1. Introduction
Colorectal cancer (CRC) is a malignancy of the gastrointestinal tract that arises either from the colon or the rectum. Although colon cancer and rectal cancer can be defined as separate diseases, common biological and clinical features determine to study colorectal cancer as a self-standing entity (1).
The epidemiology of colorectal cancer varies significantly between different areas in the world and also between different gender, racial and age groups (2). The highest incidence rates for the year 2020 were in Australia/New Zealand and European regions (40.6 per 100.000 males) and the lowest in Southern Asia and several African regions (4.4 per 100.000 females). The highest mortality was observed in Eastern Europe (20.2 per 100.000 males) and the lowest was observed in South Asia (2.5 per 100.000 females) (3).
Multiple factors have been demonstrated to be implicated in the development of colorectal cancer. They include family and personal medical history (family history and genetics, inflammatory bowel disease, colon polyps, cholecystectomy and diabetes mellitus), lifestyle risk factors (obesity, physical inactivity, cigarette smoking, alcohol consumption and several dietary patterns such as diet in red and processed meat, diet low in fiber, fruits and vegetable or diet low in calcium, vitamin D and dairy products) or various other factors (age, gut microbiota, gender, race or socioeconomic factors) (4).
2. Materials and Methods
We designed an analytical, observational, retrospective study that included patients admitted to our emergency surgical department and diagnosed with colorectal cancer.
The inclusion criteria were:
- patients admitted to our surgical department during 2020-2022 (three years);
- patients diagnosed with colorectal cancer (including the ileocecal valve);
- patients who benefited from a surgical procedure, either in emergency or elective.
The exclusion criteria consisted of:
- patients with benign lesions in the colorectal segment;
- patients admitted with the diagnosis of colorectal cancer but did not benefit from a surgical procedure or refused it;
- incomplete data was recovered from the patients’ files.
Data was obtained from the operative protocols and the patient’s files as various types of nominal and scale variables that compiled our database in Microsoft Office Excel® and afterward were analyzed using IBM SPSS Statistics 20®.
3. Results
Our study group consisted of 153 patients, divided into 87 male patients (56.9%) and 66 female patients (43.1%).
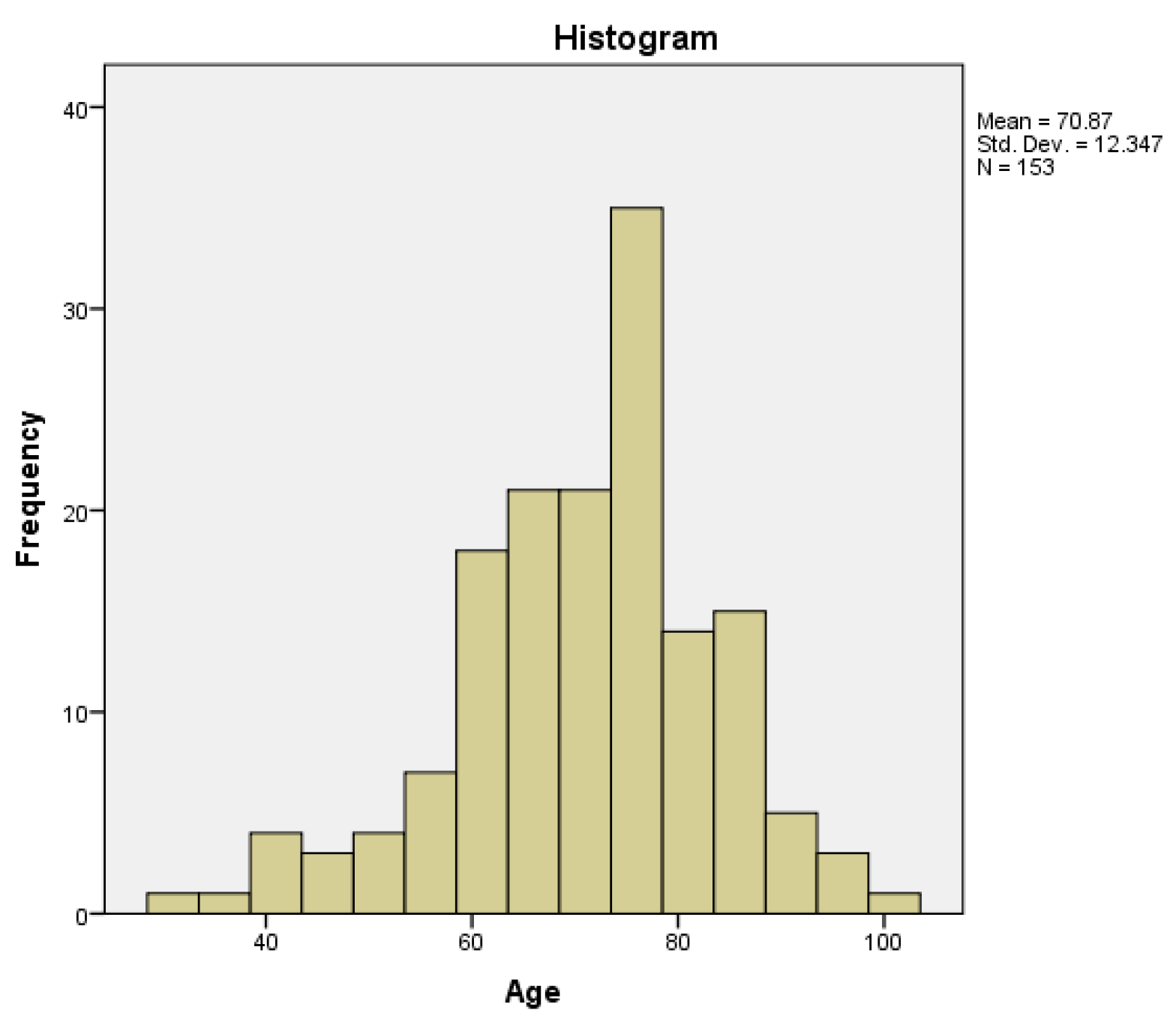
The minimum age was 31 years and the maximum was 100 years, with a mean of 70.87 years and a median of 72 years (95% CI (confidence interval)). The age distribution had a normal (gaussian) distribution (histogram –
Figure 1), with a skewness of -0.548, a kurtosis of 0.582 and a p-value of 0.026 obtained by Kolmogorov-Smirnov normality test.
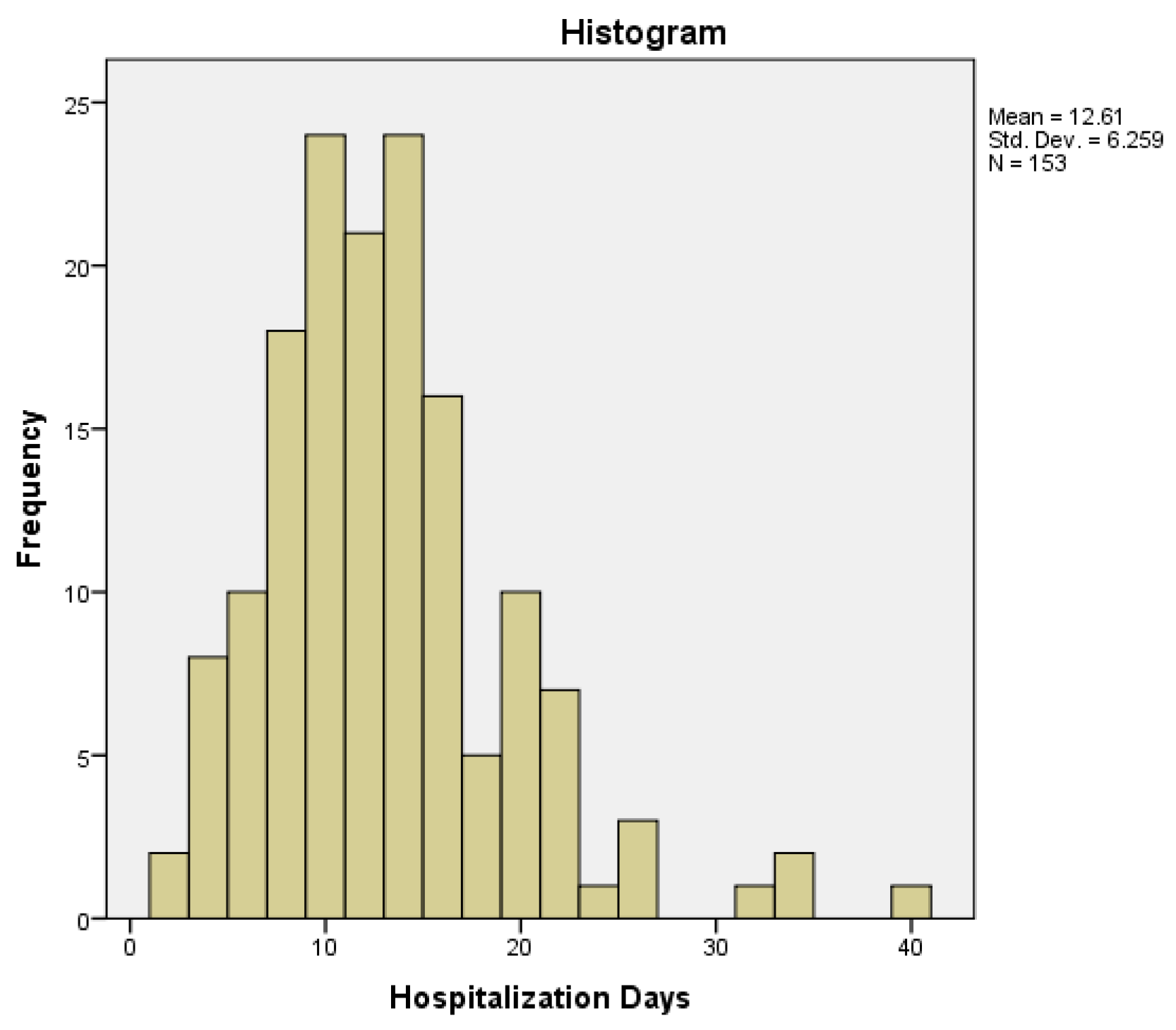
The minimum number of hospitalization days was two days, while the maximum number was 39 days, with a mean of 12.61 days, a median of 11 days (95% CI) and an interquartile range of 7. The distribution of hospitalization days was non-normal (histogram –
Figure 2), with a moderate skewness of 1.236 (tail to the right), a kurtosis of 2.726 and a p-value < 0.001 obtained by Kolmogorov-Smirnov normality test.
Regarding the distribution of age among the two types of gender, the minimum age for women was 36 years, while the maximum was 100 years. The mean value was 70.85 years, the median value was 72.50 years and the distribution was normal, with a skewness of -0.420, a kurtosis of 0.143 and a p-value for the Kolmogorov-Smirnov normality test of 0.2. For the male population, the minimum value was 31 years, while the maximum was 94 years. The mean value was 70.89 years, the median value was 72 years and the distribution was also normal, with a skewness of -0.719, a kurtosis of 1.094 and a p-value for the Kolmogorov-Smirnov normality test of 0.66. Therefore, we performed a parametric two-sample t-test, that obtained a p-value of 0.986, with no statistical significance between the two genders regarding age.
The duration of hospitalization among genders had a minimum value of two days for men and three days for women, while the maximum value was 39 days for men and 26 days for women. The mean values were 13.03 days for men and 12.06 for women, while the distribution was non-normal for men. Therefore, we determined the median values of both categories, with a median value of 12 days for men and respectively of 11 days of women, both of them with an interquartile range of 7. We performed a non-parametric Mann-Whitney U test, obtaining a p-value of 0.717, therefore the distribution of hospitalization days was the same across both genders, with no statistical significance.
A number of 99 patients (64.7% of the study group) came from the urban area, while 54 patients (35.3% of the study group) came from the rural area. There was no significant difference regarding gender and area of provenance (chi-square test, p = 0.865). The mean age of patients from urban areas was 71.74, while the mean age for patients from rural areas was 69.28. The mean number of hospitalization days for patients from urban areas was 11.94 days, while the mean number of hospitalization days for patients from rural areas was 13.85 days. There was no correlation between the area of provenance and the age of the patients (two-sample t-test, p = 0.240) or the length of hospitalization (Mann-Whitney U test, p = 0.187).
In our study group, 69 patients were operated in emergency (45.1%), while 84 patients (54.9%) benefited from elective surgery. The mean value for age regarding the patients operated in emergency (71.25 years) was similar to the mean value of the age of the patients operated electively (70.56 years). Regarding hospitalization days, the median value for emergency patients (12 days) was similar to the median value for elective patients (11 days).
We analyzed the relationship between the emergency/elective presentation of the patient and several other parameters (age, hospitalization days, gender and the area of origin – urban/rural), but found no statistical association (
Table 1).
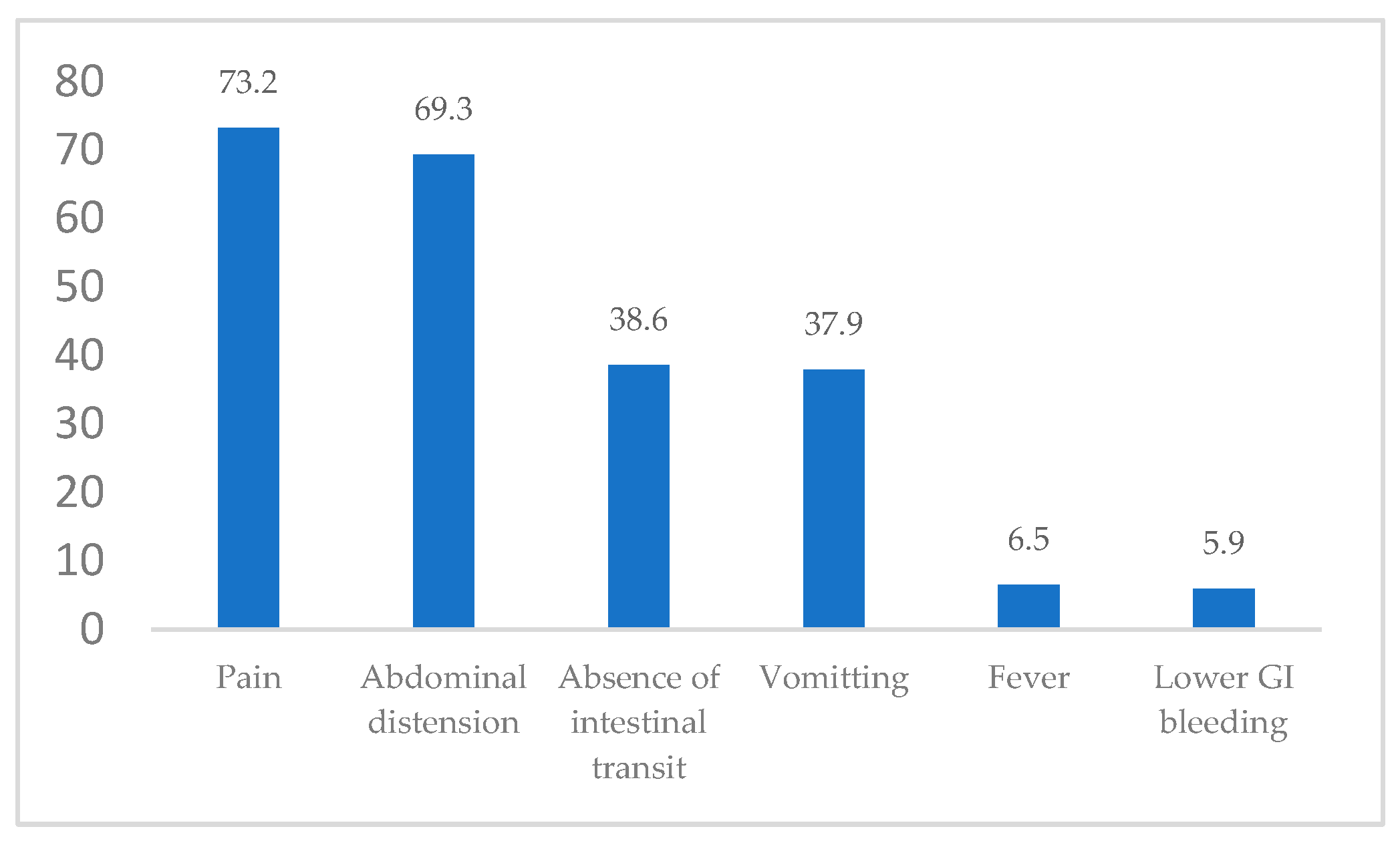
The clinical manifestations of admittance to our general surgery department included pain, nausea/vomiting, abdominal distension, absence of intestinal transit, lower GI (gastrointestinal) bleeding and fever/shivers (
Figure 3).
The most common clinical manifestations were represented by pain – 112 patients (73.2% of the study group), followed by abdominal distension – 106 patients (69.3% of the study group) and absence of intestinal transit – 59 patients (38.6% of the study group).
An abdominal CT (Computer Tomography) scan was performed preoperatively in 85 patients (55.6% of the study group) (
Table 2).
The patients who benefited from an emergency surgical procedure had an abdominal CT scan in 44 out of 69 cases (63.8%) (
Figure 4A–H). In comparison, the patients who received an elective surgical procedure had an abdominal CT in 41 out of 84 cases (48.8%). We applied a Chi-square test, with a p-value of 0.073, just over the threshold of 0.05, therefore there was no statistical correlation between an emergency surgical procedure and the preoperative CT abdominal CT scan.
Preoperatively, a complete set of biological markers was determined for all the patients in the study. The WBC (white blood cell) count varied between 1,000 and 30,000/mm
3, with a mean value of 10,300/mm
3 (normal values for our laboratory are 4,800-10,800 WBC/mm
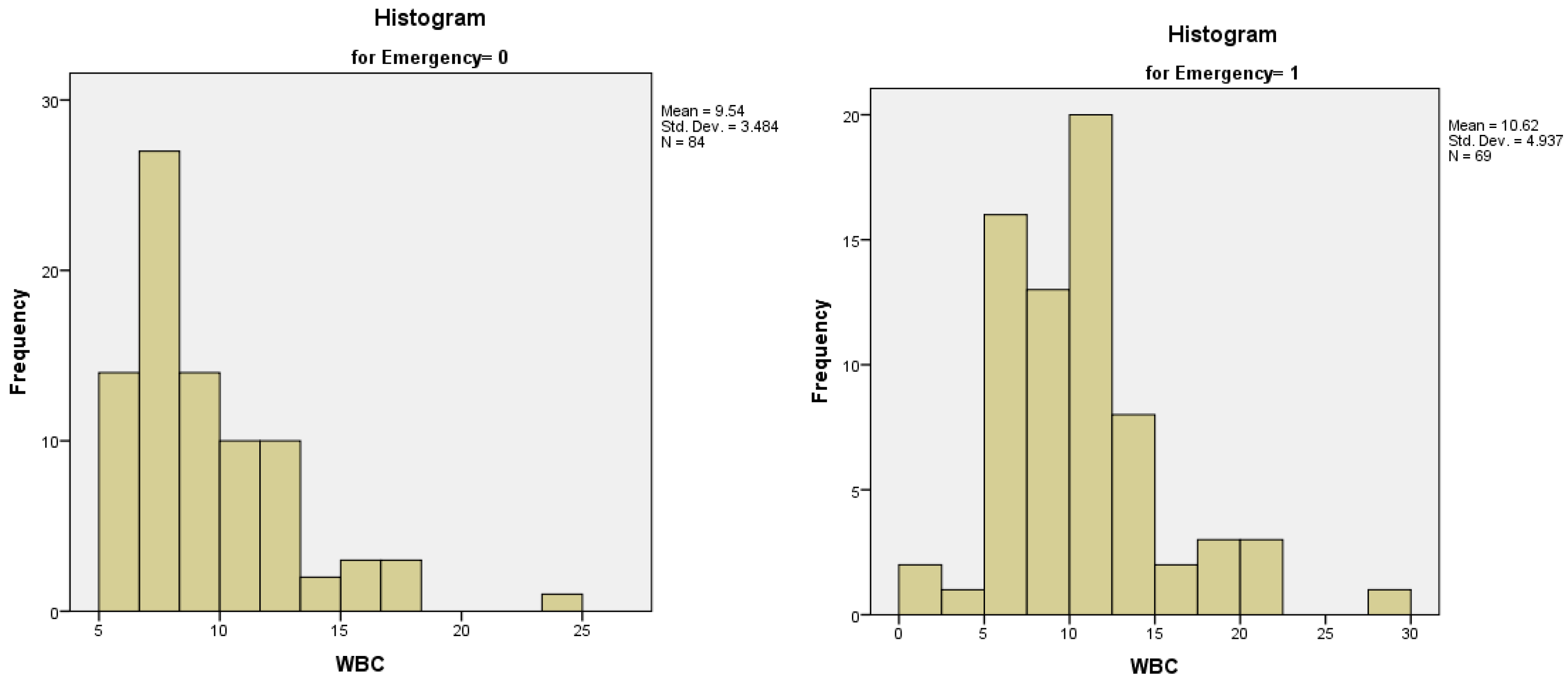
3). We analyzed the distribution of WBC count between the groups of patients who received or did not receive an emergency surgical procedure – both of the distributions were not normal (
Figure 5A,B). The median value of WBC count for patients who were operated electively was 8.67, with an interquartile range of 4, while the median value of WBC count for patients who were operated in emergency was 10.00, with an interquartile range of 5.
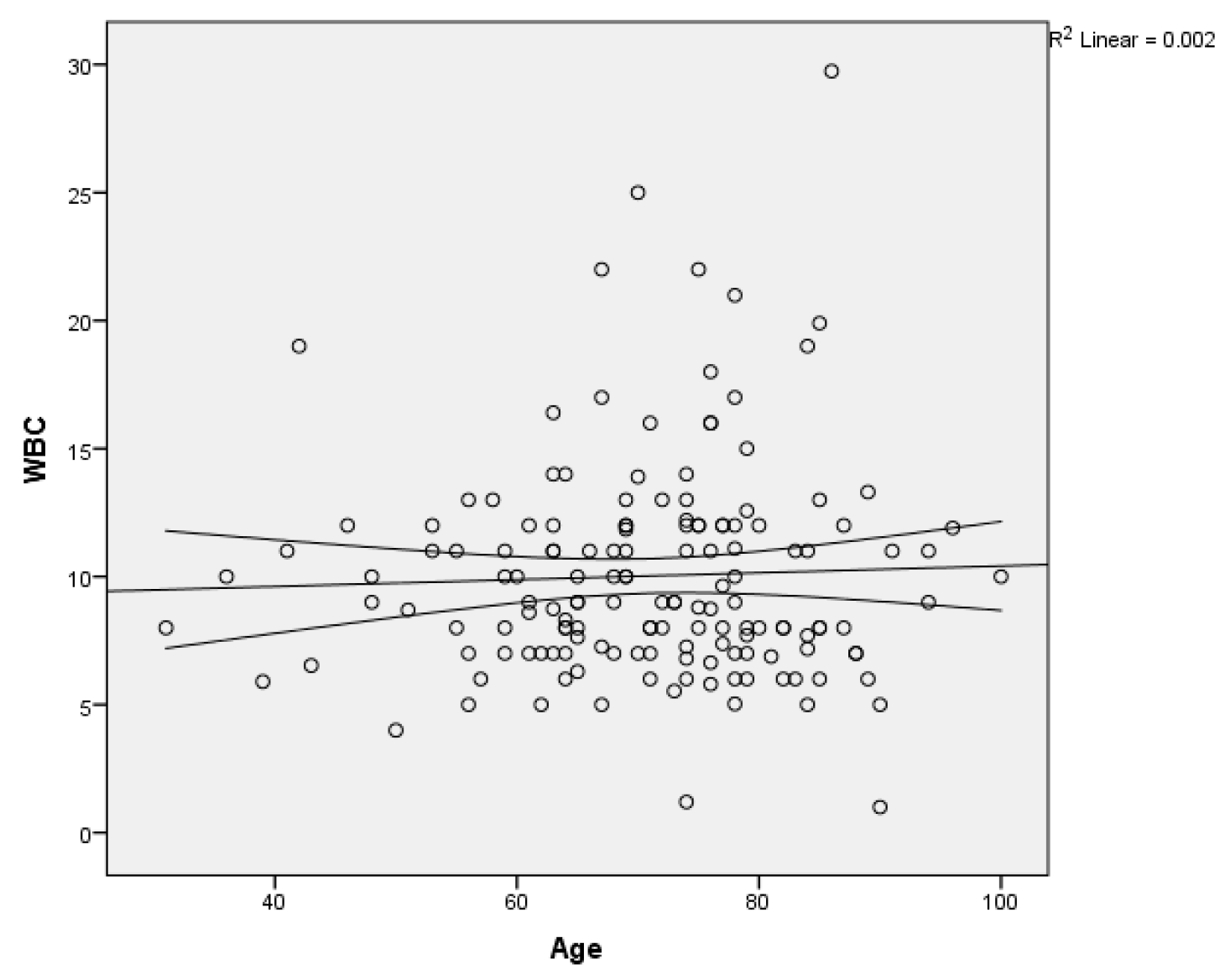
We calculated also the relationship between the age of the patients in the study group and the WBC count using linear regression, without finding any linear relationship between the two variables (
Figure 6).
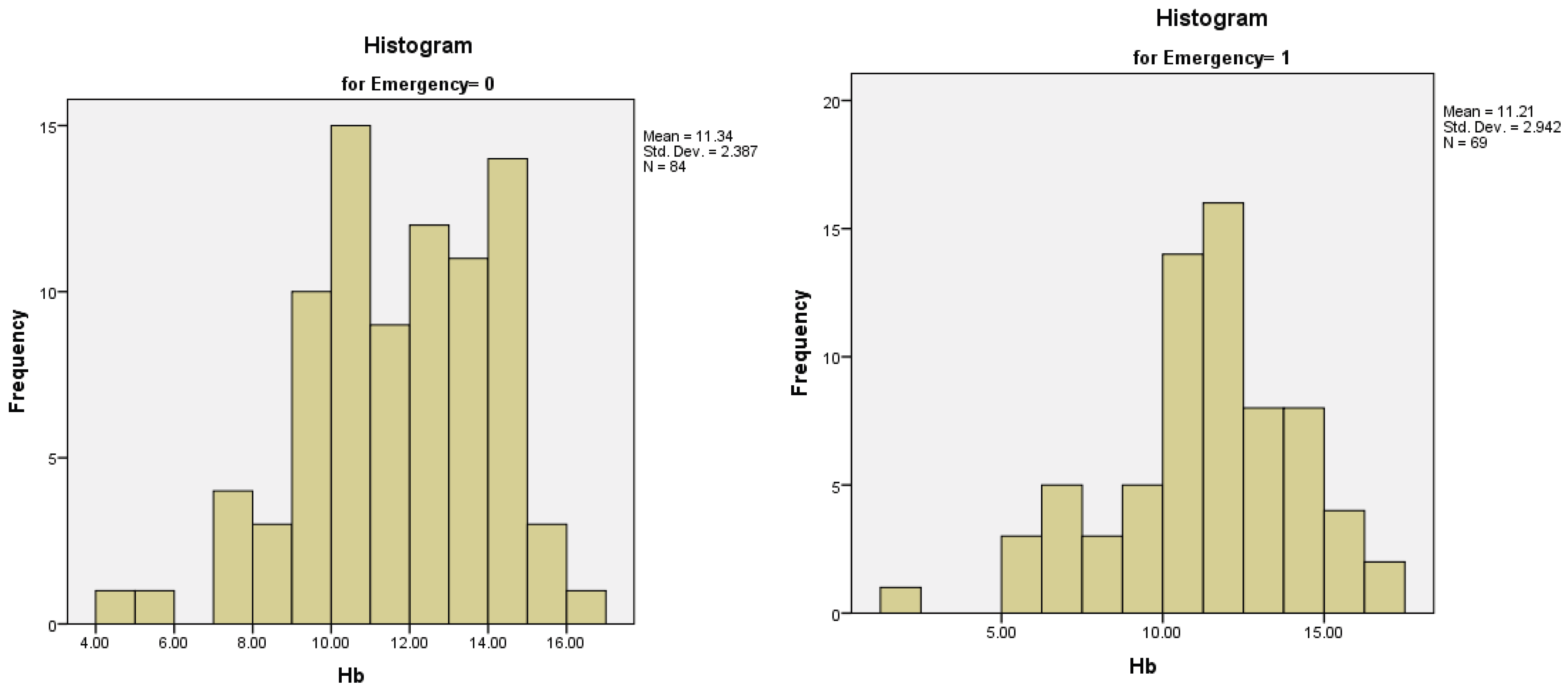
The Hb (Hemoglobin) levels varied between 2 g/dl and 17.00 g/dl, with a mean value of 11.28 (normal values for our laboratory are between 12-16 g/dl). For the patients operated electively the mean value was 11.33 g/dl, with a standard deviation of 2.38, while for patients who were operated in emergency, the mean value was 11.21 g/dl, with a standard deviation of 2.94. Both distributions were normal, with skewness and kurtosis values in the (-1;1) range (
Figure 7A,B).
Regarding gender, the mean value of Hb in women was 11.13 g/dl, with a standard deviation, of 2.53, while the mean value of Hb in men was 11.38, with a standard deviation of 2.73. Both distributions were also normal. Therefore, we performed a parametrical, two-sample t-test, with a p-value of 0.56, describing no statistical association of Hb values and gender in the patients in our group study.
We used linear regression to analyze the relationship between the age of our patients and the preoperative Hb values, with a p-value of 0.248, above the 0.05 threshold. Also, linear regression was performed to determine if the preoperative Hb influenced the number of days of hospitalization. The p-value obtained was 0.181, so the null hypothesis was accepted, that there is no correlation between these two parameters. The mean Hb value for the patients who presented with lower GI bleeding was 10.77 g/dl.
In the case of preoperative glycemia, the maximum value in our study group was 117.57 mg/dl (normal values for our laboratory are 74-106 mg/dl). Both distribution among female and male patients were non-normal. For female patients, the median value was 106.50 and the interquartile range was 31, while for male patients the median value was 110 mg/dl, with an interquartile range of also 31. The non-parametrical test Mann Whitney U determined a p-value of 0.743. The distribution among patients who needed or not an emergency procedure was also non-normal, with a median for patients operated in emergency of 116.93 mg/dl and a median for elective patients of 118.43, respectively. The interquartile range was 42 for patients operated in emergency, while the interquartile range for elective patients was 26. The linear regression used to analyze the correlation between glycemia and age obtained a p-value of 0.096.
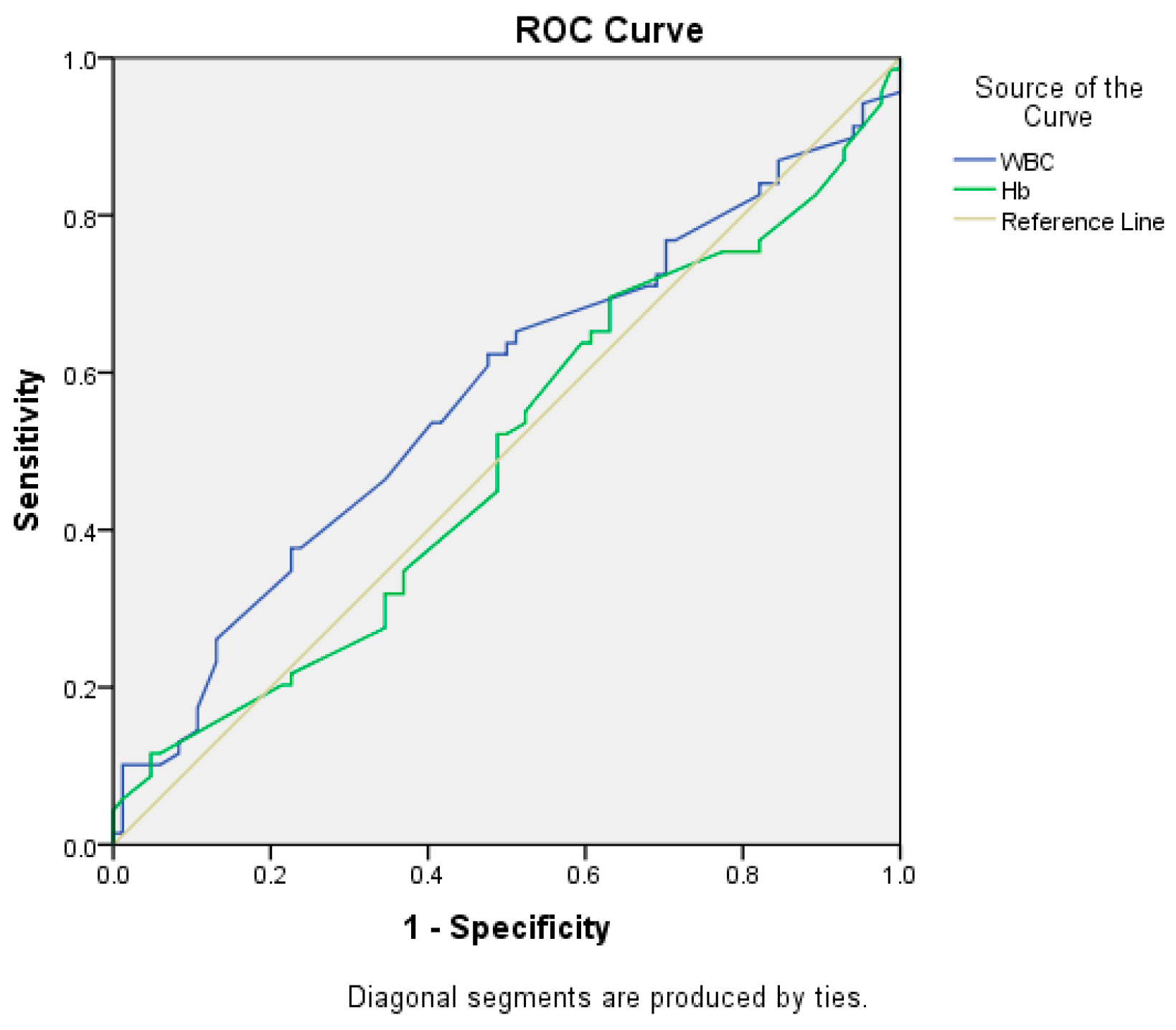
We tried to determine if the preoperative values of WBC count and hemoglobin could predict the necessity for an emergency surgical procedure. We computed the Receiver Operating Characteristic (ROC) curve (
Figure 8). Although the WBC count determined a higher value for the area under the curve in comparison to Hb (0.572 vs 0.494), the p-values for the two variables were 0.124 and 0.895 respectively, therefore neither of the two biological parameters was a significant predictor for the necessity of an emergency surgical procedure (the 95% confidence intervals contained the value 0.5).
Further, we analyzed the topography of colorectal cancer, measuring the frequency of these categories of patients regarding the type of surgical procedure (emergency/elective) (
Table 3).
The most frequent topography of the tumor in our study group was the sigmoid colon, with 30 patients (19.60% of the study group), followed by the colorectal junction, with 17 patients (15.68% of the study group) and superior rectum and inferior rectum, with 17 patients in each subcategory (11.11% of the study group). We performed a Chi-square test for each of the topographies concerning the type of surgery that was performed. The p-values obtained were all above the 0.05 threshold, except one. In the case of sigmoid colon topography, Fisher’s exact test indicated that there was a significant difference in the type of surgery between patients with or without sigmoid cancer.
We grouped the topographies of the tumors into three sub-categories:
- right colon (including ileocecal valve, cecum, ascending colon, hepatic flexure and transverse colon), consisting of 39 patients, 25.5% of the study group;
- left colon (including splenic flexure, descending colon and sigmoid colon), consisting of 47 patients, 30.7% of the study group (
Figure 9);
- rectum (including rectosigmoidian junction (
Figure 10), superior rectum, medium rectum, inferior rectum and anus), consisting of 67 patients, 43.8% of the study group.
We analyzed these three topographical sub-categories in relation with the type of emergency/elective surgery that the patients received (
Table 4):
We designed a 2x3 Chi-square table that revealed a difference in percentages especially regarding the emergency procedure in the three topographical segments – 66.0% for the left colon, compared to only 38.5% for the right colon and 45.1% for the rectum. Since every value from our cells was greater than 5, we retained the Pearson Chi-Square p-value of 0.002, which is statistically significant for a difference in the three topographical sub-categories regarding the type of surgery performed.
We also designed a 2x3 Chi-square table relating the three topographical subcategories and the gender of the patients, but the p-value obtained from the Pearson Chi-Square test did not determine a statistical association between these two variables, with a p-value of 0.927.
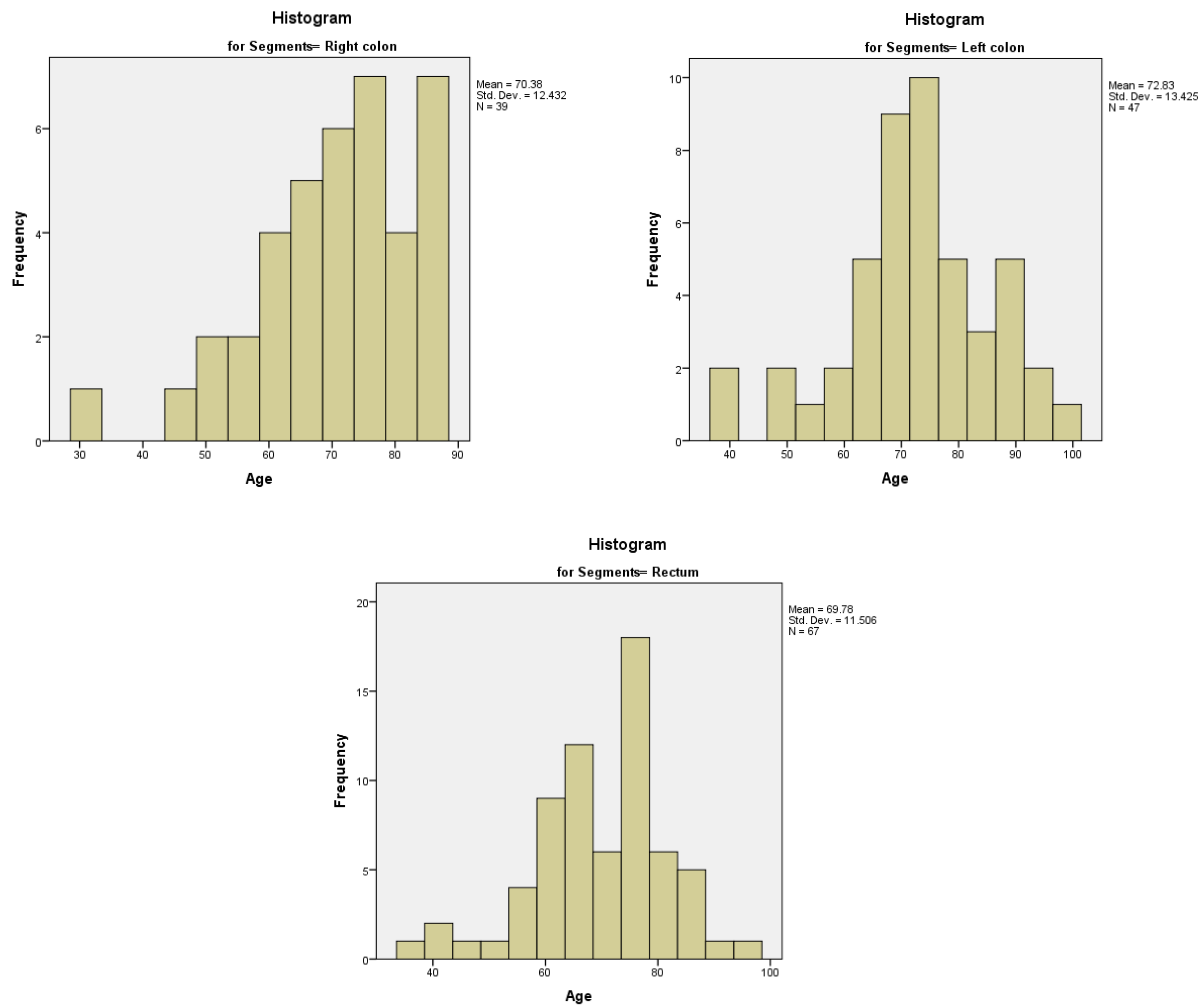
We analyzed the relationship between the age of the patients and the topography of the tumor. The age distribution for the right colon was non-normal, left-tailed, with a median of 71.00 years and an interquartile range of 17. The age distributions for the left colon and the rectum were normal, with a mean value of 72.83 years (95% CI) and a standard deviation of 12.432 for the left colon, respectively a mean value of 69.78 years (95% CI) and a standard deviation of 11.506 for the rectum (
Figure 11A–C). We calculated the Spearman’s correlation coefficient, with a rho value of 0.498, a strong relationship between age of the patients in the study group and the topography of the tumor.
We tried to determine the association between the topography of the tumor and the values of WBC count and Hb. The distribution of the biological markers was non-normal. The median value of WBC count was 8.7 x 103/mm3 for the right colon, 10.0 x 103/mm3 for the left colon and 9.0 x 103/mm3 for the rectum, respectively. The median value for Hb was 11.5 g/dl for the right colon, 12.0 g/dl for the left colon and 11.0 g/dl for the rectum, respectively. We applied a non-parametrical test, Kruskall-Wallis, with both p-values for WBC and Hb being above the threshold, 0.452 and 0.359, therefore the distribution of WBC and Hb levels was the same across the categories of patients with tumors in the three colorectal segments.
The number of hospitalization days was similar for the patients classified by the localization of the tumor – the median values of 12 days for the right colon, 11 days for the left colon and 11 days for the rectum, respectively, with a non-normal distribution. The Kruskall-Wallis non-parametrical test determined a p-value of 0.716, therefore the distribution of hospitalization days was the same across categories of patients with tumors in the three colorectal segments.
The type of surgical procedure that we performed can be seen in
Table 5. The most frequent type of procedure was right hemicolectomy, in 33 patients (21.6% of the study group), followed by rectosigmoid resection, in 32 patients (20.9% of the study group) and tumoral biopsy, in 24 patients (15.7% of the study group). The biopsies were performed for rectal cancers and in 12 cases were associated with the creation of an upward ostomy on the sigmoid colon, as a first step in the oncological multimodal management of the rectal cancer, before the radiotherapy. In our study group, the surgical procedure was finished by performing an anastomosis in 75 patients (49%), an ostomy in 66 patients (43.1%), and in 12 patients (7.8%) neither of the mentioned above, being about a tumoral biopsy.
We analyzed the relationship between the type of surgical procedure that was performed and the emergency regimen of the procedure, using a Chi-square test. The results can be seen in
Table 5 and show a statistical association for the segmentary resections of transverse and sigmoid tumors, which were performed more frequently in emergency, with p-values of 0.020 and 0.037, respectively. Also, we analyzed the relationship between the type of surgical procedure and the result of the procedure (ostomy, anastomosis, neither of them) using a 2x3 Chi-square test. The results, which also can be seen in
Table 5, determined p-values below the threshold for right hemicolectomy, which was finalized mostly with an anastomosis (p=0.002), for segmentary resection of sigmoid colon, which was finalized more frequently with an ostomy (p=0.044) and for rectosigmoid resection, which was finalized more frequently with an anastomosis (p=0.046).
We tried to determine if gender has any influence on the type of outcome of the surgical procedure (ostomy/anastomosis/neither). The 2x3 Chi-square test revealed similar frequency values, with no statistical association, a p-value of 0.882.
Also, we tried to find if the age of the patients is a risk factor for the outcome of the surgical procedure. We eliminated the 12 patients who had neither an ostomy nor an anastomosis. We compiled the ROC curve for the remaining subgroup of 141 patients, also without any statistical association, with a determined p-value of 0.238. For the same subgroup of 141 patients, we asked if the type of outcome could determine the number of hospitalization days. The distribution of the hospitalization days in relation to the binary variable of surgical outcome was non-normal, with a median of 11 days and interquartile range of 7 for patients with an ostomy, while for patients with an anastomosis had a median of 13 and an interquartile range of 6. Therefore, we performed a non-parametrical Mann Whitney U Test that determined the p-value of 0.024, statistically significant, rejecting the null hypothesis and stating that the distribution of hospitalization days is not the same across patients that received an ostomy or anastomosis.
Early reintervention was performed in six patients. The early postoperative complications included wound abscess (17 patients), fistula of the anastomosis (three patients), abdominal evisceration (two patients), and stenosis of the ostomy in one patient.
We performed a Chi-square test to determine if there is any correlation between the type of surgery performed (elective/emergency) and the development of wound abscess but obtained a p-value of 0.800.
In our study group, eight patients died, with a mortality of 5.22%. The mean age of the deceased patients was 69.75 years, and the median value was 70.50 years, with a minimum of 55 years and a maximum of 84 years. We created a ROC curve to determine the influence of the age of the patients on mortality, obtaining a p-value of 0.673. In addition, the correlation between the gender of the patients and mortality determined a p-value of 0.467.
The mean number of hospitalization days for the deceased patients was 13.50 days, with a median of 11.00, a minimum value of 3 days, and a maximum value of 39 days.
Regarding the character of the surgical procedure, seven patients out of the eight patients that died were operated in emergency, the Chi-square test obtaining a p-value of 0.023, statistically significant. There was no statistical association between the localization of the tumor (right colon, left colon, rectum) and mortality, with a p-value of 0.427.
The list of deceased patients, regarding age, gender number of hospitalization days, type of surgical procedure performed, topography of the tumor and surgical procedure can be seen in
Table 6:
4. Discussion
According to research by the American Cancer Society, men have a 30% higher risk of developing colorectal cancer than women. Also, men who are diagnosed with colorectal cancer have 40% higher mortality and a worse prognosis than women (4,5). However, women are more often diagnosed with cancer of the right colon, which tends to be found in more advanced stages of evolution and seems to be more aggressive than cancer of the left colon (6,7). Mechanisms for these disparities are not fully understood, but theories might suggest that certain risk factors (alcohol consumption, tobacco), dietary patterns and sex hormones might be involved (8).
In our study group, consisting of 153 patients, we accounted also for more male patients diagnosed with CRC than females, as many as 87 patients (56.9%). Furthermore, out of the eight patients from the study group that died, six were males (75%).
The risk for colorectal cancer increases with age, being more frequent in patients over 50 years. According to global statistics for the year 2018. the median age is 68 years for men and 72 years for women. The number of colorectal cancer cases increases gradually, being between 1.5-5.4 per 100.000 cases for the interval 25-39 years (1.4-5.3 in men and 1.6-5.5 in women), between 10.3-18.5 per 100.000 cases for the interval 40-49 years (10.2-18.9 in men 10.4-18.1 in women), between 34.3-62.5 per 100.000 cases for the interval 50-64 years (37.4-72.7 in men and 31.2-53.2 in women), between 92.6-212.2 per 100.000 cases for the interval 65-85 years (107.7-229.7 in men and 79.2-200.4 in women), and up to 240.9 in patients over 85 years (264.0 in men and 229.2 in women) (1).
Older age is considered one of the most important risk factors in the development of colorectal cancer, with approximately 90% of the cases occurring in patients over 50 years old (4,9,10). Other studies estimate that patients over the age of 65 years have a risk around three times higher to develop a CRC than patients with ages between 50-64 years and 30 times higher than patients with ages between 25-49 years (11). The relationship between age and colorectal cancer is obvious in developed countries, where higher life expectancy determines an increased number of old people (12). However, recent studies show that in Europe and the United States, the incidence of colorectal cancer has increased in younger groups of adults, between 20-49 years old, therefore the recommendations for the screening of colorectal cancer start with the age of 50 years (13,14).
This data is similar to the results from our study group of 153 patients, in which the mean value of patients’ age was 70.87 years and the median was 72 years. The mean age regarding gender was similar, with a value of 70.85 years for women and 70.89 years for men, with no statistical significance between the two genders regarding age.
Costs involving colorectal cancer are extensive and account not only for the number of days of hospitalization with all the medical care involved but also the complementary oncological treatments and the impact of this disease on the quality of life of the patients and their families and the number of years lost due to its high number of related deaths. In our group, the mean value of hospitalization days was 12.61 days, with a median value of 11 days. The distribution of hospitalization days was non-gaussian, with most of the values around the mean value, but there were a few higher values (with a maximum of 39 hospitalization days) due to important comorbidities, complications that occurred postoperatively and the need for treatment in the intensive care unit.
Clinical presentation of colorectal cancer may involve one of three pathways: asymptomatic patients (early CRC being usually asymptomatic (15)), suspicious symptoms and signs, or emergency presentation involving bowel obstruction, digestive perforation, or lower gastrointestinal bleeding. Most of colorectal cancers, 70% up to 90% are diagnosed after the onset of the clinical manifestations. Symptoms of colorectal cancer usually appear due to the growth of the tumor in the lumen or due to the spread of the tumor to the surrounding structures, therefore most of the symptomatic cases are related to an advanced CRC (16,17). Several large studies have revealed the frequency of the clinical manifestations in colorectal cancer. In a retrospective cohort of over 29,000 patients referred by their general practitioners to an outpatient colorectal surgery department over 22 years, the 1626 patients that were eventually diagnosed with CRC presented: change in bowel habits (74%), lower digestive bleeding (71%), rectal mass (24.5%), abdominal mass (12.5%), iron deficiency anemia (9.6%) and abdominal pain as a single symptom (3.8%) (18). Another recent study that evaluated 388 patients diagnosed with CRC that determined a diagnostic colonoscopy presented lower digestive bleeding in 37% of the cases, abdominal pain in 34% of the cases and anemia in 23% of the cases. Symptoms also vary on the location of the tumor. Bowel obstruction symptoms and changes in bowel habits suggest the topography of the left colon, anemia can raise the suspicion for a tumor of the right colon (cecal and ascending colon tumors can lead to four times higher blood loss than other colorectal tumors) and rectal tumors usually present tenesmus, rectal pain and diminished caliber of stools. The pain can occur no matter the topography of the tumor and can be caused by intestinal perforation, obstruction or peritoneal metastases (19–21).
In our group, the most common clinical manifestations were pain (73.2% of the study group), followed by abdominal distension (69.3% of the study group) and absence of intestinal transit (38.6% of the study group). The explanation for these values and the fact that pain was the main clinical manifestation was the large number of patients that presented to our department and required an emergency surgical procedure. The mean Hb value for the patients who presented with lower GI bleeding was 10.77 g/dl, slightly lower than the entire group of patients’ average. However, the low number of patients who presented with lower GI bleeding suggested that trying to make a correlation between these patients and the Hb value is highly predisposed to statistical error. Also, there was no statistical association between Hb values and type of emergency/elective surgery, gender or age of the patients.
Despite efforts to implement screening methods, according to several studies, approximately 30-33% of patients with colorectal cancer will present with clinical manifestations requiring an emergency surgical procedure (22–25). Emergency colorectal procedures are associated with higher morbidity and mortality rates than elective procedures (26–28). Several studies have suggested that emergency colorectal resections are associated with poor oncological outcomes (27,28), due to a more advanced tumoral stage, higher histological grade, a higher probability to present lympho-vascular invasion or metastases, but also due to physiological abnormalities or neglected comorbidities, characteristic for patients presenting an emergency (29–31). On the other hand, some studies claim that emergency curative resection for colorectal cancer has similar outcomes regarding survival to elective surgical procedures (32).
In our study group, 69 patients were operated in emergency (45.1%), while 84 patients (54.9%) benefited from elective surgery. We analyzed the correlation between the patients with colorectal cancer who benefited from an emergency procedure and several general parameters (age, gender, number of hospitalization days, or area of origin – urban/rural), but found no statistical association. We also tried to determine if the preoperative values of WBC count and hemoglobin levels could predict an emergency colorectal operation. However, both the p-values for the two variables were over the threshold, therefore neither of the two biological parameters was a significant predictor for the necessity of an emergency surgical procedure.
Although initial classifications of the topography of colorectal cancer define three regions, proximal colon, distal colon and rectum (33–35), current definitions use the right colon (derived from the embryologic midgut) which includes the caecum, ascending colon, hepatic flexure and the proximal two-thirds of the transverse colon, respectively left colon (derived from the embryologic hindgut), which includes the distal third of the transverse colon, splenic flexure, descending colon, sigmoid colon and rectum. Some classifications define the rectum as a separate entity (36). Colorectal cancer is localized in the distal or left colon in approximately 70% of the cases. However, several studies suggest that left-sided colorectal cancer has been observed less frequently and that cancer of the right colon has had an upward trend in recent years (37,38).
There are several well-documented differences between right and left colon cancer. The microbiome is different (39–41), and recently published data has shown that colorectal cancer-associated bacterial clusters are differentially correlated with mucosal gene expression profiles, while a few clusters are associated partially with the expression of pro-inflammatory genes in the mucosa, which may result to colorectal cancer in the future (42). Several molecular and chromosomal differences have been reported (43–47), while chromosomal instability has been detected in approximately 30% of the right colon cancer and 70% of left colon cancer (48,49). The median age of patients with right colon cancer is higher compared to patients with left colon cancer, while a greater proportion of patients with right colon cancer are females (44,50,51). Right colon cancer is likely to have a more advanced stage at the initial presentation compared with left colon cancer (44,50,52). Also, right colon cancer often gives metastases to the peritoneum, while a greater proportion of left colon cancer cases will give metastases to the liver and lung (47).
In our study group, the most frequent topography of the tumor was the sigmoid colon, with 19.60% of the patients, followed by the colorectal junction, with 15.68% of the patients and superior rectum and inferior rectum, with 11.11% of the patients in each subcategory. The same as the data from the literature, the frequency of cancer on the left side of the colorectal segment is higher. Statistical analysis indicated that there was a significant difference in the type of surgery (elective/emergency) between patients with or without sigmoid cancer, this type of disease being associated with an emergency surgical procedure. We grouped the patients into three categories regarding the topography of the tumor (right colon, left colon, rectum) and analyzed the types of surgical procedures for each category. Emergency surgical procedures were performed in 38.5% of the patients with right colon cancer, 66% of the patients with left colon cancer, and 34.3% of the patients with rectal cancer, obtaining a statistically significant p-value of 0.002. The relationship between age and topography showed a normal Gaussian distribution for patients with left colon cancer and rectal cancer and a non-normal distribution, left-tailed, for patients with right colon cancer. The statistical analysis showed a strong relationship between the age of the patients in the study group and the topography of the tumor. We also analyzed the relationship between the topography of the tumor and gender, number of hospitalization days, WBC count and hemoglobin levels at admission, but obtained no correlation between these parameters.
The main “pillar” of the management of colorectal cancer is surgical resection, traditionally providing the only curative treatment (53,54). The type of resection depends on the stage of the tumor. For T0 and T1 colorectal cancers, in the absence of prognostic factors that may indicate a high risk of lymph node involvement, endoscopic mucosal resection/submucosal dissection can be used for local excision of the tumor (55,56). Where resection is performed, the location of the tumor and the corresponding vessels and lymph nodes define the segment that should be resected and the surgical procedure (e.g. right hemicolectomy, left hemicolectomy, segmentary colectomy of the transverse colon or sigmoid colon, proctocolectomy, abdominoperineal rectum amputation, etc) (54). The surgical principles of mesocolic and mesorectal dissection, described by Hohenberger et al and Heald et al, respectively, have resulted in significant improvements in oncological outcomes (57,58).
Regarding non-surgical treatment, neoadjuvant radio-chemotherapy is used for locally advanced rectal cancer to increase the rate of complete resection and decrease the possibility of recurrence (59,60). Furthermore, recent trials have proved that neoadjuvant chemotherapy can be used for a subgroup of patients with locally advanced, resectable colorectal cancer (61). Adjuvant chemotherapy is recommended for patients with stage III colorectal cancer to reduce the risk of tumor recurrence. Also, systemic adjuvant therapy is used for patients with emergency presentation, anastomotic leakage, T4 tumors, inadequate node sampling or poorly differentiated histology (62,63). Recently, neoadjuvant immunotherapy has presented great results for patients with locally advanced or metastatic colorectal cancer, but only for a subgroup with a deficient MMR (Mismatch Repair) system. However, only about 15% of patients with colorectal cancer have this characteristic, most of them with cancers of the right colon, while for patients with rectum topography, this value drops under 5% (64–66).
Regarding our group of patients, the most frequent type of procedure was right hemicolectomy (21.6% of the study group), followed by rectosigmoid resection (20.9% of the study group) and tumoral biopsy (15.7% of the study group). The surgical procedure was finished by performing an anastomosis in 49% of the patients, an ostomy in 43.1% of the patients, while for 7.8% of the patients, a tumoral biopsy was performed. The segmentary resections of the transverse and sigmoid colon were statistically associated with an emergency surgical procedure, with p-values of 0.020 and 0.037, respectively. Also, statistically significant associations were determined for right hemicolectomy, which was finalized mostly with an anastomosis (p=0.002), for segmentary resection of the sigmoid colon, which was finalized more frequently with an ostomy (p=0.044) and for rectosigmoid resection, which was finalized more frequently with an anastomosis (p=0.046). There was no statistical association between surgical outcome (anastomosis/ostomy/biopsy) and gender or between the age of the patients as a risk factor and the surgical outcome. However, we obtained a correlation between the number of hospitalization days and the type of surgical outcome (a median value of 13 days for patients with anastomosis and a median value of 11 days for patients with an ostomy), with a p-value of 0.024.
Colorectal cancer is the third most common cancer and the second cause of deaths related to cancer worldwide (2). In 2020, there were estimated about 930,000 deaths due to colorectal cancer and approximately 1.9 million new cases in the world. Projections suggest that by the year 2040 there will be 3.2 million new cases of CRC and 1.6 million deaths related to CRC each year, most cases predicted to occur in high or very high human development index (HDI) countries (3). Several studies suggest that this increase is a result of environmental changes, like a more sedentary lifestyle, obesity, consumption of highly processed food, red meat consumption, alcohol and an increase in overall life expectancy (4,67,68).
In our group, we had a mortality of 5.22%. The mean age of the deceased patients was 69.75 years, and the median value was 70.50 years, with a minimum of 55 years and a maximum of 84 years. There was no statistical association between age or gender and mortality, with p-values of 0.673 and 0.467, respectively. Furthermore, we determined that seven out of eight patients who died in our group benefited from emergency surgery, statistically significant, with a p-value of 0.023. On the other side, tumor topography did not influence mortality, with a p-value of 0.427.
5. Conclusion
Colorectal cancer remains one of the most frequent cancers in the world, with a heavy burden that involves high mortality, alterations in the quality of life of the patients and their families, but also the financial costs of the medical systems.
Significant developments have been made in recent years regarding the understanding of the role of several risk factors involved in this disease, the genetic and molecular mechanisms of tumoral growth and new therapeutic measures.
In addition, great efforts are being made regarding screening methods and more personalized treatments that involve multidisciplinary teams to reduce the impact of colorectal cancer.
Author Contributions
Conceptualization, I.S.C, and V.T.G.; methodology, V.E.C..; software, C.G.F.; validation, C.B.; formal analysis, P.A.R.; investigation, M.L.; resources, G.P.G.; data curation, R.C.V.; writing—original draft preparation, I.S.C.; writing—review and editing, I.E.P.; visualization, D.A.C.; supervision, A.E.; project administration, V.T.G.; funding acquisition, I.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this paper was supported by the University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania, through the institutional program “Publish not Perish”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be available upon request to the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019 Nov;7(21):609–609. [CrossRef]
- Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021 Nov 19. [CrossRef]
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023 Feb 1;72(2):338–44. [CrossRef]
- Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers (Basel). 2021 May 1;13(9). [CrossRef]
- Bluegrass Coalition for Colorectal Screening; KMA Cancer Committee. Colorectal cancer facts. J Ky Med Assoc. 2005 Mar;103(3of):118.
- Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015 May 7;21(17):5167–75. [CrossRef]
- Schmuck R, Gerken M, Teegen EM, Krebs I, Klinkhammer-Schalke M, Aigner F, et al. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch Surg. 2020 Feb 1;405(1):71–80. [CrossRef]
- Keum NN, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019 Dec 1;16(12):713–32. [CrossRef]
- Amersi F, Agustin M, Ko CY. Colorectal cancer: epidemiology, risk factors, and health services. Clin Colon Rectal Surg. 2005 Aug;18(3):133–40. [CrossRef]
- Thélin C, Sikka S, Thélin C, Sikka S. Epidemiology of Colorectal Cancer — Incidence, Lifetime Risk Factors Statistics and Temporal Trends. Screening for Colorectal Cancer with Colonoscopy. 2015 Dec 2, Editor Rajunor Ettarh, IntechOpen.
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. [CrossRef]
- Goodarzi E, Beiranvand R, Naemi H, Momenabadi V, Khazaei Z. Worldwide Incidence and Mortality of Colorectal Cancer and Human Development Index (HDI): An Ecological Study. World Cancer Research Journal. 2019;6.
- Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018 Jul 15;124(14):2964–73. [CrossRef]
- Vuik FER, Nieuwenburg SAV, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10). [CrossRef]
- Simon, K. Colorectal cancer development and advances in screening. Clin Interv Aging [Internet]. 2016 Jul 19;11:967–76. [CrossRef]
- Moiel D, Thompson J. Early detection of colon cancer-the kaiser permanente northwest 30-year history: how do we measure success? Is it the test, the number of tests, the stage, or the percentage of screen-detected patients? Perm J. 2011 Dec.
- Moreno CC, Mittal PK, Sullivan PS, Rutherford R, Staley CA, Cardona K, et al. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin Colorectal Cancer. 2016 Mar 1;15(1):67–73. [CrossRef]
- Thompson MR, O’Leary DP, Flashman K, Asiimwe A, Ellis BG, Senapati A. Clinical assessment to determine the risk of bowel cancer using Symptoms, Age, Mass and Iron deficiency anaemia (SAMI). Br J Surg. 2017 Sep 1;104(10):1393–404. [CrossRef]
- Hreinsson JP, Jonasson JG, Bjornsson ES. Bleeding-related symptoms in colorectal cancer: a 4-year nationwide population-based study. Aliment Pharmacol Ther. 2014 Jan 1;39(1):77–84. [CrossRef]
- Yoo RN, Cho HM, Kye BH. Management of obstructive colon cancer: Current status, obstacles, and future directions. World J Gastrointest Oncol. 2021 Dec 12;13(12):1850. [CrossRef]
- Muldoon, RL. Bowel Obstructions: Malignant Large Bowel Obstruction. Clin Colon Rectal Surg. 2021 Jul 1;34(4):251. [CrossRef]
- Baer C, Menon R, Bastawrous S, Bastawrous A. Emergency Presentations of Colorectal Cancer. Surgical Clinics of NA. 2017;97:529–45. [CrossRef]
- Barnett, A.; Cedar, A.; Siddiqui, F.; Herzig, D.; Fowlkes, E.; Thomas, C.R. Colorectal Cancer Emergencies. J. Gastrointest. Cancer 2013, 44, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson H, Holm T, Ekholm A, Olsson LI. Emergency presentation of colon cancer is most frequent during summer. Colorectal Disease. 2011 Jun 1;13(6):663–8. [CrossRef]
- Elmessiry MM, Mohamed EA. Emergency curative resection of colorectal cancer, do it with caution. A comparative case series. Annals of Medicine and Surgery. 2020 Jul 1;55:70–6. [CrossRef]
- Pavlidis TE, Marakis G, Ballas K, Rafailidis S, Psarras K, Pissas D, et al. Does emergency surgery affect resectability of colorectal cancer? Acta Chir Belg. 2008;108(2):219–25. [CrossRef]
- Costa G, Tomassini F, Tierno S, Venturini L, Frezza B, Cancrini G, Mero A, Lepre L. Emergency colonic surgery: analysis of risk factors predicting morbidity and mortality. Chir Ital. 2009;61(5-6):565-71.
- Sjo OH, Larsen S, Lunde OC, Nesbakken A. Short term outcome after emergency and elective surgery for colon cancer. Colorectal Disease. 2009;11(7):733–9. [CrossRef]
- Bayar B, Yilmaz KB, Akinci M, Şahin A, Kulaçoʇlu H. An evaluation of treatment results of surgery in emergency versus elective colorectal cancer patients. Turk J Surg. 2016;32(1):011–7. [CrossRef]
- Alvarez JA, Baldonedo RF, Bear IG, Truán N, Pire G, Alvarez P. Presentation, treatment, and multivariate analysis of risk factors for obstructive and perforative colorectal carcinoma. The American Journal of Surgery. 2005 Sep 1;190(3):376–82. [CrossRef]
- Bass G, Fleming C, Conneely J, Martin Z, Mealy K. Emergency first presentation of colorectal cancer predicts significantly poorer outcomes: a review of 356 consecutive Irish patients. Dis Colon Rectum. 2009 Apr;52(4):678–84. [CrossRef]
- Biondo S, Kreisler E, Millan M, Marti-Rague J, Fraccalvieri, D, Golda T, De Oca J, Osorio A, Fradera R, Salazar R, Rodriguez-Moranta F, Sanjuan, X. Long-term results of emergency surgery for colon cancer compared with elective surgery. Cir Esp. 2007;82(2):89-98. [CrossRef]
- Bufill, JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990 Nov 15;113(10):779–88. [CrossRef]
- Gervaz P, Bucher P, Morel P. Two colons-two cancers: Paradigm shift and clinical implications. J Surg Oncol. 2004 Dec 15;88(4):261–6. [CrossRef]
- Iacopetta, B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–8. [CrossRef]
- Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017 Oct 1;84:69–80. [CrossRef]
- Topdaği Ö, Timuroglu A. Eighteen Years’ Retrospective Review of Colorectal Cancer Cases in Eastern Population. Eurasian J Med. 2018 Feb 1;50(1):19. [CrossRef]
- Dubé C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. The use of aspirin for primary prevention of colorectal cancer: A systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007 Mar 6;146(5):365–75. [CrossRef]
- Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6(FEB). [CrossRef]
- Kohoutova D, Smajs D, Moravkova P, Cyrany J, Moravkova M, Forstlova M, et al. Escherichia coli strains of phylogenetic group B2 and D and bacteriocin production are associated with advanced colorectal neoplasia. BMC Infect Dis. 2014 Dec 24;14(1). [CrossRef]
- Zhang Y, Hoffmeister M, Weck M, Chang-Claude J, Brenner H. Helicobacter pylori Infection and Colorectal Cancer Risk: Evidence From a Large Population-based Case-Control Study in Germany. Am J Epidemiol. 2012;175(5):441-50. [CrossRef]
- Flemer B, Lynch DB, Brown JMR, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633-643. [CrossRef]
- Nitsche U, Stögbauer F, Späth C, Haller B, Wilhelm D, Friess H, et al. Right sided colon cancer as a distinct histopathological subtype with reduced prognosis. Dig Surg. 2016;33(2):157-63. [CrossRef]
- Sinicrope F, Mahoney M, Yoon H, Smyrk T, Thibodeau S, Goldberg R, Nelson G, Sargent D, Alberts S, Alliance for Clinical Trials in Oncology. Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147 (Alliance). Clin Cancer Res; 2015;21(23):5294-304. [CrossRef]
- Mouradov D, Sloggett C, Jorissen R, Love C, Li S, Burgess A, Arango D, Strausberg R, Buchanan D, Wormald S, O’Connor L, Wilding J, Bicknell D, Tomlinson I, Bodmer W, Mariadson J, Sieber O. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014;74(12):3238-47. [CrossRef]
- Sugai T, Habano W, Nakamura SI, Sato H, Uesugi N, Takahashi H, et al. Genetic alterations in DNA diploid, aneuploid and multiploid colorectal carcinomas identified by the crypt isolation technique. Int J Cancer; 2000;88(4):614-9. [CrossRef]
- Missiaglia E, Jacobs B, D’ario G, Di Narzo A, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Pan Y, Roth A, Klingbiel D, Bosman F, Delorenzi M, Tejpar S. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995-2001. [CrossRef]
- Shen H, Yang J, Huang Q, Jiang M, Tan Y, Fu J, Zhu L, Fang X, Yuan Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21(21):6470-6478. [CrossRef]
- Alius C, Cirstoveanu CG, Badiu CD, Ardeleanu V, Dumitru VA. Immunohistochemical pattern– a prognostic factor for synchronous gastrointestinal cancer. Journal of Mind and Medical Sciences. 2020 Sep 29;7(2):250–6. [CrossRef]
- Hemminki K, Santi I, Weires M, Thomsen H, Sundquist J, Bermejo JL. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BMC Cancer. 2010 Dec 21;10. [CrossRef]
- Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus M, Antoniotti C, Langer C, Scherer S, Muller T, Hurwitz H, Saltz L, Falcone A, Lenz H. J Natl Cancer Inst. 2015;107(3):dju247.
- Weiss J, Pfau P, O’Connor E, King J, LoConte N, Kennedy G, Smith M. Mortality by stage for right-versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results – Medicare data. J Clin Oncol. 2011;29(33):4401-9. [CrossRef]
- Morris VK, Kennedy EB, Baxter NN, Benson AB, Cercek A, Cho M, et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023 Jan 20;41(3):678–700. [CrossRef]
- Dohrn N, Klein MF. Colorectal cancer: current management and future perspectives. British Journal of Surgery. 2023 Sep 6;110(10):1256–9. [CrossRef]
- You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020 Sep 1;63(9):1191–222. [CrossRef]
- Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022 Feb 1;65(2):148–77. [CrossRef]
- Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11(4):354–64. [CrossRef]
- Heald R, Ryall, R. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986 Jun 28;1(8496):1479-82. [CrossRef]
- Liscu H, Miron A, Rusea A, Oprea A, Mitre R, Herdea A, et al. Short-Course Radiotherapy versus Long-Course Radio-Chemotherapy as Neoadjuvant Treatment for Locally Advanced Rectal Cancer: Meta-Analysis from a Toxicity Perspective. Maedica (Bucur). 2021 Sep 15;16(3):382. [CrossRef]
- Alexandrescu ST, Dumitru AV, Babiuc RD, Costea RV. Assessment of clinical and pathological complete response after neoadjuvant chemoradiotherapy in rectal adenocarcinoma and its therapeutic implications. Romanian Journal of Morphology and Embryology. 2021 Apr 1;62(2):411–25. [CrossRef]
- Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023 Mar 10;41(8):1541–52. [CrossRef]
- Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2022 Mar 10;40(8):892–910. [CrossRef]
- Liscu HD, Liscu BR, Mitre R, Anghel IV, Antone-Iordache IL, Balan A, et al. The Conditioning of Adjuvant Chemotherapy for Stage II and III Rectal Cancer Determined by Postoperative Pathological Characteristics in Romania. Medicina 2023, Vol 59, Page 1224. 2023 Jun 29;59(7):1224. [CrossRef]
- Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020 Apr 1;26(4):566–76. [CrossRef]
- Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022 Jun 23;386(25):2363–76. [CrossRef]
- Jin Z, Sinicrope FA. Mismatch Repair-Deficient Colorectal Cancer: Building on Checkpoint Blockade. J Clin Oncol. 2022;40(24). [CrossRef]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. [CrossRef]
- Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol. 2021 May 1;19(5):955-966.e61. [CrossRef]
Figure 1.
Age distribution in the study group.
Figure 1.
Age distribution in the study group.
Figure 2.
Hospitalization days distribution in the study group.
Figure 2.
Hospitalization days distribution in the study group.
Figure 3.
Distribution of clinical manifestations of the study group on admittance (%).
Figure 3.
Distribution of clinical manifestations of the study group on admittance (%).
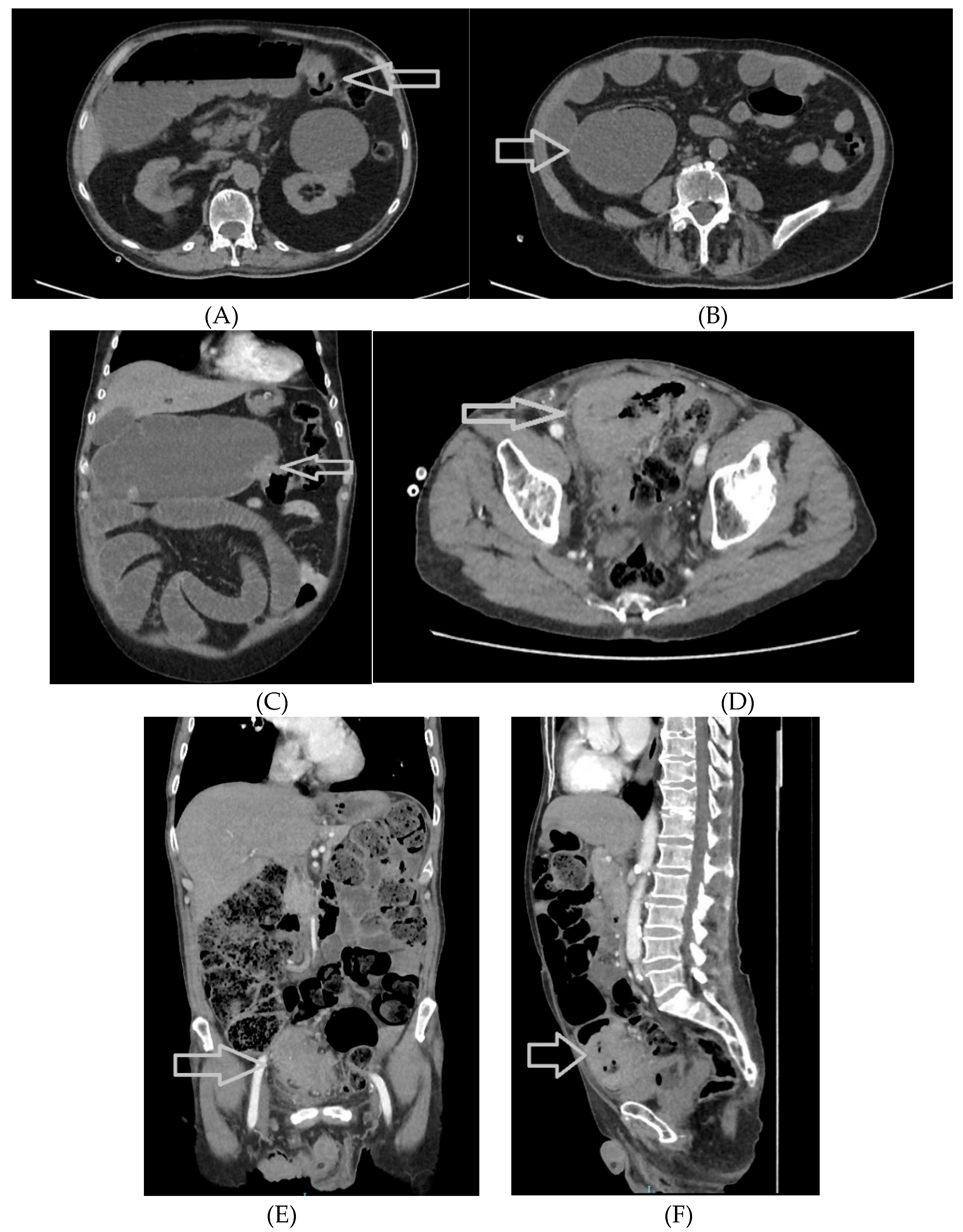
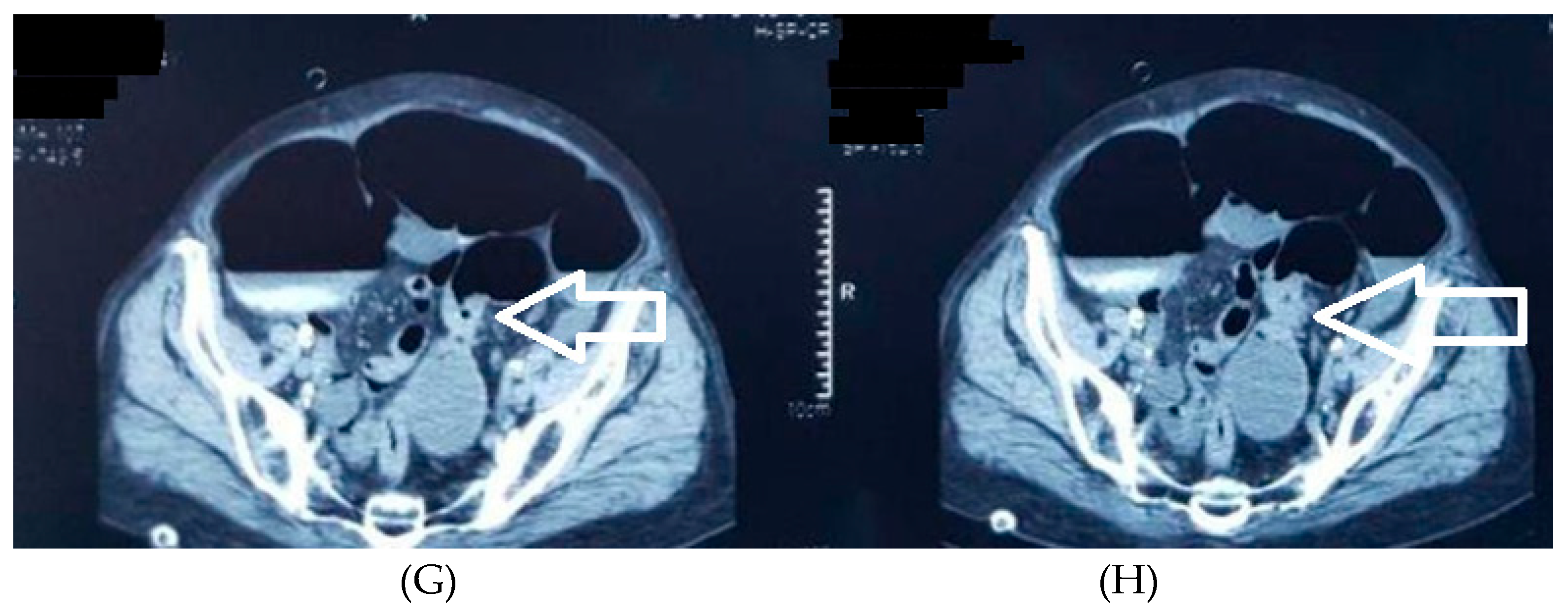
Figure 4.
A-4H. Abdominal and pelvic CT scans of colorectal tumors. A 77-year-old male patient with a stenotic tumor on the transverse colon and a distended proximal colon, with air-fluid level, axial section (
Figure 4A, tumor highlighted with a white arrow) and coronal section (4C, white arrow)); in addition, a distended cecum with a high risk of perforation (4B, white arrow). Another 77-year-old male patient with a sigmoid tumor with invasion in the urinary bladder, highlighted with a white arrow – axial (4D), coronal (4E), and sagittal (4F) section.
Figure 4G and
Figure 4H reveal a stenotic tumor of the colorectal junction (white arrow), with upper distension of the digestive tract .
Figure 4.
A-4H. Abdominal and pelvic CT scans of colorectal tumors. A 77-year-old male patient with a stenotic tumor on the transverse colon and a distended proximal colon, with air-fluid level, axial section (
Figure 4A, tumor highlighted with a white arrow) and coronal section (4C, white arrow)); in addition, a distended cecum with a high risk of perforation (4B, white arrow). Another 77-year-old male patient with a sigmoid tumor with invasion in the urinary bladder, highlighted with a white arrow – axial (4D), coronal (4E), and sagittal (4F) section.
Figure 4G and
Figure 4H reveal a stenotic tumor of the colorectal junction (white arrow), with upper distension of the digestive tract .
Figure 5.
A-5B. Distribution of WBC count in patients with an elective surgical procedure (5A) and patients with an emergency surgical procedure (5B).
Figure 5.
A-5B. Distribution of WBC count in patients with an elective surgical procedure (5A) and patients with an emergency surgical procedure (5B).
Figure 6.
Scatter plot of WBC count on age with regression line and 95% mean confidence interval.
Figure 6.
Scatter plot of WBC count on age with regression line and 95% mean confidence interval.
Figure 7.
A-7B. Distribution of Hb serum levels in patients with an elective surgical procedure (7A) and patients with an emergency surgical procedure (7B).
Figure 7.
A-7B. Distribution of Hb serum levels in patients with an elective surgical procedure (7A) and patients with an emergency surgical procedure (7B).
Figure 8.
ROC curve used to determine the possibility of WBC count and Hb values as predictors for the necessity of an emergency surgical procedure.
Figure 8.
ROC curve used to determine the possibility of WBC count and Hb values as predictors for the necessity of an emergency surgical procedure.
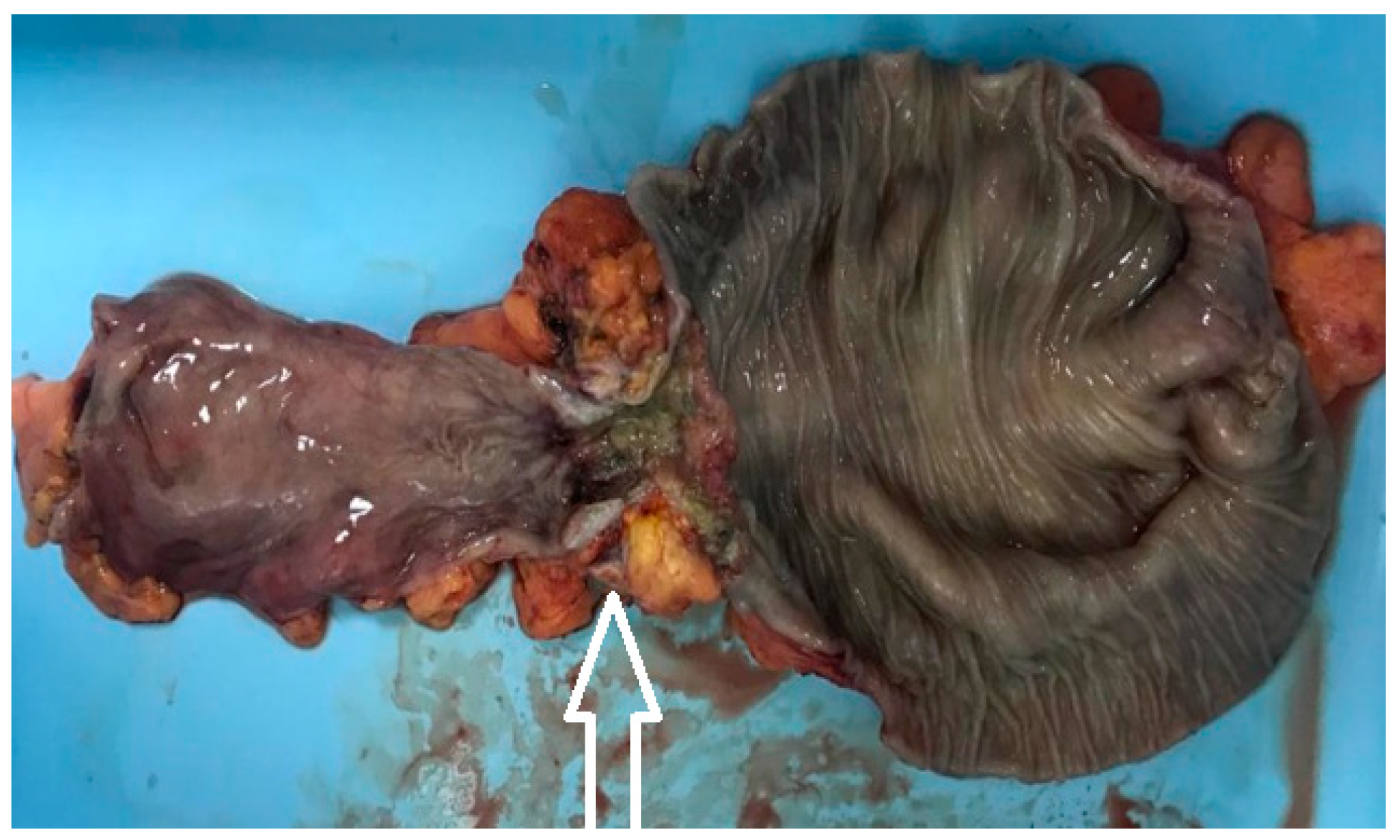
Figure 9.
The postoperative aspect of the resected specimen – stenotic tumor of the left colon – pathology result of tubular adenocarcinoma (tumor highlighted with white arrow).
Figure 9.
The postoperative aspect of the resected specimen – stenotic tumor of the left colon – pathology result of tubular adenocarcinoma (tumor highlighted with white arrow).
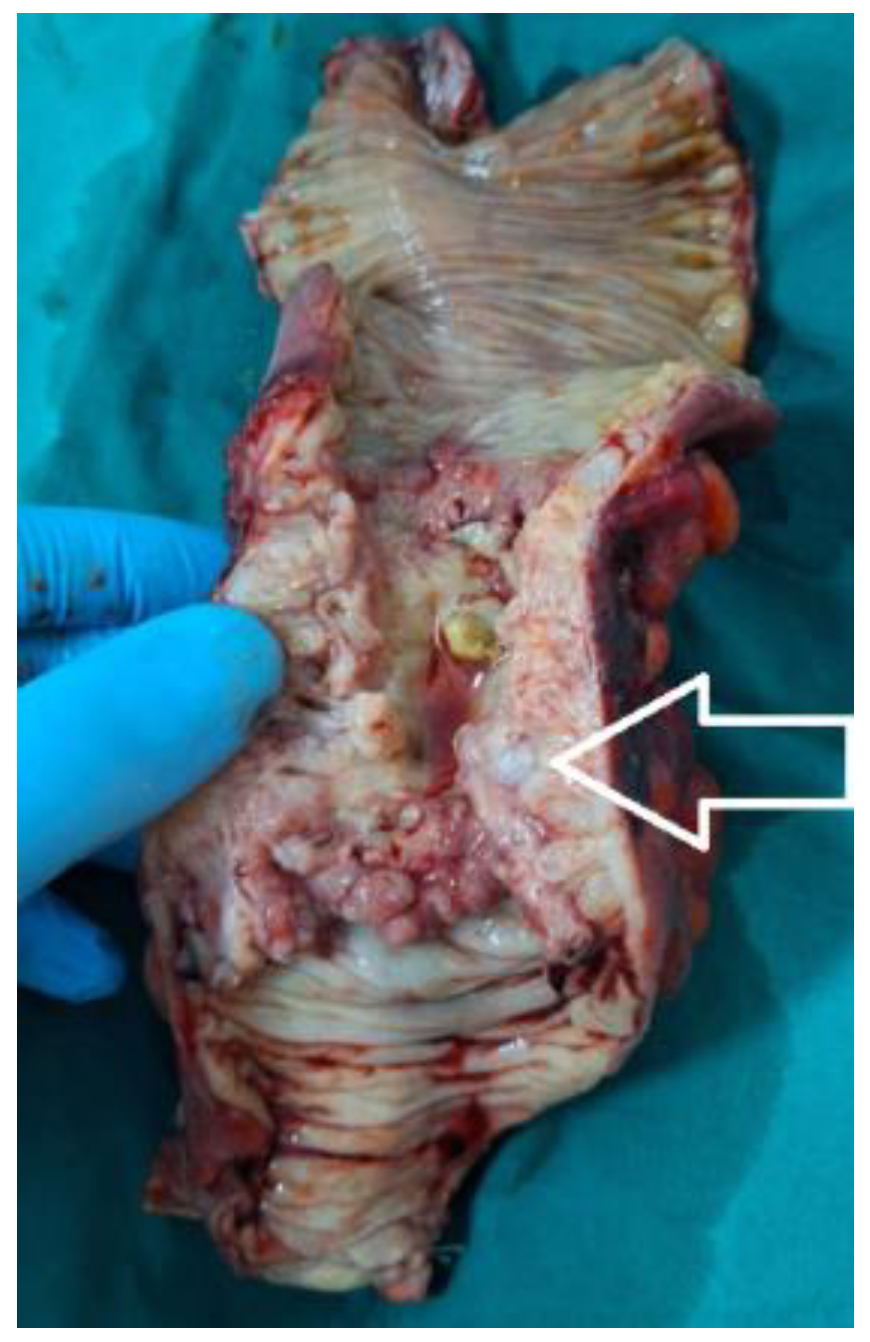
Figure 10.
The postoperative aspect of a tubular adenocarcinoma of colorectal junction (white arrow), with peritumoral abscess, perforation and secondary generalized peritonitis.
Figure 10.
The postoperative aspect of a tubular adenocarcinoma of colorectal junction (white arrow), with peritumoral abscess, perforation and secondary generalized peritonitis.
Figure 11.
A-11C. Distribution of age with various topographies of the tumor – right colon (11A), left colon (11B) and rectum (11C).
Figure 11.
A-11C. Distribution of age with various topographies of the tumor – right colon (11A), left colon (11B) and rectum (11C).
Table 1.
Correlation between type of presentation and several general parameters of the study group.
Table 1.
Correlation between type of presentation and several general parameters of the study group.
| Type of Variables Analyzed |
Type of Statistical Test |
p-Value |
| Emergency vs Age |
Two-sample t-test |
0.733 |
| Emergency vs Hospitalization days |
Mann Whitney U test |
0.823 |
| Emergency vs Gender |
Chi-square test |
0.414 |
| Emergency vs Urban/Rural |
Chi-square test |
0.497 |
Table 2.
Distribution of clinical manifestations of the study group on admittance (%).
Table 2.
Distribution of clinical manifestations of the study group on admittance (%).
| |
Preoperative CT scan |
|
| No |
Yes |
Total |
| Emergency procedure |
No |
43 |
41 |
84 |
| Yes |
25 |
44 |
69 |
| |
Total |
68 |
85 |
153 |
Table 3.
Distribution of patients with colorectal cancer regarding their topography, their type of surgery and the statistical association.
Table 3.
Distribution of patients with colorectal cancer regarding their topography, their type of surgery and the statistical association.
| Topography |
Number of patients |
Number of patients by type of surgery (Em=Emergency; El=Elective) |
P-value |
| Ileocecal valve |
13 |
Em=7; El=6 |
0.568 |
| Cecum |
3 |
El=1; Em=2 |
1.000 |
| Ascending colon |
8 |
Em=1; El=7 |
0.074 |
| Hepatic flexure |
6 |
Em=1; El=5 |
0.223 |
| Transverse colon |
9 |
Em=5; El=4 |
0.732 |
| Splenic flexure |
8 |
Em=6; El=2 |
0.141 |
| Descending colon |
9 |
Em=4; El=5 |
1.000 |
| Sigmoid colon |
30 |
Em=21; El=9 |
0.004 |
| Rectosigmoidian junction |
24 |
Em=10; El=14 |
0.824 |
| Superior rectum |
17 |
Em=7; El=10 |
0.800 |
| Medium rectum |
9 |
Em=2; El=7 |
0.186 |
| Inferior rectum and anus |
17 |
Em=3; El=14 |
0.072 |
Table 4.
2x3 Chi-square table analyzing the relation between the localization of the tumor and the type of surgery performed.
Table 4.
2x3 Chi-square table analyzing the relation between the localization of the tumor and the type of surgery performed.
| |
Type surgery |
|
p-value |
| Elective |
Emergency |
Total |
Topographical segment
|
Right colon
% within right colon
|
24
61.5% |
15
38.5% |
39
100.0% |
0.002 |
Left colon
% within left colon
|
16
34.0% |
31
66.0% |
47
100% |
Rectum
% within rectum
|
44
65.7% |
23
34.3% |
67
100% |
| |
Total
% within total
|
84
54.9% |
69
45.1% |
153
100% |
Table 5.
Distribution of surgical procedures in the study group and the statistical association with the type of emergency regimen and the result of surgical procedure.
Table 5.
Distribution of surgical procedures in the study group and the statistical association with the type of emergency regimen and the result of surgical procedure.
| |
Type of surgery |
Result of surgical procedure |
| Elective |
Emergency |
Total |
p-value |
Ostomy |
Anastomosis |
Neither |
Total |
p-value |
| Type of surgical procedure |
Right hemicolectomy
% within
|
22
66.7% |
11
33.3% |
33
100% |
0.167 |
8
24.2% |
25
75.8% |
0 |
33
100% |
0.002 |
Left hemicolectomy
% within
|
11
45.8% |
13
54.2% |
24
100% |
0.367 |
10
41.7% |
14
58.3% |
0 |
24
100% |
0.253 |
Segmentary resection of transverse colon
% within
|
3
23.1% |
10
76.9% |
13
100% |
0.020 |
5
38.5% |
8
61.5% |
0 |
13
100% |
0.440 |
Segmentary resection of sigmoid colon
% within
|
7
33.3% |
14
66.7% |
21
100% |
0.037 |
14
66.7% |
7
33.3% |
0 |
21
100% |
0.044 |
Rectosigmoid resection
% within
|
21
65.6% |
11
34.4% |
32
100 |
0.231 |
11
34.4% |
21
65.6% |
0 |
32
100% |
0.046 |
Upward ostomy on sigmoid colon
% within
|
7
58.3% |
5
41.7% |
12
100% |
1.000 |
7
100% |
0 |
0 |
7
100% |
- |
Abdominoperineal resection
% within
|
6
75% |
2
25% |
8
100% |
0.295 |
8
100% |
0 |
0 |
8
100% |
- |
Tumoral biopsy
% within
|
17
70.8% |
7
29.2% |
24
100% |
0.118 |
12
50% |
0 |
12
50% |
24
100% |
- |
Table 6.
General characteristics of deceased patients.
Table 6.
General characteristics of deceased patients.
| Number |
Age |
Gender (M=male, F=female) |
Hospitalization days |
Emergency (Em)/Elective (El) |
Topography of the tumor |
Surgical procedure |
| 1. |
74 |
M |
13 |
Em |
Sigmoid colon |
Colectomy with ostomy |
| 2. |
67 |
M |
4 |
Em |
Descending colon |
Left hemicolectomy with ostomy |
| 3. |
84 |
M |
39 |
Em |
Transverse colon |
Colectomy with ostomy |
| 4. |
63 |
M |
12 |
Em |
Splenic flexure |
Colectomy with ostomy |
| 5. |
78 |
F |
21 |
Em |
Colorectal junction |
Left rectohemicolectomy with ostomy |
| 6. |
55 |
F |
10 |
El |
Colorectal junction |
Rectosigmoid resection with ostomy |
| 7. |
78 |
M |
3 |
Em |
Transverse colon |
Extended right hemicolectomy with ileostomy |
| 8. |
59 |
M |
6 |
Em |
Sigmoid colon |
Colectomy with ostomy |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).