Submitted:
16 February 2024
Posted:
19 February 2024
You are already at the latest version
Abstract
Keywords:
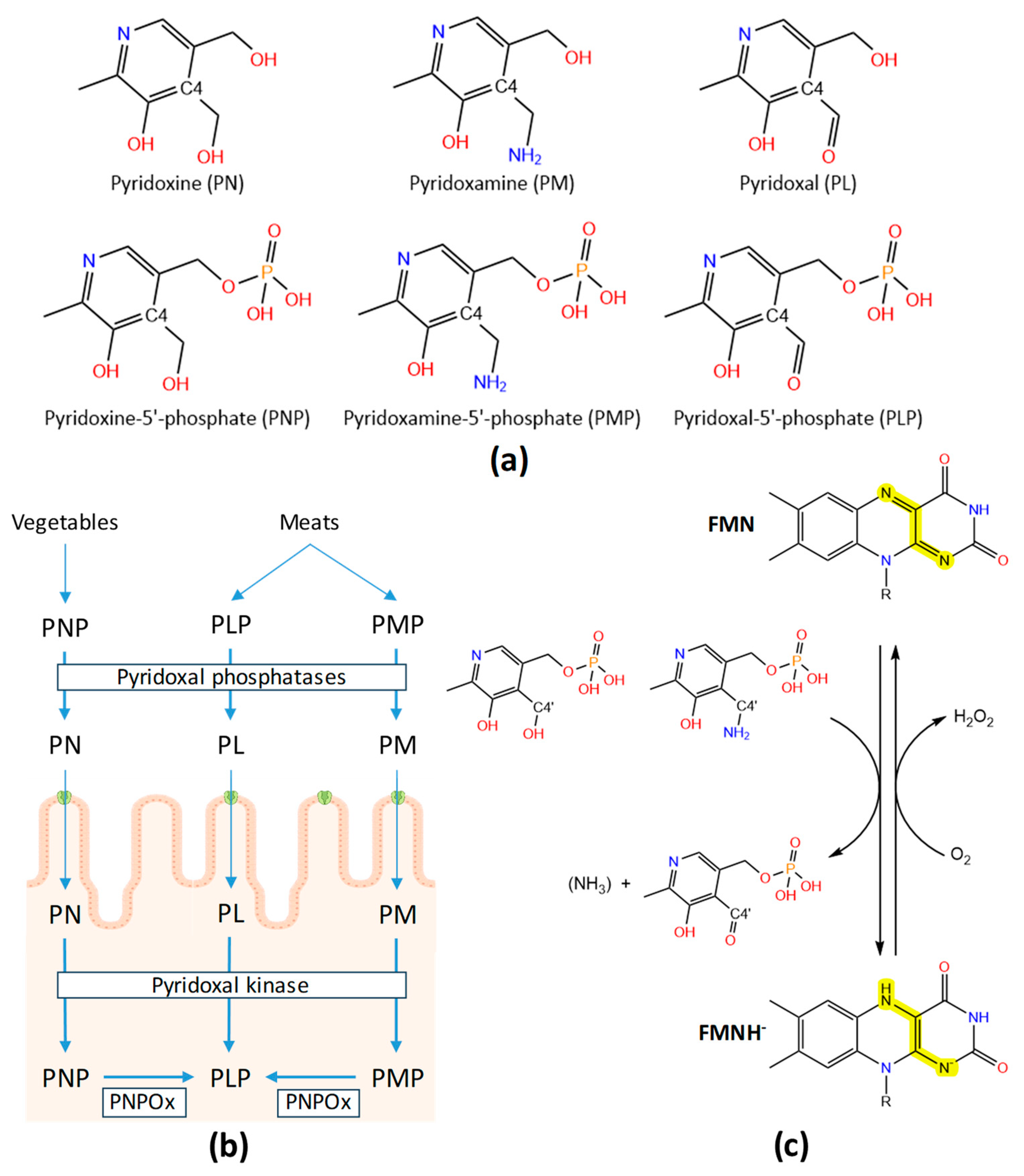
1. Vitamin B6: vitamers and metabolism
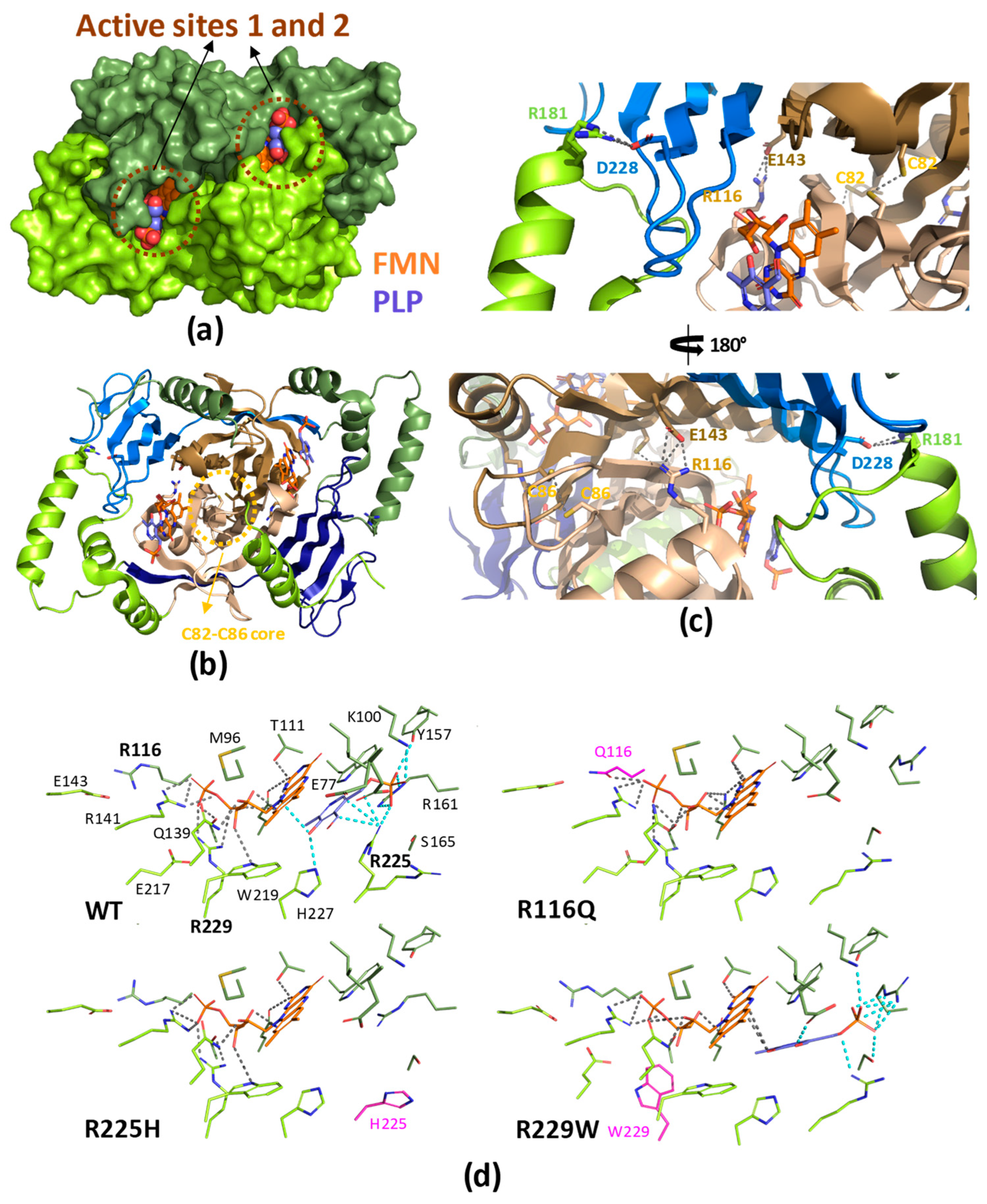
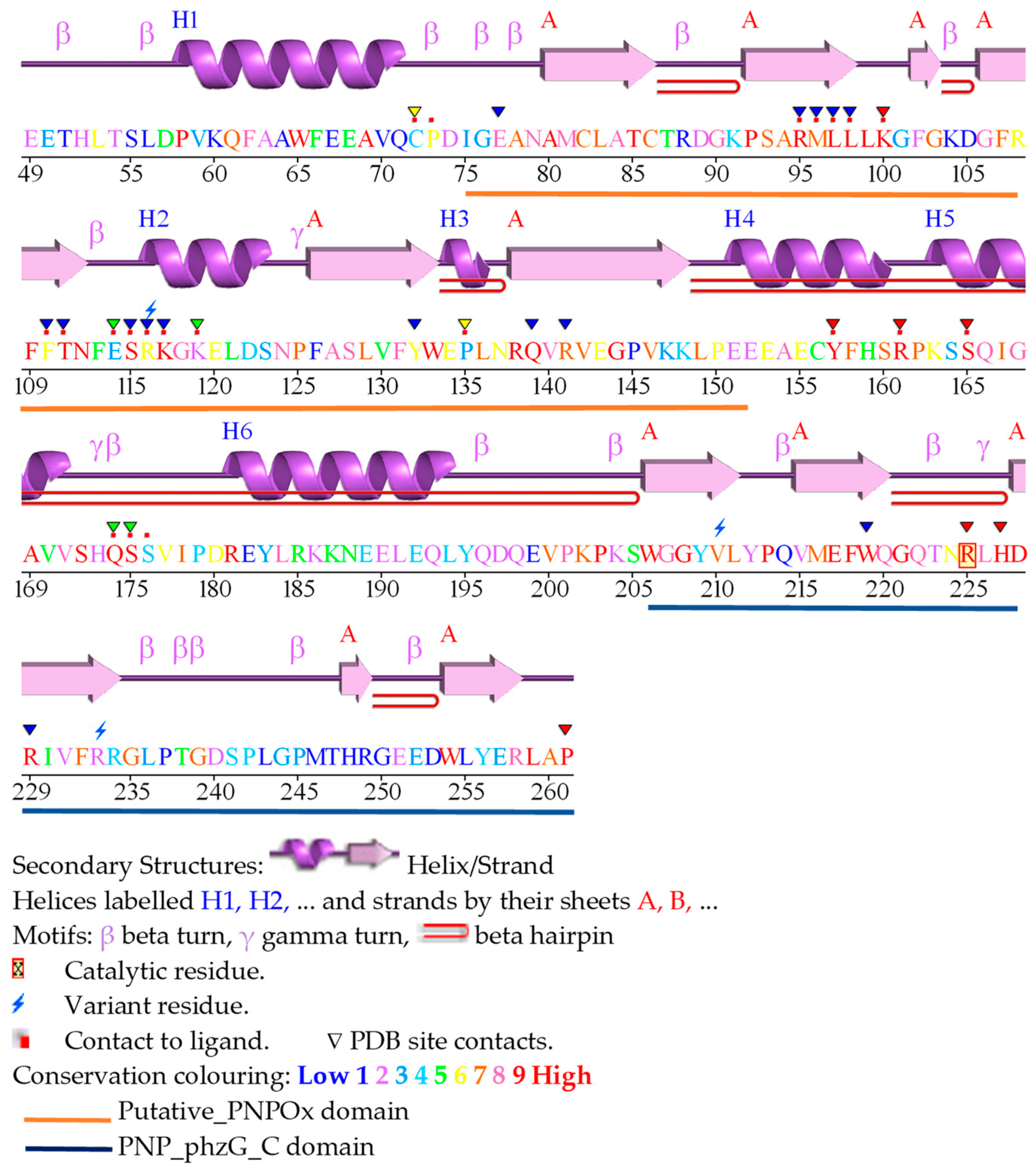
2. Functional and structural features of human PNPOx
| Species | Conditions | kcatPNP (s-1) |
KMPNP (µM) |
kcat/KMPNP (µM-1 s-1) |
kcatPMP (s-1) |
KMPMP (µM) | kcat/KMPMP (µM-1 s-1) |
KIPLP (µM) |
KIPNP (µM) | KdPLP (µM) | KdFMN (nM) |
Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. sapiens | 50 mM Tris/HCl pH 7.6 37˚ 1 |
0.20±0.01 | 1.8 | 0.11 | 0.20 | 1.0 | 0.2 | 3.2 | [21] | |||
| 50 mM Tris/BES pH 7.6 37˚ |
0.20±0.01 | 2.4±0.2 | 0.08 | 14±22 |
[22] | |||||||
| 50 mM Tris/HCl pH 7.6 37˚ |
0.06±0.01 | 2.6±0.2 | 0.02 | 0.6±0.1 | 13±2 | [23] | ||||||
| 50 mM HEPES pH 7.6 37˚ |
0.06±0.01 | 2.8±0.2 | 0.02 | 0.9±0.1 | [23] | |||||||
| 50 mM Tris/HCl pH 7.6 37˚ |
0.20±0.01 | 2.4±0.2 | 0.08 | [24] | ||||||||
| E. coli | 50 mM Tris pH 7.6 37˚ |
0.3 | 2 | 0.15 | [25] | |||||||
| NaPi/HEPES pH 7.6 37˚ |
0.2 | 2 | 0.1 | [25] | ||||||||
| 200 mM Tris-HCl, 200 mM KPi pH 8.5 37˚ |
0.8 | 2 | 0.4 | 1.7 | 105 | 0.02 | 8 | [26] | ||||
| 50 mM Na/HEPES pH 7.6 | 0.25±0.01 | 1.6±0.4 | 0.2 | 0.28±0.01 | 0.15 | [27] | ||||||
| Sheep brain | 100 mM KPi pH 8.0 25˚ |
0.2 | 13 | 0.01 | 4 | [28] | ||||||
| 100 mM KPi pH 7.4 25˚ |
2 | [29] | ||||||||||
| Rabbit liver | 200 mM Tris pH 8.0 37˚ |
30 | 10 | 40 | [30] | |||||||
| 200 mMTris/HCl pH 8.0 25˚ |
0.7 | 8.2 | 0.1 | 0.1 | 3.6 | 0.03 | 50 | 45 | [31] | |||
| Pig brain | 100 mM PPi pH 8.4 25˚ |
0.09 | 13 | 0.007 | 0.009 | 100 | 0.00009 | [32] |
3. The human PNPOx enzyme in disease
4. Functional and structural features of Escherichia coli PNPOx further contributed to understand enzyme regulation
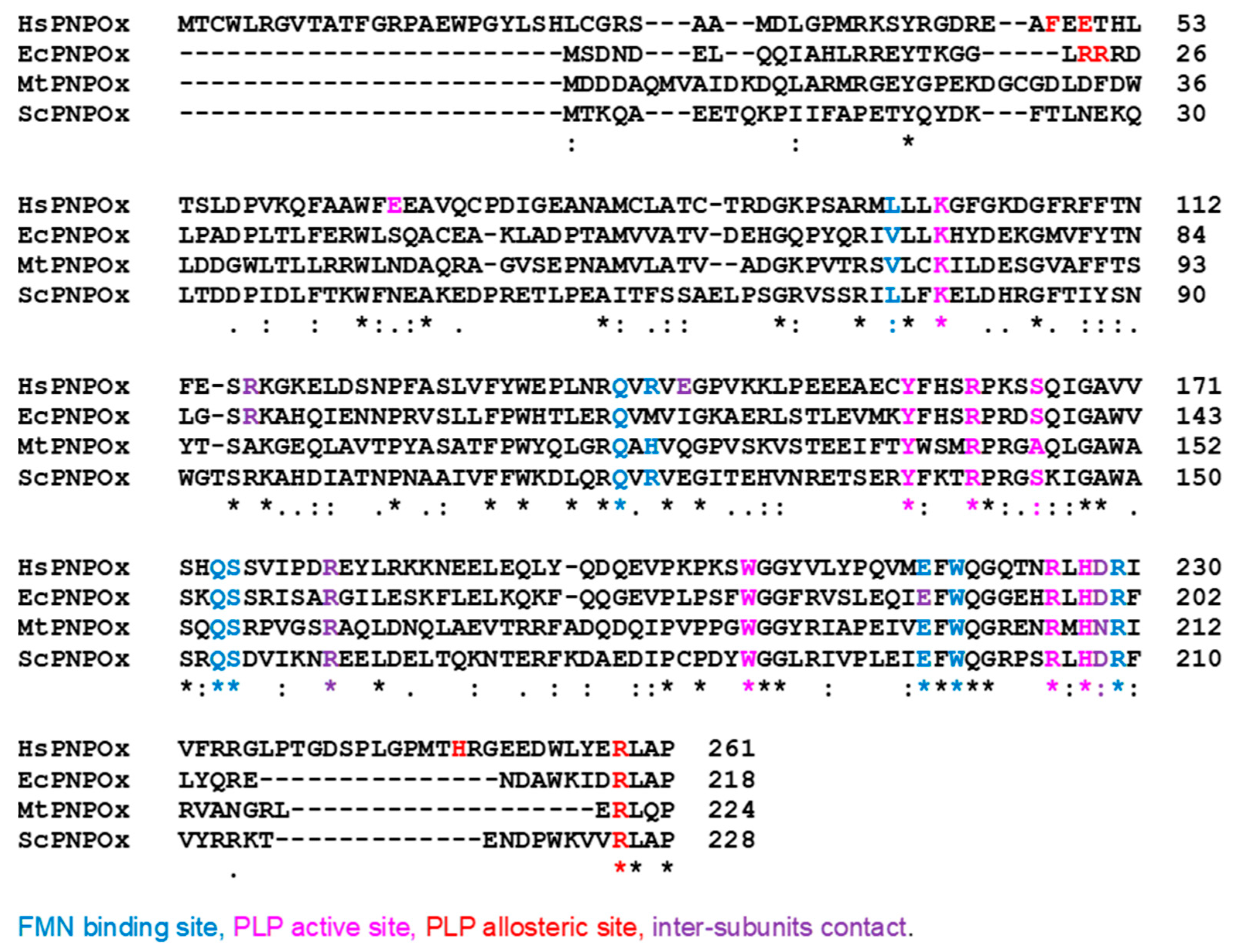
5. PNPOx species-specific features
6. PNPOx and cofactor channelling
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, M.P.; Plecko, B.; Mills, P.B.; Clayton, P.T. Disorders affecting vitamin B metabolism. J Inherit Metab Dis 2019, 42, 629–646. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B₆ and Its Role in Cell Metabolism and Physiology. Cells 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Patel, A.A.; Walsh, B.; Baumer, F.M.; Shah, A.S.; Peters, J.M.; Rodan, L.H.; Agrawal, P.B.; Pearl, P.L.; Takeoka, M. Systemic Manifestations in Pyridox(am)ine 5'-Phosphate Oxidase Deficiency. Pediatr Neurol 2017, 76, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Percudani, R.; Peracchi, A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 2003, 4, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Percudani, R.; Peracchi, A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics 2009, 10, 273. [Google Scholar] [CrossRef]
- Gachon, F.; Fonjallaz, P.; Damiola, F.; Gos, P.; Kodama, T.; Zakany, J.; Duboule, D.; Petit, B.; Tafti, M.; Schibler, U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev 2004, 18, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Laber, B.; Maurer, W.; Scharf, S.; Stepusin, K.; Schmidt, F.S. Vitamin B6 biosynthesis: formation of pyridoxine 5'-phosphate from 4-(phosphohydroxy)-L-threonine and 1-deoxy-D-xylulose-5-phosphate by PdxA and PdxJ protein. FEBS Lett 1999, 449, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T.B. Vitamin B6 biosynthesis in higher plants. Proc Natl Acad Sci U S A 2005, 102, 13687–13692. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Safo, M.K.; Contestabile, R. Biomedical aspects of pyridoxal 5'-phosphate availability. Front Biosci (Elite Ed) 2012, 4, 897–913. [Google Scholar] [CrossRef]
- Said, H.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J 2011, 437, 357–372. [Google Scholar] [CrossRef]
- Denise, R.; Babor, J.; Gerlt, J.A.; de Crécy-Lagard, V. Pyridoxal 5'-phosphate synthesis and salvage in Bacteria and Archaea: predicting pathway variant distributions and holes. Microb Genom 2023, 9. [Google Scholar] [CrossRef]
- Gregory, J.F. Nutritional Properties and significance of vitamin glycosides. Annu Rev Nutr 1998, 18, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Said, Z.M.; Subramanian, V.S.; Vaziri, N.D.; Said, H.M. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am J Physiol Cell Physiol 2008, 294, C1192–1197. [Google Scholar] [CrossRef]
- Suvorova, I.A.; Rodionov, D.A. Comparative genomics of pyridoxal 5'-phosphate-dependent transcription factor regulons in. Microb Genom 2016, 2, e000047. [Google Scholar] [CrossRef]
- Said, H.M.; Ortiz, A.; Ma, T.Y. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol 2003, 285, C1219–1225. [Google Scholar] [CrossRef]
- Miyake, K.; Yasujima, T.; Takahashi, S.; Yamashiro, T.; Yuasa, H. Identification of the amino acid residues involved in the species-dependent differences in the pyridoxine transport function of SLC19A3. J Biol Chem 2022, 298, 102161. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.; Vielreicher, M. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J Biol Chem 2003, 278, 18990–18996. [Google Scholar] [CrossRef]
- Barile, A.; Battista, T.; Fiorillo, A.; di Salvo, M.L.; Malatesta, F.; Tramonti, A.; Ilari, A.; Contestabile, R. Identification and characterization of the pyridoxal 5'-phosphate allosteric site in Escherichia coli pyridoxine 5'-phosphate oxidase. J Biol Chem 2021, 296, 100795. [Google Scholar] [CrossRef] [PubMed]
- Al Mughram, M.H.; Ghatge, M.S.; Kellogg, G.E.; Safo, M.K. Elucidating the Interaction between Pyridoxine 5'-Phosphate Oxidase and Dopa Decarboxylase: Activation of B6-Dependent Enzyme. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef]
- Kang, J.H.; Hong, M.L.; Kim, D.W.; Park, J.; Kang, T.C.; Won, M.H.; Baek, N.I.; Moon, B.J.; Choi, S.Y.; Kwon, O.S. Genomic organization, tissue distribution and deletion mutation of human pyridoxine 5'-phosphate oxidase. Eur J Biochem 2004, 271, 2452–2461. [Google Scholar] [CrossRef]
- Musayev, F.N.; Di Salvo, M.L.; Ko, T.P.; Schirch, V.; Safo, M.K. Structure and properties of recombinant human pyridoxine 5'-phosphate oxidase. Protein Sci 2003, 12, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Musayev, F.N.; Di Salvo, M.L.; Saavedra, M.A.; Contestabile, R.; Ghatge, M.S.; Haynes, A.; Schirch, V.; Safo, M.K. Molecular basis of reduced pyridoxine 5'-phosphate oxidase catalytic activity in neonatal epileptic encephalopathy disorder. J Biol Chem 2009, 284, 30949–30956. [Google Scholar] [CrossRef] [PubMed]
- Barile, A.; Nogués, I.; di Salvo, M.L.; Bunik, V.; Contestabile, R.; Tramonti, A. Molecular characterization of pyridoxine 5'-phosphate oxidase and its pathogenic forms associated with neonatal epileptic encephalopathy. Sci Rep 2020, 10, 13621. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.; Boneta, S.; Novo, N.; Velázquez-Campoy, A.; Polo, V.; Medina, M. Riboflavin kinase and pyridoxine 5'-phosphate oxidase complex formation envisages transient interactions for FMN cofactor delivery. Front Mol Biosci 2023, 10, 1167348. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, M.; Yang, E.; Zhao, G.; Winkler, M.E.; Schirch, V. Expression, purification, and characterization of recombinant Escherichia coli pyridoxine 5'-phosphate oxidase. Protein Expr Purif 1998, 13, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Winkler, M.E. Kinetic limitation and cellular amount of pyridoxine (pyridoxamine) 5'-phosphate oxidase of Escherichia coli K-12. J Bacteriol 1995, 177, 883–891. [Google Scholar] [CrossRef]
- Barile, A.; Tramonti, A.; di Salvo, M.L.; Nogués, I.; Nardella, C.; Malatesta, F.; Contestabile, R. Allosteric feedback inhibition of pyridoxine 5'-phosphate oxidase from. J Biol Chem 2019, 294, 15593–15603. [Google Scholar] [CrossRef]
- Kim, Y.T.; Churchich, J.E. Sequence of the cysteinyl-containing peptides of 4-aminobutyrate aminotransferase. Identification of sulfhydryl residues involved in intersubunit linkage. Eur J Biochem 1989, 181, 397–401. [Google Scholar] [CrossRef]
- Choi, S.Y.; Churchich, J.E.; Zaiden, E.; Kwok, F. Brain pyridoxine-5-phosphate oxidase. Modulation of its catalytic activity by reaction with pyridoxal 5-phosphate and analogs. J Biol Chem 1987, 262, 12013–12017. [Google Scholar] [CrossRef]
- Kazarinoff, M.N.; McCormick, D.B. Rabbit liver pyridoxamine (pyridoxine) 5'-phosphate oxidase. Purification and properties. J Biol Chem 1975, 250, 3436–3442. [Google Scholar] [CrossRef]
- Choi, J.D.; Bowers-Komro, M.; Davis, M.D.; Edmondson, D.E.; McCormick, D.B. Kinetic properties of pyridoxamine (pyridoxine)-5'-phosphate oxidase from rabbit liver. J Biol Chem 1983, 258, 840–845. [Google Scholar] [CrossRef]
- Churchich, J.E. Brain pyridoxine-5-phosphate oxidase. A dimeric enzyme containing one FMN site. Eur J Biochem 1984, 138, 327–332. [Google Scholar] [CrossRef]
- Safo, M.K.; Musayev, F.N.; Schirch, V. Structure of Escherichia coli pyridoxine 5'-phosphate oxidase in a tetragonal crystal form: insights into the mechanistic pathway of the enzyme. Acta Crystallogr D Biol Crystallogr 2005, 61, 599–604. [Google Scholar] [CrossRef]
- Barile, A.; Graziani, C.; Antonelli, L.; Parroni, A.; Fiorillo, A.; di Salvo, M.L.; Ilari, A.; Giorgi, A.; Rosignoli, S.; Paiardini, A.; et al. Identification of the pyridoxal 5'-phosphate allosteric site in human pyridox(am)ine 5'-phosphate oxidase. Protein Sci 2024, 33, e4900. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. PyMOL: an open-source molecular graphics tool. CCP4 Newsletter On Protein Crystallography 2002, 40, 82–92. [Google Scholar]
- Ghatge, M.S.; Al Mughram, M.; Omar, A.M.; Safo, M.K. Inborn errors in the vitamin B6 salvage enzymes associated with neonatal epileptic encephalopathy and other pathologies. Biochimie 2021, 183, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Barile, A.; Mills, P.; di Salvo, M.L.; Graziani, C.; Bunik, V.; Clayton, P.; Contestabile, R.; Tramonti, A. Characterization of Novel Pathogenic Variants Causing Pyridox(am)ine 5'-Phosphate Oxidase-Dependent Epilepsy. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Bashiri, F.A.; Abdelhakim, M.; Adly, N.; Jamjoom, D.Z.; Sumaily, K.M.; Alghanem, B.; Arold, S.T. Phenotypic and molecular spectrum of pyridoxamine-5'-phosphate oxidase deficiency: A scoping review of 87 cases of pyridoxamine-5'-phosphate oxidase deficiency. Clin Genet 2021, 99, 99–110. [Google Scholar] [CrossRef]
- Guerin, A.; Aziz, A.S.; Mutch, C.; Lewis, J.; Go, C.Y.; Mercimek-Mahmutoglu, S. Pyridox(am)ine-5-Phosphate Oxidase Deficiency Treatable Cause of Neonatal Epileptic Encephalopathy With Burst Suppression: Case Report and Review of the Literature. J Child Neurol 2015, 30, 1218–1225. [Google Scholar] [CrossRef]
- Mills, P.B.; Camuzeaux, S.S.; Footitt, E.J.; Mills, K.A.; Gissen, P.; Fisher, L.; Das, K.B.; Varadkar, S.M.; Zuberi, S.; McWilliam, R.; et al. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain 2014, 137, 1350–1360. [Google Scholar] [CrossRef]
- Khayat, M.; Korman, S.H.; Frankel, P.; Weintraub, Z.; Hershckowitz, S.; Sheffer, V.F.; Elisha, M.B.; Wevers, R.A.; Falik-Zaccai, T.C. PNPO deficiency: an under diagnosed inborn error of pyridoxine metabolism. Mol Genet Metab 2008, 94, 431–434. [Google Scholar] [CrossRef]
- Plecko, B.; Paul, K.; Mills, P.; Clayton, P.; Paschke, E.; Maier, O.; Hasselmann, O.; Schmiedel, G.; Kanz, S.; Connolly, M.; et al. Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology 2014, 82, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.; Abeling, N.G.; Salomons, G.S.; Struys, E.A.; Simas-Mendes, M.; Geukers, V.G.; Poll-The, B.T. Pyridoxine responsive epilepsy caused by a novel homozygous PNPO mutation. Mol Genet Metab Rep 2016, 6, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.F.; Schmitt, B.; Windfuhr, M.; Wagner, N.; Strehl, H.; Bagci, S.; Franz, A.R.; Mills, P.B.; Clayton, P.T.; Baumgartner, M.R.; et al. Pyridoxal 5'-phosphate may be curative in early-onset epileptic encephalopathy. J Inherit Metab Dis 2007, 30, 96–99. [Google Scholar] [CrossRef]
- Ware, T.L.; Earl, J.; Salomons, G.S.; Struys, E.A.; Peters, H.L.; Howell, K.B.; Pitt, J.J.; Freeman, J.L. Typical and atypical phenotypes of PNPO deficiency with elevated CSF and plasma pyridoxamine on treatment. Dev Med Child Neurol 2014, 56, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.B.; Surtees, R.A.; Champion, M.P.; Beesley, C.E.; Dalton, N.; Scambler, P.J.; Heales, S.J.; Briddon, A.; Scheimberg, I.; Hoffmann, G.F.; et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5'-phosphate oxidase. Hum Mol Genet 2005, 14, 1077–1086. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Mastrangelo, M.; Nogués, I.; Tolve, M.; Paiardini, A.; Carducci, C.; Mei, D.; Montomoli, M.; Tramonti, A.; Guerrini, R.; et al. Biochemical data from the characterization of a new pathogenic mutation of human pyridoxine-5'-phosphate oxidase (PNPO). Data Brief 2017, 15, 868–875. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Mastrangelo, M.; Nogués, I.; Tolve, M.; Paiardini, A.; Carducci, C.; Mei, D.; Montomoli, M.; Tramonti, A.; Guerrini, R.; et al. Pyridoxine-5'-phosphate oxidase (Pnpo) deficiency: Clinical and biochemical alterations associated with the C.347g>A (P.·Arg116gln) mutation. Mol Genet Metab 2017, 122, 135–142. [Google Scholar] [CrossRef]
- Yang, E.S.; Schirch, V. Tight binding of pyridoxal 5'-phosphate to recombinant Escherichia coli pyridoxine 5'-phosphate oxidase. Arch Biochem Biophys 2000, 377, 109–114. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Ko, T.P.; Musayev, F.N.; Raboni, S.; Schirch, V.; Safo, M.K. Active site structure and stereospecificity of Escherichia coli pyridoxine-5'-phosphate oxidase. J Mol Biol 2002, 315, 385–397. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Intracellular trafficking of the pyridoxal cofactor. Implications for health and metabolic disease. Arch Biochem Biophys 2016, 592, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, M.S.; Contestabile, R.; di Salvo, M.L.; Desai, J.V.; Gandhi, A.K.; Camara, C.M.; Florio, R.; González, I.N.; Parroni, A.; Schirch, V.; et al. Pyridoxal 5'-phosphate is a slow tight binding inhibitor of E. coli pyridoxal kinase. PLoS One 2012, 7, e41680. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.W.; Rhee, S.; Davies, D.R. The molecular basis of substrate channeling. J Biol Chem 1999, 274, 12193–12196. [Google Scholar] [CrossRef]
- Ghatge, M.S.; Karve, S.S.; David, T.M.; Ahmed, M.H.; Musayev, F.N.; Cunningham, K.; Schirch, V.; Safo, M.K. Inactive mutants of human pyridoxine 5'-phosphate oxidase: a possible role for a noncatalytic pyridoxal 5'-phosphate tight binding site. FEBS Open Bio 2016, 6, 398–408. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kwok, F.; Churchich, J.E. Interactions of pyridoxal kinase and aspartate aminotransferase emission anisotropy and compartmentation studies. J Biol Chem 1988, 263, 13712–13717. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Fong, C.C.; Ng, K.T.; Lam, W.C.; Leung, Y.C.; Tsang, C.W.; Yang, M.; Wong, M.S. Interaction between pyridoxal kinase and pyridoxal-5-phosphate-dependent enzymes. J Biochem 2003, 134, 731–738. [Google Scholar] [CrossRef]
- Anoz-Carbonell, E.; Rivero, M.; Polo, V.; Velázquez-Campoy, A.; Medina, M. Human riboflavin kinase: Species-specific traits in the biosynthesis of the FMN cofactor. FASEB J 2020, 34, 10871–10886. [Google Scholar] [CrossRef]
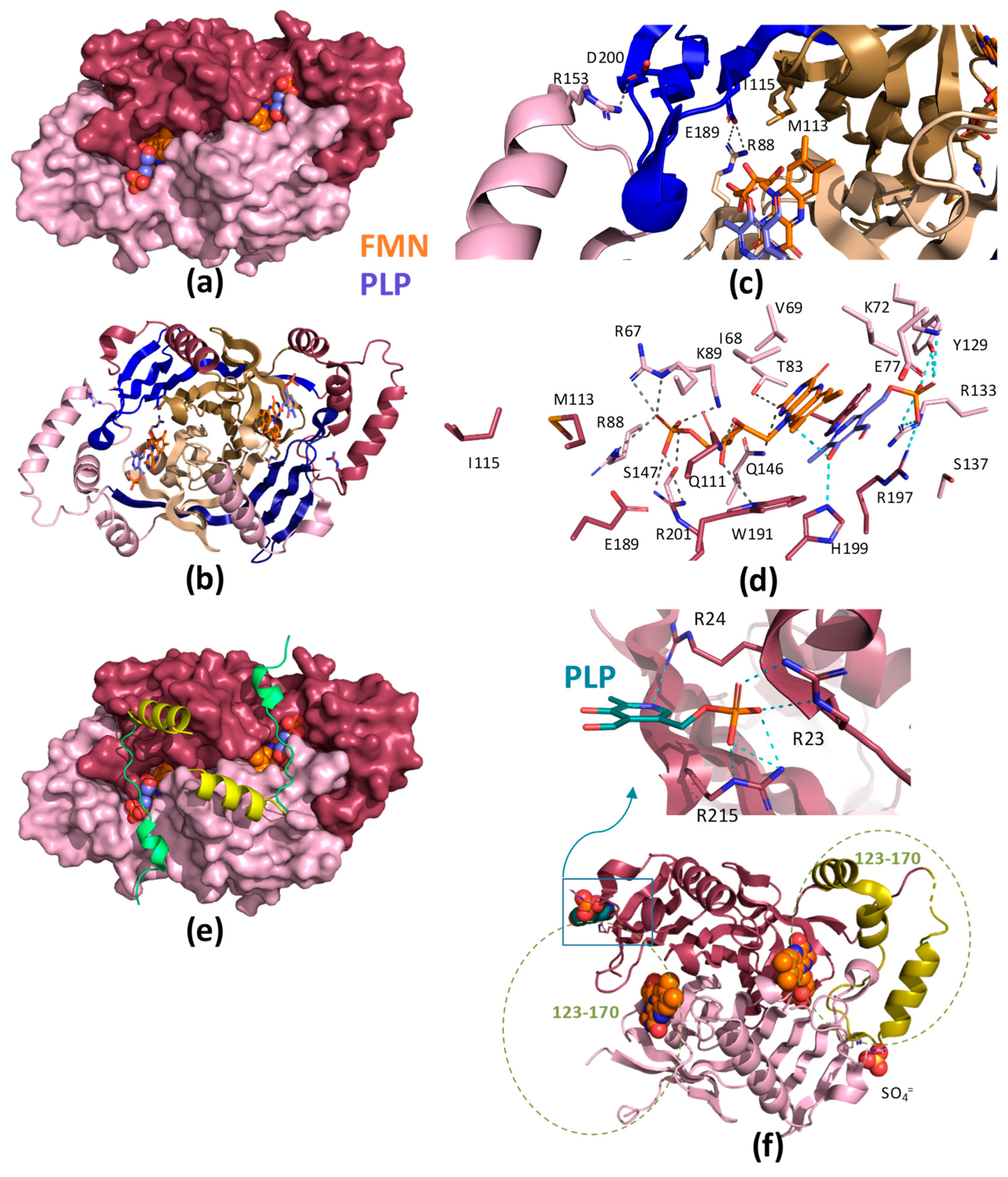
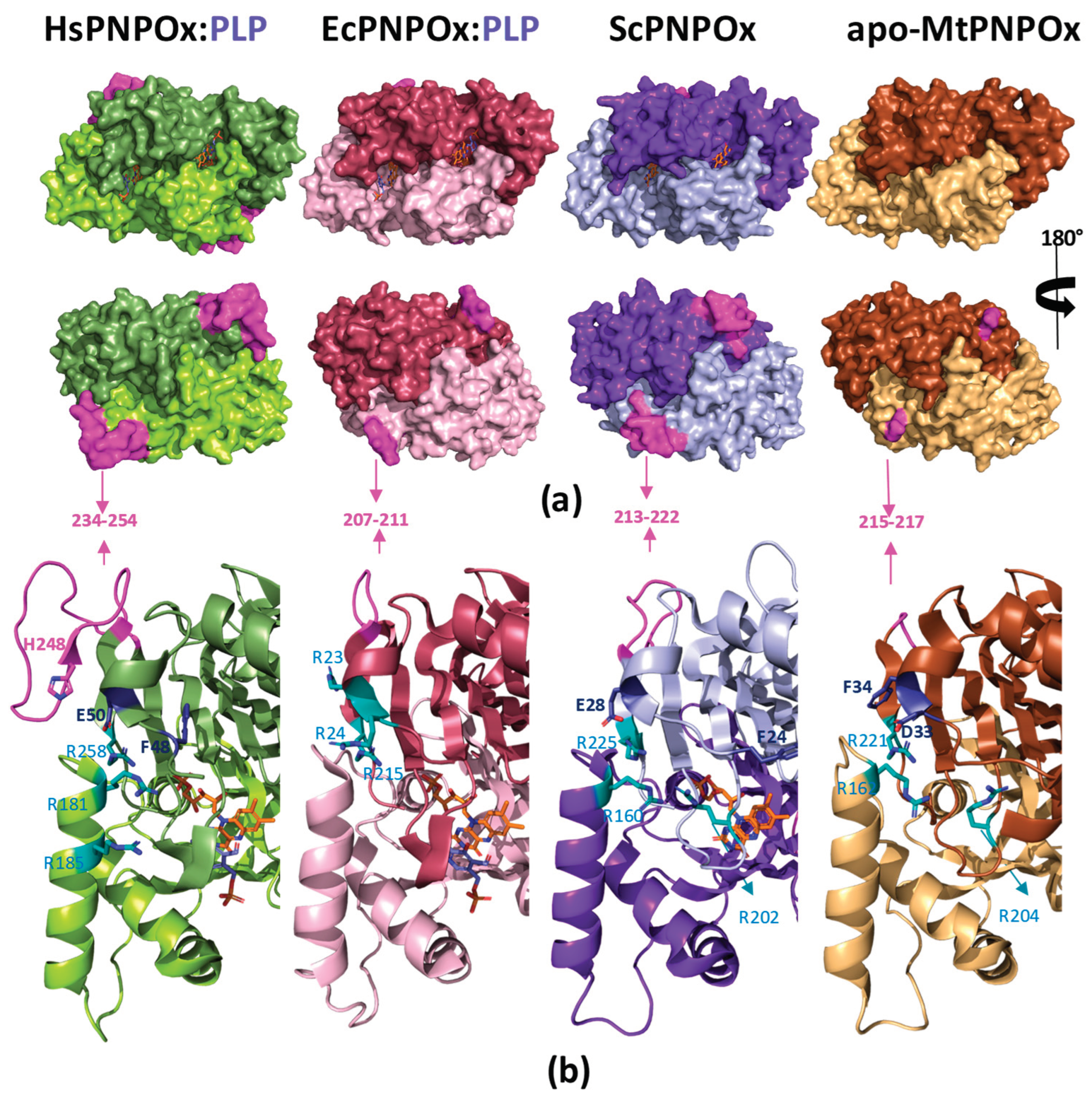
| Species | HsPNPOx | EcPNPOx | MtPNPOx | ScPNPOx | Rabbit | Pig | Sheep |
| HsPNPOx | 100 | ||||||
| EcPNPOx | 43.8 | 100 | |||||
| MtPNPOx | 38.1 | 41.5 | 100 | ||||
| ScPNPOx | 38.1 | 41.6 | 37.8 | 100 | |||
| Rabbit | 91.2 | 40.5 | 38.4 | 39.7 | 100 | ||
| Pig | 89.7 | 41.5 | 38.0 | 38.0 | 87.0 | 100 | |
| Sheep | 89.7 | 43.0 | 42.5 | 41.2 | 87.0 | 88.9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





