1. Introduction
Bat feces were first recorded as a traditional Chinese medicine in Shennong's
Classic of Materia Medica [
1] and were called Luminous Sand in the Song Dynasty's
Rihuazi Materia Medica [
2]. Luminous sands are oblong particles with slightly pointed ends, 5 to 7 mm in length, and about 2 mm in diameter. They have a rough surface and are in the form of small brown particles or powder. When observed under a microscope, brown or yellow-brown shiny insect body fragments can be seen. The ancient book
Compendium of Materia Medica reported that bat feces have the medicinal effect of reducing heat, improving eyesight, activating blood circulation, and eliminating metabolic accumulation [
3]. Modern medicine believes that bat feces contain vitamin A, which can be used to treat night blindness and relieve eye bleeding [
4].
Compared to other mammals, bats have the greatest variability in dietary strategies, spanning insectivorous, carnivorous, or frugivorous types. The food types of most bats are insects and other small arthropods. In addition, these diverse dietary habits of bats provide high-quality ecological and environmental services to the ecosystem [
5]. For example, insectivorous bats eat a large number of pests and can be used as natural pesticide [
6]. Since bats consume a rich and nutritionally diverse diet, the variability in different dietary strategies also appears to influence the composition of bat feces [
7,
8]. The Luminous Sand reported by ancient Chinese medicinal materials matches the description of the feces of insectivorous bats. It was reported that the active ingredient in insectivorous bat feces for treating eye diseases may be insect eyes, which are rich in vitamin A and have the effect of reducing heat and improving eyesight [
9]. However, our observations revealed that the main component of insectivorous bat feces is undigested insect body fragments, and the number of insect eyes contained in the feces is very small. Therefore, it is not accurate to say that insect eyes are the active ingredients of bat feces, and their reported eye-improving mechanism needs further research.
In this study, we used 2,2-diphenyl-1-picrylhydrazyl (DPPH) and liquid chromatography/tandem mass spectrometry (LC/MS/MS) to analyze the antioxidant capacity of and the types of vitamins in insectivorous bat feces. We hoped to screen the pharmacological components of bat feces, and to explain the role that these components may play in the treatment of visual deterioration.
2. Materials and Methods
2.1. Bat feces preparation
Fecal samples of Hipposideros armiger terasensis and Miniopterus fuliginosus, the most common species in Taiwan, were collected at three locations in northern Taiwan. Hipposideros armiger terasensis is the largest insectivorous bat among the Chiropterans in Taiwan. They use ultrasonic waves to search for large insects such as scarabs and beetles. In addition to flying insects, they also prey on insects perched on tree trunks or leaves. The types of insects they feed on will vary with the season and whether they reproduce or not. These bats are commonly found in natural caves, artificial tunnels, or abandoned buildings, in low- and medium-altitude areas. Miniopterus fuliginosus feeds on small insects and is a typical cave bat that uses caves or tunnels as its main habitat. During the summer, both species total as many as hundreds or thousands of individuals. Fecal samples were collected from dung piles beneath bat colonies in caves. We mainly collected fresh fecal pellets, avoiding the contamination of the samples with old feces. We collected fecal samples using sterile forceps into sterile microcentrifuge tubes, kept them on ice, transported them to the laboratory, and processed them within 24 hours. In the laboratory, we prepared pooled fecal samples for QC by combining and homogenizing approximately 5 g of thawed aliquots from 10 individual fecal samples. These samples were shaken at 4 °C for 5 min and sonicated in ice water for 5 min to obtain low-, medium-, and high-level QC fecal homogenates. Aliquots (50 μL) of the homogenate were then placed into a series of 1.5 mL Eppendorf tubes and stored at −80 °C until analysis.
2.2. Preparation of standards
For LC/MS/MS, ultrapure water, methanol, and acetonitrile were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (nicotinamide), vitamin B3 (nicotinic acid), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B8 (biotin), vitamin B9 (folic acid) , vitamin B12 (cyanocobalamin), vitamin C (ascorbic acid), vitamin A (retinol), vitamin D3 (cholecalciferol), vitamin E (α-tocopherol), vitamin K1 (phylloquinone), and LC–MS ultrapure formic acid were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). All standards were prepared under UV-shielded light. The calibration standard solutions of vitamins were prepared in 10% methanol in the concentration range 5–1,000 ng/mL (ppb) and stored at − 80 °C until use.
2.3. Determination of antioxidant capacity of fecal samples
In this study, we evaluated the antioxidant activity of the fecal samples using 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH, D9132, Sigma-Aldrich Co., St. Louis, MO, USA). DPPH is a stable free radical that can be used to measure the free radical scavenging activity of antioxidants. The DPPH method can be used in aqueous and non-polar organic solvents and can be used to examine hydrophilic and lipophilic antioxidants. We first diluted fecal samples with distilled water to prepare solutions of different concentrations, then added 100 μL of 1.5 mM/mL DPPH (Sigma-Aldrich Co.) to each well of a 96-well plate, and then added fecal samples of different concentrations. We incubated a mixture of stool samples with DPPH for 30 minutes at room temperature. Subsequently, we measured the absorbance at 517 nm using a microplate spectrophotometer (μQuant, Biotek Intruments, Inc., VT, USA). To obtain more objective experimental data, we performed three separate DPPH determinations for each concentration of fecal samples. To benchmark the antioxidant function of fecal samples, we measured the absorbance of blank methanol and L-ascorbic acid (A5960, Sigma-Aldrich) as controls. The antioxidant activity of fecal samples was calculated using the formula to calculate DPPH scavenging activity, as follows: antioxidant activity of fecal samples (%) = 100 × [(absorbance of fecal samples + DPPH)-( absorbance of fecal samples)]/[( absorbance of DPPH)-( absorbance of methanol)].
2.4. Vitamin analysis
2.4.1. Water-soluble Vitamins
- (A)
-
Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
Quaternary pump: Shimadzu LC-20AD;
- (2)
Autosampler: Shimadzu SIL-20AC;
- (3)
Photodiode array detector: Shimadzu SPD-M20;
- (4)
Mass detector: Shimadzu LCMS-8040.
- (B)
-
Sample preparation:
- (1)
Place 1 g of sample powder into a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 minute, and then ultrasonic for 15 minutes.
- (2)
Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 minute.
- (3)
Centrifuge at 3,500 rpm for 10 minutes, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
-
LC/MS/MS analysis method:
Chromatography column: Raptor Biphenyl (2.7 um, 100 x 2.1 mm);
Column temperature: 35°C.
Moving phase:
A: 5 mM ammonium acetate, 0.1% formic acid in water;
B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
Mobile phase gradient:
| Time (min) |
A,% |
B,% |
| Initial |
100 |
0 |
| 1.00 |
100 |
0 |
| 6.80 |
0 |
100 |
| 8.80 |
0 |
100 |
| 9.00 |
100 |
0 |
| 12.00 |
100 |
0 |
Flow rate: 0.4 mL/min.
Injection volume: 15 μL.
Mass spectrometry conditions:
Ion source: electrospray ionization (ESI+);
Ion source interface voltage (probe voltage): 4.5 kV;
Nebulizing gas flow: nitrogen, 3.0 mL/min;
Drying gas flow: 15.00 L/min;
Collision gas: argon, 230 kPa;
Desolventization tube temperature (DL temp.): 250°C;
Heating module temperature (heat block temp.): 400°C.
Ion pairs
| Vitamin |
Quantitative ion pair |
Qualitative ion pair |
| Precursor ion (m/z) > Product ion (m/z) |
Precursor ion (m/z) > Product ion (m/z) |
| B1 (thiamine) |
265.00>122.10 |
265.00>144.10 |
| B2 (riboflavin) |
377.20>243.10 |
377.20>198.10 |
| B3 (nicotinamide) |
123.00>80.10 |
123.00>96.10 |
| B3 (nicotinic acid) |
123.90>80.10 |
123.90>78.10 |
| B5 (pantothenic acid) |
220.20>90.10 |
220.20>202.10 |
| B6 (pyridoxine) |
170.00>152.10 |
170.00>134.10 |
| B8 (biotin) |
245.20>227.20 |
245.20>123.10 |
| B9 (folic acid) |
441.90>295.10 |
441.90>176.20 |
| B12 (cyanocobalamin) |
678.60>147.10 |
678.60>359.20 |
2.4.2. Vitamin C
- (A)
-
Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
Quaternary pump: Shimadzu LC-20AD;
- (2)
Autosampler: Shimadzu SIL-20AC;
- (3)
Photodiode array detector: Shimadzu SPD-M20;
- (4)
Mass detector: Shimadzu LCMS-8040.
- (B)
-
Sample preparation:
- (1)
Place 1 g of sample powder into a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 minute, and then ultrasonic for 15 minutes.
- (2)
Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 minute.
- (3)
Centrifuge at 3,500 rpm for 10 minutes, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
-
LC/MS/MS analysis method:
LC analysis conditions:
Chromatography column: Raptor Biphenyl (2.7 um, 100 x 2.1 mm);
Column temperature: 35°C.
Moving phase:
A: 5 mM ammonium acetate, 0.1% formic acid in water;
B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
Mobile phase gradient:
| Time (min) |
A,% |
B,% |
| Initial |
100 |
0 |
| 2.40 |
100 |
0 |
| 4.40 |
89 |
11 |
| 4.60 |
70 |
30 |
| 6.50 |
68 |
32 |
| 6.70 |
0 |
100 |
| 7.00 |
100 |
0 |
Flow rate: 0.2 mL/min.
Injection volume: 5 μL.
Mass spectrometry conditions:
Ion source: electrospray ionization (ESI+);
Ion source interface voltage (probe voltage): 4.5 kV;
Nebulizing gas flow: nitrogen, 3.0 mL/min;
Drying gas flow: 15.00 L/min;
Collision gas: argon, 230 kPa;
Desolventization tube temperature (DL temp.): 250°C;
Heating module temperature (heat block temp.): 400°C.
Ion pairs:
| Vitamin |
Quantitative ion pair |
Qualitative ion pair |
| Precursor ion (m/z) > Product ion (m/z) |
Precursor ion (m/z) > Product ion (m/z) |
| C |
177.10>95.10 |
177.10>141.20 |
2.4.3. Fat-soluble Vitamins
- (A)
-
Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
Quaternary pump: Shimadzu LC-20AD;
- (2)
Autosampler: Shimadzu SIL-20AC;
- (3)
Photodiode array detector: Shimadzu SPD-M20;
- (4)
Mass detector: Shimadzu LCMS-8040.
- (B)
-
Sample preparation:
- (1)
Take 0.25 g of sample powder and place it in a 15 mL centrifuge tube, add 1.5 mL of pure water and 1.5 mL of methanol (methanol), shake with a vortex mixer for 1 minute, and then shake with ultrasonic for 20 minutes.
- (2)
Add another 10 mL of n-Hexane to the centrifuge tube and vortex for 5 minutes.
- (3)
Centrifuge at 3,500 rpm for 10 minutes, and place 1 mL of supernatant into a glass centrifuge tube.
- (4)
Blow dry with nitrogen in a 40°C water bath, add 1 mL of methanol (methanol) to dissolve and mix evenly.
- (5)
Filter with a 0.22 μm filter membrane and use the filtrate as the test solution.
- (C)
-
LC/MS/MS analysis method:
Chromatography column: Raptor Biphenyl (2.7 um, 100 x 2.1 mm);
Column temperature: 35°C.
Moving phase:
A: 5 mM ammonium acetate, 0.1% formic acid in water;
B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
Mobile phase gradient:
| Time (min) |
A,% |
B,% |
| Initial |
100 |
0 |
| 2.40 |
100 |
0 |
| 4.40 |
89 |
11 |
| 4.60 |
70 |
30 |
| 6.50 |
68 |
32 |
| 6.70 |
0 |
100 |
| 7.00 |
100 |
0 |
Flow rate: 0.4 mL/min.
Injection volume: 5 μL.
Mass spectrometry conditions:
Ion source: electrospray ionization (ESI+);
Ion source interface voltage (probe voltage): 4.5 kV;
Nebulizing gas flow: nitrogen, 3.0 mL/min;
Drying gas flow: 15.00 L/min;
Collision gas: argon, 230 kPa;
Desolventization tube temperature (DL temp.): 250°C;
Heating module temperature (heat block temp.): 400°C.
Ion pairs:
| Vitamin |
Quantitative ion pair |
Qualitative ion pair |
| Precursor ion (m/z) > Product ion (m/z) |
Precursor ion (m/z) > Product ion (m/z) |
| A |
269.30>93.10 |
269.30>119.10 |
| D3 |
385.20>367.40 |
385.20>259.30 |
| E |
431.10>165.20 |
431.10>137.10 |
| K1 |
451.30>187.20 |
451.30>185.10 |
2.5. Heavy metals analysis
- (A)
-
Inductively coupled plasma mass spectrometry (ICP-MS) conditions:
Agilent 7500a.
- (B)
-
Sample preparation:
- (1)
Take 0.4 g of the sample powder and place it in a microwave digestion bottle, add 8 mL of nitric acid, let it stand for about 10 minutes, and then digest it in a microwave digester. The operating conditions of microwave digestion are as shown in the table below.
| Stage # |
Max Power (W) |
Ramp (min) |
Temperature (°C) |
Hold (min) |
| 1 |
1200 |
15 |
175 |
05:00 |
| 2 |
1200 |
5 |
200 |
15:00 |
- (2)
After the digestion is completed, cool to room temperature and transfer to a 100 mL quantitative flask. Wash the microwave digestion flask with pure water. Put the washing liquid into the quantitative flask, dilute it with pure water to a constant volume, mix evenly, and filter with a 0.45 μm filter membrane. The filtrate is the finished product sample solution, and this solution is used as the test solution.
- (C)
-
ICP-MS analysis method.
- (1)
-
Method settings:
Acquisition mode: spectrum;
Peak pattern: full quant;
Every mass integration time: 0.33 sec;
Repetition: three times.
- (2)
-
Peristaltic pump program:
Uptake speed: 0.35 rps;
Uptake time: 30 sec;
Stabilization time: 30 sec.
- (3)
-
Analysis conditions.
Plasma Parameters:
Plasma radio frequency power: 500~1600 W, normal setting 1200 W;
Sampling depth: 3.0~23.0 mm, normal setting 10 mm;
Carrier gas flow rate: 0.00~2.00 L/min, normal setting is 1 L/min;
Auxiliary gas flow rate: 0.00~2.00 L/min, normal setting is 0.22 L/min;
Nebulizer pump: 0.00~0.50 rps, normal setting is 0.1 rps;
Premix chamber temperature (S/C temp): 2°C.
Ion Lenses:
Extract 1: -200~10 V, normal setting -120 V;
Extract 2: -200~0 V, normal setting -39 V;
Einzel 1,3: -200~100 V, normal setting -80 V;
Einzel 2: -200~100 V, normal setting 8 V;
Omega bias: -200~100 V, normal setting -41 V;
Omega (+): -200~100 V, normal setting 9 V;
Omega (-): -200~100 V, normal setting 9 V;
QF focus: -200~100 V, normal setting 9 V;
Plate bias: -50~50 V, normal setting -10 V.
2.5. Statistical analysis
All data were shown as mean ± standard error of the mean (SEM). Differences among different groups were assessed using a one-way analysis of variance (ANOVA). Student–Newman–Keuls multiple comparisons post hoc test was performed if a significant F-value was obtained. Significance was defined as P < 0.05.
3. Results
3.1. Antioxidant capacity of bat feces treatment
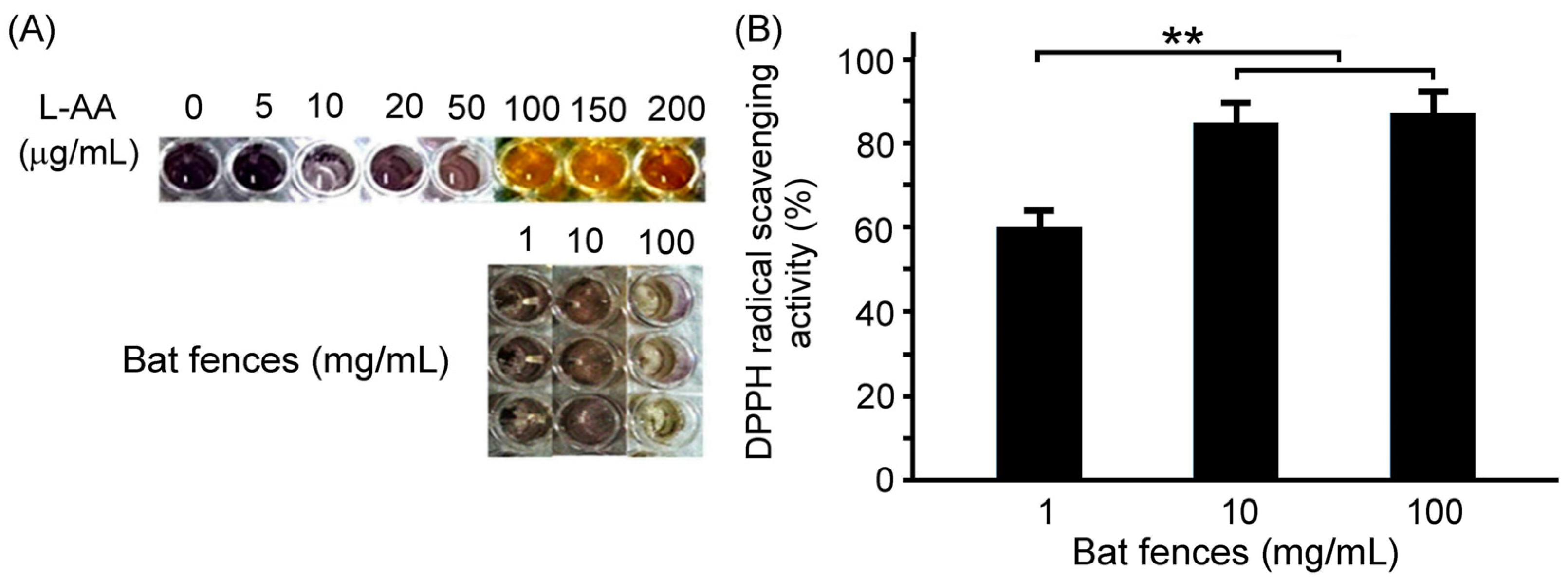
We determined the antioxidant capacity of bat feces using the DPPH assay, as shown in
Figure 1. Our results show that the free radical scavenging activity exceeds 50% when the bat feces treatment concentration is in the range of 1-100 mg/mL. The results indicate that bat feces have very good antioxidant capacity at appropriate concentrations and can effectively eliminate free radical damage.
3.2. Quantification of vitamins in bat feces using LC/MS/MS analysis
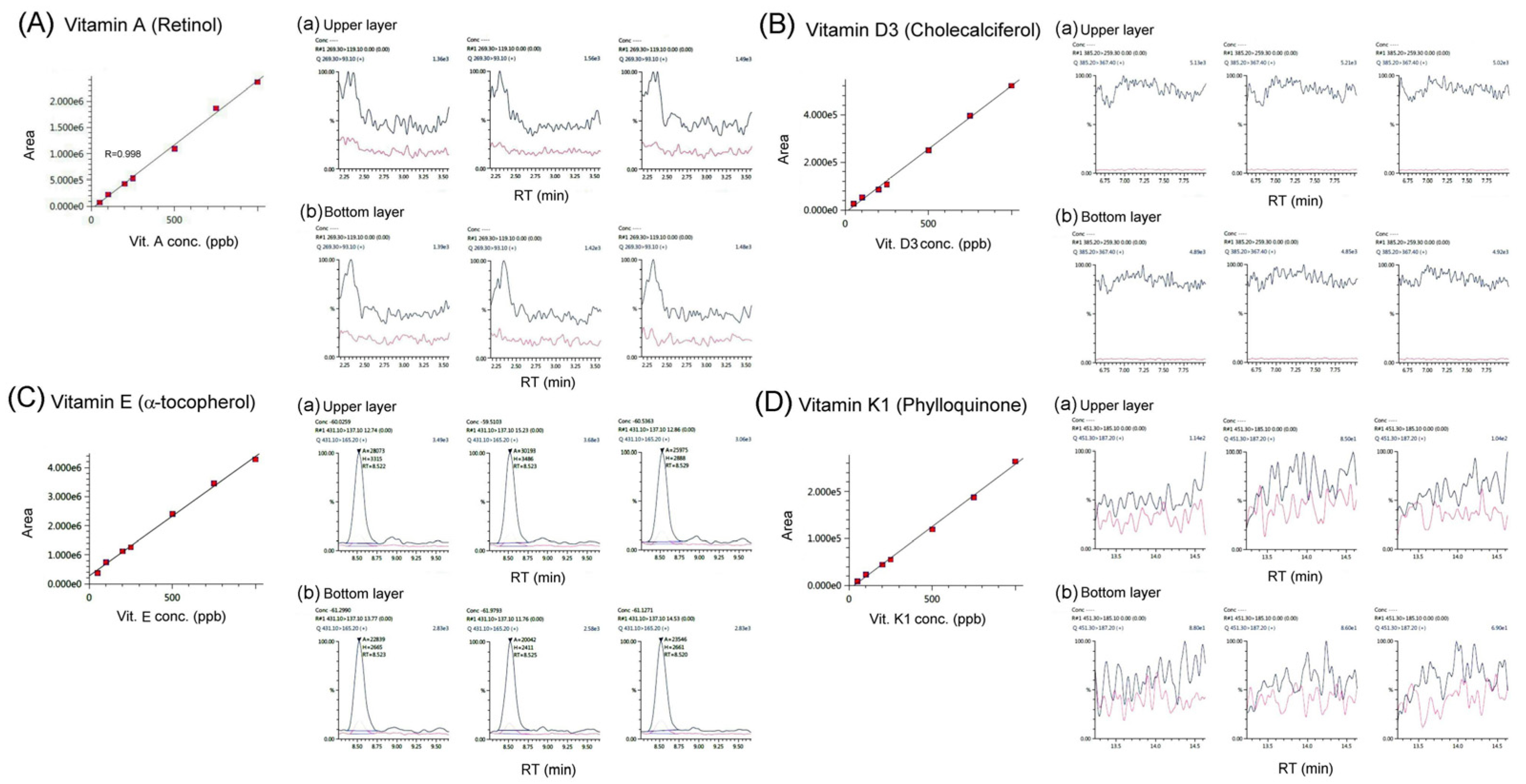
We determined whether the fat-soluble vitamins A, D3, E, and K1 were present in bat feces using LC/MS/ MS analysis, as shown in
Figure 2. The results showed that there were no obvious residues of the fat-soluble vitamins A, D3, E, or K1 in the bat feces samples. We found that bat feces contain very low amounts of fat-soluble vitamins A, D3, E, and K1, if any, as the contents of these vitamins in bat feces were all below the detection threshold and could not be detected using LC/MS/MS analysis.
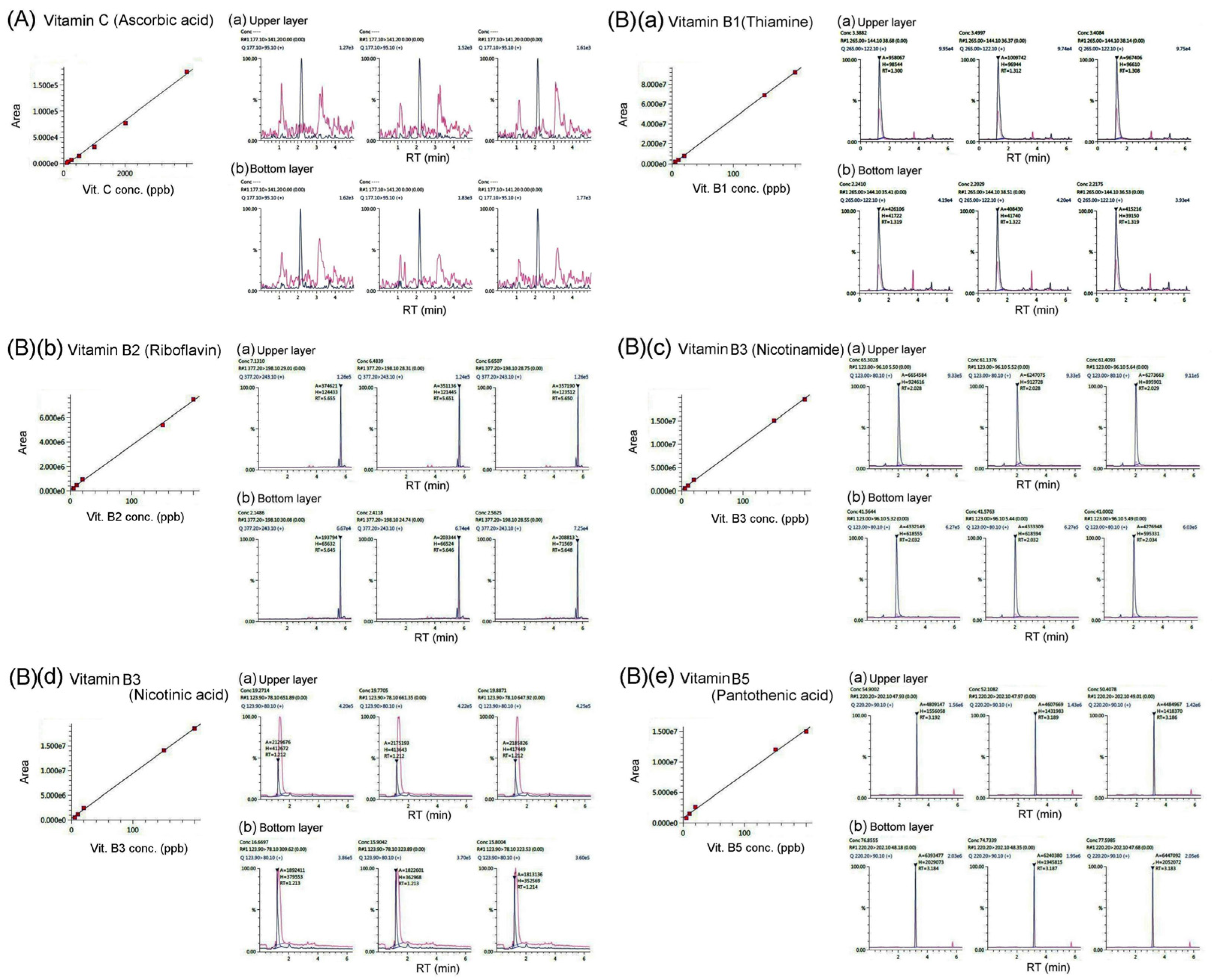
We determined whether the water-soluble vitamin C was present in bat feces using the LC/MS/ MS analysis in
Figure 3A. The results showed that the vitamin C content in bat feces was below the detection threshold and could not be detected.
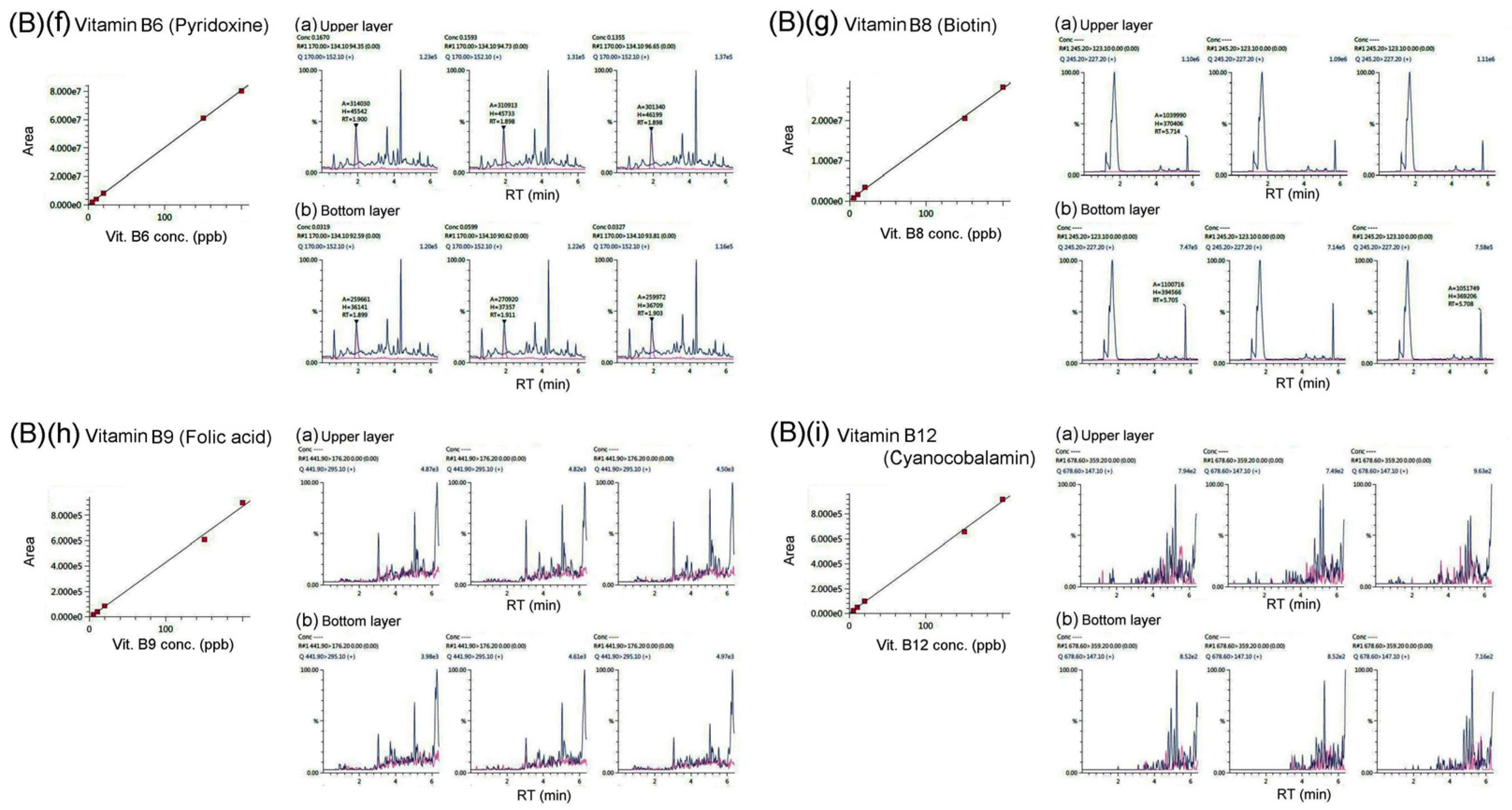
We determined the potential presence of water-soluble B vitamins in bat feces using LC/MS/ MS analysis, as shown in
Figure 3 and
Figure 4 B(a)-(i). Our research results found that bat feces contain detectable amounts of vitamins B1 (thiamine), B2 (riboflavin), B3 (nicotinamide), B3 (nicotinic acid), B5 (pantothenic acid), and B6 (pyridoxine); however, B8 (biotin), B9 (folic acid), and B12 (cyanocobalamin) were below the detection threshold of LC/MS/MS analysis and could not be detected.
Our research found that bat feces can be divided into upper and lower layers using an aqueous solution.
Table 1 shows vitamins contained in the upper and lower layers of bat feces, as determined by LC/MS/MS analysis. Our results showed that, regardless of being in the upper or lower layers of bat feces, the content of vitamins B3 (nicotinic acid) , B3 (nicotinamide), and B5 (pantothenic acid) is much higher than the content of the other B vitamins. Moreover, except for vitamin B3 (nicotinamide), the content of vitamins B1, B2, B3 (nicotinic acid), B5, and B6 in the lower layer of bat feces is slightly higher than in the upper layer of bat feces. As demonstrated by our LC/MS/MS analysis, vitamins A, C, B8, B9, B12, D3, E, and K1 were below the detection threshold of LC/MS/MS and could not be detected.
3.3. Quantification of heavy metals in bat feces using ICP/MS analysis
Bat feces are an ancient traditional method of treating visual degradation, so it is important to test the heavy metal content in them to ensure they are safe for consumption. We used ICP/MS to analyze the contents of seven heavy metals, including chromium (Cr), manganese (Mn), copper (Cu), arsenic (As), cadmium (Cd), mercury (Hg) , and lead (Pb), in bat feces samples. As reported in
Table 2, we detected Cr, Mn, Cu, As, Cd, Hg, and Pb in most bat feces samples. Among these detected heavy metals, we detected extremely high levels of Mn and Cu, with concentrations of 55.53 and 46.25 ppm, respectively. Although traditional Chinese medicine does not regulate standards for Mn and Cu content, the testing standards stipulate that the unsubdivided heavy metal limit benchmark specification must be less than 20 ppm. The bat feces samples have far exceeded the heavy metal standards of traditional Chinese medicine. In addition, as can be seen from the results in
Table 2, the As content of the bat feces samples is much higher than the heavy metal standards of TCM. Arsenic is a widely distributed and toxic metalloid element in nature. Therefore, the use of bat feces in TCM to treat visual degradation has considerable health risks.
4. Discussion
Bats, as flying mammals, have highly diverse ecological characteristics. Compared with other mammals, bats have the most variable dietary strategies, as they can be insectivores, carnivores, or various frugivores. This variability in dietary strategies may affect the composition of bat feces. A previous study suggested that bat feces mainly contain urea, uric acid, cholesterol, a small amount of vitamin A, and heavy metals such as zinc, manganese, and copper [
11]. Our results did not show the presence of vitamin A in bat feces (
Figure 2;
Table 1). Vitamins are essential for life-sustaining functions, including enzymes that promote fat and carbohydrate metabolism, and have direct and indirect antioxidant properties. Except for vitamin D, living organisms cannot produce sufficient amounts of vitamins; therefore, most vitamins must be obtained from the diet [
12]. Furthermore, most vitamins obtained from the diet are mainly absorbed from the proximal small intestine and dietary vitamins should, therefore, not reach the distal intestine and feces; thus, it is reasonable that there is no or very little vitamin A in bat feces.
Our results showed that bat feces contain vitamins B1 (thiamine), B2 (riboflavin), B3 (nicotinamide), B3 (nicotinic acid), and B5 (pantothenic acid) (
Figure 3 and
Figure 4, Table 1). Why do bat feces contain significant amounts of B vitamins? Living organisms require miniscule amounts of B vitamins, which cannot be obtained from the diet. It is reported that B vitamins can be self-synthesized by the intestinal microbiota [
13]. Furthermore, many B vitamins have been shown to be self-synthesized in the human body [
14]. B vitamins in the distal intestine also play a crucial role in maintaining intestinal microbiota homeostasis and host health through multiple mechanisms. Vitamin B1 is currently widely used to treat neuropathic pain [
15], and past studies have shown that food supplements containing vitamin B1 can improve dry eye symptoms in glaucoma patients [
16,
17]. Vitamin B2 can act as an antioxidant in the body, and its deficiency can cause visual disorders, such as conjunctivitis and cataracts [
18]. Oral vitamin B3 has a protective effect in treating or preventing glaucoma and other aging-related neurodegenerative diseases [
19]. B vitamins are known to prevent age-related macular degeneration (AMD), and a high-dose vitamin B5 intake can help reduce AMD and alleviate vision loss caused by AMD [
20]. Although these B vitamins may help maintain the health of the optic nerve and cornea, their content in bat feces is not high.
Whether bat feces are suitable as Chinese medicinal materials is still a controversial issue, because bat feces may be effective vectors and natural reservoirs for many infectious viruses, bacteria, and fungi. Bats are known to be natural hosts of many zoonotic viruses and may be responsible for numerous outbreaks, including the ongoing COVID-19 pandemic [
21]. In addition, bat feces are optimal substrates for the propagation and spread of fungi, including pathogenic histoplasmosis and fatal cryptococcosis [
22]. Our results also showed that the heavy metal content in bat feces, determined by ICP-MS analysis, exceeded the limitation standards, which may also cause harm to human health. There are many disadvantages to using bat feces as Chinese medicinal materials. For example, the chemical composition and content of bat feces are not fixed, and they can be affected by many factors, such as diet, health status, age, food contamination, and disease. When the composition and content of bat feces are uncertain, its clinical efficacy is difficult to determine. Additionally, bat feces also do not meet sanitary standards. Epidemiological surveys in the past have shown that the occurrence of diseases such as hepatitis, enteritis, dysentery, cholera, and parasites is closely related to feces-based Chinese medicinal materials. Therefore, various places have strengthened the management of feces-based Chinese medicinal materials, as the use of bat feces is not in line with people's hygienic habits.
5. Conclusions
Our country has a long history of the clinical use of bat feces-based traditional Chinese medicine. There are records of the use of bat feces in ancient Chinese medicine classics, such as Huangdi Neijing, Treatise on Febrile Diseases, and Synopsis of the Golden Chamber. Our research results found that bat feces contain vitamins B1 (thiamine), B2 (riboflavin), B3 (nicotinic acid), B3 (nicotinic acid), and B5 (pantothenic acid), but the content of these vitamins is very low. Furthermore, the heavy metal content in bat feces exceeds the standard. Therefore, we recommend that the use of bat feces as traditional Chinese medicine to improve vision should be strictly restricted.
Author Contributions
Conceptualization and methodology, K.-T. C. and H.-W. T.; investigation, L.-W. C.; writing—original draft preparation, K.-T. C. and H.-W. T.; writing—review and editing, C.-H.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to MPDI English editing service (English editing ID: english-76904) for editing and revising this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin R, Zhang B, Xue CM, Liu SM, Zhao Q, Li K. [Classification of 365 Chinese medicines in Shennong's Materia Medica Classic based on a semi-supervised incremental clustering method]. Zhong Xi Yi Jie He Xue Bao. 2011; 9(6):665-674. Chinese. [CrossRef]
- Li SZ. Compendium of Materia Medica: Bencao Gangmu. Ed. by Luo X. W. Beijing Foreign Languages Press. 2003; ISBN 7119032607.
- Wu Y. Rihuazi Materia Medica. Anhui Science and Technology Press. 2005; ISBN13:9787533732455.
- Dierenfeld ES, Seyjagat J. Plasma fat-soluble vitamin and mineral concentrations in relation to diet in captive pteropodid bats. J Zoo Wildl Med. 2000; 31(3):315-321. [CrossRef]
- Boyles JG, Cryan PM, McCracken GF, Kunz TH. Conservation. Economic importance of bats in agriculture. Science. 2011; 332(6025):41-42. [CrossRef]
- Russo D, Bosso L, Ancillotto L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: research frontiers and management implications. Agricult Ecosyst Environ. 2018; 266:31–38. [CrossRef]
- Emerson JK, Roark AM. Composition of guano produced by frugivorous, sanguivorous, and insectivorous bats. Acta Chiropterol. 2007; 9:261–267. [CrossRef]
- Banskar S, Mourya DT, Shouche YS. (2016) Bacterial diversity indicates dietary overlap among bats of different feeding habits. Microb Res. 2016; 182:99–108. [CrossRef]
- Du H, Kuang TT, Qiu S, Xu T, Gang Huan CL, Fan G, Zhang Y. Fecal medicines used in traditional medical system of China: a systematic review of their names, original species, traditional uses, and modern investigations. Chin Med. 2019;14:31.. [CrossRef]
- Yang JY, Sang Z, Wang R, Shi YH, Huang YR. Characterization and Comparison Analysis of WHO Monographs on Selected Medicinal Plants and ISO Standards of Chinese Materia Medica. Chin J Integr Med. 2023; 29(6):540-548. [CrossRef]
- Gao A, Gong J, Zheng H, Jia X, Wang M, Wu Y, Zhang X, Chen G, Ni S. Research on the traditional Chinese medicine bat feces. Jilin Journal of Traditional Chinese Medicine. 2012 ; 32 (10) 1047-1049.
- Steinert RE, Lee YK, Sybesma W. Vitamins for the Gut Microbiome. Trends Mol Med. 2020; 26(2):137-140. [CrossRef]
- Xia Y, Ji C, Li M, Zhang W, Cheng X, Qiu Y, Ge W. Simultaneous quantification of seven B vitamins in human feces by stable isotope label-based high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2024; 237: 115784. [CrossRef]
- Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015; 6:148. [CrossRef]
- Alemanno F, Ghisi D, Westermann B, Bettoni A, Fanelli A, La Colla L, Danelli G, Cesana BM. The use of vitamin B1 as a perineural adjuvant to middle interscalene block for postoperative analgesia after shoulder surgery. Acta Biomed. 2016 May 6;87(1):22-27.
- Nebbioso M, Rusciano D, Pucci B, Zicari AM, Grenga R, Pescocolido N. Treat-ment of glaucomatous patients by means of food supplement to reduce the ocular dis-comfort: a double blind randomized trial. Eur Rev Med Pharmacol Sci. 2013 Apr;17(8):1117-1122.
- Ren X, Chou Y, Wang Y, Jing D, Chen Y, Li X. The Utility of Oral Vitamin B1 and Mecobalamin to Improve Corneal Nerves in Dry Eye Disease: An In Vivo Confocal Microscopy Study. Nutrients. 2022; 14(18):3750. [CrossRef]
- Pereira A, Adekunle RD, Zaman M, Wan MJ. Association Between Vitamin Deficiencies and Ophthalmological Conditions. Clin Ophthalmol. 2023; 17:2045-2062. [CrossRef]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017; 355(6326):756-760. [CrossRef]
- Merle BMJ, Barthes S, Féart C, Cougnard-Grégoire A, Korobelnik JF, Rougier MB, Delyfer MN, Delcourt C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients. 2022 Jul 8;14(14):2821. [CrossRef]
- Das T, Sikdar S, Chowdhury MHU, Nyma KJ, Adnan M. SARS-CoV-2 prevalence in domestic and wildlife animals: A genomic and docking based structural comprehensive review. Heliyon. 2023; 9(9):e19345. [CrossRef]
- Gerbáčová K, Maliničová L, Kisková J, Maslišová V, Uhrin M, Pristaš P. The Faecal Microbiome of Building-Dwelling Insectivorous Bats (Myotis myotis and Rhinolophus hipposideros) also Contains Antibiotic-Resistant Bacterial Representatives. Curr Microbiol. 2020; 77(9):2333-2344. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).