolev, R.; Kochetkova, A. On the Role of Beeswax Components in the Regulation of Sunflower Oil Oleogel Properties. Food Biophys. 2023, 18, 262–272. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and External Factors Affecting the Crystallization, Gelation and Applicability of Wax-Based Oleogels in Food Industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Pakseresht, S.; Tehrani, M.M.; Farhoosh, R.; Koocheki, A. The Monoglyceride Oleogel Characteristics Modified by Carnauba Wax. LWT 2023, 185, 115156. [Google Scholar] [CrossRef]
- Öğütcü, M.; Yılmaz, E. Comparison of the Pomegranate Seed Oil Organogels of Carnauba Wax and Monoglyceride. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Pakseresht, S.; Tehrani, M.M.; Farhoosh, R.; Koocheki, A. Rheological and Thermal Properties of Reinforced Monoglyceride-Carnauba Wax Oleogels. J. Sci. Food Agric. 2023, 103, 4184–4194. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chemists Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Sintang, M.D.B.; Danthine, S.; Van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: A Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2017, 12, 97–108. [Google Scholar] [CrossRef]
- Öğütcü, M.; Yılmaz, E. Characterization of Hazelnut Oil Oleogels Prepared with Sunflower and Carnauba Waxes. Int. J. Food Prop. 2015, 18, 1741–1755. [Google Scholar] [CrossRef]
- Prodromidis, P.; Biliaderis, C.G.; Katsanidis, E.; Moschakis, T. Effect of Tween 20 on Structure, Phase-Transition Behavior and Mechanical Properties of Monoglyceride Oleogels. Food Struct. 2023, 38, 100345. [Google Scholar] [CrossRef]
- Lupi, F.R.; Greco, V.; Baldino, N.; Cindio, B. de; Fischer, P.; Gabriele, D. The Effects of Intermolecular Interactions on the Physical Properties of Organogels in Edible Oils. J. Colloid Interface Sci. 2016, 483, 154–164. [Google Scholar] [CrossRef]
- López-Martínez, A.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Comparing the Crystallization and Rheological Behavior of Organogels Developed by Pure and Commercial Monoglycerides in Vegetable Oil. Food Res. Int. 2014, 64, 946–957. [Google Scholar] [CrossRef]
- Palla, C.A.; Dominguez, M.; Carrín, M.E. An Overview of Structure Engineering to Tailor the Functionality of Monoglyceride Oleogels. Compr. Rev. Food Sci. Food Saf.. 2022, 21, 2587–2614. [Google Scholar] [CrossRef]
- Martini, S.; Tan, C.Y.; Jana, S. Physical Characterization of Wax/Oil Crystalline Networks. J. Food Sci. 2015, 80, C989–C997. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chemists Soc. 2012, 89, 639–647. [Google Scholar] [CrossRef]
- Vereecken, J.; Meeussen, W.; Foubert, I.; Lesaffer, A.; Wouters, J.; Dewettinck, K. Comparing the Crystallization and Polymorphic Behaviour of Saturated and Unsaturated Monoglycerides. Food Res. Int. 2009, 42, 1415–1425. [Google Scholar] [CrossRef]
- Chen, C.H.; Terentjev, E.M. Aging and Metastability of Monoglycerides in Hydrophobic Solutions. Langmuir 2009, 25, 6717–6724. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S.; Gillman, J.D.; Winkler-Moser, J.K.; Kim, S.; Singh, M.; Byars, J.A.; Evangelista, R.L. Properties of Oleogels Formed With High-Stearic Soybean Oils and Sunflower Wax. J. Am. Oil Chemists Soc. 2018, 95, 557–569. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M. A Novel Propolis Wax-Based Organogel: Effect of Oil Type on Its Formation, Crystal Structure and Thermal Properties. J. Am. Oil Chemists Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The Role of Hydrogen Bonds in TAG Derivative-Based Oleogel Structure and Properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, J.; Lu, X.; Xu, Y.; Regenstein, J.M.; Zhang, Y.; Wang, F. Development and Characterization of Monoglyceride Oleogels Prepared with Crude and Refined Walnut Oil. LWT 2022, 154, 112769. [Google Scholar] [CrossRef]
- Alfutimie, A.; Curtis, R.; Tiddy, G.J.T. Gel Phase (Lβ) Formation by Mixed Saturated and Unsaturated Monoglycerides. Colloids Surf. A Physicochem. 2014, 456, 286–295. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Wang, M.; Bi, Y.; Zhang, H.; Xu, X. Development and Characterization of Compound Oleogels Based on Monoglycerides and Edible Waxes. ACS Food Sci. Technol. 2022, 2, 302–314. [Google Scholar] [CrossRef]
- Tanislav, A.E.; Pușcaș, A.; Mureșan, V.; Mudura, E. The Oxidative Quality of Bi-, Oleo- and Emulgels and Their Bioactives Molecules Delivery. Crit. Rev. Food Sci. Nutr. 2023, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Samui, T.; Goldenisky, D.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The Development and Characterization of Novel In-Situ Bigel Formulation. Food Hydrocoll. 2021, 113, 106416. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. CODEX STAN 210-1999. Rev. 2021. Codex Standard for Edible Fats and Oils Non Covert by Individual Standards.; Italy: FAO/ WHO, 2021. [Google Scholar]
- Park, C.; Bemer, H.L.; Maleky, F. Oxidative Stability of Rice Bran Wax Oleogels and an Oleogel Cream Cheese Product. J. Am. Oil Chemists Soc. 2018, 95, 1267–1275. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Fhaner, M.; Winkler-Moser, J.K.; Liu, S.X. Oxidation of Fish Oil Oleogels Formed by Natural Waxes in Comparison With Bulk Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700378. [Google Scholar] [CrossRef]
- Orhan, N.O.; Eroglu, Z. Structural Characterization and Oxidative Stability of Black Cumin Oil Oleogels Prepared with Natural Waxes. J. Food Process. Preserv. 2022, 46, e17211. [Google Scholar] [CrossRef]
- Katsanidis, E.; Zampouni, K. Development of a Novel Steam Distillation TBA Test for the Determination of Lipid Oxidation in Meat Products. Foods 2023, 12. [Google Scholar] [CrossRef]
| Sample | Physicochemical parameters | Color parameters | |||
|---|---|---|---|---|---|
| Hardness | Cohesiveness | L* | a* | b* | |
| 6% SW | 7.36 ± 0.42 e,f | 0.168 ± 0.013 a,b | 76.1 ± 1.1 d | -8.9 ± 0.1 a | 30.9 ± 0.6 a |
| 6% [SW + MGs] | 3.51 ± 0.24 f | 0.182 ± 0.025 a | 72.2 ± 1.2 e | -7.8 ± 0.2 a | 28.2 ± 0.7 c |
| 8% SW | 13.27 ± 0.65 c,d,e | 0.091 ± 0.003 b,c | 76.4 ± 1.4 d | -8.5 ± 0.2 a | 27.6 ± 0.8 c,d |
| 8% [SW + MGs] | 10.20 ± 1.69 d,e,f | 0.095 ± 0.026 b,c | 75.8 ± 1.0 c,d | -8.3 ± 1.7 a | 30.0 ± 0.5 a,b |
| 10% SW | 15.32 ± 0.77 c,d | 0.081 ± 0.010 c | 80.0 ± 0.9 a,b | -8.4 ± 0.1 a | 26.8 ± 0.5 d,e |
| 10% [SW + MGs] | 19.98 ± 2.5 c | 0.043 ± 0.012 c | 77.2 ± 2.2 c,d | -8.6 ± 0.2 a | 27.1 ± 0.8 d,e |
| 12% SW | 27.32 ± 1.45 b | 0.070 ± 0.012 c | 81.0 ± 0.9 a | -8.3 ± 0.1 a | 26.6 ± 0.3 e |
| 12% [SW + MGs] | 35.74 ± 0.09 a | 0.024 ± 0.001 c | 78.7 ± 1.4 b,c | -9.1 ± 0.2 a | 29.5 ± 0.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





