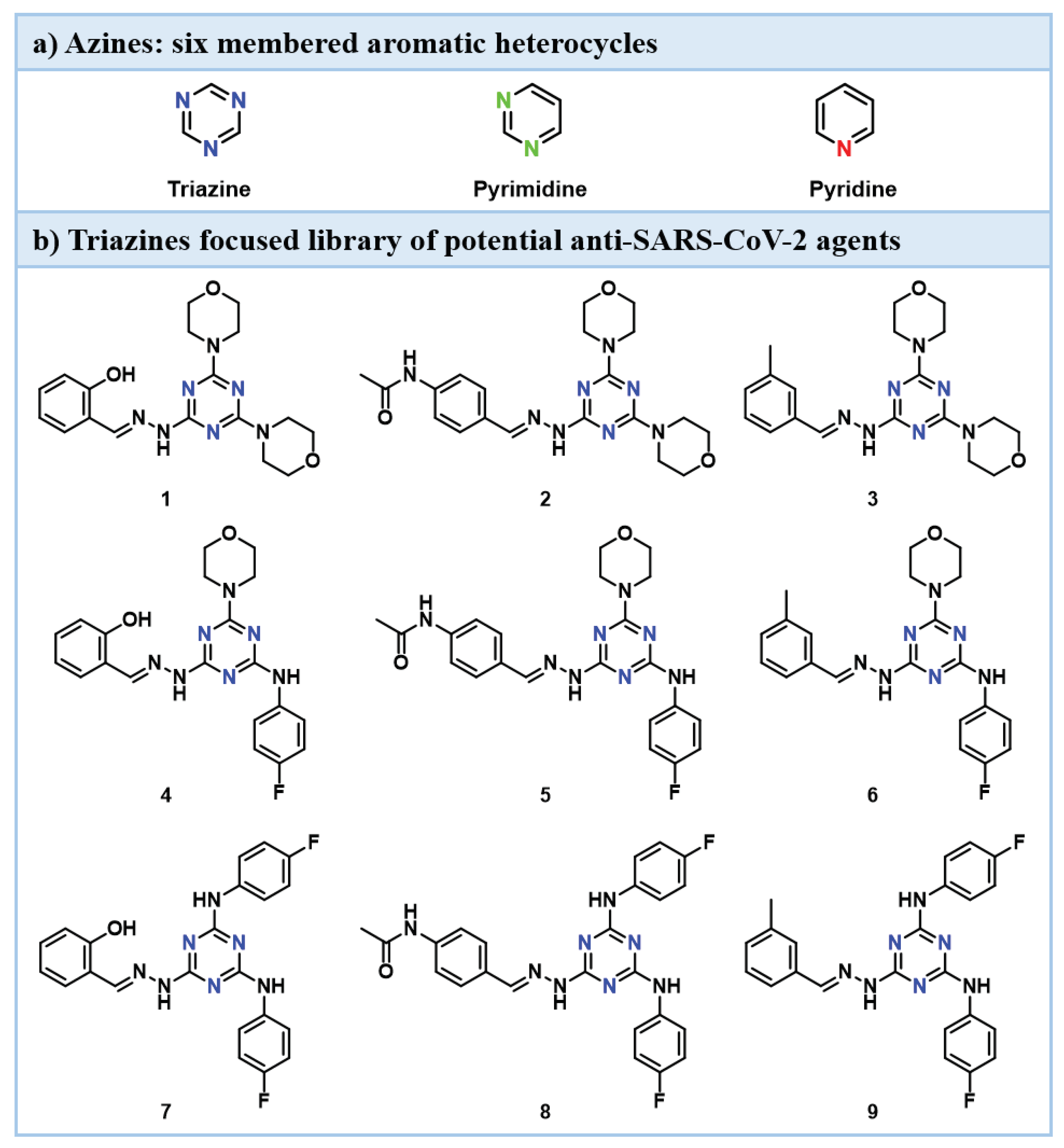
3.2. Chemistry – Experimental procedures and Compound characterization
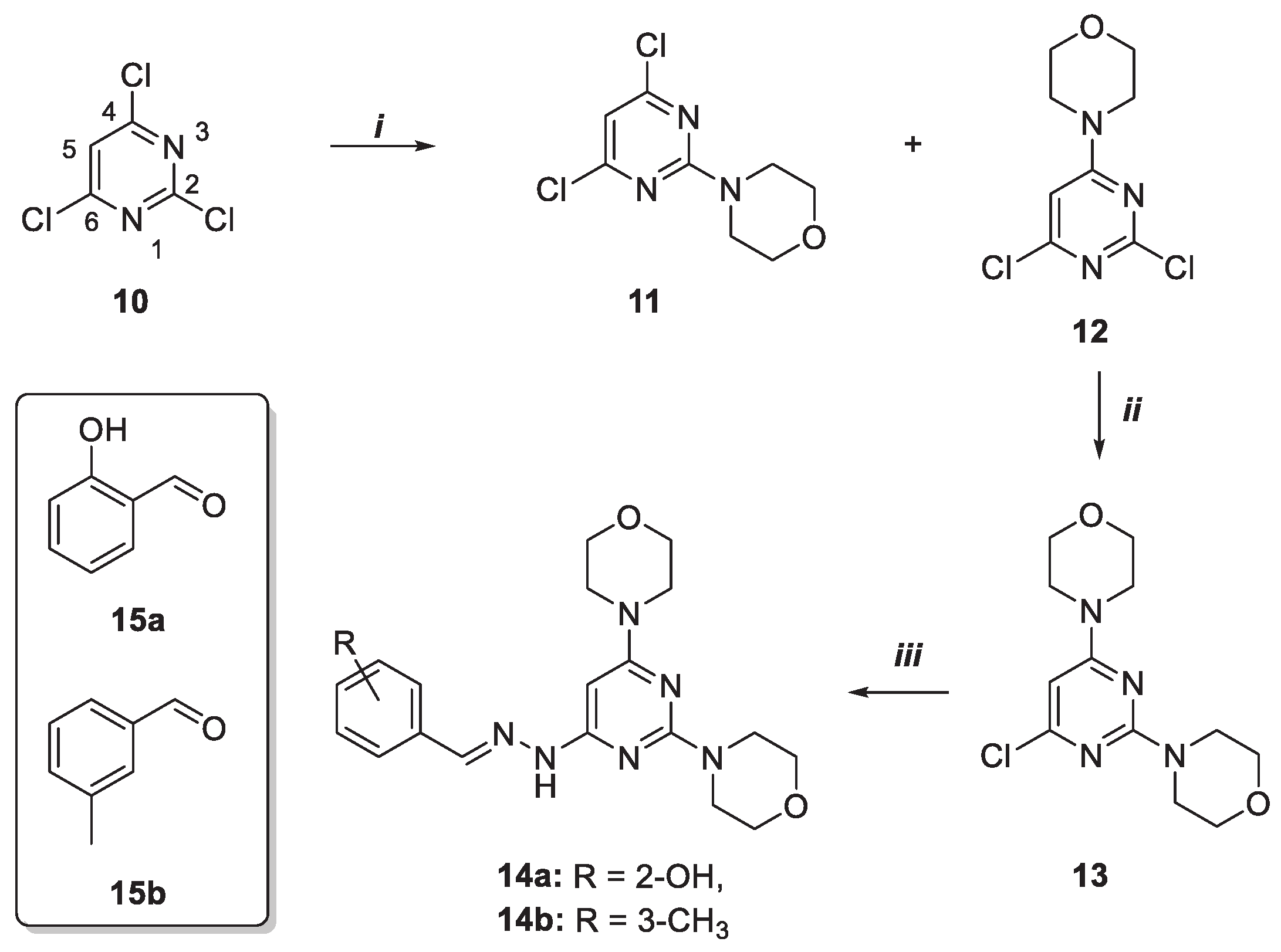
Procedure for the synthesis of monosubstituted pyrimidine derivatives 11 and 12:
To a solution of 2,4,6-trichloropyrimidine 10 (1 equiv.) in CH2Cl2 (10 mL) at 0 °C, morpholine (1 equiv.) was slowly added, followed by DIPEA (1 equiv.). The reaction mixture was stirred at 0 °C for 5h, and then warmed up to 25 °C. The mixture was washed with H2O and brine. The organic phase was dried over anhydrous Na2SO4 and evaporated to dryness. The resulting residue was purified by column chromatography using a mixture of petroleum ether (PET)/ethyl acetate (EtOAc) 4:1 to give the desired products 11 and 12 with 19% and 68% yield, respectively.
Compound 11
Rf= 0.29 (PET/EtOAc 4:1); 1H NMR (400 MHz, CDCl3) δ 6.57 (s, 1H), 3.83 (d, J=5.2 Hz, 4H), 3.76 (d, J=4.8 Hz, 4H) ppm. 13C NMR (100 MHz, CDCl3) δ 161.8, 160.6, 108.3, 66.6, 44.4 ppm. MS m/z for C8H10Cl2N3O, (ESI+) m/z: 234.0 [M+H]+.
Compound 12
Rf= 0.26 (PET/EtOAc 4:1); 1H NMR (400 MHz, CDCl3) δ 6.41 (s, 1H), 3.79-3.77 (m, 4H), 3.65 (s, 4H) ppm. 13C NMR (100 MHz, CDCl3) δ 163.3, 160.7, 159.9, 99.7, 66.2, 44.6 ppm. MS m/z for C8H10Cl2N3O, (ESI+) m/z: 234.0 [M+H]+.
Procedure for the synthesis of disubstituted pyrimidine derivative 13
Compound 12 (1 equiv.) was dissolved in 1,4-dioxane (10 mL) and morpholine (2.5 equiv.) was added. Then, were slowly added few drops of HCl. The reaction was conducted under microwave irradiation for 40 minutes at 110 °C. After cooling to 25 °C, the mixture was washed with H2O and brine. The organic phase was dried over anhydrous Na2SO4 and evaporated to dryness. The solid residue obtained was purified by column chromatography using diethyl ether (Et2O)/PET 2:1 as eluant to give the disubstituted intermediate 13 with 90% yield.
Rf = 0.26 (Et2O/PET); 1H NMR (400 MHz, CDCl3) δ 5.87 (s, 1H), 3.76-3.73 (m, 8H), 3.56-3.54 (m, 8H) ppm. 13C NMR (100 MHz, CDCl3) δ 163.5, 160.8, 160.6, 91.1, 66.8, 66.5, 44.4, 44.3 ppm. MS m/z for C12H18ClN4O2, (ESI+) m/z: 285.1 [M+H]+.
General Procedure for the synthesis of trisubstituted pyrimidine derivatives 14a-b
Compound 13 (1 equiv.) was dissolved in 1,4-dioxane (1 mL) and hydrazine hydrate (20 equiv.) was added. The reaction was stirred under microwave irradiation for 4 h at 100 °C. After this time, the reaction mixture was diluted with EtOAc and then was washed with H2O and brine, dried over anhydrous Na2SO4 and evaporated to dryness. The crude residue was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and a drop of acetic acid were added. The mixture was stirred for 24 h at 25 °C. The precipitate formed was filtered-off and dried under high vacuum giving the desired products 14a-b with 55 and 65% yield, respectively.
Compound 14a
Rf = 0.19 (hexane (Hex)/EtOAc 1:1). 1H-NMR (400 MHz, CDCl3) δ 10.54 (s, 1H), 8.40 (s,1H), 8.06 (s 1H), 7.32 (d, J=7.2 Hz, 1H), 7.23 (d, J=7.6 Hz, 1H), 7.00-6.93 (m, 2H), 5.55 (s 1H), 3.79 (d, J=8.4 Hz, 8H), 3.63 (s, 8H) ppm. 13C NMR (100 MHz, CDCl3) δ 164.0, 160.6, 157.3, 143.9, 130.9, 130.0, 119.7, 118.0, 116.6, 72.8, 66.8, 66.6, 44.5, 44.5 ppm. MS m/z for C19H25N6O3, (ESI+) m/z: 385.2 [M+H]+.
Compound 14b
Rf = 0.25 (Hex/EtOAc 1:1). 1H NMR (400 MHz, CDCl3) δ 8.14 (s, 1H), 7.68 (s, 1H), 7.47 (d, J=7.2 Hz, 2H), 7.30-7.26 (m, 2H), 7.17 (d, J=7.2 Hz, 1H), 5.92 (s, 1H), 3.81-3.79 (m, 4H), 3.74-3.72 (m, 8H), 3.63 (d, J=5.2 Hz, 4H), 2.39 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.2, 162.5, 161.1, 140.5, 138.3, 134.5, 130.0, 128.5, 127.2, 123.9, 73.8, 66.9, 66.7, 44.6, 44.4, 21.4 ppm. MS m/z for C20H27N6O2, (ESI+) m/z: 383.2 [M+H]+.
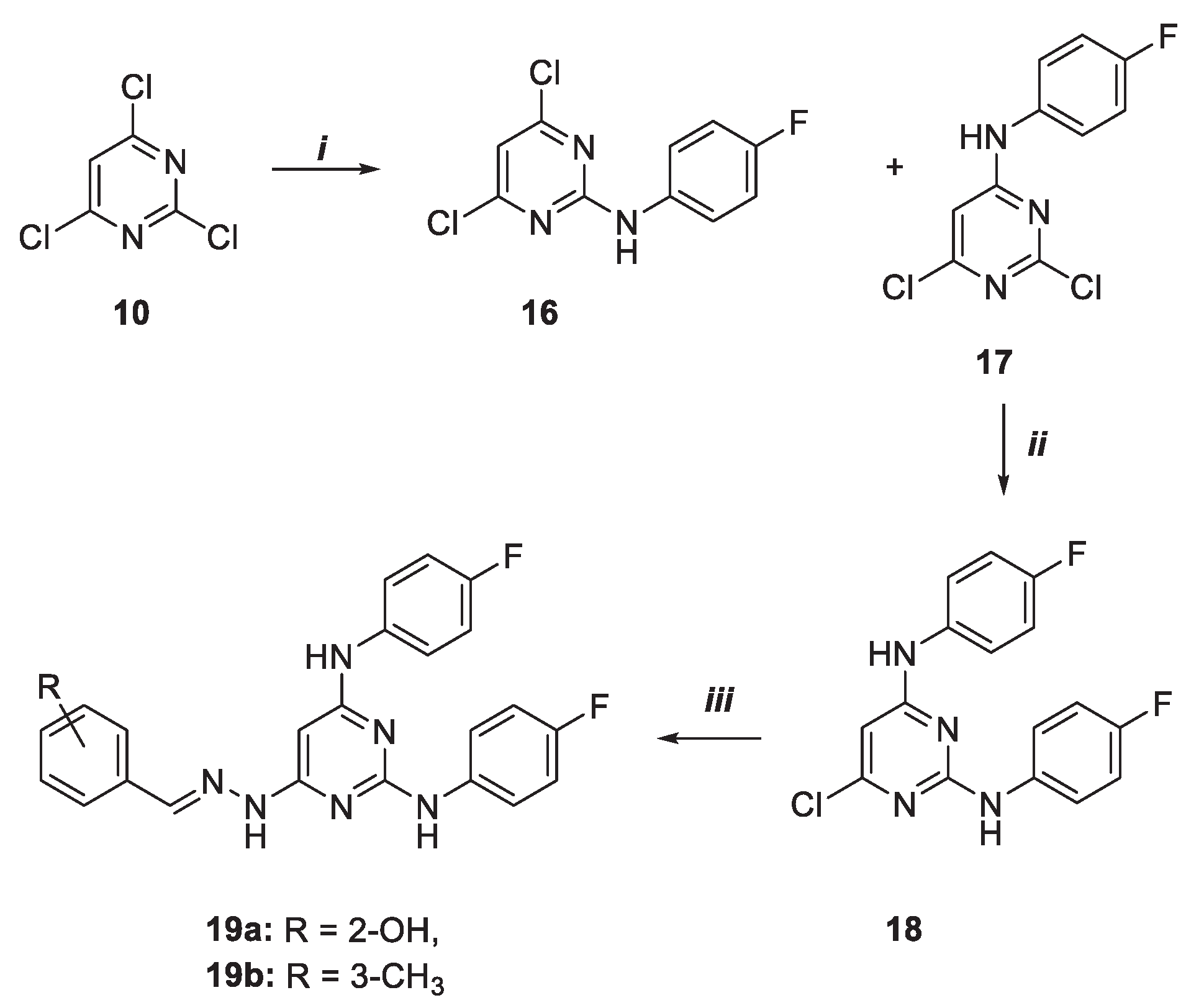
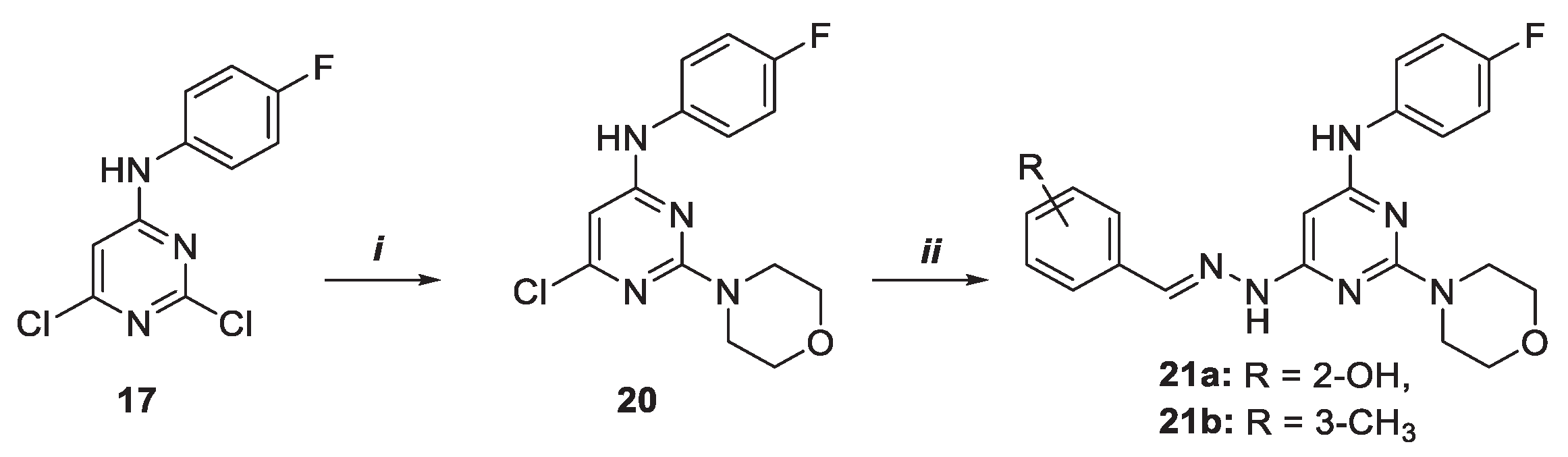
Procedure for the Synthesis of monosubstituted pyrimidine intermediate 16 and 17
To a solution of 2,4,6-trichloropyrimidine 10 (1 equiv.) in CH2Cl2 (10 mL) at 0 °C, 4-fluoroaniline (1 equiv.) was slowly added, followed by DIPEA (1 equiv.). The obtained reaction mixture was stirred at 0 °C for 5h, and then warmed up to 25 °C. The mixture was washed with water, dried over N2SO4 and the solvent removed under reduced pressure. The crude residue was purified by column chromatography PET/EtOAc 4:1 to give the desired products 16 and 17 with 14 and 75% yield, respectively.
Compound 16
Rf= 0.29 (PET/EtOAc 4:1). 1H NMR (400 MHz, CDCl3) δ 7.53-7.50 (m, 2H), 7.09-7.04 (m, 2H), 6.80 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3) δ 162.0, 159.8 (d, J= 149.0 Hz), 158.1, 133.7, 121.9 (d, J= 7.9 Hz), 115.8 (d, J= 22.5 Hz), 115.7, 111.3 ppm. MS m/z for C10H7Cl2FN3, (ESI+) m/z: 258.0 [M+H]+.
Compound 17
Rf= 0.22 (PET/EtOAc 4:1).1H NMR (400 MHz, CDCl3) δ 7.30-7.27 (m, 2H), 7.17-7.13 (m, 2H), 6.44 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3) δ 163.6, 161.8 (d, J= 116.0 Hz), 160.2, 160.0, 132.1, 126.4 (d, J= 7.1 Hz), 116.9 (d, J= 22.7 Hz), 100.7 ppm. MS m/z for C10H7Cl2FN3, (ESI+) m/z: 258.0 [M+H]+.
Procedure for the synthesis of disubstitutedpyrimidine intermediate 18
Compound 17 was dissolved in 1,4-dioxane (10 mL) and 4-fluoroaniline (2.5 eq) was added. Then few drops of HCl were slowly added to the mixture. The reaction was conducted under microwave irradiation for 40 minutes at 110 °C. The mixture was washed with water, brine and dried over N2SO4.The residue was purified by column chromatography with CH2Cl2 as eluant. The purified material was dried in vacuo to afford the desired product with 62% yield.
Rf = 0.20 (CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.49-7.46 (m, 2H), 7.31-7.27 (m, 2H), 7.11-7.06 (m, 2H), 7.03-6.99 (m, 2H), 6.98 (s, 1H), 6.06 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3) δ 162.7, 159.9 (d, J= 100.1 Hz), 157.6, 134.8, 133.5, 125.4 (d, J= 8.0 Hz), 121.8 (d, J= 7.7 Hz), 116.3 (d, J= 22.6 Hz), 115.5 (d, J= 22.3 Hz), 94.4 ppm. MS m/z for C16H12ClF2N4, (ESI+) m/z: 333.1 [M+H]+.
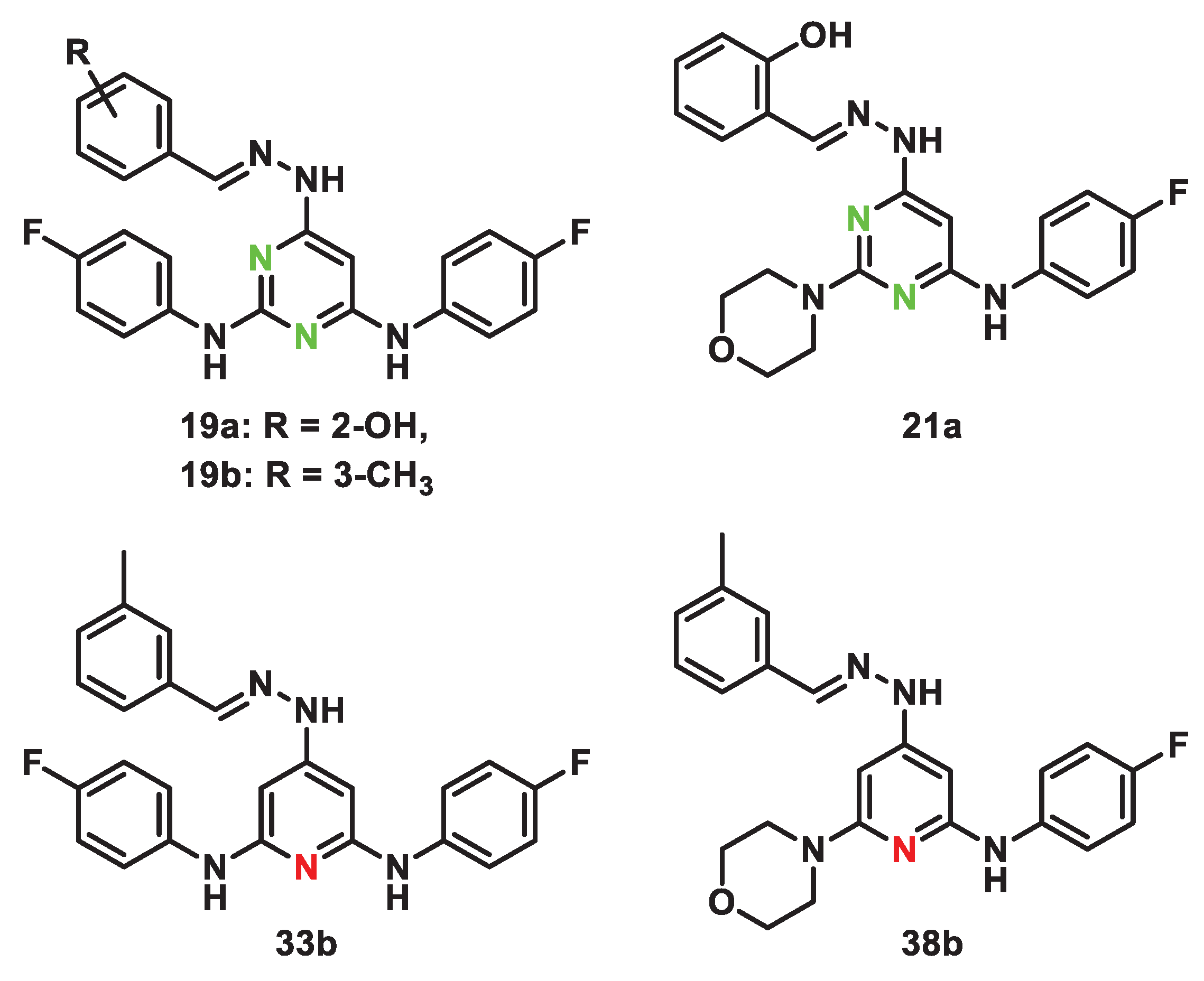
General procedure for the synthesis of trisubstituted pyrimidine derivatives 19a-b
Compound 18 (1 equiv.) was dissolved in 1,4-dioxane (1 mL) and hydrazine hydrate (20 equiv.) was added. The reaction was stirred under microwave irradiation for 4 h at 100 °C. After this time, the reaction mixture was diluted with EtOAc and then washed with H2O and brine, and dried over N2SO4. The crude residue was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and a drop acetic acid were added. The mixture was stirred for 48 h at 25 °C. The precipitate formed was filtered-off and dried under high vacuum giving the desired products 19a-b with 53 and 61% yield, respectively.
Compound 19a
Rf = 0.42 (Hex/EtOAc 1:1) 1H-NMR (400 MHz, CD3OD) δ 8.11 (s, 1H), 7.69-7.65 (m, 3H), 7.56-7.53 (m, 3H), 7.29-7.28 (d, J=6.4 Hz, 1H), 7.27-7.23 (d, 1H), 7.04-7.00 (m, 4H), 6.98-6.91 (m, 4H), 5.76 (s, 1H) ppm. 13C NMR (100 MHz, CD3OD) δ 162.4, 160.5 (d, J= 145.2 Hz), 159.1, 157.1, 156.7, 143.5, 137.0, 136.6, 130.0, 129.4, 122.4 (d, J= 7.6 Hz), 120.9 (d, J= 7.4 Hz), 119.2, 118.8, 115.9, 114.7 (d, J= 22.3 Hz), 114.3 (d, J= 22.2 Hz), 76.1 ppm. MS m/z for C23H19F2N6O, (ESI+) m/z: 433.2 [M+H]+.
Compound 19b
Rf = 0.30 (Hex/EtOAc 1:1). 1H NMR (400 MHz, CD3OD) δ 7.87 (s, 1H),7.67-7.64 (m, 2H), 7.57-7.54 (m, 2H), 7.51 (s, 1H), 7.44 (d, J=7.6 Hz, 1H), 7.27 (d, J=7.6 Hz, 1H), 7.16 (d, J= 7.6, 2H), 7.04-6.97 (m, 4H), 2.34 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ 162.3, 160.9 (d, J= 257.2 Hz), 159.1, 157.2, 156.7, 140.9, 138.0, 135.3, 129.4, 128.1, 126.5, 123.5, 122.3 (d, J= 7.5 Hz), 121.0 (d, J= 7.4 Hz), 114.6 (d, J= 22.3 Hz), 114.3 (d, J= 22.2 Hz), 76.9, 20.0 ppm. MS m/z for C24H21F2N6, (ESI+) m/z: 431.2 [M+H]+.
Procedure for the synthesis of disubstituted pyrimidine 20
To a solution of compound 17 (1 equiv.) in EtOH (10 mL), morpholine (1 equiv.) and triethyl amine (1.5 equiv.) were added. The reaction mixture was stirred at 80 °C for 18 h. After this time, the solvent was evaporated under reduced pressure and the residue was washed with water, brine and dried over Na2SO4. The crude obtained was purified by column chromatography using CH2Cl2/MeOH (10 mL:100 μL) as eluant. The purified material was dried in vacuo to afford the desired product with 62% yield.
Rf = 0.20 (CH2Cl2/MeOH 10mL:100 μL). 1H NMR (400 MHz, CDCl3) δ 7.29-7.26 (m, 2H), 7.08-7.04 (m, 2H), 6.55 (s,1H), 5.89 (s, 1H), 3.75 (d, J=4 Hz, 8H) ppm. 13C NMR (100 MHz, CDCl3) δ 164.3, 161.6 (d, J=200.2 Hz), 158.0, 135.5, 124.2 (d, J=7.1 Hz), 115.9 (d, J=22.4 Hz), 73.9, 67.0, 66.6, 44.6, 44.5 ppm. MS m/z for C14H15ClFN4O, (ESI+) m/z: 309.1 [M+H]+.
Procedure for the synthesis of disubstituted pyrimidine 21a-b
Compound 20 (1 equiv.) was dissolved in 1,4-dioxane (1 mL) and hydrazine hydrate (20 equiv.) was added. The reaction was stirred under microwave irradiation for 6 h at 100 °C. After this time, the reaction mixture was diluted with EtOAc and then washed with H2O and brine, and dried over N2SO4. The crude residue was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and a drop acetic acid were added. The mixture was stirred for 48 h at 25 °C. The precipitate formed was filtered-off and dried under high vacuum giving the desired products 21a-b with 46% and 45% yield, respectively.
Compound 21a
Rf = 0.18 (CH2Cl2/MeOH 10:0,2). 1H-NMR (400 MHz, CDCl3) δ 10.48 (s, 1H), 7.88 (s, 1H), 7.36-7.33 (m, 2 H), 7.33-7.27 (m, 2H), 7.17 (d, J=7.6 Hz, 1H), 7.10-7.05 (m, 2 H), 7.00-6.98 (m, 1H), 6.94-6.92 (m, 1 H), 6.44 (s, 1H), 5.66 (s, 1H), 3.76 (s, 8H) ppm. 13C-NMR (100 MHz, CDCl3) δ 175.4, 162.3, 161.0, 159.3 (d, J= 251.4 Hz), 157.4, 144.2, 135.0, 131.0, 130.1, 123.6 (d, J= 8.0 Hz), 119.6, 117.9, 116.8, 115.9 (d, J= 22.4 Hz), 74.6, 66.9, 44.6 ppm. MS m/z for C21H22FN6O2, (ESI+) m/z: 409.2 [M+H]+.
Compound 21b
Rf: 0.29 (CH2Cl2/MeOH 10:0,2) 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 1H), 7.44 (s, 1H), 7.41 (s, 1H), 7.39-7.35 (m, 2H), 7.29 (s, 1H), 7.18 (d, J=7.6 Hz, 1H), 7.09-7.04 (m, 2H), 6.39 (s, 1H), 6.02 (s, 1H), 3.75 (s, 8H), 2.38 (3H) ppm. 13C NMR (100 MHz, DMSO-d6) δ 162.4, 161.8, 160.0 (d, J= 309.8 Hz), 156.1, 140.8, 138.4, 138.0, 135.6, 130.0, 129.1, 126.8, 123.9, 121.1 (d, J= 7.4 Hz), 115.5 (d, J= 21.8 Hz), 76.5, 66.6, 44.7, 21.5 ppm. MS m/z for C22H24FN6O, (ESI+) m/z: 407.2 [M+H]+.
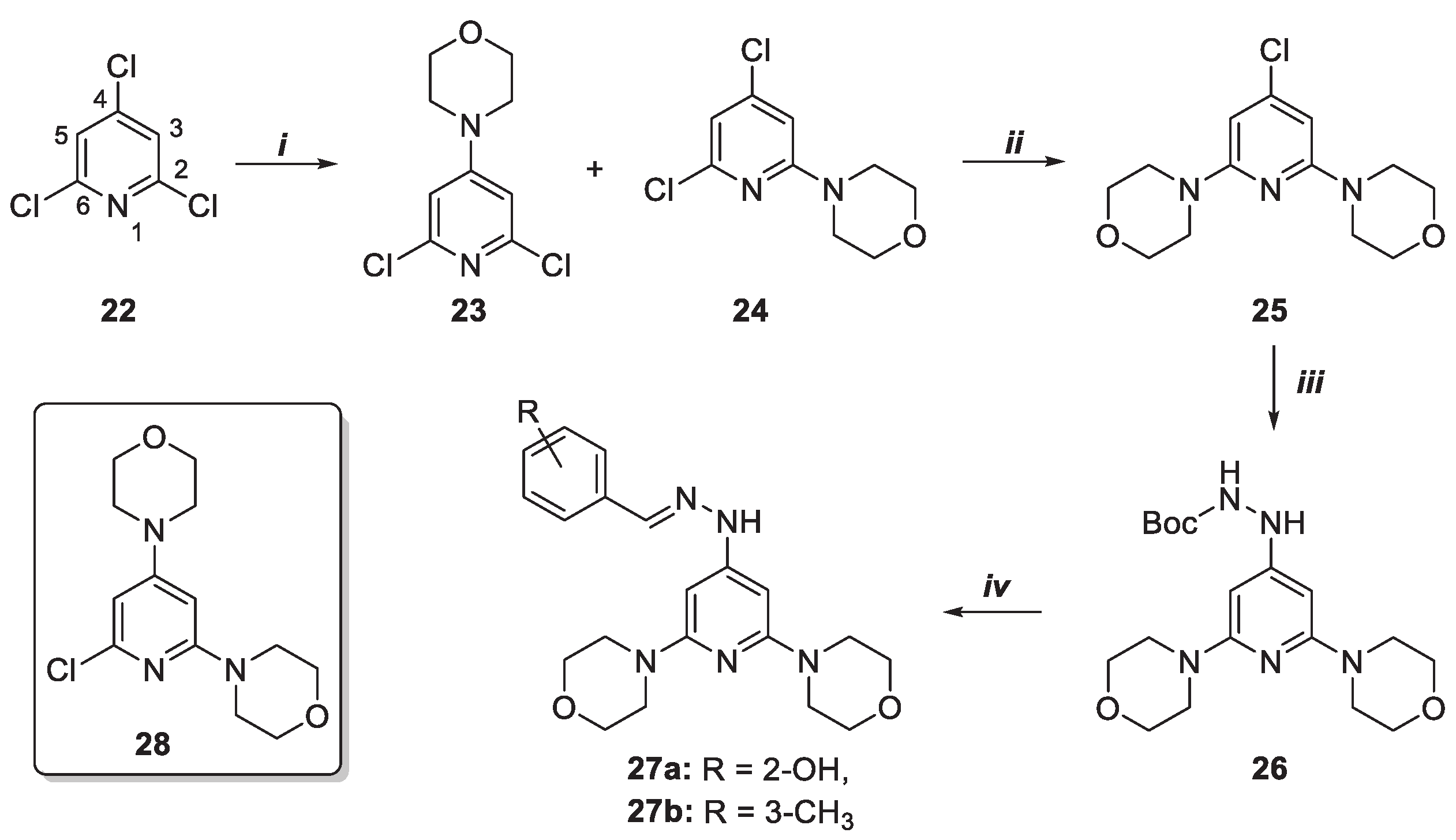
Procedure for the synthesis of monosubstituted pyridine derivatives 23 and 24
To a solution of morpholine (1 equiv.) and Et3N (2.5 equiv.) in dry THF (5 mL) was added a solution of 2,4,6-trichlorpyridine 22 (1 equiv.) in THF (2 mL). The reaction mixture was stirred 24 h at 65 °C. After cooling to 25 °C, the reaction was concentrated to remove THF and then diluted with Et2O and washed with water and brine, dried over anhydrous Na2SO4, filtered and evaporated in vacuo. The crude mixture was purified by column chromatography using Hex/EtOAc 5:1 as eluant to give 23 and 24 with 15 and 30 % yield, respectively.
Compound 23
Rf = 0.17 (Hex/EtOAc 5:1). 1H NMR (400 MHz, CDCl3) δ 6.57 (s, 2H), 3.81 (t, J=4 Hz, 4H), 3.30 (t, J=4 Hz, 4H) ppm. 13C NMR (100 MHz, CDCl3) δ 158.0, 151.2, 106.2, 66.0, 46.1 ppm. MS m/z for C9H11Cl2N2O, (ESI+) m/z: 233.0 [M+H]+.
Compound 24
Rf = 0.40 (Hex/EtOAc 5:1). 1H NMR (400 MHz, CDCl3) δ = 6.66 (d, J=1.2 Hz, 1H), 6.47 (d, J=4 Hz, 1H), 3.79 (t, J=4.8 Hz, 4H), 3.53 (t, J=4 Hz, 4H) ppm. 13C NMR (100 MHz, CDCl3) δ 159.2, 150.2, 146.3, 112.6, 104.4, 66.4, 45.1 ppm. MS m/z for C9H11Cl2N2O, (ESI+) m/z: 233.0 [M+H]+.
Procedure for the Synthesis of disubstituted pyridine derivatives 25 and 28
To a solution of monosubstituted pyridine 24 (1 equiv.) in DMF (2 ml) was added morpholine (10 equiv.) and DIPEA (3 equiv.). The mixture was warmed at 120 °C for 48 h. After cooling to 25 °C, the reaction mixture was diluted with CH2Cl2 and washed with aqueous solution of 3% LiCl. The organic phase was dried over anhydrous Na2SO4, filtered and evaporated in vacuo to afford a crude mixture of two regioisomers. Products 25 and 28 can be separated by column chromatography using a mixture of Hex/EtOAc 2:1, and recovered with 58 and 18% yield, respectively.
Compound 25
Rf = 0.45 (Hex/EtOAc 2:1)] 1H NMR (400 MHz, CDCl3) δ 6.0 (s, 2H), 3.79 (d, J=4 Hz, 8H), 3.47 (d, J=8 Hz, 8H) ppm. 13C NMR (100 MHz, CDCl3) δ 158.2, 146.8, 97.0, 66.7, 49.3 ppm. MS m/z for C13H19ClN3O2, (ESI+) m/z: 284.1 [M+H]+.
Compound 28
Rf = 0.13 (Hex/EtOAc 2:1). 1H NMR (400 MHz, CDCl3) δ 6.20 (d, J=4 Hz, 1H), 5.77 (d, J=1,2 Hz, 1H), 3.82-3.78 (m, 8H), 3.46 (t, J=4 Hz, 4H), 3.25 (t, J=4 Hz, 4H) ppm. 13C NMR (100 MHz, CDCl3) δ 159.2, 150.2, 146.3, 112.6, 104.4, 66.4, 45.1 ppm. MS m/z for C13H19ClN3O2, (ESI+) m/z: 284.1 [M+H]+.
Procedure for the synthesis of Boc-protected trisubstituted pyridine carbazate 26
In a microwave tube were added under argon compound 25 (1 equiv.), tert-butyl carbazate (BocNHNH2, 10 equiv.), Pd2(dba)3 (0,04 equiv.), DPPF (0,12 equiv.), Cs2CO3 (2 equiv.) and dry toluene (1 mL). The reaction mixture was stirred at 150°C for 10 minutes under microwave irradiation. The mixture was filtered through celite, rinsed with EtOAc and concentrated. The crude was purified by column chromatography with CH2Cl2/EtOAc 4:1 as eluant to give the product 26 with 65% yield.
Rf= 0.43 (Hex/EtOAc 2:1)] 1H NMR (400 MHz, CDCl3) δ 6.2 (s, 2H), 3.98-3.85 (m, 8H), 3.81 (t, J=4.8 Hz, 4H), 3.50 (t, J=4.4 Hz, 4H), 1.49 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ 159.0, 154.4, 153.2, 89.9, 82.6, 66.9, 46.0, 28.3 ppm. MS m/z for C18H30N5O4, (ESI+) m/z: 380.2 [M+H]+.
Procedure for the synthesis of trisubstituted pyridine derivatives 27a-b
Boc-protected pyridine 26 (1 equiv.) was dissolved in anhydrous CH2Cl2 (430µL) and cooled to 0°C. TFA (290µL) was added and the reaction mixture was warmed to 25 °C and stirred for 1h. After this time, the mixture was concentrated at reduced pressure. The residue obtained was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and few drops of acetic acid were added. The mixture was stirred for 24 h at 25 °C and concentrated under vacuum. The residue was purified by column chromatography (Hex/EtOAc 2:1) furnishing the desired products 27a-b with 39% and 18% yield, respectively.
Compound 27a
Rf = 0.40 (Hex/EtOAc 1:1). 1H NMR (400 MHz, CDCl3) δ 10.92 (s, 1H), 8.39 (s, 1H), 7.86 (s, 1H), 7.27 (d, J=12.0 Hz, 1H), 7.16-7,14 (dd, J=1.6 Hz, J=7.6 Hz, 1H), 7.00 (d, J=8.0 Hz, 1H), 6.91 (s, 1H), 5.96 (d, J=1.6 Hz, 1H), 5.60 (d, J=1.2 Hz, 1H), 3.85-3.78 (m, 8H), 3.44 (t, J=4.0 Hz, 4H), 3.31 (t, J=4.0 Hz, 4H) ppm. 13C NMR (CDCl3, 100 Hz) δ 160.1, 159.6, 157.1, 155.3, 141.7, 130.1, 129.5, 119.5, 118.5, 116.5, 84.5, 82.1, 66.8, 47.0, 46.2 ppm. MS m/z for C20H26N5O3, (ESI+) m/z: 384.2 [M+H]+.
Compound 27b
Rf = 0.55 (Hex/EtOAc 2:1). 1H NMR (400 MHz, CDCl3) δ = 8.11 (s, 1H), 7.66 (s, 1H), 7.47 (d, J=8.0 Hz, 2H), 7.27 (t, J=8.0 Hz, 1H), 7.14 (d, J=8.0 Hz, 1H), 6.31(d, J=4.0 Hz, 1H), 5.57 (d, J=2.0 Hz, 1H), 3.86-3.80 (m, 8H), 3.42 (t, J=8.0 Hz, 4H), 3.33 (t, J=8.0 Hz, 4H), 2.39 (s, 3H) ppm. 13CNMR (CDCl3, 100 Hz) δ 159.1, 138.4, 134.2, 130.6, 128.2, 127.9, 124.1, 82.7, 81.1, 66.2, 48.2, 46.3, 31.2, 29.7, 21.3 ppm. MS m/z for C21H28N5O2, (ESI+) m/z: 382.2 [M+H]+.
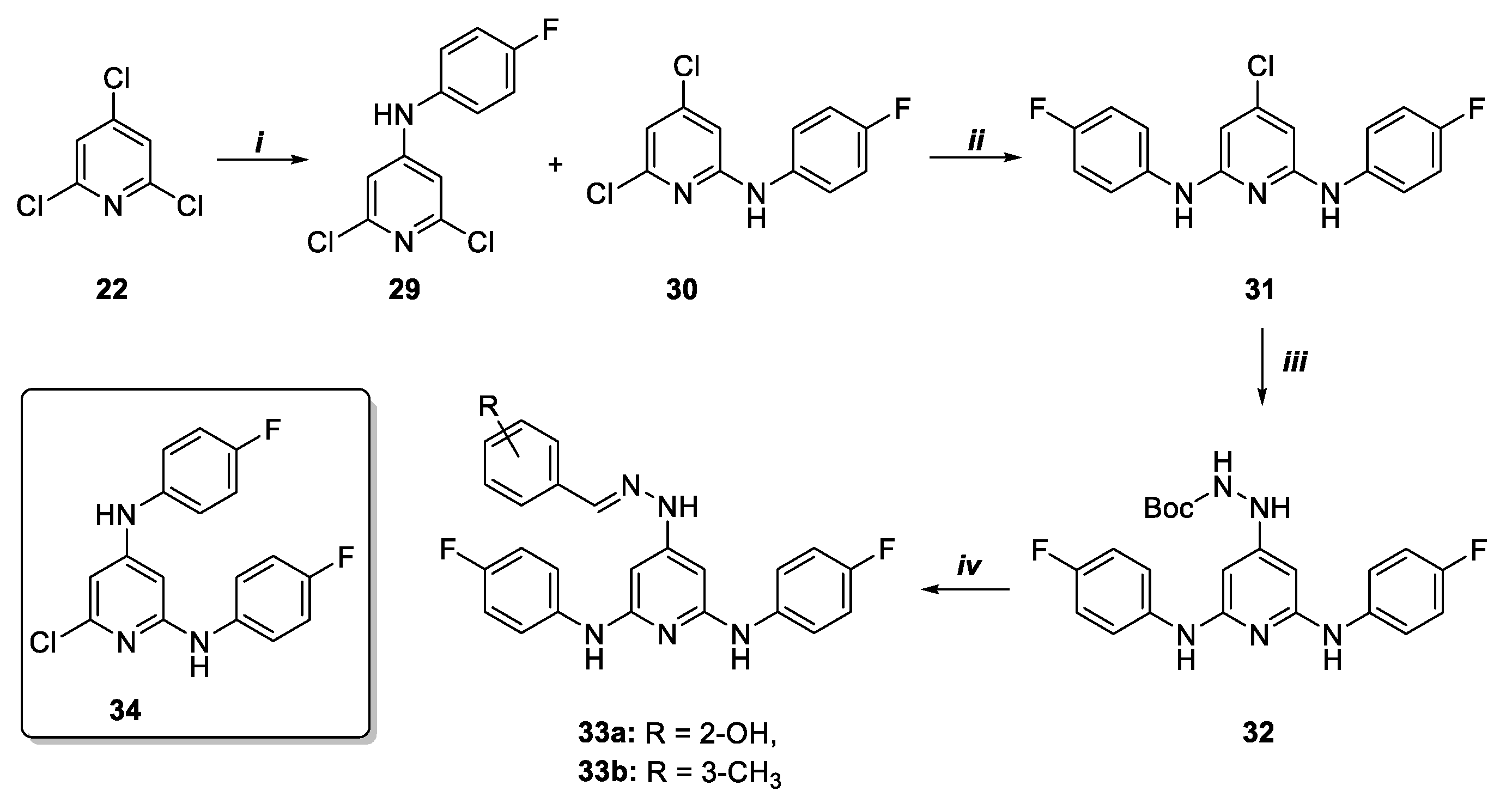
Procedure for the synthesis of monosubstituted pyridine derivatives 29 and 30
In a two necks flask equipped with molecular sieves, 2,4,6-tricloropyridine 22 (1 equiv.), Cs2CO3 (1 equiv.), Pd(PPh3)4 (0,02 equiv.) and dry toluene (5 mL) were added. Subsequently, 4-fluoroaniline (1,6 equiv.) was added under argon atmosphere and the reaction mixture was stirred for 24h at 150 °C. After this time, the mixture was filtered through celite, rinsed with EtOAc and concentrated. The residue so obtained was diluted with EtOAc, washed with a saturated aqueous solution of NaHCO3 and brine, dried over Na2SO4 and concentrated under reduced pressure. The crude was purified by column chromatography with Pet/EtOAc 4:1 as eluant to give the products 29 and 30 with 19 and 50% yield, respectively.
Compound 29
Rf = 0.50 (Pet/EtOAc 4:1). 1H NMR (400 MHz, CDCl3) δ 7.27 (m, 2H), 7.12 (t, J=8.4 Hz, 2H), 7.00 (s, 1H), 6.7 (s, 2H) ppm. 13C (100 MHz, CDCl3) δ 159.3 (d, J=241.9 Hz), 156.2, 146.3, 135.6, 123.8 (d, J=6.9 Hz), 116.0 (d, J=22.3 Hz), 98.0 ppm. MS m/z for C11H8Cl2FN2, (ESI+) m/z: 257.0 [M+H]+.
Compound 30
Rf = 0.65 (Pet/EtOAc 4:1). 1H NMR (400 MHz, CDCl3) δ 7.26 (m, 2H), 7.09 (t, J=8.8 Hz, 2H), 7.00 (s, 1H), 6.73 (d, J=1.2 Hz, 1H), 6.54 (d, J=1.2 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3) δ 160.0 (d, J=240.3 Hz), 157.2, 150.5, 146.6, 134.5, 124.8 (d, J=8.1 Hz), 116.4 (d, J=22.6 Hz), 114.2, 104.9 ppm. MS m/z for C11H8Cl2FN2, (ESI+) m/z: 257.0 [M+H]+.
Procedure for the synthesis of disubstituted pyridine derivatives 31 and 34
Pd(OAc)2 (0.02 equiv.) and BINAP (0.02 equiv.) were dissolved in anhydrous toluene (1 mL) and the mixture was kept under argon for 10 minutes. At the same time, in a Schenck tube were added compound 30 (1 equiv.), anhydrous toluene (1 mL), Cs2CO3 (3.5 equiv.), and 4-fluoroaniline (1.5 equiv.) and then the solution of Pd(OAc)2 and BINAP above described. The reaction mixture was warmed at 160°C and left under stirring for 4 h. After this time, the mixture was filtered through celite, rinsed with EtOAc and concentrated. The crude was purified by column chromatography with Pet/EtOAc 3:1 as eluant to give the products 31 and 34 with 36 and 13% yield, respectively.
Compound 31
Rf = 0.63 (Pet/EtOAc 3:1). 1H NMR (400 MHz, CDCl3) δ 7.29 (m, 4H), 7.06 (m, 4H), 6.65 (s, 1H), 6.13 (s, 2H) ppm. 13C NMR (100 MHz, CDCl3) δ 159.2 (d, J=260.2 Hz), 156.0, 146.6, 135.4, 123.9 (d, J=7.9 Hz), 116.1 (d, J=22.4 Hz), 98.0 ppm. MS m/z for C17H13ClF2N3, (ESI+) m/z: 332.1 [M+H]+.
Compound 34
Rf = 0.44 (Pet/EtOAc 3:1). 1H NMR (400 MHz, CDCl3) δ 7.21 (m, 4H), 7.11 (m, 4H), 6.45 (s, 1H), 6.24 (d, J=1.6 Hz, 1H), 6.0 (d, J=1.6 Hz, 1H), 5.9 (s, 1H) ppm. 13C (100 MHz, CDCl3) δ 159.2 (d, J=270.4 Hz), 156.8, 148.1, 141.5, 136.5, 123.4 (d, J=7.4 Hz), 116.3 (d, J=22.4 Hz), 100.0, 94.0 ppm. MS m/z for C17H13ClF2N3, (ESI+) m/z: 332.1 [M+H]+.
Procedure for the synthesis of Boc-protected trisubstituted pyridine carbazate 32
In a microwave tube were added under argon compound 31 (1 equiv.), BocNHNH2 (10 equiv.), Pd2(dba)3 (0,04 equiv.), DPPF (0,12 equiv.), Cs2CO3 (2 equiv.) and dry toluene (1 mL). The reaction mixture was stirred at 150°C for 10 minutes under microwave irradiation. The mixture was filtered through celite, rinsed with EtOAc and concentrated. The crude was purified by column chromatography with Hex/EtOAc 2:1 as eluant to give the product 32 with 59% yield.
Rf=0.37 (Hex/EtOAc 2:1). 1H NMR (400 MHz, CDCl3): δ 7.31 (m, 4H), 7.03 (m, 4H), 6.61 (s, 2H), 5.32 (s, 1H), 4.24 (s, 1H), 1.49 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ 158.8 (d, J=240.2 Hz), 155.6, 154.2, 153.3, 136.5, 123.0 (d, J=7.7 Hz), 115. (d, J=22.2 Hz), 90.9, 83.1, 28.2 ppm. MS m/z for C22H24F2N5O2, (ESI+) m/z: 428.2 [M+H]+.
Procedure for the synthesis of trisubstituted pyridine derivativities 33a-b
Boc-protected pyridine 32 (1 equiv.) was dissolved in anhydrous CH2Cl2 (430µL) and cooled to 0°C. TFA (290µL) was added and the reaction mixture was warmed to 25 °C and stirred for 1h. After this time, the mixture was concentrated at reduced pressure. The residue obtained was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and few drops of acetic acid were added. The mixture was stirred for 24 h at 25 °C and concentrated under vacuum. The residue was purified by column chromatography (Hex/EtOAc 1:1) furnishing the desired products 33a-b with 29% and 21% yield, respectively.
Compound 33a
Rf = 0.37 (Hex/EtOAc 1:1). 1H NMR (400 MHz, CDCl3) δ 10.47 (s, 1H), 7.81 (s, 1H), 7.30 (m, 4H), 7.06 (m, 6H), 6.93 (m, 2H), 6.36 (s, 2H), 5.80 (s, 2H), 5.63 (s, 1H) ppm. 13C (100 MHz, CDCl3) δ 162.5, 158.8 (d, J=240.2 Hz) 157.1, 155.3, 143.0, 140.3, 130.2, 129.6, 122.6 (d, J= 7.7 Hz), 119.2, 117.3, 116.5, 116.2 (d, J= 22.0 Hz), 90.9 ppm. MS m/z for C24H20F2N5O, (ESI+) m/z: 432.2 [M+H]+.
Compound 33b
Rf = 0.49 (Hex/EtOAc 2:1). 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 7.43-7.21 (m, 8H), 7.05 (m, 4H), 6.33 (s, 1H), 5.96 (d, J=11.6 Hz, 2H), 2.39 (s, 3H) ppm. 13C (100 MHz, CDCl3) δ 161.6, 159.0 (d, J=241.0 Hz), 155.6, 154.0, 140.1, 138.4, 136.2, 134.3, 130.2, 128.6, 127.1, 123.5 (d, J= 7.8 Hz), 115.8 (d, J= 22.4 Hz), 106.9, 21.3 ppm. MS m/z for C25H22F2N5, (ESI+) m/z: 430.2 [M+H]+.
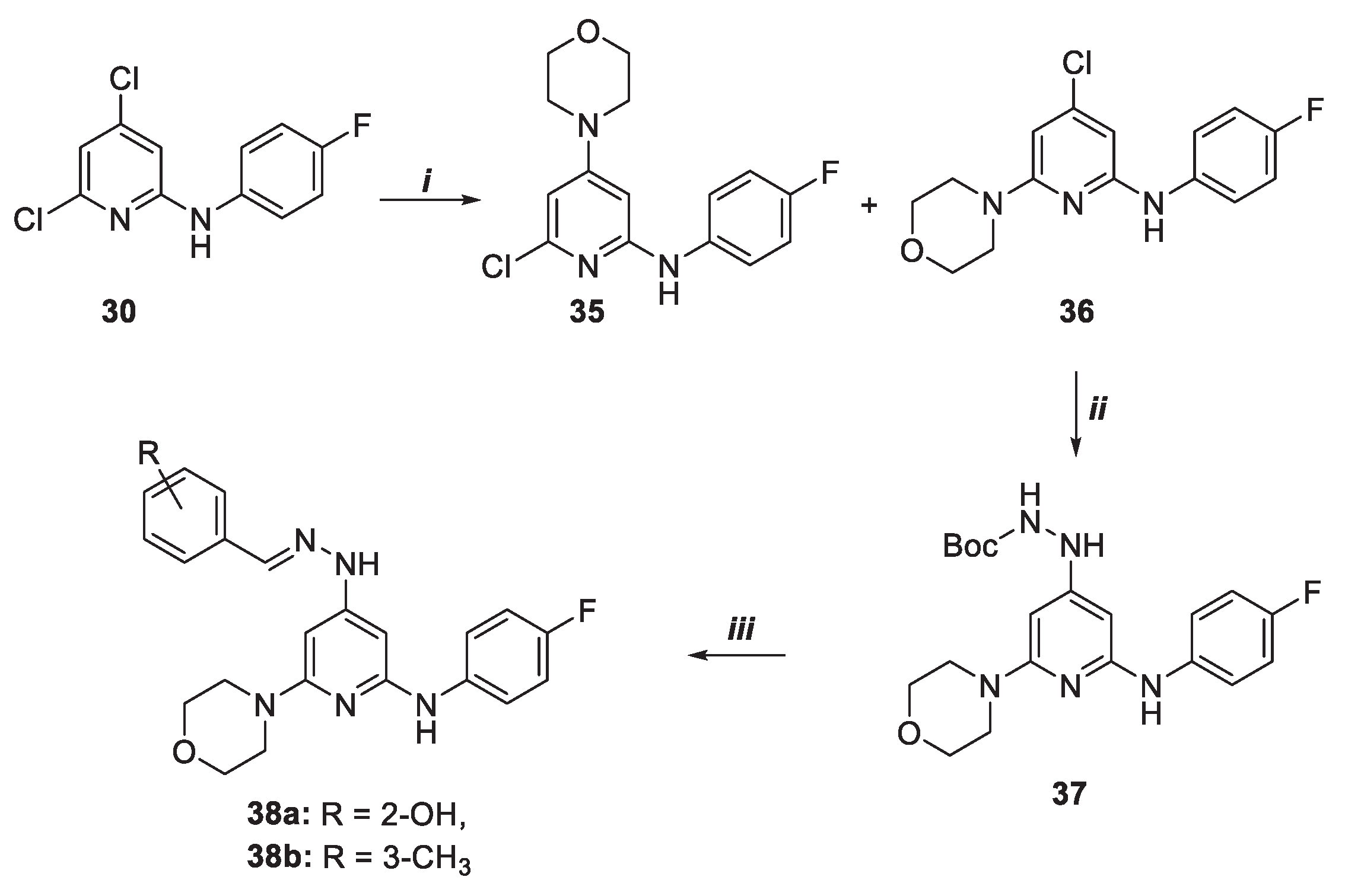
Procedure for the synthesis of disubstituted pyridine derivatives 35 and 36
In a microwave tube equipped with a stir bar were added the monosubstituted pyridine 30 (1 equiv.) dissolved in anhydrous 1,4-dioxane (1.5 mL), morpholine (5 equiv.) and DIPEA (1,5 equiv.). The reaction mixture was irradiated at 160°C for 10h in a microwave oven. Then, the mixture was extracted with EtOAc, washed with brine, dried over anhydrous Na2SO4 and concentrated under vacuum. The crude was purified by column chromatography using PET/EtOAc 5:1 as eluant to give products 35 and 36 with 19 and 46% yield, respectively.
Compound 35
Rf = 0.08 (PET/EtOAc 5:1). 1H NMR (400 MHz, CDCl3) δ = 7.21 (m, 2H), 7.11 (m, 2H), 6.24 (d, J=1.6 Hz, 1H), 6.0 (d, J=1.6 Hz, 1H), 5.9 (s, 1H), 3.82-3.78 (m, 4H), 3.46 (t, J=4 Hz, 2H), 3.25 (t, J=4 Hz, 2H) ppm. 13C (100 MHz, CDCl3) δ 161.1, 157.6 (d, J=197.8 Hz), 148.0, 134.8, 124.9 (d, J=6.6 Hz), 124.6, 116.4 (d, J=22.6 Hz), 100.0, 87.3, 68.8, 46.4 ppm. MS m/z for C15H16ClFN3O, (ESI+) m/z: 308.1 [M+H]+.
Compound 36
Rf = 0.37 (PET/EtOAc 5:1). 1H NMR (400 MHz, CDCl3) δ 7.27 (m, 2H), 7.05 (t, J=8.8 Hz, 2H), 6.24 (d, J=9.2 Hz, 1H), 6.08 (d, J=4.8 Hz, 1H), 3.81 (t, J=4.8 Hz, 2H), 3.49 (t, J=4Hz, 2H) ppm. 13C NMR (100 MHz, CDCl3) δ 159.3, 159.1 (d, J=240.2 Hz), 155.7, 146.4, 135.9, 123.4 (d, J=6.7 Hz), 115.9 (d, J=22.4 Hz), 97.4, 96.9, 66.7, 45.4 ppm. MS m/z for C15H16ClFN3O, (ESI+) m/z: 308.1 [M+H]+.
Procedure for the synthesis of Boc-protected trisubstituted pyridine carbazate 37
In a microwave tube were added under argon compound 36 (1 equiv.), BocNHNH2 (10 equiv.), Pd2(dba)3 (0,04 equiv.), DPPF (0,12 equiv.), Cs2CO3 (2 equiv.) and dry toluene (1 mL). The reaction mixture was stirred at 150°C for 10 minutes under microwave irradiation. The mixture was filtered through celite, rinsed with EtOAc and concentrated. The crude was purified by column chromatography with Hex/EtOAc 2:1 as eluant to give the product 37 with 42% yield.
Rf=0.38 (Hex/EtOAc 2:1). 1H NMR (400 MHz, CDCl3) δ 7.31 (m, 2H), 7.0 (t, J=8.4 Hz, 2H), 6.54 (s, 1H), 6.24 (s, 1H), 3.84 (t, J=4.8 Hz, 2H), 3.51 (t, J=4.8 Hz, 2H) ppm. 13C (100 MHz, CDCl3) δ 160.3, 159.6 (d, J= 240.2 Hz), 155.7, 154.2, 146.2, 135.8, 123.4 (d, J= 6.8 Hz), 115.9 (d, J= 22.2 Hz), 97.4, 96.8, 80.5, 66.9, 45.9, 28.3 ppm. MS m/z for C20H27FN5O3, (ESI+) m/z: 404.2 [M+H]+.
Procedure for the synthesis of trisubstituted pyridine derivativities 38a-b
Boc-protected pyridine 37 (1 equiv.) was dissolved in anhydrous CH2Cl2 (430µL) and cooled to 0°C. TFA (290µL) was added, and the reaction mixture was warmed to 25 °C and stirred for 1h. After this time, the mixture was concentrated at reduced pressure The residue obtained was enough pure to be subjected to the next step.
To a solution of hydrazine derivative (1 equiv.) in MeOH (10 mL), the opportune aldehyde (1 equiv.) and few drops of acetic acid were added. The mixture was stirred for 24 h at 25 °C and concentrated under vacuum. The residue was purified by column chromatography (Hex/EtOAc 1:1) furnishing the desired products 38a-b with 37% and 33% yield, respectively.
Compound 38a
Rf = 0.31 (Hex/EtOAc 1:1). 1H NMR (400 MHz, CDCl3). δ 10.63 (s, 1H), 7.84 (s, 1H), 7.31 (m, 2H), 7.03 (m, 4H), 6.94 (m, 2H), 5. 72 (s, 1H), 5.32 (s 1H), 3.79 (t, J=4.4 Hz, 2H), 3.49 (t, J=4.4 Hz, 2H) ppm. 13C (100 MHz, CDCl3) δ 160.3, 156.7 (d, J=113.7 Hz), 152.4, 142.5, 136.7, 130.7, 129.7, 123.9, 122.8 (d, J=7.5 Hz), 119.6, 118.0, 116.7, 116.5, 115.7 (d, J=22.3 Hz), 81.8, 81.7, 66.8, 45.7 ppm. MS m/z for C22H23FN5O2, (ESI+) m/z: 408.2 [M+H]+.
Compound 38b
Rf = 0.43 (Hex/EtOAc 1:1)]. 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H), 7.44 (d, J=8.8 Hz, 2H), 7.30 (m, 4H), 7.18 (d, J=7.6 Hz, 1H), 7.03 (m, 2H), 6.0 (s, 2H), 3.84 (t, J=4.8 Hz, 2H), 3.51 (t, J=4.8 Hz, 2H), 2.42 (s, 3H) ppm. 13C (100 MHz, CDCl3) δ 162.5, 161.6, 159.2 (d, J=227.6 Hz), 155.6, 154.5, 148.9, 138.4, 134.2, 130.3, 128.6, 127.3, 123.9 (d, J=6.7 Hz), 123.7, 115.8 (d, J=22.3 Hz), 97.6, 97.0, 66.6, 46.4, 21.3 ppm. MS m/z for C23H25FN5O, (ESI+) m/z: 406.2 [M+H]+.